Abstract
Radioiodine therapy, the most effective form of systemic radiotherapy available, is currently useful only for thyroid cancer because of the thyroid-specific expression of the human sodium iodide symporter (hNIS). Here, we explore the efficacy of a novel form of gene therapy using prostate-specific membrane antigen (PSMA) promoter-mediated hNIS gene transfer followed by radioiodine administration for the treatment of castration-resistant prostate cancer (CRPC). The androgen-dependent C33 LNCaP cell line and the androgen-independent C81 LNCaP cell line were transfected by adenovirus. PSMA promoter-hNIS (Ad.PSMApro-hNIS) or adenovirus.cytomegalovirus–hNIS containing the cytomegalovirus promoter (Ad.CMV-hNIS) or a control virus. The iodide uptake was measured in vitro. The in vivo iodide uptake by C81 cell xenografts in nude mice injected with an adenovirus carrying the hNIS gene linked to PSMA and the corresponding tumor volume fluctuation were assessed. Iodide accumulation was shown in different LNCaP cell lines after Ad.PSMApro-hNIS and Ad.CMV-hNIS infection, but not in different LNCaP cell lines after adenovirus.cytomegalovirus (Ad.CMV) infection. At each time point, higher iodide uptake was shown in the C81 cells infected with Ad.PSMApro-hNIS than in the C33 cells (P < 0.05). An in vivo animal model showed a significant difference in 131I radioiodine uptake in the tumors infected with Ad.PSMApro-hNIS, Ad.CMV-hNIS and control virus (P < 0.05) and a maximum reduction of tumor volume in mice infected with Ad.PSMApro-hNIS. These results show prostate-specific expression of the hNIS gene delivered by the PSMA promoter and effective radioiodine therapy of CRPC by the PSMA promoter-driven hNIS transfection.
Keywords: genetic therapy, prostate-specific membrane antigen (PSMA), prostatic neoplasms, sodium-iodide symporter
INTRODUCTION
The human sodium iodide symporter (hNIS) is an intrinsic membrane glycoprotein with 13 putative transmembrane domains. hNIS is responsible for the ability of the thyroid gland to transport and concentrate iodide at levels approximately 20–40-fold above plasma concentration.1,2 Recently, the mechanism mediating active iodide transport across the basolateral membrane of the thyroid follicular cells has been clarified by cloning and characterization of hNIS.3,4,5 The expression of hNIS in thyroid cancer cells allows effective therapy with radioiodine, even in cases of advanced disease with distant metastases. This type of therapy contributes to the improved prognoses of these patients.6 A novel form of cytoreductive cancer gene therapy, using hNIS gene transfer to induce radioiodine accumulation activity in cancer cells, would extend the application of carrier-free radioiodine and the extensive experience with radioiodine in thyroid cancer therapy to the treatment of non-thyroidal cancer.7,8,9,10
Advanced prostate cancer (PCa) is initially sensitive to endocrine therapy, but easily becomes castration resistant with long-term endocrine therapy. When progressing to a castration-resistant stage, the PCa cells also acquire radiation resistance.11 At present, there is no curative therapy for castration-resistant PCa (CRPC). Previous reports have shown tissue-specific iodide uptake activity in PC cells in vitro and in vivo following adenovirus-mediated hNIS gene delivery.12 In this study, the androgen-independent human prostatic adenocarcinoma cell line LNCaP was stably transfected with the hNIS gene under the control of the prostate-specific membrane antigen (PSMA) promoter and prostate-specific iodide uptake activity, and the therapeutic response to the accumulated 131I were investigated.
MATERIALS AND METHODS
Cells and cell culture
The parental androgen-sensitive human LNCaP cell line was kindly donated by Prof. Jung Klaus (Department of Urology, University Hospital Charité, Humboldt University, Berlin, Germany) and cultured in Rosewell Park Memorial Institute (RPMI) 1640 medium (Life Technologies, Carlsbad, California, USA) supplemented with 10% fetal bovine serum (FBS) (Life Technologies, Carlsbad, USA) at 37°C in a 5% CO2 environment. The LNCaP cells were fed twice per week and split once per week with trypsinization as one passage. The passage number of LNCaP cells was designated as C-33 when the passage number was less than 33 and C-81 the passage number was greater than 80.13 For the steroid-reduced conditions, the LNCaP cells were grown in phenol red-free RPMI 1640 medium supplemented with 10% charcoal/dextran-treated FBS serum (CDS) (Life Technologies, Carlsbad, USA).
