Abstract
Loud low-frequency sounds can induce temporary oscillatory changes in cochlear sensitivity, which have been termed the ‘bounce’ phenomenon. The origin of these sensitivity changes has been attributed to slow fluctuations in cochlear homeostasis, causing changes in the operating points of the outer hair cell mechano-electrical and electro-mechanical transducers. Here, we acquired three objective and subjective measures resulting in a comprehensive dataset of the bounce phenomenon in each of 22 normal-hearing human subjects. We analysed the level and phase of cubic and quadratic distortion product otoacoustic emissions and the auditory thresholds before and after presentation of a low-frequency stimulus (30 Hz sine wave, 120 dB SPL, 90 s) as a function of time. In addition, the perceived loudness of temporary, tinnitus-like sensations occurring in all subjects after cessation of the low-frequency stimulus was tracked over time. The majority of the subjects (70 %) showed a significant, biphasic change of quadratic, but not cubic, distortion product otoacoustic emissions of about 3–4 dB. Eighty-six percent of the tested subjects showed significant alterations of hearing thresholds after low-frequency stimulation. Four different types of threshold changes were observed, namely monophasic desensitisations (the majority of cases), monophasic sensitisations, biphasic alterations with initial sensitisation and biphasic alterations with initial desensitisation. The similar duration of the three bounce phenomenon measures indicates a common origin. The current findings are consistent with the hypothesis that slow oscillations of homeostatic control mechanisms and associated operating point shifts within the cochlea are the source of the bounce phenomenon.
Keywords: cochlea, bounce phenomenon, endolymphatic hydrops, Ménière’s disease, tinnitus
INTRODUCTION
The term ‘bounce’ phenomenon (BP) was originally coined by Hirsh and Ward (1952) for a temporary deterioration of hearing thresholds in humans occurring after presentation of a loud, long-duration, low-frequency (LF) sound. Hirsh and Ward (1952) also mentioned the perception of tinnitus by the subjects during the deterioration of the hearing threshold. Later, Hughes (1954) reported a sensitisation of absolute thresholds in humans occurring at about 1 min after the offset of a LF sound. Zwicker and Hesse (1984) measured alterations of hearing thresholds during and after presentation of LF sound and suggested that the recorded threshold fluctuations were due to LF sound-induced oscillations of cochlear, not central nervous system origin.
The discovery of otoacoustic emissions (Kemp 1978) enabled objective measures of the BP: Kemp (1986) and Kemp and Brill (2009) reported a slow oscillatory change of the level of transient-evoked otoacoustic emission after loud LF sound stimulation in humans, which followed a time course similar to changes of auditory thresholds after LF sound presentation. They also reported temporary changes of spontaneous otoacoustic emissions after loud LF stimulation and mentioned that most subjects also reported a ‘roaring’ tinnitus during the BP period. Patuzzi and Wareing (2002) analysed tinnitus levels in humans during BP periods and found their time course to be slightly different from BP periods of hearing threshold changes.
Kirk and Patuzzi (1997) and Kirk et al. (1997) carried out a detailed analysis of the effect of the BP on cochlear potentials and otoacoustic emissions in guinea pigs, supporting the earlier results of Kemp (1986). They found temporary sensitisations of compound action potentials, an increase in the levels of even-order (e.g. quadratic, f2 − f1), but not odd-order (e.g. cubic, 2f1 − f2) distortion product otoacoustic emissions (DPOAEs), a reduction of cochlear microphonic amplitudes and an endolymphatic potential increase with a time course similar to the changes found in human studies. Kirk and Patuzzi (1997) and Kirk et al. (1997) also showed that the symptoms of the BP in their preparations are cochleogenic, not neural, in origin.
We aimed to provide a comprehensive dataset on both objective and subjective measures of the BP in the same human subjects, based on the hypothesis that they reflect a LF sound-induced, temporary deviation from cochlear homeostasis (Patuzzi 2011). The latter may be associated with changes of ion concentrations in cochlear fluids and has been suggested as an explanation for the development of endolymphatic hydrops, a pathological volume increase of the potassium-rich endolymph in the scala media of the cochlea of hitherto unknown genesis (e.g. Salt and Plontke 2010, for review). Endolymphatic hydrops is the primary histopathological correlate of Ménière’s disease, a condition affecting the inner ear and causing hearing loss, recurrent, spontaneous episodic vertigo, aural fullness and tinnitus (Monsell et al. 1995). It is, however, unclear if endolymphatic hydrops represents cause or effect of Ménière’s disease (Merchant et al. 2005; Berlinger 2011).
In this paper, we show that LF sound can temporarily induce changes in humans consistent with a transient imbalance of cochlear homeostasis. A complete description of the BP in humans is therefore relevant to our understanding of control systems (and pathologies thereof) enabling the cochlea to maintain its function during periods of mechanical or ionic disturbances.
METHODS
Subjects
Twenty-two (11 males, 11 females) normal-hearing subjects (mean age, 22; range, 21–27) participated in this study. All subjects were screened for eligibility before enrolment in the study. No histories of chronic middle or inner ear diseases were reported. Hearing thresholds for screening purposes were assessed using the ‘Békésy tracking’ procedure for test tones between 0.25 and 8 kHz, and thresholds were considered normal if the hearing loss was not worse than 10 dB HL. In addition, subjects were only included if tympanometric assessment gave normal results. Tympanometric results were considered normal when the peak admittance was found to be between −150 and +100 daPa with peak levels between 0.2 and 2.5 millimhos. All screened subjects fulfilled these criteria and were eligible for the study.
Only one ear of the subjects was tested, which was chosen randomly. If quadratic DPOAEs (QDP) levels were too small to allow for a minimum signal-to-noise ratio of 6 dB in one ear, recordings were repeated in the other ear. In this study, 11 left and 11 right ears were examined. Subjects were seated in a comfortable recliner during all experimental procedures and were advised to remain still and quiet during measurements. All experiments were carried out in a double-walled, sound-attenuated booth.
Subjects completed the three different experiments usually in three separate sessions of about 60–90 min within 1 week. Only a single session was permitted on a given day. The order of the experiments was arbitrary.
The ethics committee of the University Hospital of the Ludwig-Maximilians University Munich, Germany, in agreement with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, approved the procedures, and all subjects gave their informed consent. This included a statement that we cannot exclude potential short- and long-term harm to the inner ear caused by the sound levels involved. We also stated that we considered the risk not greater than the one caused by the sound levels one is routinely exposed to in daily life (e.g. with personal sound systems).
The A-weighted sound level of the LF stimulus was about 80 dB. Accumulated daily LF sound exposure was monitored and controlled to be well within the daily limit for normal working environments in Germany.
Signal generation and data acquisition
An ER-10C DPOAE probe system (Etymotic Research Inc., Elk Grove Village, IL, USA) was used for recording and delivery of all sounds, with the exception of the LF tone (30 Hz sine wave, 120 dB sound pressure level (SPL), 90 s, including 0.1 s raised-cosine ramps) that was supplied by a separate loudspeaker (NSW1-205-8A, Aura Sound Inc., Santa Fe Springs, CA, USA). This loudspeaker was connected to a 50-cm-long polyethylene tube (inner diameter 1 mm); the tip of which was fed through the foam ear tip of the ER-10C DPOAE probe and was driven by an RB-960BX power amplifier (Rotel, Worthing, UK).
Signal generation and data acquisition was carried out with a RME Fireface UC 24-bit external sound card (RME, Audio AG, Haimhausen, Germany), operated with a sampling rate of 44.1 kHz. The recorded signal was amplified 30 dB by the preamplifier of the external soundcard. Scripts written in MatLab 7.5 (MathWorks, Natick, MA, USA) and run on an ASUS G60 VX laptop (ASUSTeK Computer Inc., Taipei, Taiwan) controlled the external sound card. The SoundMexPro sound application (HörTech, Oldenburg, Germany) was employed to use low-latency multi-channel ASIO interfacing in the MatLab environment.
Calibration was carried out in situ (i.e. the transducers were calibrated for constant sound pressure at the probe microphone membrane, not at the tympanic membrane), with the known drawback of sound pressure level deviations between the tympanic membrane and the probe microphone due to quarter wavelength standing waves at frequencies higher than about 3 kHz (e.g. Siegel and Hirohata 1994; Drexl et al. 2012). The calibration of the primary tones for DPOAE recordings and the probe tones for threshold detection was carried out by stimulating the ear canal with a chirp (3 s, 0.5 to 9 kHz). The frequency response of the loudspeakers was recorded in situ with the microphone of the ER-10C probe. An impulse response was calculated by cross-correlating the input and output of the sound system. The result was compared with a synthesised impulse response representing a uniform frequency response, and, by comparing the two, a compensation impulse response was generated. Convolving the actual stimuli with this compensation impulse response resulted in a uniform frequency response. The success of this procedure was immediately tested before the start of each trial by convolving the initial chirp (which was used for calibration), with the compensation impulse response and replaying the result of this procedure, which gave a flat spectrum for the calibrated frequency range. If this failed, the probe was checked for clogging, cleaned if needed, repositioned and the calibration procedure was repeated. No system distortion above the noise floor at QDP and cubic distortion product otoacoustic emission (CDP) frequencies could be detected for the frequencies and levels of the primary tones used in this study as measured in an artificial ear (B&K 4157, Brüel & Kjær Sound & Vibration Measurement A/S, Denmark).
Calibration procedures were carried out before each trial. In addition to this, a probe-fit-check procedure preceded and concluded each trial by presenting a band-stop noise consisting of a low- and a high-frequency band and analysing the ear response using a Fourier transform analysis. If the probe-fit-check procedure at the end of a trial indicated that the probe position had changed, the trial was rejected and repeated.
For calibration of the LF tone, the amplitude response of the probe microphone was compared to the amplitude response of the measuring microphone of an artificial ear (B&K 4157, Brüel & Kjær Sound & Vibration Measurement A/S, Denmark) and was corrected accordingly. The level of the first harmonic of the LF tone was at least 50 dB lower than the LF tone level. The level of the LF stimulus was monitored continuously during presentation.
All analysis, statistics and visualisation were carried out with scripts written in MatLab 7.5 (MathWorks, Natick, MA, USA).
