Abstract
Sex and sex hormones play a major role in lung physiology. It has been proposed that the ratio of the second to fourth digits (digit ratio) is correlated with fetal sex hormones. We therefore hypothesized that digit ratio might help predict lung function. We investigated the relationship between digit ratio and pulmonary function test (PFT) findings. A total of 245 South Korean patients (162 male, 83 female) aged from 34 to 90 years who were hospitalized for urological surgery were prospectively enrolled. Before administering the PFTs, the lengths of the second and fourth digits of the right hand were measured by a single investigator using a digital Vernier caliper. In males (n = 162), univariate and multivariate analysis using linear regression models showed that digit ratio was a significant predictive factor of forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) (FVC: r = 0.156, P = 0.047; FEV1: r = 0.160, P = 0.042). In male ever-smokers (n = 69), lung functions (FVC and FEV1) were correlated with smoking exposure rather than digit ratio. In female never-smokers (n = 83), lung functions (FEV1 and FEV1/FVC ratio) were positively correlated with digit ratio on univariate analysis (FEV1: r = 0.242, P = 0.027; FEV1/FVC ratio: r = 0.245, P = 0.026). Patients with lower digit ratios tend to have decreased lung function. These results suggest that digit ratio is a predictor of airway function.
Keywords: airway function, digit ratio, sex hormone
INTRODUCTION
Sex and sex hormones play a major role in lung physiology.1 With respect to lung function, males tended to be superior to females with the same anthropometric characteristics. However, after controlling for lung size, female performance surpassed that of males.2 In animal studies, the conducting airways of female mice have been proposed to be larger than those of males.3 In the male rat, combined administration of a synthetic potent progestin and estradiol for 5 days significantly increased tidal volume and minute expiratory ventilation.4
Sex hormones have been increasingly recognized as regulators of fetal and postnatal lung development.5 Fetal determinants of airway function may predispose individuals to adult-onset lung diseases such as asthma, chronic obstructive pulmonary disease (COPD) and cystic fibrosis.6 Lung morphogenesis is a highly regulated process that may be affected by the both genetic and environmental factors in utero. Importantly, all known sex steroid hormone receptors have been shown to be expressed in lung tissue.7 Sex hormones appear to exert regulatory effects on human lung development before and during the neonatal period. The androgen receptor is expressed in mesenchymal and epithelial cells of the lung throughout the human lifespan, and branching morphogenesis of the human lung may be regulated in part by androgen.7
The Hox gene is essential for normal development of the embryonic body plan.8 Several Hox genes are expressed at different time points in the developing lung8 and adult lung.8,9 Furthermore, several studies showed that Hox genes control the development of digits as well as the differentiation of testes and ovaries,10 and that the ratio of second to fourth digit length (digit ratio) might be related to Hox gene expression.11 Digit ratios differ between males and females. The mean digit ratio is lower in males than females.12,13,14 This gender-associated difference may be due to changes in prenatal steroid concentrations.15 Thus, digit ratio is primarily determined during embryonic development15,16 and changes little after sexual maturation.16,17 Recently, it has been reported that digit ratio was associated with testosterone levels in infertile men18 and the risk of prostate cancer19 and penile length.20,21
A previous study reported that the digit ratio of the right hand is negatively correlated with the fetal testosterone to fetal estradiol ratio.15 A high level of fetal testosterone relative to fetal estradiol would therefore result in a low digit ratio. Also, Zheng and Cohn22 reported that the digit ratio is controlled by the balance of androgen to estrogen signaling during digit development.22,23
From the above findings, we could speculate that the patterns of digit formation may be associated with lung development and airway physiology. We consequently hypothesized that digit ratio might be related to lung function. Therefore, the aim of the current study was to investigate the relationships between digit ratio and pulmonary function test (PFT) findings.
MATERIALS AND METHODS
Study participant selection
The study was approved by the Gachon University Gil Hospital Institutional Review Board (GIRBA2821–2012). A total of 245 South Korean patients (162 male, 83 female) aged from 34 to 90 years who were hospitalized for urological surgery at a single tertiary academic center were prospectively enrolled in our investigation. Informed consent was obtained from all participants. Patients with abnormal chest X-ray findings as well as those who had fingers amputated were excluded. Based on the smoking histories, ‘never-smokers’ were defined as those who had smoked on average <1 cigarette per day for <6 months or had never smoked. For the ever-smokers, pack-years were calculated to quantify tobacco use in which 1 pack-year was equivalent to smoking an average of 20 cigarettes per day for 1 year.
Digit measurement
As described in our previous study,20 lengths of the second and fourth digits of the right hand were measured prior to the PFTs by a single investigator using a digital Vernier caliper accurate to 0.01 mm directly on the ventral surface of the fingers, from the crease proximal to the palm at the base of each digit to the digit tip. To minimize measurement errors, the mean values of two digit ratios that were calculated based on duplicate measurements were used for the analysis. The intraclass correlation coefficient of two repeated digit ratio measurements by a single investigator was 0.949. This finding demonstrated that direct measurement of the digit ratio using a digital Vernier caliper has acceptable repeatability.
