Abstract
With recent advances in the instrumentation and with increased expertise the results of microvascular surgery are getting better. Complications though, cannot be completely avoided. This paper gives a brief introduction to the possible complications at various stages of free tissue transfer. With careful planning and execution and vigilant postoperative care the overall success rate can be improved.
KEY WORDS: Free tissue transfer, re-exploration, unfavourable results
INTRODUCTION
Microsurgical free tissue transfer has vastly expanded the reconstructive surgeon's repertoire and enabled us to think of a variety of reconstructive possibilities, which would have been impossible to conceive in the pre-microsurgical era. Most large series of free flaps published over the last two decades have cited success rates in the range of 95-99%.[1–7] Nevertheless, microsurgical free tissue transfer is a technically demanding exercise which requires meticulous attention to planning and execution. In spite of improving equipment, better training facilities and a large amount of experience; failures and complications still do occur and these can be a source of considerable heartburn to the surgical team as well as a great deal of anguish to patients and their well-wishers.
The aim of free tissue transfer is restoration of anatomy to near normal; with the least amount of trauma and scarring. Though the concept of ‘ideal donor site’ or ‘ideal wound healing’ is still elusive, optimum coverage/healing of the defect and minimum residual scarring and functional problems in the donor site are the goals.[7,8] Any deviation from these expected end results can be termed ‘unfavourable’. For the sake of order, we can divide unfavourable results into six categories:
Complete flap failure: Essentially failure of the microvascular anastomosis
Partial flap failure: Part of the flap is lost
The flap survives, but fails to achieve the desired goal completely
Unacceptable/significant donor site morbidity
Other medical complications
Unsatisfied patients: Where all the intended surgical goals have been achieved, but the patient is not satisfied. Essentially a failure of communication.
COMPLETE FLAP FAILURE
Complete flap loss is a dramatic event. The failure may be attributable to patient-related factors or to surgery/anaesthesia related factors. Sometimes, the aetiology may be multi-factorial and at other times, a flap may fail without any discernible cause. Meticulous planning and execution at different stages will maximize the chances of a successful outcome.
Pre-operative flap-planning and patient optimization is the most important step. Most free tissue transfers (unlike re-plantations) are elective procedures and the available time should be utilized to optimise the general condition of the patient. Even systemic conditions not directly related to the flap may affect the results in specific conditions and should be carefully looked into.
Patient factors
Age
There is enough evidence in the literature that free-flap survival is not significantly different at extremes of age; however it is also well-known that in patients of advanced age, non-surgical complications including mortality are higher.[9,10]
Diabetes
Diabetes, per se, is not related to higher flap failure, but diabetes is often associated with peripheral-vascular disease, particularly in the lower limbs. Such patients need a more thorough vascular evaluation, which may include Doppler studies and/or angiography.[7,11,12]
Other systemic conditions
Patients with liver disease or following chemotherapy may have coagulopathies predisposing them to bleeding and haematoma formation. Same may be the case in patients on anti-coagulants.[13]
Tobacco use
There is evidence, both experimental and clinical, that smoking leads to vascular spasm and is associated with higher flap failure rates. It is strongly recommended that patients refrain from smoking during the period 2 weeks before and 2 weeks after free flap surgery.[14,15]
Alcohol abuse
Although consumption of alcohol is not directly related to flap survival, some people who are regular heavy drinkers are susceptible to developing acute alcohol withdrawal symptoms when they suddenly stop drinking. These symptoms typically manifest on the 3rd to 5th day after withdrawal. Such patients may become extremely uncooperative and even violent. It is prudent to advise patients to cut down alcohol use for a couple weeks pre-operatively. In case of emergency free flaps, one needs to be aware of this problem and plan appropriate treatment, including the use of medications for psychiatric illnesses. Such patients should also be routinely evaluated for liver functions.[16]
Flap selection and planning
A surgeon doing regular free-flap surgery ought to be familiar with a variety of free flap transfers to suit all occasions and requirements.
Skin and fascio-cutaneous flaps are the commonly used free flaps. Skin flaps have advantages like; they tolerate ischemia better,[17] have better stability and appearance and secondary surgeries are easier. Limitations of skin flaps are that only moderate sized flaps will allow primary donor site closure and they do not fill cavities and irregular defects easily.
It is important to visualise the likely donor site scar and explain to the patient accordingly, as the scar remains with the patient forever. All efforts should be made to create a donor scar, which is least obvious and easily camouflaged. A flap like anterolateral thigh (ALT) is preferred over radial/ulnar forearm flaps for this reason. ALT also has a long and large pedicle and can be primarily thinned. On the flipside, the thigh skin is often hairy and larger flaps will need Skin grafting for the donor site. We routinely use para-scapular[18] flap especially in children and thin adults. The vascular pedicle is very reliable, up to 4-6 cm long, with an average calibre of 2-2.5 mm (artery). The donor scar is easily hidden by ordinary clothing.