Recording of DPOAE level and phase after LF sound stimulation
QDP (f2 − f1) and CDP (2f1 − f2) levels and phases were extracted from the same recording and followed over time before and after LF stimulation. QDPs and CDPs were evoked with two pure-tone primaries, f1 and f2. f2 was set to 4 or 5 kHz, depending on which f2 frequency gave larger QDP levels. The primary sound levels, l1 and l2, were both set to 65 dB SPL. The f2/f1 frequency ratio was optimised for each subject to yield maximum QDP levels and was within 1.14 and 1.54. The duration of the primary tones was set to 250 ms including 10 ms raised-cosine ramps. DPOAE recordings were synchronised to the stimulus presentation. Stimulus presentation and recording were repeated at least 16 times and up to 32 times (with an inter-stimulus interval of 0.5 s) to achieve a fixed number of 16 valid recordings, depending on how many recordings were rejected due to noise (see Drexl et al. 2012, for details on noise rejection procedures). This resulted in a variable duration of typically about 10–15 s for each DPOAE measurement, depending on the number of rejected recordings. DPOAE measurements were derived by averaging the recordings in the time domain to reduce random noise. DPOAE measurements consisting of less than 16 valid recordings, or showing DPOAE levels less than 6 dB above noise floor after averaging, were not included in the analysis. The averaged time domain signals were high-pass filtered with a digital filter (tenth order, corner frequency 500 Hz, 60 dB/octave). A subsequent Fourier transform analysis, where the Fourier transform size was equal to the length of the recorded signal, transformed the signal from the time domain into the frequency domain. The spectral magnitudes and phase of the CDP and QDP were then extracted from the positive frequency half of the spectrum. The mean noise floor was estimated by averaging the magnitudes of three spectral lines above and three below the spectral line of the CDP or QDP.
A trial consisted of 15 DPOAE measurements before the start of the LF sound and 30 DPOAE measurements after the end of the LF sound. Thus, a trial took about 9 to 12 min including 90 s LF sound exposure. A trial with LF sound stimulation was alternated with a trial without LF sound exposure, but including a period of silence the same duration as the LF sound presentation. For each subject, four trials were obtained in a single session, two with LF sound stimulation and two without. Only data of subjects where the two trials with LF stimulation were qualitatively similar (i.e. both trials with LF sound exposure showed the BP and showed the same qualitative effect, e.g. enhancement followed by suppression) were included in the quantitative analysis and counted as ‘bouncers’. For quantitative analysis, a cubic spline interpolation was applied on the data to achieve a temporal resolution of 5 s. A two-sided t test, applied on sliding analysis windows (window length = 9 interpolated DPOAE values), was carried out to find DPOAE measurements in the post-exposure period where level and phase were independently statistically different (p ≤ 0.01) from the period before the presentation of LF sound (the pre-exposure period). All quantitative analyses were based on those DPOAE values. Level and phase alterations are expressed relative to the mean of the pre-exposure period. Changes were considered to be LF sound induced when they started within 10 s after LF stimulus offset (as cochlear changes induced by the BP are known to build up right after LF stimulus offset or even during LF stimulation) and lasted for at least 75 s. These criteria were introduced to avoid detection of random changes by the analysis algorithm not caused by the BP. The minimum duration requirement of 75 s is significantly longer than the spontaneous changes observed in the control recordings without preceding LF sound stimulation. Moreover, because these changes were not related to the BP, they were uncorrelated across both control trials. Short episodes of non-significant DPOAE measurements (less than three interpolated DPOAE values) can occur during the ‘zero-crossing’ of the oscillation of DPOAE levels or phase. These were ignored for purposes of the calculation of the BP duration.
Recording of tinnitus-like sensations after LF sound stimulation
Subjects received standardised, written instructions before the experiments and were asked to track the loudness of a noise sensation perceived after LF sound stimulation over a period of 240 s. The subjects were not informed about the nature and origin of the sound they might hear. They were left under the impression that they listened to randomly occurring noise being played to them. In a training session before the actual experiment, subjects learned by visual feedback to correlate physically presented pink noise stimuli of different sound pressure levels with a defined pressure they needed to exert on a Peleus ball connected via airtight tubing to a custom-built pressure sensor. The pressure sensor produced a DC voltage that was proportional to the pressure applied by the subjects and therefore considered a relative measure of the perceived loudness. DC voltages were sampled with a data acquisition card (Measurement Computing, Norton, MA, USA) at a sampling rate of 5 Hz.
After the training, the subjects continued to receive real-time visual feedback during the actual recording about the pressure they exerted. The purpose of this feedback was to ensure that a tinnitus sound with a constant perceived loudness was tracked as such. As for the DPOAE measures, four trials were obtained in a single session, two with LF stimulation and two without. Only data in the second set of trials, where subjects were familiar with the procedure and were able to track periods of tinnitus reliably, were further analysed. For analysis, adjusted pressures of the trial without LF stimulation were subtracted from the trial with LF stimulation to exclude pre-existing tinnitus-like phenomena not related to the BP. To describe the time course of the tinnitus-like sensation, the starting point was considered to be the first positive pressure difference that was maintained for at least 10 s. The end point was set when the average pressure difference across a 10-s interval following the starting point was not positive any more. For the purpose of this paper, we refer to the noise-like sensation subjects perceived after LF sound exposure as ‘tinnitus’ and follow therefore the nomenclature introduced by Patuzzi (2002) who classified these events as ‘non-oscillatory, subjective, cochlear tinnitus’.
Recording of hearing thresholds after LF sound stimulation
The subjects were asked to track their hearing thresholds before and after the presentation of LF sound using the Békésy tracking procedure. Subjects received standardised, written instructions before the start of the experiment.
Pulsed test tones with a frequency of 1, 2 or 4 kHz were presented with a duration of 300 ms (including 10 ms raised-cosine ramps) and a repetition rate of 2 Hz, resulting in a temporal resolution of 500 ms. After starting the tracking procedure, test tone levels increased at a rate of 1 dB/s unless the subjects indicated by pressing a hand switch that the threshold for detecting the test tone had been reached. Then, the test tones decreased at the same rate as they increased before. Using this procedure, patients tracked their thresholds for each of the three test tone frequencies for 90 s before and for 240 s after the presentation of LF sound. Thresholds were not tracked during LF sound exposure.
As with the other measures, a trial with LF sound stimulation was alternated with a trial without LF sound exposure and this sequence was repeated once. Only the second trial was used in the analysis, however, under the condition that threshold changes of both sets were qualitatively similar (i.e. both trials with LF sound exposure showed the BP and showed the same qualitative effect, e.g. enhancement followed by suppression), if not, the subject was counted as not bouncing. A two-sided t test applied on a sliding analysis window (window length = 4.5 s) was carried out to find data points in the post-exposure period statistically different (p ≤ 0.01) from the period before the presentation of the LF sound. All quantitative analyses were based on those data points. Consistent with the DPOAE measurements (see ‘Recording of Tinnitus-Like Sensations After LF Sound Stimulation’), statistically significant threshold alterations were considered to be LF sound induced when they started within 10 s after cessation of LF sound stimulation and when they lasted for at least 75 s. For consistency, the minimum duration requirements are the same as for the DPOAE measures (see ‘Recording of Tinnitus-Like Sensations After LF Sound Stimulation’). Within these temporal boundaries, short segments of non-significant threshold changes (with durations less than 1.5 s) occurring during the ‘zero-crossings’ of the oscillation of hearing thresholds were ignored for purposes of the calculation of the bounce duration.
All quantitative analyses were based only on data segments showing statistically significant alterations according to these criteria. In addition, subjects were not counted as ‘bouncing’ if the data from the control trials indicated strong fluctuations of the hearing threshold.
All classifications and measurements were made automatically by MatLab scripts based on these criteria.
Qualitative modelling
The behaviour of QDP and CDP levels during the BP was simulated with a model based on a single saturating non-linearity, the Boltzmann function
 |
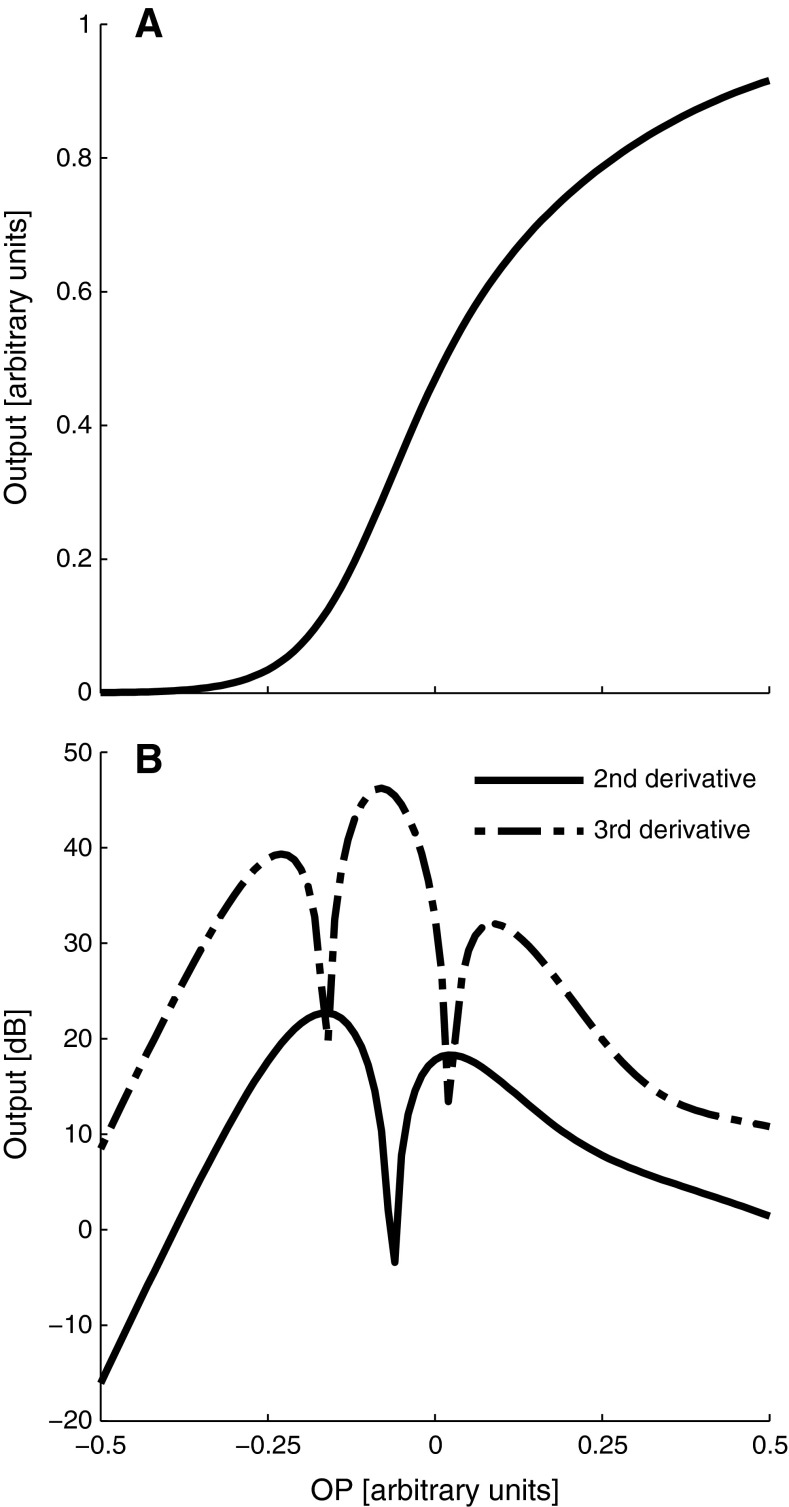
with a1 = 3a2 = 12.8, x1 = x2 = −0.06 (e.g. Frank and Kossl 1995, 1996; Abel et al. 2009). The behaviour of QDP and CDP levels as a function of the operating point (OP) of the mechano-electrical transducer (MET) transfer functions was simulated by the absolute value of the second and third derivative (e.g. Lukashkin and Russell 2005 and Fig. 1B) of the Boltzmann function (Fig. 1A), respectively. Hearing threshold changes were simulated with the first derivative (i.e. the slope) of the Boltzmann function, representing outer hair cell efficiency as a function of OP changes (Kirk and Patuzzi 1997). LF sound-induced shifts of the OP were assumed to initially move towards the hyperpolarising side of the transfer function, which corresponds to a movement of the organ of Corti towards scala tympani. This assumption is arbitrary and all results can equally be modelled by inverting the direction of the modelled OP shift and the location of the initial OP relative to the inflection point of the MET transfer function (the point of maximum sensitivity/slope). However, experimental evidence from guinea pigs (Kirk et al. 1997), investigating cochlear microphonics before and during the BP, found initial shifts of the OP during the BP in the hyperpolarizing direction, in agreement with a movement of the organ of Corti towards scala tympani.