Pulmonary function tests
All 245 patients underwent spirometry before urological surgery. The tests were performed following the American Thoracic Society (ATS) guidelines.24,25 We did not measure post-bronchodilator responses.
Spirometry is a physiological test that measures how an individual inhales or exhales volumes of air as a function of time. The most important aspects of spirometry are the forced vital capacity (FVC), which is the volume delivered during an expiration made as forcefully and completely as possible starting from full inspiration, and the forced expiratory volume in one second (FEV1), which is the volume delivered in the first second of an FVC maneuver. The predicted value is the result of a formula that takes into account the race, sex, age, height and weight of the person. The presence of a post-bronchodilator FEV1/FVC ratio less than 0.70 confirms the presence of persistent airflow limitation and thus of COPD.24,25
Statistics
Relationships between the study variables were analyzed using Pearson's linear correlation. To identify the independent predictive factors influencing lung function, univariate and multivariate analyses were performed using linear regression modeling. Student's t-test was used to compare variables of the two study groups. All analyses were performed using Statistical Package for Social Sciences 12.0 (SPSS Inc., Chicago, IL, USA) and P < 0.05 was considered statistically significant.
RESULTS
Participant characteristics
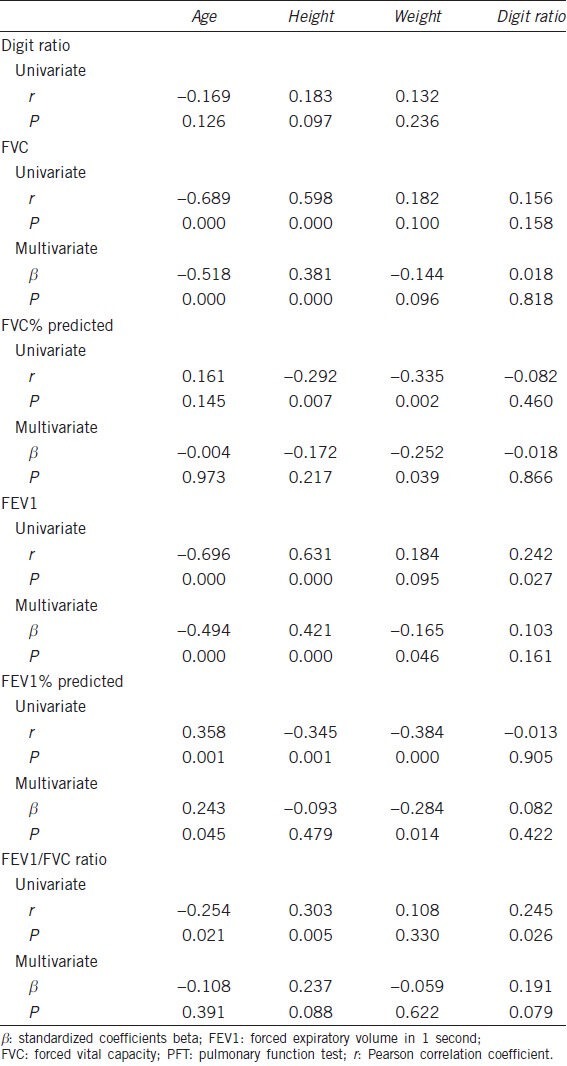
The characteristics of all 245 participants (162 male, 83 female) are summarized in Table 1. There were significant differences in study variables between male vs female. Men were younger than women (63.1 ± 7.0 vs 67.7 ± 9.6, P = 0.000). Digit ratios of men were higher than those of women (0.955 ± 0.035 vs 0.936 ± 0.030, P = 0.000). Of the PFT findings, FVC and FEV1 of men were higher than those of women. FVC% predicted, FEV1% predicted and FEV1/FVC ratio of men were lower than those of women (Table 1).
Table 1.