The groin flap is another good donor site. Based on the superficial circumflex iliac artery system, this flap can be easily made up to 20 cm long and 10 cm broad; with direct closure of the donor site. The skin is mostly hairless and fairly thin. In our experience of over a hundred flaps, we have found that; we can always find a reliable artery of 1.2-1.5 mm calibre, if we follow the vascular dissection to the femoral artery. There is dual venous drainage. The superficial circumflex iliac vein is large in calibre (2.5-3 mm) and can be traced the saphenous bulb. The only disadvantage of this flap is a short donor pedicle (3-4 cm).[19]
Myocutaneous flaps are too bulky and a muscle flap covered with a skin graft is preferred over them. The most commonly used muscle flaps are the gracilis and the latissimus dorsi (LD). Gracilis muscle is the most commonly used muscles for free transfers. Gracilis virtually leaves no donor site morbidity while LD has few disadvantages like the necessity of lateral position, general anaesthesia (GA), a long scar on the flank and frequent seromas in the donor site. Less commonly used muscles include the vastus lateralis, rectus femoris and gastrocnemius. The vastus lateralis can provide a flap of up to 8 cm × 20 cm, with a long pedicle (descending branch of the lateral circumflex).
All muscles are less tolerant of ischaemia and this decreases their chance of salvage in case of anastomotic failure. An ischaemia time of more than 3-4 h is likely to lead to complications.
Recipient site: Location and dimensions
There is some evidence that flaps transferred to specific areas (like lower limbs) have higher failure rates.[1,4] Secondary reconstructions have a higher failure rates possibly due to already traumatized vessels.[4] If the recipient bed is poorly vascularized owing to irradiation, infection or residual tumour, the chances of complications are high.[20]
When doing simultaneous flap harvest, one must plan for alterations in the dimensions of the defect, which may occur after debridement, release of contractures or repositioning of displaced structures. Comparing the affected area to the opposite normal side (wherever possible) will give a good estimate of the final defect. The flap dimensions should also take into account the depth of the defect and the thickness of the flap. Thus for a shallow recipient defect, a 2 cm. Thick ALT flap will need a minimum of 4 cm of additional width to allow a tension free inset. One must also account for post-operative swelling. One needs to be extra careful in ALT flaps, which include a sizable chunk of vastus lateralis muscle. The muscle tends to swell up and if the skin flap has been completely inset, may compress the pedicle.
Intra-operative factors
A free flap transfer operation should proceed in a smooth stepwise fashion culminating in a flap perfectly fitting the defect without tension, anastomoses carried out between well-matching healthy vessels with good flow, with the pedicle sitting comfortably without tension, torsion, redundancy or kinking. Complications and failures are likely to occur whenever any of these steps does not go according to the script. This is a failure of execution.
Patient positioning and intra-operative planning
Ideally, to save time, all free flaps should be done as two-team operations. Thus, flaps which can be harvested in the supine position, with both teams working simultaneously are preferred. Even when a lateral position is necessary, proper planning can allow a two-team approach.
The flap should be kept attached until the recipient site dissection is complete. We prefer to prepare the recipient vessels under microscope. Once the suitable vessels and site of anastomosis is identified, the need of donor pedicle length will be clear. The flap is perfused till this time to avoid surprises.
Recipient site preparation
Debridement/ablation
For all traumatic wounds, thorough debridement is critical to the success of the flap. Nothing but viable tissue should be present in the wound at the end of debridement. One needs to be even more aggressive when dealing with old/infected wounds/electrical burns.[21]
While doing cancer reconstruction, one must follow standard oncosurgical guidelines and place free flaps only on wounds, which have been widely cleared of the tumour.
Quality of recipient vessels
The quality of vessels is probably the most important factor in the success or failure of a free flap. The recipient artery and vein(s) must be identified and prepared before the donor pedicle is divided. A good recipient artery has a smooth glistening intima and pulsatile bright red blood flow.[21,22] In traumatic cases, the recipient vessels should be prepared well away from the zone of trauma. If the artery has significant atheromatous changes, it can still be used as the recipient artery, as long as the flow is good. One needs to be extra careful to choose a site free of large plaques. The artery should be cut with sharp, fine-pointed microscissors. If the wall is thick, it may be a good idea to make a sharp transverse nick with a number 11 blade and complete the cut all around the wall using a sharp-pointed micro-scissor. The aim is to avoid creating an intimal flap or to fracture a plaque. While doing the anastomosis, one should position oneself in such a way that the needle always goes inside-out in the atheromatous vessel. Bringing the needle from outside-in is liable to fracture a plaque or lift an intimal flap. The needle should be thin and sharp. The calcium in atheromatous vessel walls tends to blunt the needle tips rapidly, so one should be ready with a plentiful supply of fresh sutures. Sometimes, while dealing with severely calcified arteries using a cutting/spatulated needle may be helpful.
Inappropriate use of clamps may also cause damage to such arteries. One should only use clamps made by reputable manufacturers and choose appropriate-sized clamps for the given vessel size. Repeated application and removal of clamps should be avoided.
Veins
An ideal recipient vein should be supple and easily compressible.[21] When it is cut, the intima should be smooth and glistening and there should be no clots inside. Blood should flow freely from the distal cut-end. Bleeding from the proximal cut-end only means that there is an active tributary distal to the next valve. What we need to know is whether the vein allows free centripetal flow of blood. This is best tested by gently pushing into the vein a bolus of warm heparinized saline through a fine (22/24G) atraumatic plastic cannula, using a frictionless syringe (the kind used by anaesthetists to give epidural injections). Ideally, the effort required to push the saline into the vein should be no more than that required to push the saline into air.