FIG. 1.
The relation between OP shifts and the magnitude of cubic and quadratic distortions, estimated by the absolute value of the second (B, simulated QDP level) and third derivative (B, simulated CDP level), respectively, of a two-exponential Boltzmann function (A) representing the transfer function of the outer hair cell MET. Note that the second derivative shows a notch when the OP is close to the inflection point (in a more symmetric position), whereas the third derivative has a maximum at this position.
RESULTS
Time course of DPOAE level and phase changes after LF sound stimulation
DPOAE data from 20 subjects were included in the analysis. In two subjects, QDP levels were too low to achieve stable recordings over time. In 11 subjects, maximum QDP levels could be evoked with f2 = 4 kHz. In the remaining nine subjects, f2 = 5 kHz resulted in higher QDP levels.
In 14 subjects (70 %), the LF sound stimulation induced an increase of the QDP level lasting for about 60 to 90 s (see Fig. 2A). QDP levels increased with a median of 3.4 dB (see Table 1). In most cases, this QDP increase was followed by a similar QDP decrease (median, −2.4 dB; see Fig. 2A and Table 1) at about 120–150 s post-exposure. This decrease slowly recovered to pre-exposure QDP levels. The median duration of the overall oscillatory change of the QDP level was 214 s (see Table 1).
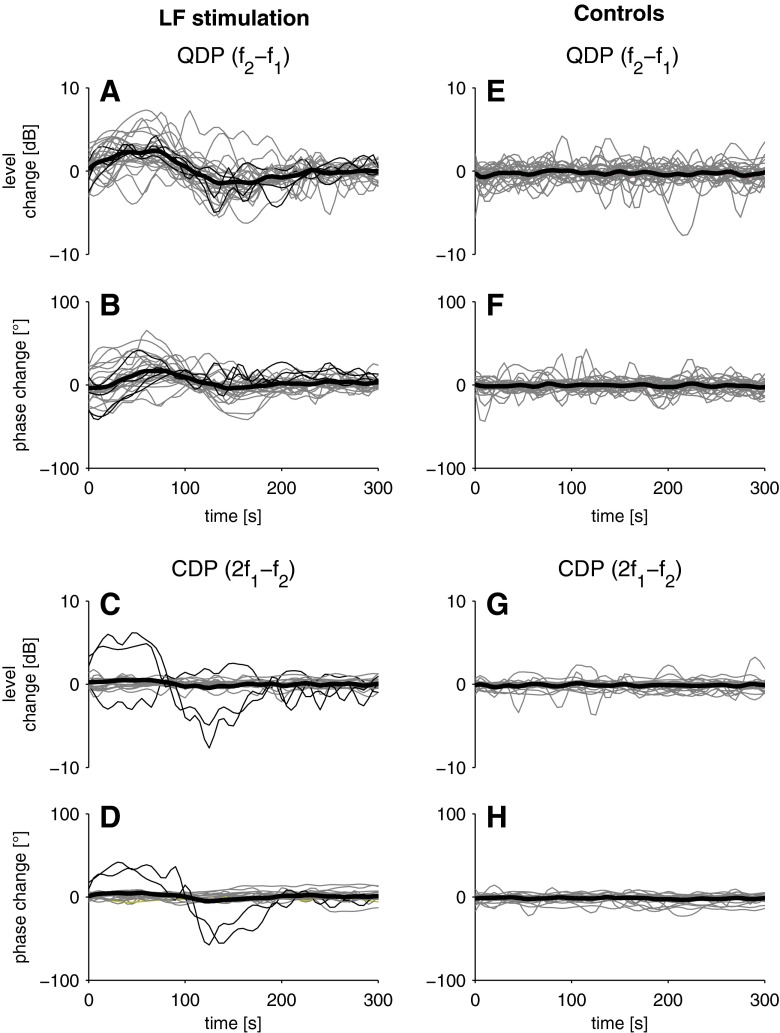
FIG. 2.
Level and phase of QDPs (f 2 − f 1; A, B, respectively) and CDPs (2f 1 − f 2; C, D, respectively) as a function of time after LF sound exposure (30 Hz sine wave, 120 dB SPL, 90 s) relative to the corresponding mean of the pre-exposure period. Bold lines represent median DPOAE measures, and grey lines are individual DPOAE measures. For QDPs, only data from subjects showing a significant level change (see ‘Recording of DPOAE Level and Phase After LF Sound Stimulation’ section, two-sided t test, t(35) = 2.8–17.6, p = 10−16–0.01) after LF sound exposure are shown (N = 28 from 14 subjects). For CDPs, only recordings from the same 14 subjects with sufficiently large CDP levels (signal-to-noise ratio ≥ 6 dB) are shown (N = 22 from 11 subjects). CDP levels were extracted from the same data as QDP levels, for which f 2/f 1 ratios were optimised. Please note that while CDPs typically do not show significant changes of level (two-sided t test, t(35) = 0.005–2.6, p = 0.011–0.99) and phase (two-sided t test, t(35) = 0.004–2.6, p = 0.011–0.99) after LF sound exposure, we observed ‘abnormal’ behaviour with significant changes (two-sided t test, t(35) = 2.8–9.4, p = 10−11–0.008) in two subjects (LS and SL, thin black lines). Controls (E–H) as above, but without LF sound exposure.
TABLE 1.
Summary of the three BP measures
| Subject | Tinnitus max. rel. level [dB] (duration [s]) | QDP min. and max. level change [dB] (duration [s]) | QDP min. and max. phase change [°] (duration [s]) | Hearing threshold max. pos. and neg. change, 1 kHz [dB] (duration [s]) | Hearing threshold max. pos. and neg. change, 2 kHz [dB] (duration [s]) | Hearing threshold max. pos. and neg. change, 4 kHz [dB] (duration [s]) | Corr. coefficient QDP change/threshold change 1/2/4 kHz |
|---|---|---|---|---|---|---|---|
| AI | 16 (97) | – | – | +5.2/−1.6 (238) | +8.3/− (120) | − | − |
| AK | 41 (92) | +4.1/−3.7 (207) | +39.3/−11.4 (208) | +7.5/− (122) | +3.8/− (125) | −3.0/−(115) | 0.48/0.63/–0.79 |
| CF | 32 (81) | – | – | +6.6/− (143) | +5.3/−3.8 (239) | − | − |
| CB | 20 (92) | +3.9/−2.2 (145) | – | +4.7/− (185) | +8.2/− (202) | − | 0.15/0.64/− |
| FK | 34 (176) | +6.8/−2.8 (222) | +36.7/−7.0 (148) | – | −3.6/+1.8 (250) | – | −/−0.45/− |
| JU | 8 (85) | – | – | −3/− (135) | – | – | − |
| KF | 16 (91) | +2.9/−5.3 (342) | +59.7/−38.3 (220) | +9.2/− (218) | – | – | 0.30/−/− |
| KB | 10 (148) | +2.7/−3.8 (237) | – | +4.6/−1.4 (179) | – | – | 0.52/−/− |
| LS a | 9 (81) | +1.5/−2.3 (295) | +12.2/−0.6 (230) | +4.1/− (206) | +1.8/− (182) | – | 0.43/–0.05/− |
| MW | 30 (76) | +3.8/−2.5 (200) | +22.0/−29.6 (190) | – | – | – | – |
| Mwi | 15 (105) | – | – | – | – | – | – |
| ML | 41 (83) | +2.5/−2.0 (138) | +1.3/−29.7 (98) | −3.1/− (136) | − | +2.8/− (144) | −0.26/−/0.08 |
| NH | 9 (71) | − | – | +5.4/− (111) | +9.8/− (192) | +3.1/− (125) | – |
| PP | 16 (96) | − | – | −4.4/− (146) | – | – | – |
| PW | 29 (109) | +2.3/−1.7 (228) | – | +3.1/− (213) | – | – | −0.15/−/− |
| RM | 2 (89) | − | – | − | − | −3.1/− (120) | − |
| RS | 23 (221) | +2.5/−1.5 (320) | +35/−0.5 (265) | – | +4.6/− (76) | −1.4/+2.1 (242) | −/0.55/−0.48 |
| SW | 23 (175) | +4.2/−1.7 (165) | − | +4.2/− (188) | − | − | 0.14/−/− |
| SJ | 13 (116) | − | − | – | – | – | – |
| SL a | 23 (92) | +3.0/−1.4 (220) | +16.3/−10 (108) | – | +4.2/− (132) | – | −/0.66/− |
| TH | 13 (92) | +6.3/−5.5 (175) | +33.3/−3.0 (98) | – | – | +2.7/− (120) | −/0.71/− |
| TS | 9 (77) | +3.8/−5.0 (148) | +24.7/−39.3 (315) | – | +3.4/− (239) | +3.0/− (229) | −/0.53/0.19 |
| Median | 16 (92) | +3.4/−2.4 (214) | +29.0/−10.7(199) | +5/−3 (179) | +4.4/-3.7 (187) | +2.8/–3 (125) | |
| Bouncers [%] | 100 | 70 | 46 | 59 | 45 | 31 |
Rows with data in bold indicate subjects with changes in all three measures
aSubjects with significant changes (two-sided t test, t(35) = 2.8–9.4, p = 10−11–0.008) of CDPs
QDP phase changes were less uniform and their duration was slightly shorter (median, 199 s; see Fig. 2B and Table 1) than QDP level changes. QDP phase changes could be detected in only 10 of the 14 subjects (86 %) with QDP level changes. In 1 of the 20 subjects with sufficient QDP levels, even though no QDP level change could be found, a phase change could be detected. Since QDP level changes were part of our criteria for the identification of the BP, this subject was not included in the quantitative analysis. Typically, phase changes also showed a biphasic behaviour with a median phase increase of +29 ° and a median phase decrease of −10.7 ° (see Table 1). No statistically significant difference (two-sided t test) could be found between QDPs evoked with f2 = 4 or 5 kHz with regard to level (t(18) = 0.28, p = 0.78) and phase (t(18) = 0.42, p = 0.67) change duration. In six subjects (30 %), no change according to the criteria above could be detected because the two consecutive trials gave inconsistent results.