Characteristics of the study population
Correlation study of males (n = 162)
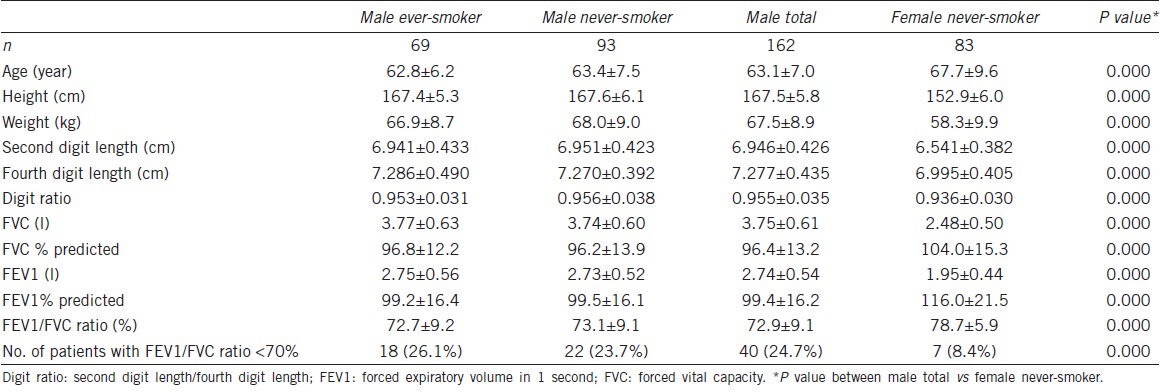
Significant negative correlations were found between age and PFT findings (FVC: r = −0.451, P = 0.000; FEV1: r = −0.555, P = 0.000; FEV1/FVC ratio: r = −0.222, P = 0.005) (Table 2). Age, height, weight and smoking history (pack-years) was not significantly correlated with digit ratio (age: r = −0.066, P = 0.404; height: r = −0.031, P = 0.697; weight: r = −0.017, P = 0.831; smoking history (pack-years): r = −0.072, P = 0.365) (Table 2). However, significant positive correlations were found between digit ratio and lung function on univariate analysis using linear regression models (FVC: r = 0.156, P = 0.047; FVC% predicted: r = 0.166, P = 0.035; FEV1: r = 0.160, P = 0.042, FEV1% predicted: r = 0.160, P = 0.042) (Table 2). Furthermore, the multivariate analysis using linear regression models showed that digit ratio was still a significant factor for predicting lung function (Table 2). Figures 1 and 2 show the relationship between digit ratio and PFT findings (FVC and FEV1). Both FVC and FEV1 were found to be positively associated with digit ratio (Figures 1 and 2).
Table 2.
Relationships between PFT findings and study variables in men (n=162)
Figure 1.
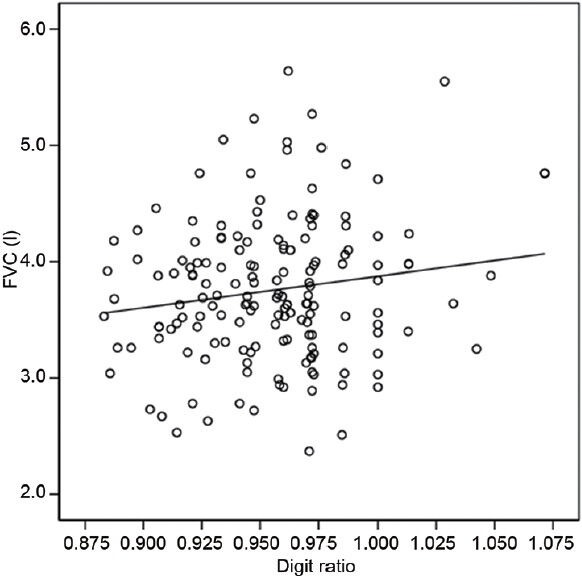
The relationship between digit ratio and forced vital capacity (FVC) in men. The digit ratio was found to be positively associated with FVC (r = 0.156, P = 0.047; y = 2.710x + 1.166, y: FVC, x: digit ratio).
Figure 2.
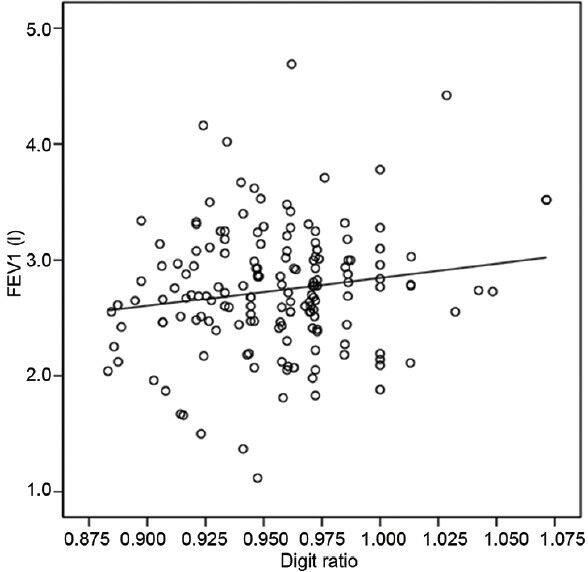
The relationship between digit ratio and forced expiratory volume in 1 second (FEV1) in men. The digit ratio was found to be positively associated with FEV1 (r = 0.160, P = 0.042; y = 2.446x + 0.402, y: FEV1, x: digit ratio).
Correlation study of females (n = 83)
Significant negative correlations were found between age and PFT findings (FVC: r = −0.689, P = 0.000; FEV1: r = −0.696, P = 0.000; FEV1/FVC ratio: r = −0.254, P = 0.021) (Table 3). Age, height and weight was not significantly correlated with digit ratio (age: r = −0.169, P = 0.126; height: r = 0.183, P = 0.097; weight: r = 0.132, P = 0.236) (Table 3). Significant positive correlations were found between digit ratio and lung function (FEV1 and FEV1/FVC ratio) on univariate analysis using linear regression models (FEV1: r = 0.242, P = 0.027; FEV1/FVC ratio: r = 0.245, P = 0.026) (Table 3). However, the significance disappeared on multivariate analysis using linear regression models (Table 3).