Matching and positioning
The endeavour should be to have a smooth flow of blood with minimum turbulence and minimum exposure of sub-endothelial tissues. Thus, one should try to have recipient and donor vessels matching each other in calibre as closely as possible. There should be no twist and sharp angulations and the length should be just right. Too short and it leads to axial tension, sutures cutting through and leakage. Too long and there is redundancy, leading to turbulence and possible thrombosis.
Vessel preparation
Only as much adventitia need be trimmed from the vessel ends as is necessary to prevent it from getting into the lumen or getting snagged in the knots.
Anastomotic technique
There are different, well-described ways of completing a micro-vascular anastomosis.[23–26] One does need to spend a finite amount of time in the wet lab and (assisting) in the operating room before one becomes adept at the job.[27] The important points to remember are: Never handle the intima, always keep the needle tip under vision as it passes from one lumen to another. Some amount of discrepancy in the vessel size can be addressed by dilating the smaller vessel with a vessel dilator and/or sectioning it obliquely. It is a good idea to do the most difficult part of the anastomosis in the beginning and progress to easier steps. To allow full visualisation of the lumen, the last two or three stitches should be taken as a continuous running stitch and then each tied separately.
When both the arterial and venous anastomoses are completed, we should check once again for any twist or redundancy. Next, the arterial clamps should be released. If there is a major leak from the arterial anastomotic site, the arterial clamps are re-applied and an additional suture is inserted to close the leak. Minor oozing from between the sutures is best managed by covering it with a bit of autogenous fat for a few minutes. Once the arterial circulation is re-established in the flap, the donor vein should fill up. At this point, the venous clamp on the side of the flap is released and the returning blood is allowed to flow through and dilate the venous anastomosis. This manoeuvre helps to confirm a good venous return from the flap, distend the venous anastomosis thus overcoming any spasm and rule out any significant leaks on the venous side. If there is a kink or twist in the vein this manoeuvre will make it more obvious. After this, the venous clamp on the recipient side is also removed. Figures 1 and 2 show the line diagrams of few possible problems in the lie of the pedicle that may cause problems.
Figure 1.
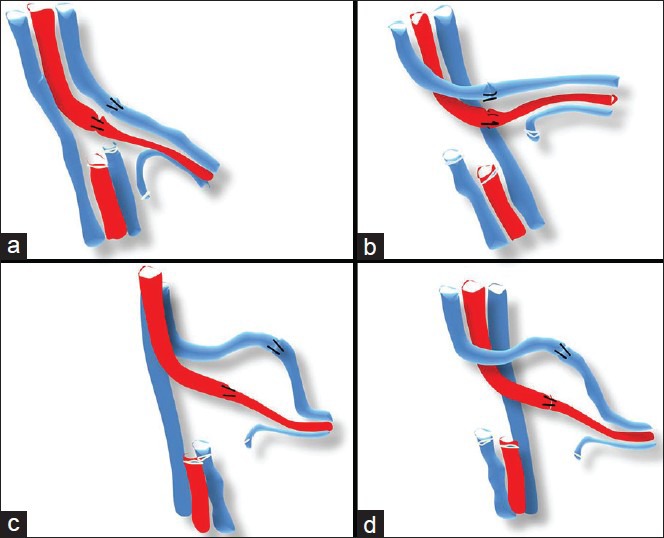
(a) Ideal arrangement of vessels, they should be of similar length and preferably lie parallel to each other. If they are crossing each other one should make sure that the artery is not compressing the vein. (b) The vein crossing the artery but the lie is comfortable. (c and d) If the length of the vein and the artery are different the longer vessel tends to kink. In the figures shown the vein on either side can have this tendency if left longer than artery
Figure 2.
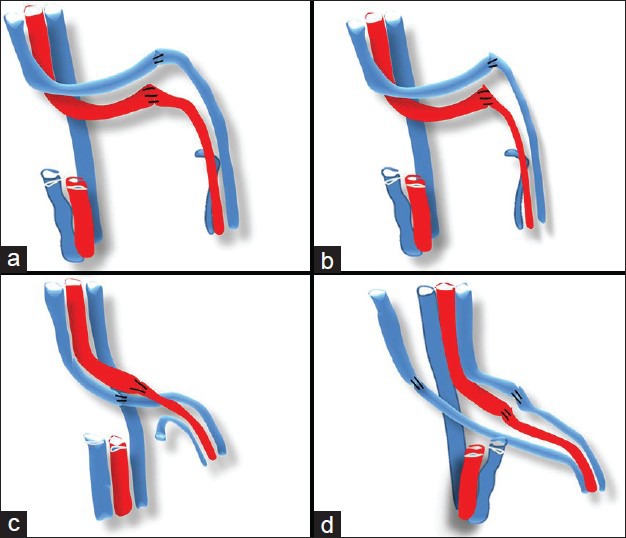
(a) If both the vessels are long and redundant then the chances of kink are much higher. (b) Long redundant vessels even tend to get twisted around themselves decreasing the flow and increasing the chances of thrombosis. (c) If the orientation is not right especially the vein as it is low pressure system is vulnerable to pressure from the overlying artery/ or between the artery and the skin. (d) When doing two veins, again the arrangement should be made as easy as possible for primary surgery as well as possible exploration
Haemostasis and wound closure
It is important to perform meticulous haemostasis in the peri-anastomotic area. The wound should be closed without tension. In case that is not possible, a small local skin flap can be mobilized to cover the pedicle, with the resultant raw area grafted.