Control recordings without LF stimulation were analysed in the same way as the recordings with LF sound exposure. No phase changes fulfilling our criteria could be detected (Fig. 2F). In about one third of the control recordings, we observed level changes showing random time courses different from the recordings after LF sound exposure. The level changes were much shorter in duration (median, 75 s) than recordings with LF sound exposure and did not exceed about 1 dB. The averaged time course of the control recordings showed the random nature of the fluctuations and did not resemble the synchronised level changes we observed after LF sound exposure (see Fig. 2E).
Even though we focused on QDPs in this study, in many cases, it was also possible to extract CDP levels and phases from the same recording (albeit f2/f1 ratios were optimised to achieve maximum QDP levels). Typically, we observed no significant changes of CDP level and phase after LF sound exposure (see Fig. 2C, D) and in control recordings (Fig. 2G, H). In one single case, we observed changes of CDP levels showing the opposite behaviour of QDP changes, i.e. while QDP levels increased, CDP levels decreased and vice versa. In another single case, we observed increases of CDP levels and changes of CDP phase after LF sound exposure accompanied by increases of QDP levels with a similar time course (see Fig. 2A–D).
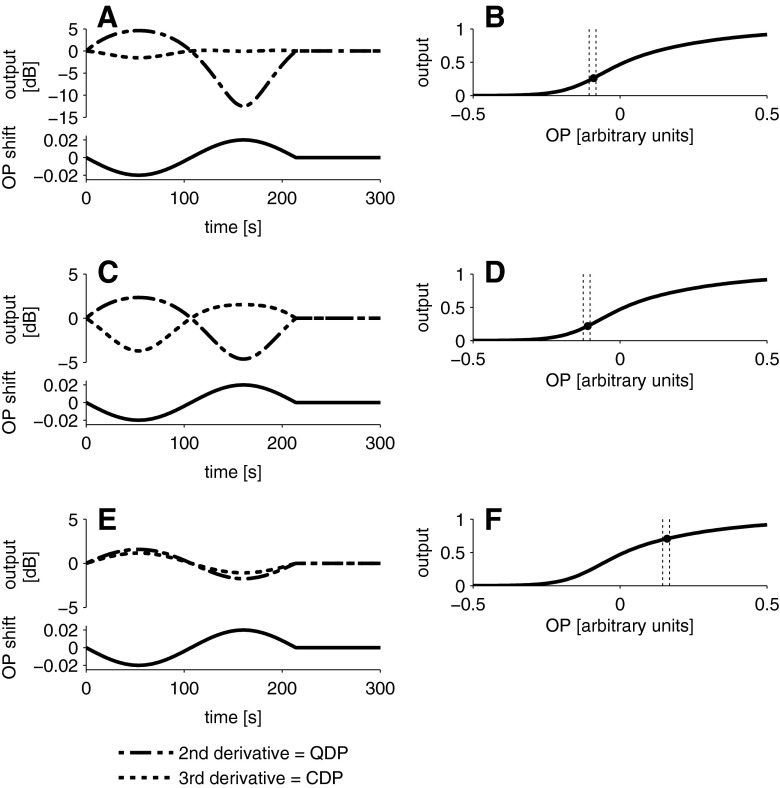
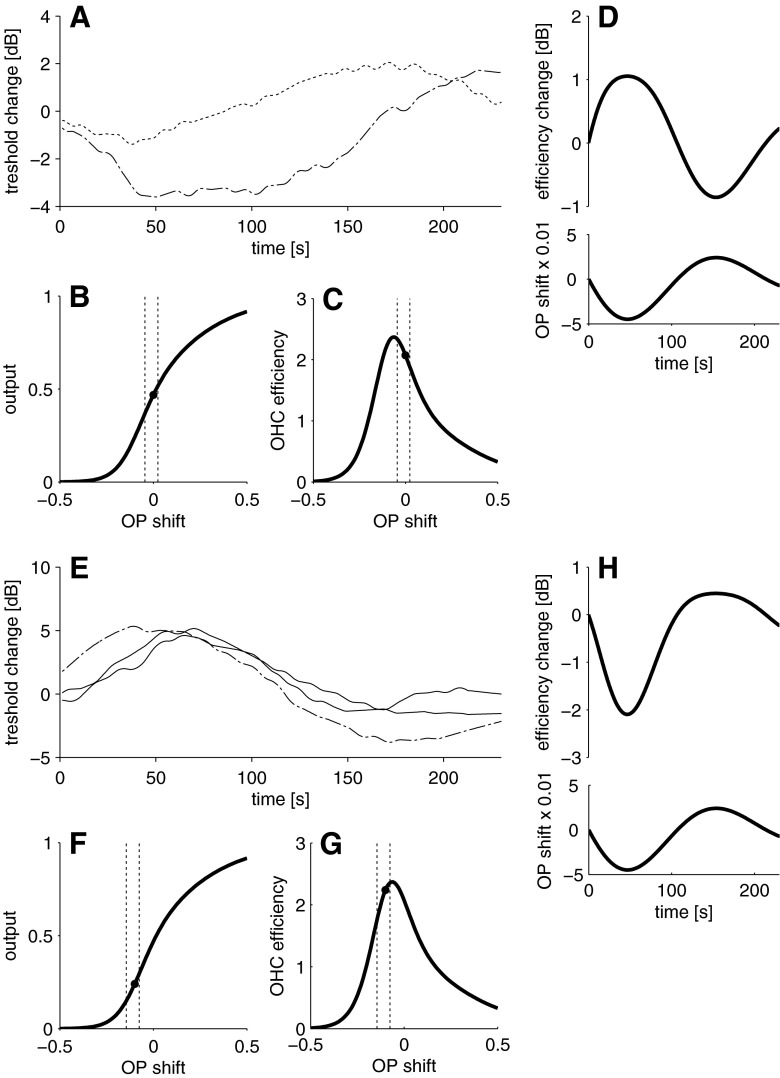
Boltzmann simulations show that if the initial OP of the MET transfer function corresponds to a displacement of the organ of Corti towards scala tympani (Fig. 3B) and if the BP causes small movements of the OP further away from the inflection point (and back), then simulated QDP, but not CDP levels, oscillate in a similar manner as the QDP data (Fig. 3A). The same model can account for the ‘irregular’ data in the two subjects mentioned previously when simulating different initial OP positions on the transfer function (Fig. 3C–F).
FIG. 3.
Simulation of the level change (relative to the mean of the pre-exposure period) of CDP and QDP as a function of sinusoidal OP changes. QDP levels change markedly while CDP remain almost unchanged (A, top panel) when the OP is moved sinusoidally (A, bottom panel) around an initial OP position (B, black symbol) near the inflection point of the Boltzmann function. QDP and CDP change with opposite sign (C, top panel) when the OP is sinusoidally changed (C, bottom panel) around an initial OP position further away from the inflection point (D). QDP and CDP change with the same sign (E, top panel), when the OP is changed sinusoidally (E, bottom panel) around an initial OP position far away from the inflection point (F). Dashes lines in B, D and F indicate the range of the OP shift. See also Fig. 1 for the behaviour of QDP and CDP levels as a function of OP shifts.