Table 3.
Relationships between PFT findings and study variables in women (n=83)
Smoking
Out of the 162 men, 69 (42.6%) were ever-smokers. Mean smoking exposure of ever-smokers (n = 69) was 31.2 ± 13.4 pack-years. However, all the females were never-smokers. When the male patients were divided into two groups according to smoking habits (ever-smoker, n = 69; never-smoker, n = 93), no significant differences in age, height, weight, digit ratio, FVC, FEV1 or FEV1/FVC ratio between two groups were observed (Table 1).
Among ever-smokers (69 male), digit ratio was not found to be statistically associated with FVC or FEV1, and there was no evidence of a dose-response relationship between tobacco use and airflow obstruction. However, smoking volume (in pack-years) was significantly correlated with a decline in lung function (FVC: r = −0.335, P = 0.009; FEV1: r = −0.361, P = 0.005) (Table 4).
Table 4.
Relationship between digit ratio and PFT findings of the study participants according to smoking history
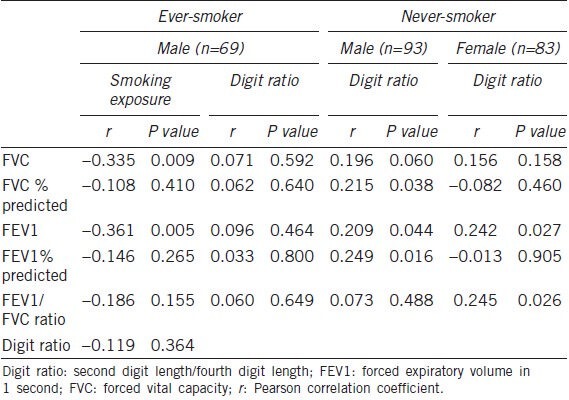
Among never-smokers (93 male, 83 female), significant positive correlations were found between digit ratio and lung function (Male: FVC: r = 0.196, P = 0.060; FVC % predicted: r = 0.215, P = 0.038; FEV1: r = 0.209, P = 0.044; FEV1% predicted: r = 0.249, P = 0.016; female: FEV1: r = 0.242, P = 0.027; FEV1/FVC ratio: r = 0.245, P = 0.026) (Table 4).
DISCUSSION
In the present study, we found that lung function was related to digit ratio, which is reflective of prenatal steroid hormone activity in an individual. However, we observed that this relationship was modified by smoking history. A lack of correlation between the digit ratio and adult lung function among ever-smokers, unlike never-smokers, indicates that smoking per se rather than digit ratio has a stronger relation to lung function. In general, it is well-known that increasing age and smoking are major risk factors for airway obstruction.26 Furthermore, the association between smoking, accelerated loss of lung function and COPD is well-established.27
Although cigarette smoking is known to be the major risk factor for COPD, 5%–10% of nonsmoking young adults and up to 30% of nonsmoking adults aged 60 or more have symptoms of COPD.28 Coupled with the finding that only a portion of smokers develops COPD with emphysema, these data clearly demonstrate that genetic susceptibility must play a significant part in COPD pathogenesis.6 Individuals who enter adult life with lung function deficits are more likely to develop COPD during the later adulthood.29 Therefore, poor airway function presenting shortly after birth is a risk factor for airflow obstruction development in early adult life, and it is possible that fetal determinants of airway function may predispose individuals to airflow obstruction and COPD.30 However, factors that determine normal or impaired pulmonary development in utero are not well-understood.
COPD has been traditionally regarded as a male-specific disease due to the difference in the prevalence of smokers between the sexes.31 Although there is controversy about the contribution of sex according to country,32,33 male sex was an independent risk factor for COPD after adjustment for smoking in Korea (prevalence of COPD in nonsmoker: male 14.6% vs female 7.6%).34 Furthermore, accumulating evidence suggests that gender impacts the incidence, susceptibility and severity of COPD. Many studies have addressed the role of sex hormones in the pathogenesis of COPD. It is now known that human airway smooth muscle is actively involved in the inflammatory process crucial for COPD pathogenesis. Androgens appear to be detrimental in the pathogenesis of lipopolysaccharide-induced airway inflammation and hyperresponsiveness.35 On the other hand, estrogen suppresses lung inflammatory responses through its effects on vascular cell adhesion molecules and proinflammatory cytokines.36 In other words, male sex hormones promote lung inflammatory responses, while female hormones ameliorate these processes.35,36
Several studies on human lung development have indicated that in utero milieu may influence adult lung function. Sex hormones appear to regulate human lung development before and during the neonatal period. Androgen receptors are expressed in mesenchymal and epithelial cells of the lung throughout the human lifespan, and branching morphogenesis of human lung may be regulated in part by androgens.7 Considerable experimental animal data suggest that sex hormones have a role in regulating lung development. Androgens have been shown to have inhibitory effects while estrogens were found to exert stimulatory effects.1
Respiratory distress syndrome mainly results from surfactant production deficiency related to incomplete fetal lung maturation at birth.37 In many species, lung maturation as measured by surfactant production is delayed in male fetuses compared with female fetuses.38 Maternal administration of estrogen accelerates lung maturation and stimulates surfactant production in fetal rabbits and rats.39 The earlier appearance of surfactants in female neonatal lungs favors the patency of small airways and airspaces, and may contribute to their higher airflow rate and lower airway resistance compared to neonatal males.40 Thus, a higher incidence of respiratory distress syndrome was reported in male versus female preterm neonates (1.7:1) more than 30 years ago.41 These mean that females get better results than males during lung development.5,38,39,40 Female lungs tend to be smaller and weigh less than those of males, and on average may contain fewer respiratory bronchioles at birth.42 These minor differences in lung structure and development can have major impacts on respiratory health in later life, demonstrating that gender and sex hormones are critical modulators of normal human lung development and maturation.