Poor arterial flow
If the arterial circulation appears to be sluggish, we need to check several things: The blood pressure, the core temperature of the patient and the ambient temperature. The arterial pressure on the patient should be compared with the pre-operative values. In general, one should aim for a high-normal range of blood pressure. To maintain adequate blood pressure, the patient should be well-hydrated. Occasionally, when, in spite of adequate fluid replacement, the blood pressure remains low, we can use an infusion of low-dose dopamine (3-5 mcg/kg/min).[28]
Low ambient temperature tends to cause vaso-spasm, especially in the limbs. Special precautions should be taken to maintain the patient's core temperature during surgery (warming blankets, foil and warmed intravenous fluids). If all these factors are taken care of and the arterial flow is still sluggish, it would be a good idea to clamp the arterial anastomosis on both sides and examine the inside of the lumen by removing two or three sutures. If there is a platelet plug or a clot, the arterial anastomosis should be revised.
Venous problems
Signs of venous obstruction include undue bleeding from the flap, dark and brisk bleeding when the flap is pricked or scratched and swelling. The flap vein may appear to be excessively distended if there is an obstruction at the site of venous anastomosis or in the recipient vein. If another donor vein is available, it can be anastomosed to another recipient vein.[29,30] If the problem persists the venous anastomosis needs revision. We have been regularly using subcutaneous veins as recipient veins with no complaints.
Use of vein grafts
Vein grafts should ideally be avoided by appropriate planning, as they are a linked to significantly higher flap losses.[31] One should always try to harvest an extra length of pedicle to allow ideal positioning of the flap and good approximation of the recipient and donor vessels.
The ‘no reflow’ phenomena
Occasionally, the flap fails to perfuse in spite a patent arterial anastomosis.[32] This has been attributed to ischemic injury to the intra-flap vasculature. This is particularly important for muscle/myo-cutaneous flaps when the duration of ischemia is more than 3-4 h. Before labelling it as ‘no reflow’, one should exclude correctable causes like low systemic arterial pressure and hypothermia-induced vasospasm.
Post-operative factors
Flap monitoring: All free flaps should be monitored according to a strict protocol by experienced staff. Monitoring should be at least hourly in the first 48 h and every 3-4 h subsequently.[33,34] The whole flap should be visible for monitoring. Visual inspection of the colour and turgor of the flap and the pattern of bleeding after a needle scratch are still the most commonly used monitoring techniques. Bleeding after needle scratch should come at a steady pace and be bright red in colour. Where a muscle flap has been used, a corner of the skin graft can be turned over and the same technique applied to check for bleeding. Monitoring with a handheld Doppler works very well for the artery, but not so well for the veins.
Factors which can cause venous congestion in the flap include dependency of the limb, tight circumferential dressings, dressings caked with blood leading to compression and tracheostomy tapes around the neck.
Patient monitoring
The patient's pulse, blood pressure, oxygen saturation and urine output are monitored hourly. A urine output of 0.75-1 ml/kg/h denotes good hydration. Unnecessary blood transfusions should be avoided. A haematocrit of 25-30 is supposed to be ideal to ensure adequate microcirculation.