Time course of evoked, transient tinnitus after LF sound stimulation
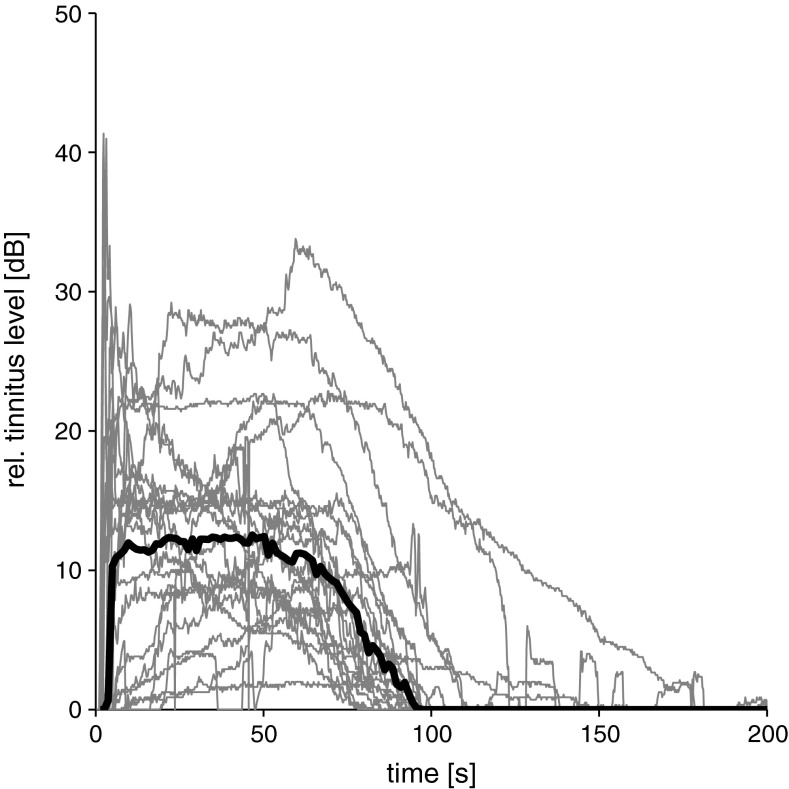
All 22 subjects reported a tinnitus-like sensation after the presentation of LF tone, which started immediately after the cessation of the LF stimulation and reached a plateau of maximum loudness about 10 to 15 s post-exposure (see Fig. 4). Typically, the perceived loudness of the tinnitus remained constant for about 1 min before it almost monotonically returned to baseline values with a median duration of 92 s, see also Table 1). None of the subjects reported oscillations of the perceived tinnitus loudness. The subjects compared the mean perceived loudness to a synthesised, band pass noise with a median maximum sound pressure level of 16 dB (re. control recording). Individually estimated maximum tinnitus levels, however, ranged from 2 to 41 dB (re. control recording, see Table 1). After completion of all trials, subjects were asked about the quality of the acoustic sensations they perceived. Most of the subjects likened the sounds to a LF-pitched, roaring noise. Some subjects noted a ‘fan-like’ quality of the sensations or compared them to the static noise of a mistuned radio. Some subjects pointed out that they also heard single tones of higher frequencies with fluctuating levels added to the noisy tinnitus previously mentioned.
FIG. 4.
Perceived loudness of tinnitus-like sensations after LF sound exposure (30 Hz sine wave, 120 dB SPL, 90 s) relative to control recordings without LF sound exposure as a function of time after LF sound exposure. Individual recordings from 22 subjects are shown in grey, the median in black.
Time course of hearing threshold changes after LF sound stimulation
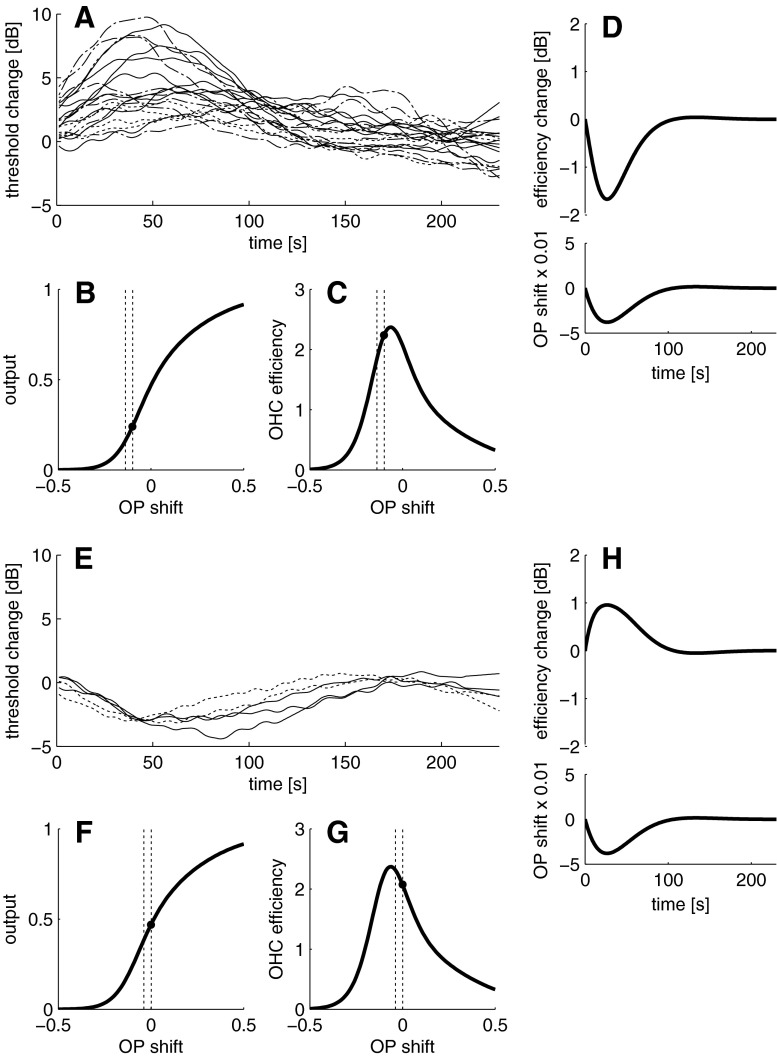
Nineteen of the 22 subjects (86 %) showed alterations of hearing thresholds at least at one of the three probe tone frequencies after LF sound stimulation. LF sound-induced changes occurred most frequently when hearing thresholds were tested with tones of 1 kHz (59 % of all tested subjects, Table 1). The number of subjects showing threshold alterations decreased with increasing test tone frequency (see Table 1). With a probe tone of 1 kHz, nine subjects were classified as not bouncing (four showed no changes, five showed changes which were not repeatable in both trials), with 2 kHz, six showed no BP and another six subjects showed changes which were not repeatable. With 4 kHz, 3 subjects showed no BP and 12 had changes which were not repeatable. Both desensitisations and sensitisations of hearing thresholds occurred (see Figs. 5A, E and 6A, E). Desensitisations of the hearing threshold were always larger (up to about 10 dB) than sensitisations (up to about −4 dB). Typically, maximum threshold alterations were reached between 40 and 70 s post-exposure, regardless of the direction of the change, and the median of the overall duration ranged from 125 to 187 s (Table 1), depending on probe tone frequency. Threshold alteration patterns were not uniform in this study. Four different types of threshold changes (see Figs. 5A, E and 6A, E) were observed, namely monophasic desensitisations (Fig. 5A, the majority of cases), monophasic sensitisations (Fig. 5E), biphasic alterations with initial sensitisation (Fig. 6A) and biphasic alterations with initial desensitisation (Fig. 6E). The occurrence of the patterns showed no correlation to the probe tone frequency (see Table 1).
FIG. 5.
Pooled monophasic changes of hearing thresholds probed with 1, 2 and 4 kHz after LF sound exposure relative to the mean of the pre-exposure period. Both desensitisation (A) and sensitisation (E) occurred, with desensitisations dominating. A simulated initial OP below (black symbol, B) or above (F) the inflection point on the Boltzmann function can produce a monophasic desensitisation (D) or sensitisation (H), if the first derivative (i.e. the slope) of the Boltzmann function (C, G) is used to simulate outer hair cell efficiency. D, H: outer hair cell efficiency as a function of an OP change following a damped oscillation, in hyperpolarising direction on the MET transducer function and back (i.e. corresponding to a movement of the organ of Corti towards scala tympani and back). Monophasic sensitisation occurred in subjects AK, JU, ML, PP and RM, and monophasic desensitisations occurred in subjects AI, AK, CF, CB, KF, LS, ML, NH, PW, RS, SW, SL, TH and TS. In A and E, solid lines = 1 kHz probe tone, dashed-dotted line = 2 kHz, dotted line = 4 kHz. Dashed lines in B, C, F and G indicate the range of the OP shift.
FIG. 6.
Pooled biphasic changes of hearing thresholds probed with 1, 2 and 4 kHz after LF sound exposure relative to the mean of the pre-exposure period. Both initial sensitisation (A) and desensitisation (E) occur. A simulated initial OP above (black symbol, B) or below (F) the inflection point on the Boltzmann function can produce a biphasic change with initial sensitisation (D) or desensitisation (H). D, H: as in Fig. 5, with a sinusoidal OP change. Biphasic changes with initial sensitisation occurred in subjects FK and RS, with initial desensitisation in the subjects AI, CF and KB. Solid lines = 1 kHz probe tone, dashed-dotted line = 2 kHz, dotted line = 4 kHz. Dashed lines in B, C, F and G indicate the range of the OP shift.
Monophasic threshold changes can be simulated with the first derivative of the Boltzmann function (Figs. 5B–D), where desensitisations require the initial OP to be located below the inflection point (if we assume that the BP caused a shift of the OP following a damped oscillation towards the hyperpolarising part of the MET transfer function and back, i.e. corresponding to a movement of the organ of Corti towards scala tympani and back), whereas sensitisations require a location of the initial OP above the inflection point (Fig. 5F–H). Biphasic changes can be simulated with a change of the OP following a sinusoidal oscillation. Depending on the location of the OP above (Fig. 6B, C) or below (Figs. 6F, G) the inflection point, biphasic changes with initial sensitisation (Fig. 6D) or desensitisation (Fig. 6H) may occur.
The qualitative results of the three BP measures are summarised in Table 1. All subjects experienced tinnitus after LF sound exposure, making it the most ‘sensitive’ measure of the BP. Fifty-nine percent of the subjects showed LF sound-induced changes in all three measures. Two subjects (9 %) experienced tinnitus only and had no indication of the BP in the other measures. DPOAE changes and hearing threshold changes did not necessarily occur together, as we found six subjects (27 %) with hearing threshold changes who did not show DPOAE changes and one subject with DPOAE changes, but no hearing threshold changes. Out of the 13 subjects showing the BP in both hearing thresholds and QDP level changes, 11 had correlations between the QDP level changes and hearing thresholds for at least one probe tone frequency with coefficients greater than 0.25 (see Table 1).
DISCUSSION
In this paper, we showed that exposure to loud, LF sound induced temporary changes of hearing thresholds and alterations of quadratic, but not cubic, DPOAEs in the majority of subjects. In addition, LF sound induced a tinnitus-like sensation. This is, to the best of our knowledge, the first study to examine all the manifestations of the BP accessible in humans in the same subjects and the first study to follow CDPs and QDPs during the BP in humans.