cDNA cloning of human sodium iodide symporter
The hNIS cDNA (2000bp) was cloned from human thyroid tissue by the reverse transcription-polymerase chain reaction (PCR) kit (TAKARA, DaLian, ShenYang, China). Total RNA was isolated from human thyroid tissue. Single-stranded oligo (dT)-primed cDNA was generated using superscript II reverse transcriptase. A PCR primer set was designed to amplify the entire coding region of the hNIS cDNA (forward, 5’-CGCGTCGACATGGAGGCCGTGGAGACCG-3’; reverse, 5’-CCAAGCTTTCAGAGGTTTG TCTCCTGCTGG-3’). PCR was carried out in a 50 μl aliquot containing 4 μl of each cDNA template, 50 pmol of each primer, 125 per μmol deoxynucleotide triphosphates, 1 unit of expand high fidelity PCR polymerase and 5 μl of a 10 × reaction buffer. The PCR amplification conditions were a 10 min initial denaturation at 95°C, followed by 40 cycles of 30 s at 95°C, 60 s at 60°C, 3 min at 72°C and a 7 min final extension at 72°C. The PCR product was ligated into the shuttle vector plasmid pShuttle.CMV using the rapid DNA ligation kit and the sequence of the insert was determined by DNA sequencing.
DNA cloning of the prostate-specific membrane antigen promoter
The PSMA promoter DNA sequence was cloned from the total DNA isolated from the androgen-sensitive human prostatic adenocarcinoma cell line LNCaP. A PCR primer set was designed to amplify the entire region of the PSMA promoter sequence (forward, 5’-GCGGTACCCTACTCAGCTGGCCCATGG-3’; reverse, 5’-CGCGTCGACTGTGCTGC TGCTCTACTGCG-3’). PCR was carried out in a 50 μl aliquot containing 1 μl of the total DNA template, 50 pmol of each primer, 150 μmol l−1 deoxynucleotide triphosphates, 1 unit of expand high fidelity PCR polymerase and 5 μl of a 10 × reaction buffer. The PCR amplification conditions were a 10 min initial denaturation at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 56°C, 2 min at 68°C and a 6 min final extension at 68°C. The PCR product was ligated into the recombinant shuttle vector plasmid pShuttle.hNIS using the rapid DNA ligation kit and the PSMApro sequence of the insert was determined by DNA sequencing.
Recombinant adenovirus production
The pShuttle.PSMApro-hNIS and pShuttle.CMV-hNIS were homologically recombined with the adenoviral backbone plasmid pAdEasy-1 in the bacteria BJ5183 (Stratagene, California, USA). The latter includes a nonspecific promoter sequence, while the former contains the PSMA promoter sequence. The positive recombinant adenoviral cosmids pAd.CMV-hNIS and pAd.PSMApro-hNIS were selected, packaged by liposomes and amplified in HEK293 cells to obtain the recombinant adenovirus. The replication-deficient human recombinant type 5 adenovirus carrying hNIS linked to the PSMA promoter (Ad.PSMApro-hNIS) and the CMV promoter (Ad.CMV-hNIS) were developed in collaboration with Vira Quest.14
Adenovirus-mediated hNIS gene delivery in LNCaP cells in vitro
LNCaP cells plated onto 12-well plates (1 × 105 cells per ml) were washed and incubated with OptiMEM (Life Technologies, Carlsbad, USA) containing the Ad.PSMApro-hNIS, Ad.CMV-hNIS or Ad.CMV for 3 h. The multiplicity of infection of PSMApro-NIS or Ad.CMV-hNIS or Ad.CMV in the different LNCaP cells was the same (30:1). The medium was replaced with fresh culture medium and the virus-infected cells were further maintained for 2-6 days before the iodide accumulation was measured to determine the levels of functional hNIS protein expression.
Iodide uptake studies in vitro
The uptake of 131I by the virus-infected LNCaP cells was determined at steady-state conditions as described by Weiss et al.15 There were four wells in each group. In brief, 2-6 days after infection, the iodide uptake studies were performed in Hank's balanced salt solution (HBSS) supplemented with 10 μ mol l−1 NaI, 0.1 μCi Na131I per ml and 10 m mol l−1 HEPES at pH 7.3. The trapped iodide was removed from the cells by 20 min incubation in 1 mol l−1 NaOH and was measured by γ-counting.
