Abstract
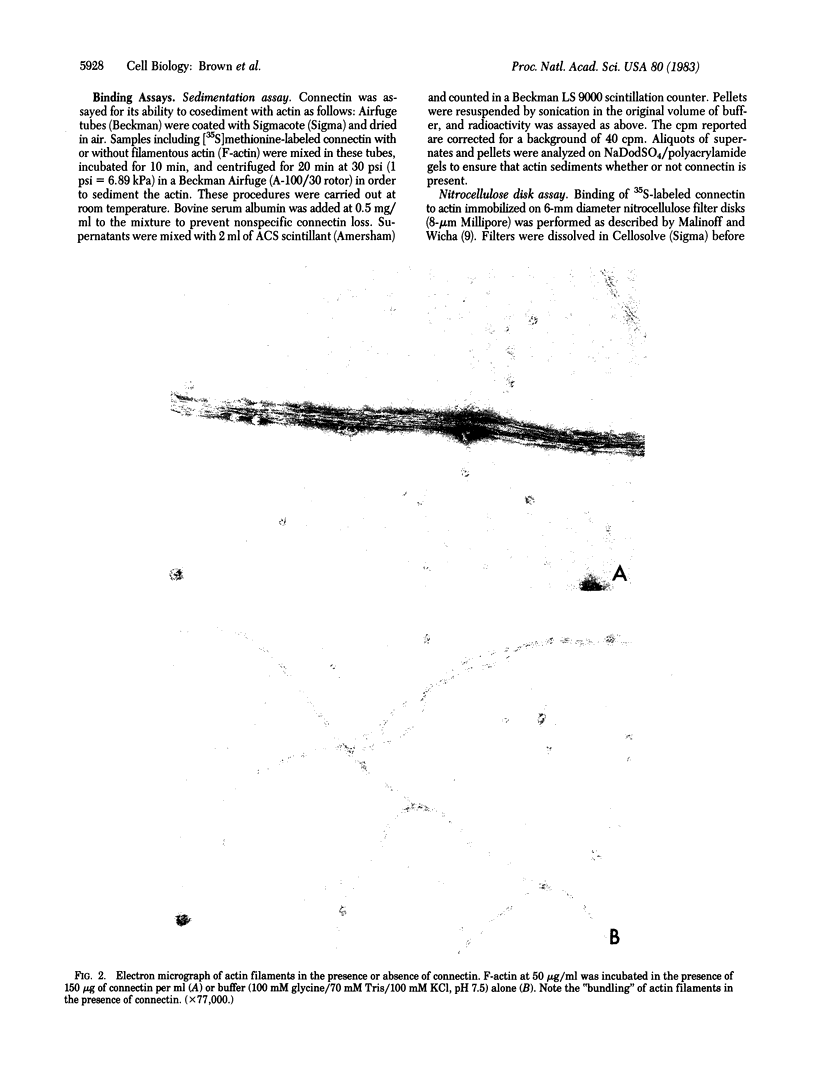
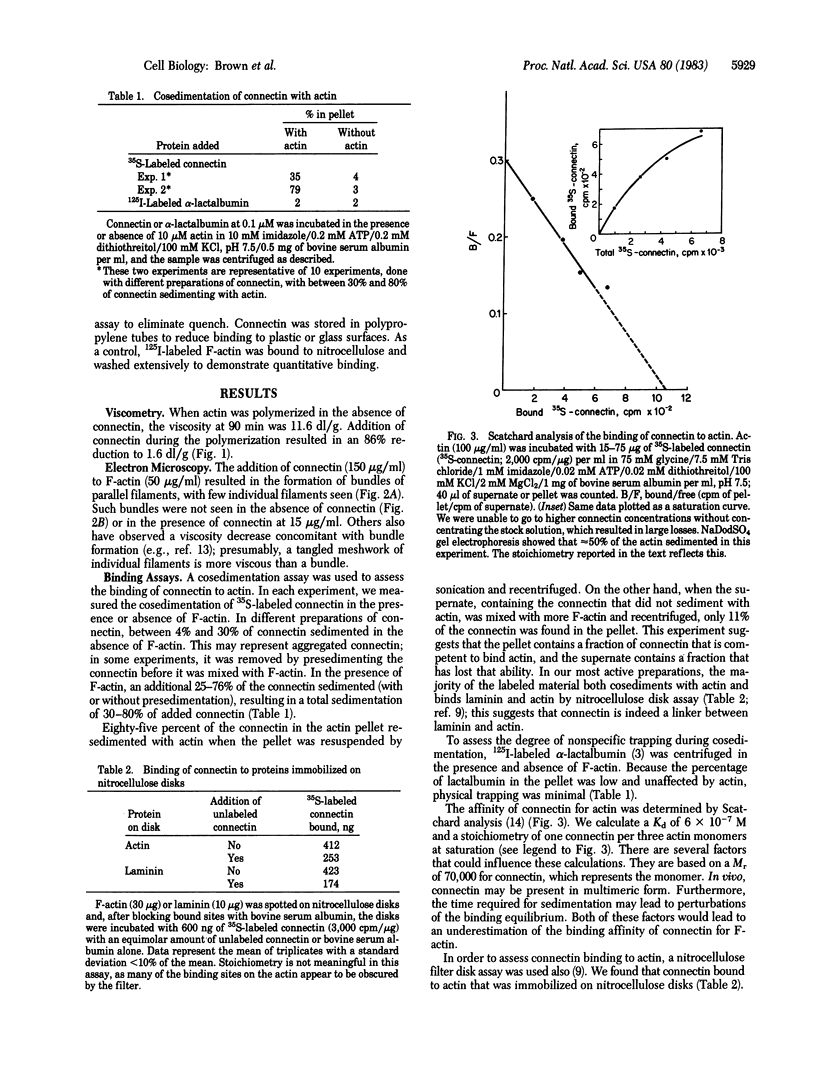
A purified cell surface receptor protein for laminin (Mr = 70,000) isolated from mouse fibrosarcoma cells binds to actin with specificity and high affinity. This binding was demonstrated both by cosedimentation of the receptor with actin and binding of the receptor to actin immobilized on nitrocellulose filters. Specificity was demonstrated by displacement of 35S-labeled receptor by unlabeled receptor. Scatchard analysis of receptor binding to actin yielded a Kd of 6 X 10(-7) M. The receptor was observed to reduce the viscosity of actin filaments. It also caused the formation of bundles of parallel filaments. This observation and the stoichiometry of binding suggest that the receptor binds along the sides of actin filaments. Based on the ability of this receptor to bind both extracellular laminin and intracellular actin, we have named this protein "connectin." Connectin may be an example of a transmembrane protein that is capable of mediating the interaction of a cell with its extracellular matrix.
Full text
PDF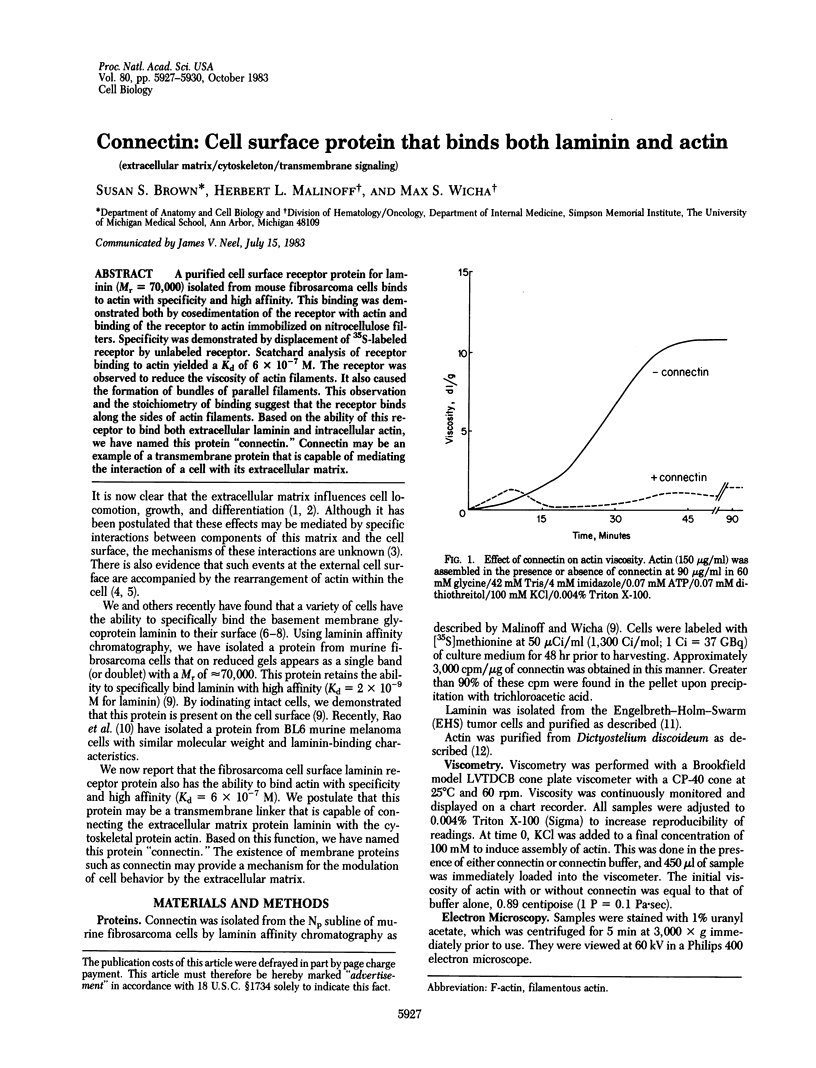

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg D. A., Rodewald R., Rebhun L. I. The visualization of actin filament polarity in thin sections. Evidence for the uniform polarity of membrane-associated filaments. J Cell Biol. 1978 Dec;79(3):846–852. doi: 10.1083/jcb.79.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L. Y., Singer S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5031–5035. doi: 10.1073/pnas.74.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway C. A., Jung G., Carraway K. L. Isolation of actin-containing transmembrane complexes from ascites adenocarcinoma sublines having mobile and immobile receptors. Proc Natl Acad Sci U S A. 1983 Jan;80(2):430–434. doi: 10.1073/pnas.80.