LF sound-induced changes of level and phase of DPOAEs
As illustrated by our qualitative simulations, we suggest an underlying OP shift that accounts for the DPOAE changes we observed. DPOAEs as measured in the ear canal are composed of a distortion source and a reflection source. The interaction of the two sources leads to quasi-periodic DPOAE level changes when observed with a high-frequency resolution, which have been termed the total DPOAE fine structure (e.g. Shera and Guinan 1999; Talmadge et al. 1999). The two components can be separated by suppression of one of the components (Kalluri and Shera 2001), but other techniques are also available (e.g. Long et al. 2008). Since we recorded the total DPOAE with both components present, LF sound-induced changes of DPOAE fine structure might theoretically represent an alternative explanation. Human total DPOAEs show a pronounced fine structure at moderate primary tone levels, and manipulations of cochlear gain (e.g. by efferent activity) can cause a frequency shift of the fine structure. This can lead to a decrease or increase of the DPOAE level, depending on the original location of the DPOAE frequency near a fine structure peak or trough (Henin et al. 2011). A frequency shift of the total DPOAE fine structure could also contribute to the effects of LF sound on DPOAEs we observed. Contributions from the DPOAE reflection source are small compared to the distortion source at the relatively high (65 dB SPL) primary tone levels we used in this study (Mauermann and Kollmeier 2004; Young et al. 2012). If the effects we observed were due to a change of the interference between the two sources, it is unlikely that the effect (if it occurs) always goes in the same direction (enhancement followed by suppression). In fact, since the primaries were optimised for QDPs, it can be assumed that the subjects were recorded at QDP fine structure peaks. This makes it unlikely that the consistently observed shifts towards larger QDP levels during the initial phase of the BP are due to fine structure changes. In addition, CDPs were recorded with f2/f1 ratios individually optimised for QDPs which implies that, especially at f2/f1 ratios lower than the usual optimum f2/f1 ratio of 1.22 in humans, a pronounced fine structure should be present. Moreover, if LF sound exposure caused fine structure frequency changes, these should be reflected not only in the QDP level, but also in the CDP level (although the primaries were not optimised for the latter). We, therefore, do not consider fine structure changes to be the major cause of our results.
In the present work, transient changes of QDPs are qualitatively similar to the results of previous animal studies (Kirk et al. 1997; Kirk and Patuzzi 1997) and showed the same oscillatory pattern with a comparable time course. Consistent with these animal studies, QDP, but not CDP, levels changed after LF sound exposure, which can be explained by the fact that QDPs are more susceptible to small OP shifts around the inflection point of the MET transfer function (see also Fig. 1).
OP shifts have also been shown during LF sound exposure, so-called LF-biasing experiments (e.g. recording of DPOAEs during LF sound stimulation) in animals (Frank and Kossl 1996, 1997; Althen et al. 2012) and in humans (Drexl et al. 2012). In these biasing experiments, QDP levels also oscillate (coupled to the LF sound period), but CDP levels remain unchanged (Abel et al. 2009). We observed a similar, differential effect on QDPs and CDPs in the present work, but the oscillation is much slower, it is not coupled to the LF sound period and the oscillation occurs after the LF sound offset. QDPs and CDPs represent different properties of MET transfer functions and are believed to reflect compression and gain of cochlear transduction, respectively (Bian 2004). This can result in opposite magnitude changes of QDPs and CDPs when the OPs are sufficiently shifted by manipulations of the cochlea, including LF biasing (Frank and Kossl 1997; Drexl et al. 2012), or by stimulation of the efferent system (Wittekindt et al. 2009; Althen et al. 2012). We observed concomitant changes of QDP and CDP levels with opposite sign in one single case. Individual differences can exist in the sensitivity of human cochleae to LF sound (Marquardt et al. 2007; Drexl et al. 2012). We therefore suggest that in this single case, the LF sound exposure was sufficient to drive the OP to a position asymmetrical enough to not only affect QDPs, but also CDPs. This can be achieved by an initial OP position located on the hyperpolarising side of the MET transfer function (Fig. 3C, D).
In another single case, we observed simultaneous increases of QDP and CDP levels in the same direction, which can be explained if the initial OP was located above the inflection point far on the depolarising side and the LF sound exposure caused a shift of the OP closer to the inflection point (Fig. 3E, F).
LF sound-induced transient sound perceptions
In the only other study focusing on the systematic investigation of the time course of tinnitus-like phenomena after exposure to LF sound we are aware of (Patuzzi and Wareing 2002), the LF sound-induced tinnitus lasted for more than 200 s before returning to baseline values in an almost exponential way, which is much longer than what we found in this work. Also, in the study of Patuzzi and Wareing (2002), maximum tinnitus loudness was reached after about 60–70 s, whereas in the present study, maximum loudness was usually reached within a few seconds and remained almost stable before slowly returning to baseline values. The duration differences might arise through the different stimuli that were used to induce the BP. Patuzzi and Wareing (2002) used varying LF sounds (40 Hz, 126 dB SPL and 100 Hz, 116 dB SPL) and exposure durations of up to 300 s, whereas in the present study, the LF sound was fixed at 30 Hz, 120 dB SPL for 90 s. In both studies, oscillatory changes of tinnitus loudness could not be shown; rather, after reaching a maximum, the tinnitus percept loudness decreased, and once no longer perceived, the tinnitus never returned for the remaining observation period.
We can only speculate on the underlying causes of the LF sound-induced tinnitus. It seems reasonable to assume that the LF sound-induced tinnitus is not due to traumatic changes of the cochlea which are usually accompanied by prominent deteriorations of hearing thresholds (e.g. Chermak and Dengerink 1987). This is supported by the findings in several other publications (Lindsay and Von Schulthess 1958; Lindsay et al. 1967; Merchant 2010) where it was reported that only about 10 % of the cochleae from Ménière’s disease patients (typically also suffering from tinnitus) showed significant loss of hair cells and cells of the spiral ganglion at the apex despite having all symptoms of Ménière’s disease. Intense LF sound (30 Hz, 100 dB SPL, presented continuously for up to 24 h) has been shown to leave spiral ganglion cells intact and to not affect cochlear function in chinchillas (Harding et al. 2007) 24 h post-exposure. This is a point in time where noise-induced damage to afferent terminals at inner hair cells is usually at a maximum (Lin et al. 2011). It is therefore unlikely that mechanisms usually associated with common noise-induced temporary threshold shifts (Robertson 1982, 1983; Wang et al. 2002) are responsible for the tinnitus-like sensations observed during the BP.
Hearing threshold changes after LF sound stimulation
The majority of publications on the BP deal with the slow, temporary changes of the hearing threshold after LF sound exposure, but the reported changes are not uniform and their time courses differ. It has been shown that exposure to intense stimuli with various frequencies can produce increased sensitivity, decreased sensitivity, oscillation between increased and decreased sensitivity (the BP) or no change in hearing thresholds in humans (Hughes 1954; Noffsinger and Olsen 1970; Noffsinger and Tillman 1970).
In the present study, the effects on hearing thresholds after LF sound exposure were also not uniform. The range of effects of the LF sound may at first appear puzzling; however, by paying close attention to the individual recording paradigms in the literature, we may be able to account for this variability. Hirsh and Ward (1952) found what they called the bounce (a transient worsening of hearing thresholds following initial recovery after LF sound exposure) in about 65 % of their subjects and only a small fraction showed initial sensitisation. In the present study, subjects tracked thresholds only after, not during, the LF sound exposure. Noffsinger and Olsen (1970) and Noffsinger and Tillman (1970) showed that additional threshold tracking during LF sound exposure can enhance the BP after the offset of the LF tone. Also, in the study by Hughes (1954), the subjects received training over a period of 18 months, whereas in our study, subjects were intentionally naïve to the procedure to avoid any biasing of the results due to informed subjects. In the present work, some subjects reported that the presence of the tinnitus during threshold tracking had a negative impact on their performance. The masking effect of the probe tone by the tinnitus can most likely be avoided by training and might explain the higher percentage of bouncing subjects in studies where subjects had been trained. The parameters of the LF sound and the probe tone used for threshold tracking seem to affect the manifestation of the BP in the hearing threshold, which may account for the diversity of effects after LF sound exposure found in the literature. Although our subjects had not been trained, we were still able to demonstrate the BP-typical biphasic changes in hearing threshold in some subjects, but not as consistently as in previous investigations with differing paradigms and subject training (e.g. Hirsh and Ward 1952).
A common origin for all three manifestations of the bounce phenomenon?
We recorded three measures of the BP in the same subjects with seemingly contradictory results: All subjects indicated the perception of a tinnitus-like phenomenon, but only 70 % showed DPOAE changes, and 85 % showed hearing threshold changes for at least one probe tone frequency. In the subjects where both DPOAE and hearing threshold changes were observed, DPOAE changes were always uniform and biphasic with initial enhancement followed by suppression, whereas hearing threshold changes were biphasic or monophasic and often uncorrelated to the DPOAE change pattern.
In the following, we will show that a LF sound-induced OP shift can qualitatively explain all the observed phenomena with one simple model under the following assumptions:
The LF sound induces an OP shift with a similar time course and always the same initial direction across listeners (a hyperpolarising shift corresponding to a movement of the organ of Corti towards scala tympani; see ‘Qualitative modelling’ section).
The initial OP is slightly different across individual subjects.
The initial OP shifts as a function of the level of the primary or probe tones and is therefore different in experiments with primary tones of moderate intensity (DPOAE recordings) and in experiments with no (tinnitus measurements) or near-threshold stimulation (hearing thresholds).
Individual differences exist in LF sound sensitivity and the ability to induce the BP (see ‘LF Sound-Induced Changes of Level and Phase of DPOAEs’).
As mentioned before, individual differences can exist in the sensitivity of human cochleae to LF sound and therefore how it affects cochlear processes. This can be the reason why some subjects only showed tinnitus and others showed changes in all three measures. When subjects showed changes in all three measures, the duration of the tinnitus matched the period within which QDP levels were significantly increased. Moreover, the duration of the hearing threshold change mostly matched the duration of the overall QDP level change (both enhancement and suppression). It seems difficult to explain the concomitant increase of QDPs, indicating a shift of the OP to a more asymmetric and hence less sensitive position, and a sensitisation of the hearing threshold, indicating an OP shift to a more sensitive position. The solution to this problem can be found in the different acoustic situations in which these two measures were recorded (Kirk and Patuzzi 1997). While hearing thresholds are obtained with acoustic stimuli at levels obviously close to hearing thresholds (usually between about 0 and 10 dB SPL in the present study), DPOAEs were evoked with primary tones of 65 dB SPL. There are indications that the OPs of outer hair cell MET transfer functions shift with stimulus level and are therefore not identical under low and high level stimulation (Dallos 1986; Cody and Russell 1995; Frank and Kossl 1997; Bian et al. 2002; Bian et al. 2004) which can explain the seemingly opposite behaviour of hearing thresholds and QDP levels. A movement of the OP (which is assumed to be located below the inflection point in the presence of the intense primary tones) away from the inflection point, towards the inflection point and back, would produce the biphasic pattern of QDPs with initial enhancement (see Fig. 3A).