Adenovirus-mediated human sodium iodide symporter gene delivery in LNCaP cell xenografts in vivo
A total of 15, 4-week-old male castrated BALB/c nude mice (SPF) weighing 13–17 g (Centre of Experimental Animal, the Second Military Medical University, Shanghai, China) were used in this study. All animal experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act of 1986 and its associated guidelines, the EEC Directive of 1986 (86/609/EEC). The study was approved by the Ethical Committee of Changhai Hospital. The xenotransplants derived from the C-81 cells were established in mice by subcutaneous injections of 1 × 106 cells suspended in 0.25 ml of phenol red-free RPMI 1640 medium supplemented with 10% CDS medium and 0.25 ml of Matrigel basement membrane matrix. Following 6–8 weeks of tumor growth, once the tumor volume became palpable and measurable by callipers (approximately 5 mm), a total volume of 150 μl (3 × 109 plaque forming units per ml in 3% sucrose/phosphate buffered saline) of a recombinant Ad.PSMApro-hNIS and Ad.CMV-hNIS or control virus Ad.CMV was injected directly into the tumors using tuberculin syringes with a 28-gauge needle. The needle was moved to various sites within the tumor during the injection to maximize the area of virus exposure. Before the injections, the mice received a low-iodine diet and T4 supplementation (5 mg l−1 in their drinking water for 2 weeks to maximize the radioiodine uptake in the tumor and reduce the iodide uptake by the thyroid gland.
Iodide uptake studies in vivo
Four days after the intratumoral injection of Ad.PSMApro-hNIS and Ad.CMV-hNIS or control virus, five mice in each group were injected intraperitoneally with 500 μCi of 131I (Xinke, Shanghai, China), and radioiodine imaging was performed using a gamma camera equipped with a low energy general-purpose collimator (Toshiba, Tokyo, Japan). Every week, the radioiodine uptake by the tumors was monitored and quantified by imaging with a gamma camera. The tumor volume fluctuation was also recorded.
Statistics
We calculated descriptive statistics for each variable. Before the analysis, we examined each variable for its distributional characteristics. All the data are shown as the means ± standard deviation (s.d.). Statistical significance was defined as P < 0.05 for a two-tailed test. The calculation was performed using Statistical Analysis Software (SAS) 9.1.
RESULTS
Cell morphological features of the different LNCaP cells
The C-81 subgroup cells were established by continuous passaging of the C-33 LNCaP parental cells in RPMI 1640 medium. Compared to the C-33 parental cells, the cell body of the C-81 subgroup cells became smaller and more rounded (Figure 1). Even in the steroid-reduced conditions, the C-81 cells still survived and were able to be continuously passaged.
Figure 1.
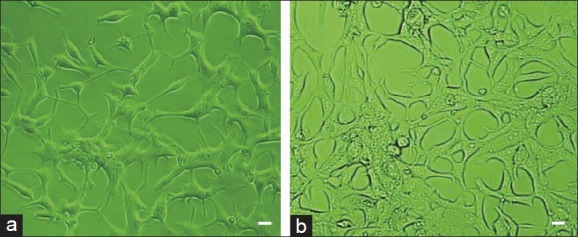
C-81 (a) cells (derived from C-33 parental cells) (b) had a smaller, rounded cell body. Scale bars = 10 μm
Iodide uptake studies in vitro
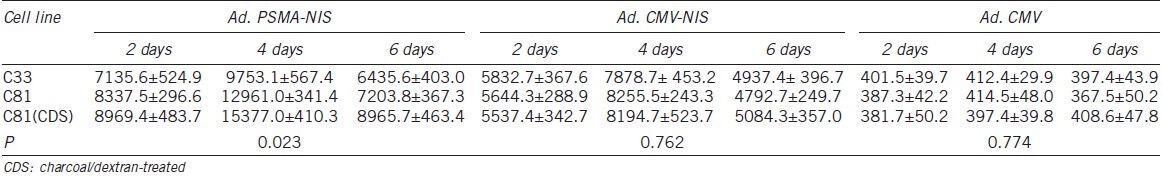
At each time point (2, 4 and 6 days) after adenovirus infection, the iodide uptake was measured (Table 1). Iodide accumulation was shown in the different LNCaP cell lines after Ad.PSMApro-hNIS or Ad.CMV-hNIS infection, but was not shown in the different LNCaP cell lines after Ad.CMV infection. At each time point, higher iodide uptake was shown in the C81 cells (FBS or CDS culture) infected with Ad.PSMApro-hNIS than in the C33 cells (P = 0.023). No difference was observed in the iodide uptake in the different LNCaP cell lines infected with Ad.CMV-hNIS (P = 0.762). The maximum iodide accumulation was shown at 4 days in the different LNCaP cells.