Vascular compromise and re-exploration
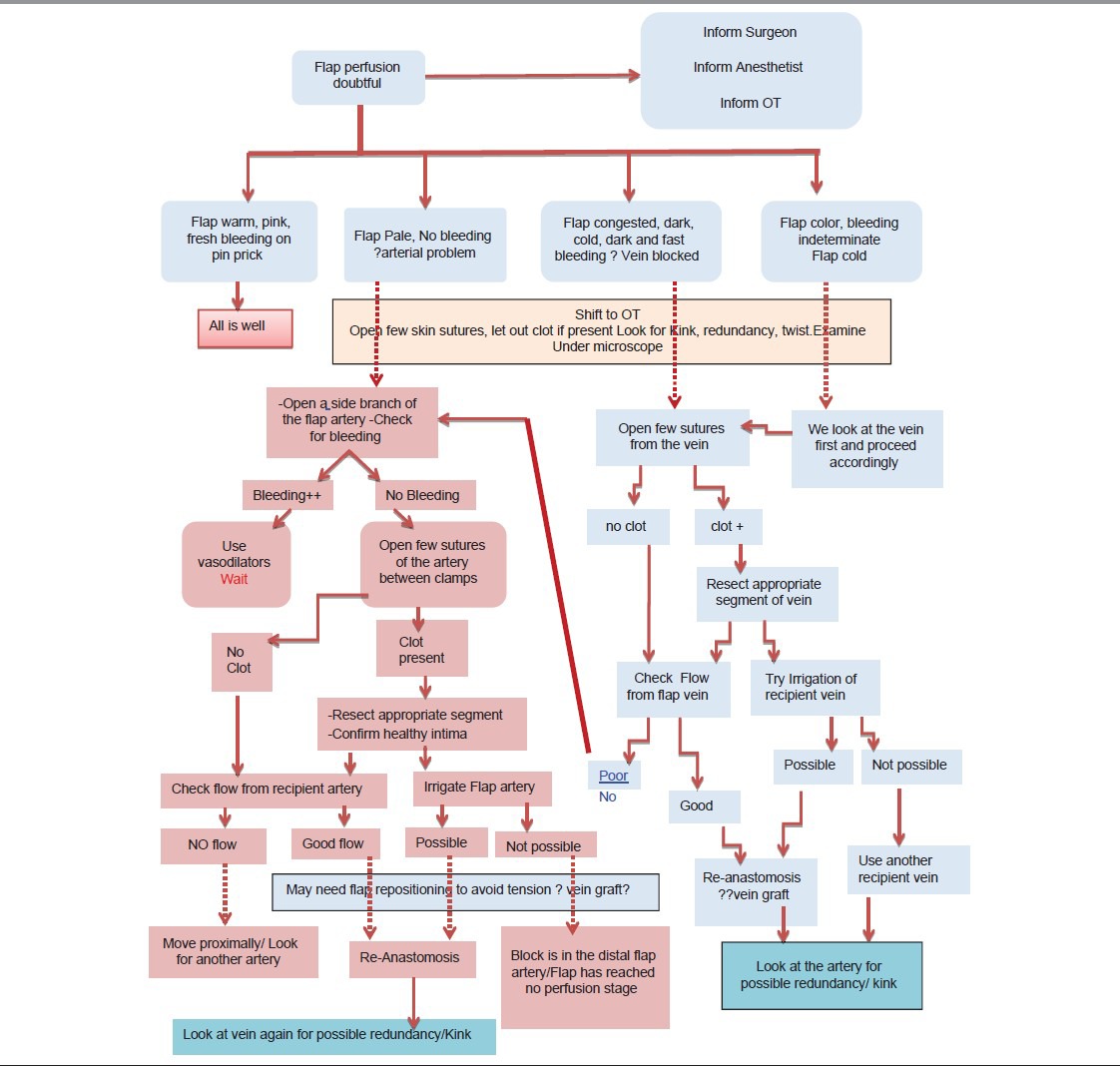
During the first 48-72 h, whenever there is the slightest doubt about the adequacy of flap perfusion and after having excluded or corrected systemic causes like hypotension/hypothermia, one should maintain a low threshold for re-exploration. If the patient is receiving continuous anaesthesia (epidural/continuous nerve block) analgesia, it may be possible to re-explore without giving GA. At re-exploration, we look for evidence of tight skin closure, a compressing haematoma and kinking/twisting/excessive redundancy of the vessels and for our step-by-step algorithm of free flap re-exploration, as shown in Table 1.
Table 1.
Algorithm for flap re-exploration
Sometimes when we feel that the recipient vein is not healthy and there is no alternative, we insert a cannula in one of the tributaries of donor vein and continuously irrigate the venous anastomotic site with low dose anticoagulant solution (5000 units of heparin in 500 ml of normal saline at 5 ml/min) for 4-5 days [Figure 3]. The cannula is pulled out after 2-3 days of stopping the infusion.
Figure 3.
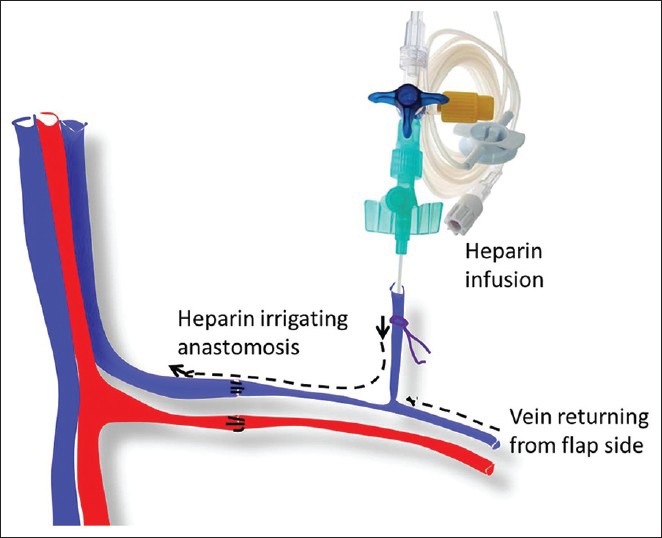
Schematic representation of the placement of cannula in a vein when there is a doubt about the health of intima and there are no other options. The heparin saline given through cannula continuously irrigates the anastomosis
People have described salvage of microsurgical flaps with leeches,[35] thrombolytic therapy,[36,37] external shunts,[38] but we do not have any experience of these methods.
Figure 4 shows a case where early exploration saved the flap. It was a case of hidradenitis of axilla with scarred skin. After debridement, a groin flap was planned and dissected for the cover. In early post-operative period the flap developed severe congestion. She was immediately taken to theatre and the sutures were let open under GA. A large hematoma was drained. On inspection under microscope the anastomosis were patent; so probably external pressure was responsible for the venous compromise. The wound was loosely closed and final in-setting was done secondarily. The flap ultimately settled well.