In our study, adult lung function was associated with digit ratio, which is reflective of in utero milieu (prenatal androgen exposures). These findings are supported by ones from studies about racial differences in in utero milieu and lung function between white and black males. The in utero milieu of black males is different from that of whites. Black women have higher maternal testosterone levels than white women.43 Additionally, a short CAG repeat length on the androgen receptor gene, which is related to high activity of androgen receptor, was associated with African-American rather than white men.44 The digit ratio of blacks is also lower than that of whites.45 Additionally, the Third National Health and Nutrition Examination Survey (1988–1994) and the Multi-Ethnic Study of Atherosclerosis Lung Study (2000–2006) showed that the mean lung function of never-smoking participants without respiratory diseases or symptoms was lower for African-Americans than whites after adjusting for age, gender and height.46 These findings indicated that the lower lung function of black men might be related to the in utero milieu of black men.
In another study, lung function during early adulthood was found to be associated with airway function measured shortly after birth.30 Between 1980 and 1984, investigators conducting the Tucson Children's Respiratory Study measured maximal expiratory flow at functional residual capacity (Vmax (FRC)) in 169 non-selected infants (mean age: 2.3 ± 1.9 months) using the chest compression technique. Lung function was again measured at least once when the study subjects were 11-, 16- and 22-years-old. Up to the age of 22, participants who had infant Vmax (FRC) values in the lowest quartile also had lower values for the FEV1/FVC ratio (−5.2%, P < 0.0001), FEF25-75 (−663 ml s−1, P < 0.0001) and FEV1 (−233 ml, P = 0.001) than those in the upper three quartiles combined after adjusting for height, weight, age and gender. These results indicated that decreased airway function observed shortly after birth might be related to airflow obstruction in young adults, and that COPD development could possibly start during the fetal stage.30
Based on the evidence above, we hypothesized that patterns of digit formation may be associated with lung development and airway physiology. One of the novel findings of our study was that adult lung function is related to digit ratio, which is reflective of prenatal steroid hormone activity among individuals. In the present study, participants with a lower digit ratio tended to have decreased lung function (Tables 2-4)]. In males, univariate and multivariate analysis using linear regression models showed that digit ratio was a significant predictive factor for FVC and FEV1 (FVC: r = 0.156, P = 0.047; FEV1: r = 0.160, P = 0.042) (Table 2). These results suggest that digit ratio is related to airway physiology influenced by sex hormones, and that the in utero milieu might impact adult lung function.
Unlike male, digit ratio, when adjusted for other factors, is not an independent predictive factor for adult lung function in women (Table 3). However, significant positive correlations were clearly found between digit ratio and lung function (FEV1 and FEV1/FVC ratio) on univariate analysis using linear regression models (FEV1: r = 0.242, P = 0.027; FEV1/FVC ratio: r = 0.245, P = 0.026) (Table 3). These results also suggest that digit ratio is related to lung function in women.