A similar OP shift as in the DPOAE experiments can, depending on the damping of the OP oscillation and the individual initial OP, cause a biphasic or monophasic hearing threshold change with sensitisation and/or desensitisation (see Figs. 5 and 6). In the hearing threshold experiments, the OP can be assumed to be not shifted by the near-threshold stimuli. If we accept that individual initial OPs are slightly different (Sirjani et al. 2004; Brown et al. 2009; Lichtenhan 2012) and can be located above or below the inflection point of the MET transfer function (Kirk and Patuzzi 1997), then a LF sound-induced OP shift can explain the different categories of threshold changes we observed. An OP located slightly above the inflection point on the MET transfer function would be shifted towards the point of maximum sensitivity (Fig. 5E–H). This resulted in a transient sensitisation before the OP returns to its initial location or even travels beyond, which would produce a biphasic change (sensitisation followed by desensitisation, the BP; Fig. 6A–D). If the OP is located below the inflection point, LF sound exposure would shift the OP away from the point of maximum sensitivity, thus causing a transient desensitisation (Fig. 5A–D) and, if travelling beyond the initial position to a place closer to the inflection point, could also cause a subsequent sensitisation (Fig. 6E–H). These OP shifts are consequently accompanied by changes of the gain of the cochlear amplifier (Frank and Kossl 1996, 1997).
For the tinnitus, the same OP shift would also move the OP towards the hyperpolarising region (and back), causing an initial decrease of the standing current through the MET ion channels. Here, the individual location of the initial OP is not important, as a hyperpolarising movement of the OP will always decrease the opening probability of the MET channels regardless the initial location of the OP. This can lead to increased endolymphatic potentials and in turn to a depolarisation of inner hair cells, increased spontaneous transmitter release and increased spontaneous activity of the auditory nerve (Patuzzi 2011) which could be perceived as tinnitus. We suggest that the tinnitus measure is the most sensitive of the BP measures, as all subjects had a tinnitus percept in this study, which might indicate that even small bounce OP changes can lead to a tinnitus percept. For the other two measures, requiring external acoustic stimulation, a minimum OP shift seems to be necessary in order to detect any effects.
Could the bounce phenomenon and Ménière’s disease be related?
It is interesting that the symptoms of Ménière’s disease and of the BP share similarities. Both phenomena seem to be localised to apical regions of the cochlea. Patients suffering from Ménière’s disease typically (but not necessarily) develop initial LF hearing loss (e.g. Belinchon et al. 2011), hinting that structures involved in LF sound processing are impaired by the unknown pathophysiological processes behind Ménière’s disease, at least in the early stages of the disease. The BP can only be evoked with LF sound (e.g. Kemp 1986; Kirk and Patuzzi 1997), which could suggest that LF tones have a greater influence on the cochlear apex than the base (Lichtenhan 2012) and that processes at the apex are responsible for the generation of the BP.
In addition, the quality of the perceived tinnitus in Ménière’s disease patients and that observed during the BP appears similar. The tinnitus that Ménière’s disease patients suffer from is usually confined to low frequencies and has been described as roaring (Douek and Reid 1968; Vernon et al. 1980; Han et al. 2009). Subjects during BP periods made similar qualitative assessments of the tinnitus they heard (e.g. Kemp 1986; this study) which indicates that both kinds of tinnitus are probably, at least in the initial stage of Ménière’s disease, related.
Finally, there are indications in Ménière’s disease patients with minimal hearing loss that the OP of the outer hair cell MET transfer function is shifted compared to unaffected ears. (Hirschfelder et al. 2005; Brown and Gibson 2011). These observations are consistent with changes in DPOAEs observed during BP periods. Since the latter have been observed in both animal models (Kirk et al. 1997; Kirk and Patuzzi 1997) and human subjects (this study), there may exist a relationship worth further investigation.
Recently, Patuzzi (2011) proposed an updated model for the mechanisms behind the BP. He suggested that the central element is a LF sound-induced oscillation of intracellular Ca2+ levels in outer hair cells. Ca2+ can trigger a plethora of events in outer hair cells (reviewed by Mammano et al. 2007), one of which is slow somatic motility. Patuzzi (2011) suggested that the increased intracellular Ca2+ level causes slow contractions of the outer hair cell soma, leading to a decreased opening probability of MET channels and therefore to a reduction of the standing current through the outer hair cell. This can cause a rise of the endolymphatic potential and potentially an accumulation of K+, which can in turn lead to water influx through aquaporins into scala media and therefore cause endolymphatic hydrops. There is experimental evidence from animal models (Kirk and Patuzzi 1997; Salt 2004) showing endolymphatic hydrops and/or increased endolymphatic potential levels after LF sound exposure. The increased endolymphatic potential could be one reason for the tinnitus-like perceptions we observed after LF sound exposure and might also contribute to increased sensitivity of hearing thresholds (Sewell 1984). Ca2+-induced contractions of outer hair cell somas inevitably cause OP shifts on the outer hair cell MET transfer function that could lead to the typical behaviour of CDPs and QDPs we observed. OP shifts (and associated changes of cochlear gain) can also account for the hearing threshold alterations.
If we accept that the mechanisms producing the BP are similar or even identical in humans and rodents, then we can assume that the exposure to LF sound produces an endolymphatic hydrops-like condition in human cochleae, similar to what has been shown in guinea pigs in vivo (Salt 2004) and in vitro (Flock and Flock 2000).
In this paper, we have shown that intense LF sound induces slow oscillations of cochlear compression and gain, causing several measures of cochlear activity to cycle through phases of increased and decreased sensitivity. Based on the phenomenological similarities between the BP and Ménière’s disease, we suggest that the two phenomena may share similar underlying mechanisms. A slow oscillation of a homeostatic control mechanism within the cochlea has been suggested as the source of the BP (Patuzzi 2011). If this control mechanism fails to operate correctly, and disturbances of cochlear homeostasis are not rectified, then inner ear functions could be compromised, leading to endolymphatic hydrops and finally Ménière’s disease.
Acknowledgments
This work was funded by a grant from the German Ministry of Science and Education to the German Center for Vertigo and Balance Disorders (IFB), project TR-F9 to R.G., E.K. and M.D., a Medical Research Council grant G0801693 to A.N.L. and T.D.W and a grant from the BCCN Munich, TP7, B3 Wiegrebe to L.W.
We would like to thank the four anonymous reviewers for their constructive comments on earlier versions of the manuscript.
We are grateful to Sybille Krall, who inspired the experiments in the present study.
Footnotes
E. Krause and R. Gürkov contributed equally to this study.
References
- Abel C, Wittekindt A, Kossl M. Contralateral acoustic stimulation modulates low-frequency biasing of DPOAE: efferent influence on cochlear amplifier operating state? J Neurophysiol. 2009;101:2362–2371. doi: 10.1152/jn.00026.2009. [DOI] [PubMed] [Google Scholar]
- Althen H, Wittekindt A, Gaese B, Kossl M, Abel C. Effect of contralateral pure tone stimulation on distortion emissions suggests a frequency-specific functioning of the efferent cochlear control. J Neurophysiol. 2012;107:1962–1969. doi: 10.1152/jn.00418.2011. [DOI] [PubMed] [Google Scholar]
- Belinchon A, Perez-Garrigues H, Tenias JM, Lopez A. Hearing assessment in Meniere’s disease. Laryngoscope. 2011;121:622–626. doi: 10.1002/lary.21335. [DOI] [PubMed] [Google Scholar]
- Berlinger NT. Meniere’s disease: new concepts, new treatments. Minn Med. 2011;94:33–36. [PubMed] [Google Scholar]
- Bian L. Cochlear compression: effects of low-frequency biasing on quadratic distortion product otoacoustic emission. J Acoust Soc Am. 2004;116:3559–3571. doi: 10.1121/1.1819501. [DOI] [PubMed] [Google Scholar]
- Bian L, Chertoff ME, Miller E. Deriving a cochlear transducer function from low-frequency modulation of distortion product otoacoustic emissions. J Acoust Soc Am. 2002;112:198–210. doi: 10.1121/1.1488943. [DOI] [PubMed] [Google Scholar]
- Bian L, Linhardt EE, Chertoff ME. Cochlear hysteresis: observation with low-frequency modulated distortion product otoacoustic emissions. J Acoust Soc Am. 2004;115:2159–2172. doi: 10.1121/1.1690081. [DOI] [PubMed] [Google Scholar]
- Brown DJ, Gibson WP. On the differential diagnosis of Meniere’s disease using low-frequency acoustic biasing of the 2f1-f2 DPOAE. Hear Res. 2011;282:119–127. doi: 10.1016/j.heares.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Brown DJ, Hartsock JJ, Gill RM, Fitzgerald HE, Salt AN. Estimating the operating point of the cochlear transducer using low-frequency biased distortion products. J Acoust Soc Am. 2009;125:2129–2145. doi: 10.1121/1.3083228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermak GD, Dengerink JE. Characteristics of temporary noise-induced tinnitus in male and female subjects. Scand Audiol. 1987;16:67–73. doi: 10.3109/01050398709042158. [DOI] [PubMed] [Google Scholar]
- Cody AR, Russell IJ. Time-varying voltage responses of mammalian hair cells to isoamplitude acoustic stimulation. Audit Neurosci. 1995;1:351–361. [Google Scholar]