Figure 4.
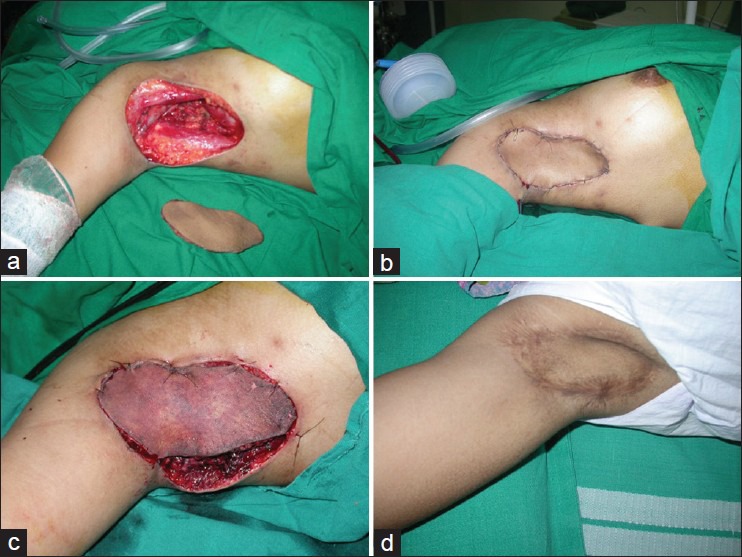
A 25-year-old girl with hydreadenitis suppurativa, with diseased and scared skin in the axilla was taken up for debridement and flap cover. (a) The defect after completion of debridement. (b) Groin flap was used for the cover. The flap was transferred to the defect. In the post-operative flap developed congestion and she had to be rushed to the OT. (c) On opening the skin sutures, there was a large hematoma that was drained. (d) Immediate exploration saved the flap and ultimately the flap settled well
PARTIAL FLAP FAILURE
A free flap developing partial necrosis is uncommon. When it does happen, it is usually due to faulty flap planning. For example, the lowermost 4-5 cm of LD muscle (just above the iliac crest) is not reliably supplied by the thoracodorsal vessels. Similarly, the lower most 5-6 cm of anterior thigh skin is not reliably served by the ALT perforator (author's experience). Including these portions in the flap transfer is likely to be risky. One can use alternate flaps or even two flaps in such situations. Occasionally, marginal necrosis may develop in a flap due to pressure by an underlying hematoma. This is best prevented by good haemostasis and adequate placement of drains. Areas of partial flap necrosis should be excised early, as soon as they have demarcated (usually within 4-5 days). For small areas of marginal necrosis, flap advancement may be sufficient. Otherwise, the defect can be managed by a local flap or a skin graft.
COMPLETE FLAP SURVIVAL; RECONSTRUCTIVE GOAL NOT FULLY ACHIEVED
This again is usually due to lack of adequate planning or inadequate assessment of the defect. Examples of such situation are heel and sole defects where it takes very long time for the native skin to demarcate. In cases of avulsion/closed degloving injuries also more skin is likely to necrose than what seems dead and aggressive debridement is warranted. In severe crush/compartment syndrome/electrical burns necrosis of deep muscles may present late with deep infection or even secondary haemorrhage. At initial debridement, good bleeding alone should not be accepted as a sign of viable muscle. Healthy muscle should bleed and contract on stimulation.[21] Though not directly related to the flap, sometimes poorly stabilized fractures/loose non-viable bone fragments, non-viable bits of muscle and unfilled cavities may become source of infection and compromise the outcome. All these should be considered during planning and execution.
SYSTEMIC COMPLICATIONS
As most free flap operations are long surgeries, these patients are prone to systemic complications like pulmonary atelectasis and deep venous thrombosis (DVT). Patients who have had flaps harvested from the chest or abdomen are more prone to pulmonary complications. Similarly, flaps like free Transverse rectus abdominis myocutaneous flap/ deep inferior epigastric artery perforator flap (DIEP), where the vascular dissection has been carried down to iliac vessels, may be at a higher risk of DVT. Appropriate physical and pharmacological measures for preventing DVT should be taken in all adults undergoing such flap transfers.[39] In paediatric patients and patients with cardiac problems the blood loss should be carefully estimated and restored to maintain a haematocrit of 25-30.
DONOR SITE COMPLICATIONS
Early donor site morbidity
Meticulous haemostasis is mandatory. We prefer to use a fine tipped monopolar cautery to do all the dissection, coagulating all the tiny bleeders meticulously. Avoid tension while closing the donor sites, when one cannot approximate donor site with a single 2-0 nylon suture, there is probably too much tension.[40] Skin graft is preferred in these circumstances. Whenever a large muscle flap like LD is harvested, we prefer obliterating the cavity with quilting sutures or use suction drains, to prevent the formation of a seroma.