Table 1.
Iodide uptake by different LNCaP cell lines after Ad.PSMApro-hNIS, Ad.CMV-hNIS or Ad.CMV infection
Iodide uptake studies in vivo
Six weeks following the subcutaneous injection of the C-81 cells, when the tumors had reached a tumor size of approximately 5 mm in diameter, an intratumoral injection of Ad.PSMApro-hNIS and Ad.CMV-hNIS or control virus was performed. Four days after the adenovirus injection with the maximum iodide accumulation, the mice were given 500 μCi of 131I by intraperitoneal injection. The 131I radioiodine uptake in the tumors, thyroid, liver and spleen is shown in (Table 2). There was a difference in the 131I radioiodine uptake in the tumors infected with Ad.PSMApro-hNIS, Ad.CMV-hNIS and control virus (P = 0.0075), but no difference was observed in the 131I radioiodine uptake in the thyroid (P = 0.892). There was no iodide accumulation in the liver or the spleen. The maximum reduction in the tumor volume occurred in the mice infected with Ad.PSMApro-hNIS, (Figure 2) and the maximum reduction in the tumor volume in the Ad.PSMApro-hNIS and Ad.CMV-hNIS groups was 60.4% and 41.4%, respectively (P = 0.0222).
Table 2.
Iodide uptake by tumors derived from C81 cells infected with Ad.PSMApro-hNIS, Ad.CMV-hNIS or Ad.CMV and iodide uptake by the thyroid, liver and spleen of castrated nude mice
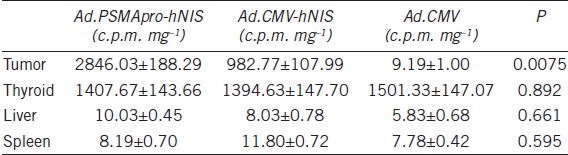
Figure 2.
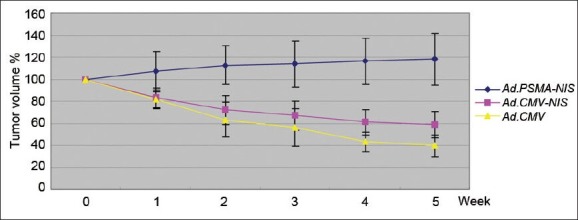
Four days following the intratumoral injection of Ad.PSMApro-hNIS and Ad.CMV-hNIS or control virus, five mice in each group were injected intraperitoneally with 500 μCi 131I. Every week, the radioiodine uptake by the tumors was monitored and quantified by imaging with a μ camera, and the tumor volume fluctuation is presented as the mean ± standard deviation (s.d)
DISCUSSION
hNIS can mediate the active transport of iodide in a variety of extrathyroidal tissues. We characterized hNIS as a novel therapeutic gene for potential use against cancers outside the thyroid gland, including breast cancer, melanoma tumors, cervical cancer, human glioma and hepatoma cell lines. As early as 2000, Spitzweg et al.14 reported radioiodine accumulation and therapeutic effectiveness of 131I in an NIS-transfected LNCaP cell line.
To avoid radiation exposure to additional organs, tissue-specific expression of hNIS is important. Thus, the strategy of transfecting hNIS cDNA under control of the prostate-specific promoter was used. Prostate-specific antigen (PSA) promoter is the most common, but has limited packaging capacity of the adenoviral vectors.12 The surviving promoter and the probasin promoter are also recommended for driving prostate-specific expression of hNIS.16,17 Few reports have disclosed the introduction of such exogenous promoters in CRPC.
In contrast to the above promoters, the PSMA promoter is highly expressed in a variety of PC cell lines, including both androgen-dependent or castration-resistant lines. The PSMA gene maps to a region of chromosome 11p11–12, with the promoter region containing a 1244 nt fragment that consists of approximately 1 kb of the 5’ flanking sequence from the most proximal start site and 200 nt of the 5’ untranslated region (5’UTR). Wright et al.18 disclosed the upregulation of PSMA in LNCaP cells after androgen deprivation treatment. Our study also found upregulation of PSMA in the C81 cells cultured in CDS medium compared with the C33 cells cultured in FBS-containing medium (data not shown). The fine prostate specificity of PSMA has been demonstrated, and less expression of PSMA in other tissues such as the normal duodenum, kidney, salivary gland and brain has been reported.19,20,21 In this study, we also found a higher iodide accumulation in the LNCaP cells infected with Ad.PSMApro-hNIS than those infected with Ad.CMV-hNIS, which further shows the fine prostate specificity of the PSMA promoter.