- Dallos P. Neurobiology of cochlear inner and outer hair cells: intracellular recordings. Hear Res. 1986;22:185–198. doi: 10.1016/0378-5955(86)90095-X. [DOI] [PubMed] [Google Scholar]
- Douek E, Reid J. The diagnostic value of tinnitus pitch. J Laryngol Otol. 1968;82:1039–1042. doi: 10.1017/S0022215100069838. [DOI] [PubMed] [Google Scholar]
- Drexl M, Gurkov R, Krause E. Low-frequency modulated quadratic and cubic distortion product otoacoustic emissions in humans. Hear Res. 2012;287:91–101. doi: 10.1016/j.heares.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Flock A, Flock B. Hydrops in the cochlea can be induced by sound as well as by static pressure. Hear Res. 2000;150:175–188. doi: 10.1016/S0378-5955(00)00198-2. [DOI] [PubMed] [Google Scholar]
- Frank G, Kossl M. The shape of 2f1-f2 suppression tuning curves reflects basilar membrane specializations in the mustached bat, Pteronotus parnellii. Hear Res. 1995;83:151–160. doi: 10.1016/0378-5955(94)00197-X. [DOI] [PubMed] [Google Scholar]
- Frank G, Kossl M. The acoustic two-tone distortions 2f1-f2 and f2-f1 and their possible relation to changes in the operating point of the cochlear amplifier. Hear Res. 1996;98:104–115. doi: 10.1016/0378-5955(96)00083-4. [DOI] [PubMed] [Google Scholar]
- Frank G, Kossl M. Acoustical and electrical biasing of the cochlea partition. Effects on the acoustic two tone distortions f2-f1 and 2f1-f2. Hear Res. 1997;113:57–68. doi: 10.1016/S0378-5955(97)00131-7. [DOI] [PubMed] [Google Scholar]
- Han BI, Lee HW, Kim TY, Lim JS, Shin KS. Tinnitus: characteristics, causes, mechanisms, and treatments. J Clin Neurol. 2009;5:11–19. doi: 10.3988/jcn.2009.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Lee SC, Salt AN. Effect of infrasound on cochlear damage from exposure to a 4 khz octave band of noise. Hear Res. 2007;225:128–138. doi: 10.1016/j.heares.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henin S, Thompson S, Abdelrazeq S, Long GR. Changes in amplitude and phase of distortion-product otoacoustic emission fine-structure and separated components during efferent activation. J Acoust Soc Am. 2011;129:2068–2079. doi: 10.1121/1.3543945. [DOI] [PubMed] [Google Scholar]
- Hirschfelder A, Gossow-Muller-Hohenstein E, Hensel J, Scholz G, Mrowinski D. Diagnosis of endolymphatic hydrops using low frequency modulated distortion product otoacoustic emissions. HNO. 2005;53:612–617. doi: 10.1007/s00106-004-1171-4. [DOI] [PubMed] [Google Scholar]
- Hirsh IJ, Ward WD. Recovery of the auditory threshold after strong acoustic stimulation. J Acoust Soc Am. 1952;24:131–141. doi: 10.1121/1.1906867. [DOI] [Google Scholar]
- Hughes JR. Auditory sensitization. J Acoust Soc Am. 1954;26:1064–1070. doi: 10.1121/1.1907450. [DOI] [Google Scholar]
- Kalluri R, Shera CA. Distortion-product source unmixing: a test of the two-mechanism model for DPOAE generation. J Acoust Soc Am. 2001;109:622–637. doi: 10.1121/1.1334597. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Otoacoustic emissions, travelling waves and cochlear mechanisms. Hear Res. 1986;22:95–104. doi: 10.1016/0378-5955(86)90087-0. [DOI] [PubMed] [Google Scholar]
- Kemp DT, Brill OJ. Slow oscillatory cochlear adaptation to brief over stimulation: cochlear homeostasis dynamics. In: Cooper NP, Kemp DT, editors. Concepts and challenges in the biophysics of hearing. Singapore: World Scientific; 2009. pp. 168–174. [Google Scholar]
- Kirk DL, Patuzzi RB. Transient changes in cochlear potentials and DPOAEs after low-frequency tones: the ‘two-minute bounce’ revisited. Hear Res. 1997;112:49–68. doi: 10.1016/S0378-5955(97)00105-6. [DOI] [PubMed] [Google Scholar]
- Kirk DL, Moleirinho A, Patuzzi RB. Microphonic and DPOAE measurements suggest a micromechanical mechanism for the ‘bounce’ phenomenon following low-frequency tones. Hear Res. 1997;112:69–86. doi: 10.1016/S0378-5955(97)00104-4. [DOI] [PubMed] [Google Scholar]
- Lichtenhan JT. Effects of low-frequency biasing on otoacoustic and neural measures suggest that stimulus-frequency otoacoustic emissions originate near the peak region of the traveling wave. J Assoc Res Otolaryngol. 2012;13:17–28. doi: 10.1007/s10162-011-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay JR, Von Schulthess G. An unusual case of labyrinthine hydrops. Acta Otolaryngol. 1958;49:315–324. doi: 10.3109/00016485809134760. [DOI] [PubMed] [Google Scholar]
- Lindsay JR, Kohut RI, Sciarra PA. Meniere’s disease: pathology and manifestations. Ann Otol Rhinol Laryngol. 1967;76:5–22. doi: 10.1177/000348946707600101. [DOI] [PubMed] [Google Scholar]
- Long GR, Talmadge CL, Lee J. Measuring distortion product otoacoustic emissions using continuously sweeping primaries. J Acoust Soc Am. 2008;124:1613–1626. doi: 10.1121/1.2949505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashkin AN, Russell IJ. Dependence of the DPOAE amplitude pattern on acoustical biasing of the cochlear partition. Hear Res. 2005;203:45–53. doi: 10.1016/j.heares.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Mammano F, Bortolozzi M, Ortolano S, Anselmi F. Ca2+ signaling in the inner ear. Physiology (Bethesda) 2007;22:131–144. doi: 10.1152/physiol.00040.2006. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Hensel J, Mrowinski D, Scholz G. Low-frequency characteristics of human and guinea pig cochleae. J Acoust Soc Am. 2007;121:3628–3638. doi: 10.1121/1.2722506. [DOI] [PubMed] [Google Scholar]
- Mauermann M, Kollmeier B. Distortion product otoacoustic emission (DPOAE) input/output functions and the influence of the second DPOAE source. J Acoust Soc Am. 2004;116:2199–2212. doi: 10.1121/1.1791719. [DOI] [PubMed] [Google Scholar]
- Merchant SN. Schuknecht’s pathology of the ear. Shelton: People’s Medical Publishing House; 2010. [Google Scholar]
- Merchant SN, Adams JC, Nadol JB., Jr Pathophysiology of Meniere’s syndrome: are symptoms caused by endolymphatic hydrops? Otol Neurotol. 2005;26:74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- Monsell E, Balkany T, Gates G, Goldenberg R, Meyerhoff W, House J. Committee on hearing and equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. Otolaryngol Head Neck Surg. 1995;111:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- Noffsinger PD, Tillman TW. Postexposure responsiveness in the auditory system. I. Immediate sensitization. J Acoust Soc Am. 1970;47:546–551. doi: 10.1121/1.1911927. [DOI] [PubMed] [Google Scholar]
- Noffsinger PD, Olsen WO. Postexposure responsiveness in the auditory system. II. Sensitization and desensitization. J Acoust Soc Am. 1970;47:552–564. doi: 10.1121/1.1911928. [DOI] [PubMed] [Google Scholar]
- Patuzzi R (2002) Outer hair cells, EP regulation and tinnitus. In: Proceedings of the Seventh International Tinnitus Seminar. (Patuzzi R ed). The University of Western Australia, Crawley.
- Patuzzi R. Ion flow in cochlear hair cells and the regulation of hearing sensitivity. Hear Res. 2011;280:3–20. doi: 10.1016/j.heares.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Patuzzi R, Wareing N (2002) Generation of transient tinnitus in humans using low-frequency tones and its mechanism. In: Proceedings of the Seventh International Tinnitus Seminar (Patuzzi R, ed), pp 16–24. The University of Western Australia, Crawley
- Robertson D. Effects of acoustic trauma on stereocilia structure and spiral ganglion cell tuning properties in the guinea pig cochlea. Hear Res. 1982;7:55–74. doi: 10.1016/0378-5955(82)90081-8. [DOI] [PubMed] [Google Scholar]
- Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hear Res. 1983;9:263–278. doi: 10.1016/0378-5955(83)90031-X. [DOI] [PubMed] [Google Scholar]
- Salt AN. Acute endolymphatic hydrops generated by exposure of the ear to nontraumatic low-frequency tones. J Assoc Res Otolaryngol. 2004;5:203–214. doi: 10.1007/s10162-003-4032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Plontke SK. Endolymphatic hydrops: pathophysiology and experimental models. Otolaryngol Clin North Am. 2010;43:971–983. doi: 10.1016/j.otc.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell WF. The effects of furosemide on the endocochlear potential and auditory-nerve fiber tuning curves in cats. Hear Res. 1984;14:305–314. doi: 10.1016/0378-5955(84)90057-1. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAES. JAcoustSocAm. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Hirohata ET. Sound calibration and distortion-product otoacoustic emissions at high-frequencies. Hear Res. 1994;80:146–152. doi: 10.1016/0378-5955(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Sirjani DB, Salt AN, Gill RM, Hale SA. The influence of transducer operating point on distortion generation in the cochlea. J Acoust Soc Am. 2004;115:1219–1229. doi: 10.1121/1.1647479. [DOI] [PubMed] [Google Scholar]
- Talmadge CL, Long GR, Tubis A, Dhar S. Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions. JAcoustSocAm. 1999;105:275–292. doi: 10.1121/1.424584. [DOI] [PubMed] [Google Scholar]
- Vernon J, Johnson R, Schleuning A. The characteristics and natural history of tinnitus in Meniere’s disease. Otolaryngol Clin North Am. 1980;13:611–619. [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekindt A, Gaese BH, Kossl M. Influence of contralateral acoustic stimulation on the quadratic distortion product f2-f1 in humans. Hear Res. 2009;247:27–33. doi: 10.1016/j.heares.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Young JA, Elliott SJ, Lineton B. Investigating the wave-fixed and place-fixed origins of the 2f(1)-f(2) distortion product otoacoustic emission within a micromechanical cochlear model. J Acoust Soc Am. 2012;131:4699–4709. doi: 10.1121/1.4707447. [DOI] [PubMed] [Google Scholar]
- Zwicker E, Hesse A. Temporary threshold shifts after onset and offset of moderately loud low-frequency maskers. J Acoust Soc Am. 1984;75:545–549. doi: 10.1121/1.390488. [DOI] [PubMed] [Google Scholar]