Our data in the present study were against a background of numerous research demonstrating negative relationships between digit ratio and sports performance.47,48,49 To date, it has been suggested that among several components of physical fitness (e.g., cardiorespiratory fitness, muscle strength and endurance, flexibility and glucose tolerance),50 athletes’ maximal ability to consume oxygen (VO2max) is an important determinant of long distance running speed.51 Hill et al.52 suggested that VO2max explained the relationship between digit ratio and sports performance. They showed that low right-left digit ratio is associated with high VO2max in a sample of teenage boys.52 However, these studies about digit ratio did not investigate the direct association with lung function but investigated the relationship with sports performance. Actually, lung function is not directly related to VO2max. FEV1 has served as an important diagnostic measurement of COPD, but has not been found to correlate with exercise capacity in patients with chronic airway obstruction.53,54 In general, it has been known that the major factors limiting VO2max is O2 transport by the circulation.55,56 Actually, alveolar ventilation is negligible in normoxia.56
Unlike previous studies about digit ratio,12,57 the women in the present study had lower digit ratios than the men (0.936 ± 0.030 vs 0.955 ± 0.035, P = 0.000) (Table 1). In addition, the mean value of the females’ digit ratio was lower than that of males in our previous studies (0.952,19 0.97,20 0.951,58 and 0.94859. However, in one study conducted among 664 (332 males, 332 females) university students (mean age: male: 22.4 years, female: 21 years), the digit ratio in Korean men was significantly lower than that of Korean women (0.96 vs 0.97, P < 0.001).60 We could not properly explain why this was. However, we think that the characteristics of the study population might be one possible explanation. The participants of the present study were not a general population but urological patients who were hospitalized for urological surgery at a single tertiary academic center. Furthermore, unlike many other studies about digit ratio,12,57 the age of the study population in the present study is very high (male: 63.1 ± 7.0 years; female: 67.7 ± 9.6 years) (Table 1). However, there are not enough data from the present study to speculate about why females have a lower digit ratio than males.
We performed this study based on the assumption that digit ratio is reflective of steroid hormone activity among individuals. Thus, we did not directly measure the steroid hormone activity in the study participants and only calculated digit ratios. In other words, we only indirectly assessed the relationship between PFT findings and steroid hormone activity indicated by the right hand digit ratio. We believe that this is a limitation of our study. Nevertheless, we are confident that our results provide sufficient evidence about the relationship between steroid hormone activity and adult lung function.
Another limitation is that we enrolled patients from a very specific setting, and this lacks generalizability of the observations to other populations, given the limited demographics of the study population. However, in our data, the prevalence of airway obstruction (FEV1/FVC ratio <70%) were 24.7% in men and 8.4% in women (Table 1), which was similar to previously reported COPD prevalence among Korean adults over the age of 45 years (men: 25.8%, women: 9.6%).34
CONCLUSIONS
Our study demonstrated that patients with lower digit ratios tend to have decreased lung function. The results suggest that digit ratio, along with smoking and increasing age, is a predictor of airway function and that the in utero milieu might impact adult lung function. These findings have important public health implications.
AUTHOR CONTRIBUTIONS
INP conceived of the study and involved in drafting the manuscript. HKY participated in the design of the study and performed the interpretation of data. SCL participated in collecting the data and performed the statistical analysis. JKO participated in evaluating the data and performed the statistical analysis. TBK conceived of the study, and participated in its coordination and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by Inje University Research Grant.
REFERENCES
- 1.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, et al. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293:L272–8. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz J, Katz SA, Fegley RW, Tockman MS. Sex and race differences in the development of lung function. Am Rev Respir Dis. 1988;138:1415–21. doi: 10.1164/ajrccm/138.6.1415. [DOI] [PubMed] [Google Scholar]
- 3.Reinhard C, Eder G, Fuchs H, Ziesenis A, Heyder J, et al. Inbred strain variation in lung function. Mamm Genome. 2002;13:429–37. doi: 10.1007/s00335-002-3005-6. [DOI] [PubMed] [Google Scholar]
- 4.Tatsumi K, Mikami M, Kuriyama T, Fukuda Y. Respiratory stimulation by female hormones in awake male rats. J Appl Physiol (1985) 1991;71:37–42. doi: 10.1152/jappl.1991.71.1.37. [DOI] [PubMed] [Google Scholar]