In this study, the C33 cells were androgen-sensitive LNCaP cells, but became androgen-independent C81 cells after continuous passage over 80 generations. The C81 cells can survive in the presence or absence of androgen. The differential iodide accumulation in the different LNCaP cells after Ad.PSMApro-hNIS infection demonstrates that the PSMA promoter is a good driver of the expression of hNIS in androgen-independent PCa. In an in vivo animal model from this study, the intratumoral injection of Ad.PSMApro-hNIS can greatly reduce the volume of tumor arising from the androgen-independent C81 cells. The above result suggests the possibility of PSMA promoter-driven hNIS for targeted radioiodine therapy of CRPC. We did not further evaluate PSMA promoter-driven hNIS for metastatic CRPC in this study. A recent study in a rat model showed that, instead of an intratumoral injection, intravenous administration is also safe and effective for transferring the hNIS gene to local and metastatic PC cells.22 The above study suggests the possibility of prostate-restricted hNIS gene transfer combined with 131I for treating metastatic CRPC in the clinic, although further work is needed.
In this study, the Ad.PSMApro-hNIS construct was able to cause the C81 cells to concentrate 131I by 2.9-fold over those infected with Ad.CMV-hNIS. This iodide concentrating ability exceeds that observed in normal thyroid cells and can explain the cytotoxic effect of radioactive iodide therapy in prostate tumors. High iodide uptake can cause potential damage to adjacent organs; however, in the in vivo animal model, no significant damage to the liver or the spleen was shown. Hypothyroidism is the most common complication of radioiodine therapy, but pretreating the patients with thyroid hormone might be advantageous because it would suppress the thyroid-stimulating hormone levels and thyroidal 131I uptake. Even in cases of hypothyroidism, the patients could be easily and inexpensively managed with thyroid hormone replacement therapy.
hNIS has been demonstrated to be a potentially therapeutic gene for human PC.23 Six men had clinically localised PCa and received an intraprostatic injection of Ad5-yCD/mutTK (SR39) rep-hNIS under transrectal ultrasound guidance. On multiple days after the adenovirus injection, the hNIS gene expression was detected in the prostate of all of the men (100%). On average, the gene was expressed throughout 45% (range 18%-83%) of the prostate volume. Although hNIS transfer as a targeted radioiodine therapy is only introduced for local PCa and no prostate-specific promoter has been used in hNIS transfer, the encouraging clinical outcomes show the promising future for the application of PSMA promoter-driven hNIS transfer for treating CRPC. In summary, transfer of the Ad.PSMApro-hNIS can result in high levels of hNIS expression in androgen-independent LNCaP cells. Because of its tissue specificity, Ad.PSMApro-hNIS is capable of limiting the radioactive iodine accumulation in the prostate tissue, thereby minimizing extratumoral cytotoxicity. When combined with 131I radioiodine therapy, Ad.PSMApro-hNIS provides a potential therapy for CRPC.
AUTHOR CONTRIBUTIONS
XFG participated in conceiving of the study and drafted the manuscript. GHC conducted the cell culture and animal model experiments and was involved in drafting the manuscript. TZ was responsible for drafting the manuscript, study design and statistics. CLX participated in the design of the study. YLD carried out the gene delivery, DNA and cDNA cloning, recombinant adenovirus production, and participated in drafting the manuscript. YHS conceived of the study and participated in its design and coordination and revised it critically for important intellectual content. All of the authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare that there are no competing interests.
ACKNOWLEDGMENTS
This project was funded by the National Natural Science Foundation of China (Nos. 30300351; 81070602).