Late donor site morbidity
This can be unsightly scars, numbness and paresthesias, weakness and contour irregularities. Stretching of scars can be minimised by meticulous multilayer closure and avoidance of tension. Scars in some places naturally tend to be worse than others. For example, when a lateral arm flap is extended beyond the epicondyle, the resulting longitudinal scar across the elbow joint often tends to hypertrophy. This may be a life-long sore point for a patient used to wearing short sleeves. One source of scarring, which is often over looked is skin graft donor sites. Ways to minimise unsightly graft donor scars include harvesting thinner rather than thicker grafts, using a dermatome (to give a uniform graft thickness), taking grafts from less exposed areas (like scalp, buttocks). Careful preservation of sensory nerves as and when possible will decrease incidences of numbness and paresthesia. Contour irregularities in after harvest of large skin flaps cannot be avoided completely; it can be corrected to certain extent at a later date.
UNSATISFIED PATIENT
This is usually the result of failure of communication. When the patient is not satisfied despite a good reconstruction it means that the surgical team has not been able to fully convey the short and long-term implications of the surgery to the patient.
Informed consent
It is assumed that the surgical team will brief the patient in detail about the nature and scope of the surgical operation, intended goals and more importantly things that cannot be achieved with that specific surgery. In microsurgery the possibility of complications and unfavourable results, need of skin, vein and nerve grafts and possible alteration of surgical plans while on table must be explained and documented. The patient should be clearly informed about the very real possibility of anastomotic blockage and the need for emergency re-operation in the early post-operative period and various alternative options in case the flap is not salvaged. It is worth repeating here, that by law, only a patient can sign consent, unless he/she is a minor or is in an altered state of consciousness or is not sound of mind. If a change of course is considered while the patient is under anaesthesia, it is not justifiable to obtain consent from the next of kin.
Financial issues
A large number of free tissue transfers in our country are performed in private hospitals. One of the major problems, leading to a lot of bitterness, is inflated bills following prolonged surgery/re-operation/surgical complications. The surgical team, along with the hospital management, need to formulate a clear policy for such situations and communicate the same to the patient well in advance.
SUMMARY
Free flap surgery is a demanding operation. Careful planning, thorough and complete communication with the patient and his/her relatives, meticulous execution and diligent post-operative care are the keys to a successful flap and a satisfied patient.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bui DT, Cordeiro PG, Hu QY, Disa JJ, Pusic A, Mehrara BJ. Free flap reexploration: Indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg. 2007;119:2092–100. doi: 10.1097/01.prs.0000260598.24376.e1. [DOI] [PubMed] [Google Scholar]
- 2.Pohlenz P, Blessmann M, Blake F, Li L, Schmelzle R, Heiland M. Outcome and complications of 540 microvascular free flaps: The Hamburg experience. Clin Oral Investig. 2007;11:89–92. doi: 10.1007/s00784-006-0073-0. [DOI] [PubMed] [Google Scholar]
- 3.Panchapakesan V, Addison P, Beausang E, Lipa JE, Gilbert RW, Neligan PC. Role of thrombolysis in free-flap salvage. J Reconstr Microsurg. 2003;19:523–30. doi: 10.1055/s-2004-815638. [DOI] [PubMed] [Google Scholar]
- 4.Nakatsuka T, Harii K, Asato H, Takushima A, Ebihara S, Kimata Y, et al. Analytic review of 2372 free flap transfers for head and neck reconstruction following cancer resection. J Reconstr Microsurg. 2003;19:363–8. doi: 10.1055/s-2003-42630. [DOI] [PubMed] [Google Scholar]
- 5.Yii NW, Evans GR, Miller MJ, Reece GP, Langstein H, Chang D, et al. Thrombolytic therapy: What is its role in free flap salvage? Ann Plast Surg. 2001;46:601–4. doi: 10.1097/00000637-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kroll SS, Schusterman MA, Reece GP, Miller MJ, Evans GR, Robb GL, et al. Timing of pedicle thrombosis and flap loss after free-tissue transfer. Plast Reconstr Surg. 1996;98:1230–3. doi: 10.1097/00006534-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Khouri RK, Cooley BC, Kunselman AR, Landis JR, Yeramian P, Ingram D, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102:711–21. doi: 10.1097/00006534-199809030-00015. [DOI] [PubMed] [Google Scholar]
- 8.Wilson JL, Morritt AN, Morrison WA. Avoiding complications. In: Wei FC, Mardini S, editors. Flaps and Reconstructive Surgery. Philadelphia: Saunders: Elsevier; 2009. pp. 117–24. [Google Scholar]