- 5.Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab. 2010;21:729–38. doi: 10.1016/j.tem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest. 2007;132:651–6. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]
- 7.Kimura Y, Suzuki T, Kaneko C, Darnel AD, Akahira J, et al. Expression of androgen receptor and 5alphareductase types 1 and 2 in early gestation fetal lung: a possible correlation with branching morphogenesis. Clin Sci (Lond) 2003;105:709–13. doi: 10.1042/CS20030236. [DOI] [PubMed] [Google Scholar]
- 8.Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 9.Bogue CW, Lou LJ, Vasavada H, Wilson CM, Jacobs HC. Expression of Hoxb genes in the developing foregut and lung. Am J Respir Cell Mol Biol. 1996;15:163–71. doi: 10.1165/ajrcmb.15.2.8703472. [DOI] [PubMed] [Google Scholar]
- 10.Zákány J, Formental-Ramian C, Warot X, Duboule D. Regulation of the number and size of the digits by posterior Hox genes: a dose-dependent mechanism with potential evolutionary implications. Proc Natl Acad Sci U S A. 1997;94:13695–700. doi: 10.1073/pnas.94.25.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugie Y, Sugie H, Fukuda T, Osawa J. Study of HOXD genes in autism particularly regarding the ratio of second to fourth digit length. Brain Dev. 2010;32:356–61. doi: 10.1016/j.braindev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Hönekopp J, Watson S. Meta-Analysis of digit ratio 2D: 4D shows greater sex difference in the right hand. Am J Hum Biol. 2010;22:619–30. doi: 10.1002/ajhb.21054. [DOI] [PubMed] [Google Scholar]
- 13.Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13:3000–4. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- 14.McFadden D, Shubel E. Relative lengths of fingers and toes in human males and females. Horm Behav. 2002;42:492–500. doi: 10.1006/hbeh.2002.1833. [DOI] [PubMed] [Google Scholar]
- 15.Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev. 2004;77:23–8. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Garn SM, Burdi AR, Babler WJ, Stinson S. Early prenatal attainment of adult metacarpal-phalangeal rankings and proportions. Am J Phys Anthropol. 1975;43:327–32. doi: 10.1002/ajpa.1330430305. [DOI] [PubMed] [Google Scholar]
- 17.Manning JT, Stewart A, Bundred PE, Trivers RL. Sex and ethnic differences in 2nd to 4th digit ratio of children. Early Hum Dev. 2004;80:161–8. doi: 10.1016/j.earlhumdev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Manning JT, Wood S, Vang E, Walton J, Bundred PE, et al. Second to fourth digit ratio (2D: 4D) and testosterone in men. Asian J Androl. 2004;6:211–5. [PubMed] [Google Scholar]
- 19.Jung H, Kim KH, Yoon SJ, Kim TB. Second to fourth digit ratio: a predictor of prostate-specific antigen level and the presence of prostate cancer. BJU Int. 2011;107:591–6. doi: 10.1111/j.1464-410X.2010.09490.x. [DOI] [PubMed] [Google Scholar]
- 20.Choi IH, Kim KH, Jung H, Yoon SJ, Kim SW, et al. Second to fourth digit ratio: a predictor of adult penile length. Asian J Androl. 2011;13:710–4. doi: 10.1038/aja.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McQuade DB. Does digit ratio (2D: 4D) predict penile length? Asian J Androl. 2011;13:667–8. doi: 10.1038/aja.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Z, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. Proc Natl Acad Sci U S A. 2011;108:16289–94. doi: 10.1073/pnas.1108312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning JT. Resolving the role of prenatal sex steroids in the development of digit ratio. Proc Natl Acad Sci U S A. 2011;108:16143–4. doi: 10.1073/pnas.1113312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 26.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–43. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannino DM, Watt G, Hole D, Gillis C, Hart C, et al. The natural history of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:627–43. doi: 10.1183/09031936.06.00024605. [DOI] [PubMed] [Google Scholar]
- 29.Weiss ST, Ware JH. Overview of issues in the longitudinal analysis of respiratory data. Am J Respir Crit Care Med. 1996;154:S208–11. doi: 10.1164/ajrccm/154.6_Pt_2.S208. [DOI] [PubMed] [Google Scholar]
- 30.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–64. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J. 1995;8:1398–420. doi: 10.1183/09031936.95.08081398. [DOI] [PubMed] [Google Scholar]
- 32.Bridevaux PO, Probst-Hensch NM, Schindler C, Curjuric I, Felber Dietrich D, et al. Prevalence of airflow obstruction in smokers and never-smokers in Switzerland. Eur Respir J. 2010;36:1259–69. doi: 10.1183/09031936.00004110. [DOI] [PubMed] [Google Scholar]
- 33.Hagstad S, Ekerljung L, Lindberg A, Backman H, Rönmark E, et al. COPD among non-smokers-report from the obstructive lung disease in Northern Sweden (OLIN) studies. Respir Med. 2012;106:980–8. doi: 10.1016/j.rmed.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Kim DS, Kim YS, Jung KS, Chang JH, Lim CM, et al. Korean Academy of Tuberculosis and Respiratory Diseases. Prevalence of chronic obstructive pulmonary disease in Korea. Am J Respir Crit Care Med. 2005;172:842–7. doi: 10.1164/rccm.200502-259OC. [DOI] [PubMed] [Google Scholar]