REFERENCES
- 1.De La Vieja A, Dohan O, Levy O, Carrasco N. Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev. 2000;80:1083–105. doi: 10.1152/physrev.2000.80.3.1083. [DOI] [PubMed] [Google Scholar]
- 2.Spitzweg C, Heufelder AE, Morris JC. Thyroid iodine transport. Thyroid. 2000;10:321–30. doi: 10.1089/thy.2000.10.321. [DOI] [PubMed] [Google Scholar]
- 3.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–60. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 4.Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, et al. Cloning of the human sodium iodide symporter. Biochem Biophys Res Commun. 1996;226:339–45. doi: 10.1006/bbrc.1996.1358. [DOI] [PubMed] [Google Scholar]
- 5.Smanik PA, Ryu KY, Theil KS, Mazzaferri EL, Jhiang SM. Expression, exon–intron organization, and chromosome mapping of the human sodium iodide symporter. Endocrinol. 1997;138:3555–8. doi: 10.1210/endo.138.8.5262. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferri EL. Carcinoma of follicular epithelium: radioiodine and other treatments and outcomes. In: Braverman LE, Utiger RD, editors. The Thyroid: A Fundamental and Clinical Text. Lippincott-Raven: Philadelphia; 1996. pp. 922–45. [Google Scholar]
- 7.Cho JY, Xing S, Liu X, Buckwalter TL, Hwa L, et al. Expression and activity of human Na+/I − symporter in human glioma cells by adenovirus-mediated gene delivery. Gene Therapy. 2000;7:740–9. doi: 10.1038/sj.gt.3301170. [DOI] [PubMed] [Google Scholar]
- 8.Shimura H, Haraguchi K, Miyazaki A, Endo T, Onaya T. Iodide uptake and experimental 131I therapyin transplanted undifferentiated thyroid cancer cells expressingthe Na+/I − symporter gene. Endocrinol. 1997;138:4493–6. doi: 10.1210/endo.138.10.5571. [DOI] [PubMed] [Google Scholar]
- 9.Mandell RB, Mandell LZ, Link CJ., Jr Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res. 1999;59:661–8. [PubMed] [Google Scholar]
- 10.Boland A, Ricard M, Opolon P. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60:3484–92. [PubMed] [Google Scholar]
- 11.Xie BX, Zhang H, Yu L, Wang J, Pang B, et al. The radiation response of androgen-refractory prostate cancer cell line C4–2 derived from androgen-sensitive cell line LNCaP. Asian J Androl. 2010;12:405–14. doi: 10.1038/aja.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spitzweg C, Zhang S, Bergert ER, Castro MR, McIver B, et al. Prostate-specific antigen (PSA) promoter driven androgen-inducible expression of sodium iodide symporter in prostate cancer cell lines. Cancer Res. 1999;59:2136–41. [PubMed] [Google Scholar]
- 13.Igawa T, Lin FF, Lee MS, Karan D, Batra SK, et al. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50:222–35. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- 14.Spitzweg C, O’Connor MK, Bergert ER, Tindall DJ, Young CY, et al. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60:6526–30. [PubMed] [Google Scholar]
- 15.Weiss SJ, Philp NJ, Grollman EF. Iodine transport in a continous line of cultured cells from rat thyroid. Endocrinol. 1984;114:1090–8. doi: 10.1210/endo-114-4-1090. [DOI] [PubMed] [Google Scholar]
- 16.Trujillo MA, Oneal MJ, McDonough S, Qin R, Morris JC. A probasin promoter, conditionally replicating adenovirus that expresses the sodium iodide symporter (NIS) for radiovirotherapy of prostate cancer. Gene Ther. 2010;17:1325–32. doi: 10.1038/gt.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang R, Zhao Z, Ma X, Li S, Gong R, et al. Targeting of tumor radioiodine therapy by expression of the sodium iodide symporter under control of the survivin promoter. Cancer Gene Ther. 2011;18:144–52. doi: 10.1038/cgt.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright GL, Jr, Grob BM, Haley C, Grossman K, Newhall K, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–34. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 19.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–11. [PubMed] [Google Scholar]
- 20.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5. [PubMed] [Google Scholar]
- 21.O’Keefe DS, Su SL, Bacich DJ, Horiguchi Y, Luo Y, et al. Mapping, genomic organization and promoter analysis of the human prostate specific membrane antigen gene. Biochim Biophys Acta. 1998;1443:113–27. doi: 10.1016/s0167-4781(98)00200-0. [DOI] [PubMed] [Google Scholar]
- 22.Rajecki M, Sarparanta M, Hakkarainen T, Tenhunen M, Diaconu I, et al. SPECT/CT imaging of hNIS-expression after intravenous delivery of an oncolytic adenovirus and 131I. PLoS One. 2012;7:e32871. doi: 10.1371/journal.pone.0032871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barton KN, Stricker H, Elshaikh MA, Pegg J, Cheng J, et al. Feasibility of adenovirus-mediated hNIS gene transfer and 131I radioiodine therapy as a definitive treatment for localized prostate cancer. Mol Ther. 2011;19:1353–9. doi: 10.1038/mt.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]