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Tyler J. M., Branton D. Spectrin-actin associations studied by electron microscopy of shadowed preparations. Cell. 1980 Oct;21(3):875–883. doi: 10.1016/0092-8674(80)90451-1. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Hök M., Rees D. A., Timpl R. Adhesion, growth, and matrix production by fibroblasts on laminin substrates. J Cell Biol. 1983 Jan;96(1):177–183. doi: 10.1083/jcb.96.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edds K. T. Microfilament bundles. I. Formation with uniform polarity. Exp Cell Res. 1977 Sep;108(2):452–456. doi: 10.1016/s0014-4827(77)80056-6. [DOI] [PubMed] [Google Scholar]
- Flanagan J., Koch G. L. Cross-linked surface Ig attaches to actin. Nature. 1978 May 25;273(5660):278–281. doi: 10.1038/273278a0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Zumbe A., Vassalli P. Actin and tubulin co-cap with surface immunoglobulins in mouse B lymphocytes. Nature. 1977 Oct 20;269(5630):697–698. doi: 10.1038/269697a0. [DOI] [PubMed] [Google Scholar]
- Giuffre R. M., Tovell D. R., Kay C. M., Tyrrell D. L. Evidence for an interaction between the membrane protein of a paramyxovirus and actin. J Virol. 1982 Jun;42(3):963–968. doi: 10.1128/jvi.42.3.963-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B. M., Isenberg G. Interaction of alpha-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci U S A. 1981 May;78(5):3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. L., Smith M. J. An association between actin and the major histocompatibility antigen H-2. Nature. 1978 May 25;273(5660):274–278. doi: 10.1038/273274a0. [DOI] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S., Tilney L. G. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol. 1975 Dec;67(3):725–743. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund R. E., Leung J. T., Kipnis D. M. Muscle actin filaments bind pituitary secretory granules in vitro. J Cell Biol. 1977 Apr;73(1):78–87. doi: 10.1083/jcb.73.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Guild B. C., Strominger J. L., Veatch W. R. Purification of HLA-A2 antigen, fluorescent labeling of its intracellular region, and demonstration of an interaction between fluorescently labeled HLA-A2 antigen and lymphoblastoid cell cytoskeleton proteins in vitro. Biochemistry. 1981 Sep 15;20(19):5625–5633. doi: 10.1021/bi00522a042. [DOI] [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Sugrue S. P., Hay E. D. Response of basal epithelial cell surface and Cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol. 1981 Oct;91(1):45–54. doi: 10.1083/jcb.91.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist K. G., Ehrnst A. Cytoskeletal control of surface membrane mobility. Nature. 1976 Nov 18;264(5583):226–231. doi: 10.1038/264226a0. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Toh B. H., Hard C. C. Actin co-caps with concanavalin A receptors. Nature. 1977 Oct 20;269(5630):695–697. doi: 10.1038/269695a0. [DOI] [PubMed] [Google Scholar]
- Tomasek J. J., Hay E. D., Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, alpha-actinin, and myosin. Dev Biol. 1982 Jul;92(1):107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Utsumi K., Okimasu E., Takehara Y., Watanabe S., Miyahara M., Moromizato Y. Interaction of cytoplasmic proteins with liposomes and their cell specificity. FEBS Lett. 1981 Feb 23;124(2):257–260. doi: 10.1016/0014-5793(81)80150-0. [DOI] [PubMed] [Google Scholar]
- Uyemura D. G., Brown S. S., Spudich J. A. Biochemical and structural characterization of actin from Dictyostelium discoideum. J Biol Chem. 1978 Dec 25;253(24):9088–9096. [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982 May;79(10):3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]