- 35.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177:621–30. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, et al. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol. 2005;288:C881–90. doi: 10.1152/ajpcell.00467.2004. [DOI] [PubMed] [Google Scholar]
- 37.Grenache DG, Gronowski AM. Fetal lung maturity. Clin Biochem. 2006;39:1–10. doi: 10.1016/j.clinbiochem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Torday JS, Nielsen HC, Fencl Mde M, Avery ME. Sex differences in fetal lung maturation. Am Rev Respir Dis. 1981;123:205–8. doi: 10.1164/arrd.1981.123.2.205. [DOI] [PubMed] [Google Scholar]
- 39.Possmayer F, Casola PG, Chan F, MacDonald P, Ormseth MA, et al. Hormonal induction of pulmonary maturation in the rabbit fetus: effects of maternal treatment with estradiol-17 beta on the endogenous levels of cholinephosphate, CDP-choline and phosphatidylcholine. Biochim Biophys Acta. 1981;664:10–21. doi: 10.1016/0005-2760(81)90024-2. [DOI] [PubMed] [Google Scholar]
- 40.Doershuk CF, Fisher BJ, Matthews LW. Specific airway resistance from the perinatal period into adulthood. Alterations in childhood pulmonary disease. Am Rev Respir Dis. 1974;109:452–7. doi: 10.1164/arrd.1974.109.4.452. [DOI] [PubMed] [Google Scholar]
- 41.Farrell PM, Avery ME. Hyaline membrane disease. Am Rev Respir Dis. 1975;111:657–88. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- 42.Thurlbeck WM. Postnatal human lung growth. Thora×. 1982;37:564–71. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troisi R, Potischman N, Roberts J, Siiteri P, Daftary A, et al. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States) Cancer Causes Control. 2003;14:347–55. doi: 10.1023/a:1023934518975. [DOI] [PubMed] [Google Scholar]
- 44.Bennett CL, Price DK, Kim S, Liu D, Jovanovic BD, et al. Racial variation in CAG repeat lengths within the androgen receptor gene among prostate cancer patients of lower socioeconomic status. J Clin Oncol. 2002;20:3599–604. doi: 10.1200/JCO.2002.11.085. [DOI] [PubMed] [Google Scholar]
- 45.Manning JT. London: Faber and Faber; 2008. The finger book: sex, behaviour and disease revealed in the fingers. [Google Scholar]
- 46.Kiefer EM, Hankinson JL, Barr RG. Similar relation of age and height to lung function among Whites, African Americans, and Hispanics. Am J Epidemiol. 2011;173:376–87. doi: 10.1093/aje/kwq417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manning JT, Hill MR. Digit ratio (2D: 4D) and sprinting speed in boys. Am J Hum Biol. 2009;21:210–3. doi: 10.1002/ajhb.20855. [DOI] [PubMed] [Google Scholar]
- 48.Manning JT, Morris L, Caswell N. Endurance running and digit ratio (2D: 4D): implications for fetal testosterone effects on running speed and vascular health. Am J Hum Biol. 2007;19:416–21. doi: 10.1002/ajhb.20603. [DOI] [PubMed] [Google Scholar]
- 49.Hönekopp J, Schuster M. A meta-analysis on 2D: 4D and athletic prowess: substantial relationships but neither hand out-predicts the other. Pers Individ Dif. 2010;48:4–10. [Google Scholar]
- 50.Bouchard C, Shepard RJ, Stephens T. Champaign: Human Kinetics Publishers; 1994. Physical activity, fitness, and health: international proceedings and consensus statement. [Google Scholar]
- 51.Foster C, Costill DL, Daniels JT, Fink WJ. Skeletal muscle enzyme activity, fiber composition and VO2 max in relation to distance running performance. Eur J Appl Physiol Occup Physiol. 1978;39:73–80. doi: 10.1007/BF00421711. [DOI] [PubMed] [Google Scholar]
- 52.Hill R, Simpson B, Millet G, Manning J, Kilduff L. Right-left digit ratio (2D: 4D) and maximal oxygen uptake. J Sports Sci. 2012;30:129–34. doi: 10.1080/02640414.2011.637947. [DOI] [PubMed] [Google Scholar]
- 53.Foglio K, Carone M, Pagani M, Bianchi L, Jones PW, et al. Physiological and symptom determinants of exercise performance in patients with chronic airway obstruction. Respir Med. 2000;94:256–63. doi: 10.1053/rmed.1999.0734. [DOI] [PubMed] [Google Scholar]
- 54.Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med. 2006;119:21–31. doi: 10.1016/j.amjmed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 56.di Prampero PE. Factors limiting maximal performance in humans. Eur J Appl Physiol. 2003;90:420–9. doi: 10.1007/s00421-003-0926-z. [DOI] [PubMed] [Google Scholar]
- 57.Zhao D, Li B, Yu K, Zheng L. Digit ratio (2D: 4D) and handgrip strength in subjects of Han ethnicity: impact of sex and age. Am J Phys Anthropol. 2012;149:266–71. doi: 10.1002/ajpa.22130. [DOI] [PubMed] [Google Scholar]
- 58.Kim TB, Oh JK, Kim KH, Jung H, Yoon SJ, et al. Dutasteride, who is it more effective for? Second to fourth digit ratio and the relationship with prostate volume reduction by dutasteride treatment. BJU Int. 2012;110:E857–63. doi: 10.1111/j.1464-410X.2012.11343.x. [DOI] [PubMed] [Google Scholar]
- 59.Oh JK, Kim KH, Jung H, Yoon SJ, Kim TB. Second to fourth digit ratio: its relationship with core cancer volume and Gleason score in prostate biopsy. Int Braz J Urol. 2012;38:611–9. doi: 10.1590/s1677-55382012000500005. [DOI] [PubMed] [Google Scholar]
- 60.Kim SI, Cho KJ. Second to Fourth Digit Ratio as a Sex Determinant in Korean. Korean J Phys Anthropol. 2012;25:137–44. [Google Scholar]