- 9.Tarsitano A, Pizzigallo A, Sgarzani R, Oranges CM, Cipriani R, Marchetti C. Head and neck cancer in elderly patients: Is microsurgical free-tissue transfer a safe procedure? Acta Otorhinolaryngol Ital. 2012;32:371–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Ozkan O, Ozgentas HE, Islamoglu K, Boztug N, Bigat Z, Dikici MB. Experiences with microsurgical tissue transfers in elderly patients. Microsurgery. 2005;25:390–5. doi: 10.1002/micr.20136. [DOI] [PubMed] [Google Scholar]
- 11.Nahabedian MY, Singh N, Deune EG, Silverman R, Tufaro AP. Recipient vessel analysis for microvascular reconstruction of the head and neck. Ann Plast Surg. 2004;52:148–55. doi: 10.1097/01.sap.0000095409.32437.d4. [DOI] [PubMed] [Google Scholar]
- 12.Colen LB, Stevenson A, Sidorov V, Potparic Z, Pacelli E, Searles J, et al. Microvascular anastomotic thrombosis in experimental diabetes mellitus. Plast Reconstr Surg. 1997;99:156–62. doi: 10.1097/00006534-199701000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Kroll SS, Miller MJ, Reece GP, Baldwin BJ, Robb GL, Bengtson BP, et al. Anticoagulants and hematomas in free flap surgery. Plast Reconstr Surg. 1995;96:643–7. doi: 10.1097/00006534-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Aköz T, Akan M, Yildirim S. If you continue to smoke, we may have a problem: Smoking's effects on plastic surgery. Aesthetic Plast Surg. 2002;26:477–82. doi: 10.1007/s00266-002-2045-3. [DOI] [PubMed] [Google Scholar]
- 15.Gu YD, Zhang GM, Zhang LY, Li FG, Jiang JF. Clinical and experimental studies of cigarette smoking in microvascular tissue transfers. Microsurgery. 1993;14:391–7. doi: 10.1002/micr.1920140608. [DOI] [PubMed] [Google Scholar]
- 16.Kuo YR, Jeng SF, Lin KM, Hou SJ, Su CY, Chien CY, et al. Microsurgical tissue transfers for head and neck reconstruction in patients with alcohol-induced mental disorder. Ann Surg Oncol. 2008;15:371–7. doi: 10.1245/s10434-007-9506-5. [DOI] [PubMed] [Google Scholar]
- 17.Torii S, Harii K, Ohmori K. Experimental study of ischemia time influencing free skin flap survival. Eur J Plast Surg. 1979;4:225–33. [Google Scholar]
- 18.Chen D, Jupiter JB, Lipton HA, Li SQ. The parascapular flap for treatment of lower extremity disorders. Plast Reconstr Surg. 1989;84:108–16. doi: 10.1097/00006534-198907000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Muresan C, Dorafshar AH, Rodriguez ED. A reappraisal of the free groin flap in aesthetic craniofacial reconstruction. Ann Plast Surg. 2012;68:175–9. doi: 10.1097/SAP.0b013e3182275d0f. [DOI] [PubMed] [Google Scholar]
- 20.Fosnot J, Fischer JP, Smartt JM, Jr, Low DW, Kovach SJ, 3rd, Wu LC, et al. Does previous chest wall irradiation increase vascular complications in free autologous breast reconstruction? Plast Reconstr Surg. 2011;127:496–504. doi: 10.1097/PRS.0b013e3181fed560. [DOI] [PubMed] [Google Scholar]
- 21.Koul AR, Patil RK, Philip VK. Early use of microvascular free tissue transfer in the management of electrical injuries. Burns. 2008;34:681–7. doi: 10.1016/j.burns.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Han SH, Lee TJ. Algorithm for recipient vessel selection in free tissue transfer to the lower extremity. Plast Reconstr Surg. 1999;103:1937–48. doi: 10.1097/00006534-199906000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Acland RD. 2nd ed. St. Louis: C. V. Mosby Company; 1989. Practice Manual for Microvascular Surgery. [Google Scholar]
- 24.Tetik C, Unal MB, Kocaoglu B, Erol B. Use of continuous horizontal mattress suture techniques in microsurgery: An experimental study in rats. J Hand Surg Am. 2005;30:587–95. doi: 10.1016/j.jhsa.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Yajima K, Yamamoto Y, Nohira K, Shintomi Y, Blondeel PN, Sekido M, et al. A new technique of microvascular suturing: The chopstick rest technique. Br J Plast Surg. 2004;57:567–71. doi: 10.1016/j.bjps.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Spector JA, Draper LB, Levine JP, Ahn CY. Routine use of microvascular coupling device for arterial anastomosis in breast reconstruction. Ann Plast Surg. 2006;56:365–8. doi: 10.1097/01.sap.0000202614.45743.34. [DOI] [PubMed] [Google Scholar]
- 27.Chan WY, Matteucci P, Southern SJ. Validation of microsurgical models in microsurgery training and competence: A review. Microsurgery. 2007;27:494–9. doi: 10.1002/micr.20393. [DOI] [PubMed] [Google Scholar]
- 28.Suominen S, Svartling N, Silvasti M, Niemi T, Kuokkanen H, Asko-Seljavaara S. The effect of intravenous dopamine and dobutamine on blood circulation during a microvascular TRAM flap operation. Ann Plast Surg. 2004;53:425–31. doi: 10.1097/01.sap.0000137133.08105.73. [DOI] [PubMed] [Google Scholar]
- 29.Sano K, Hallock GG, Rice DC. Venous “supercharging” augments survival of the delayed rat TRAM flap. Ann Plast Surg. 2003;51:398–402. doi: 10.1097/01.SAP.0000068111.83104.7F. [DOI] [PubMed] [Google Scholar]
- 30.Enajat M, Rozen WM, Whitaker IS, Smit JM, Acosta R. A single center comparison of one versus two venous anastomoses in 564 consecutive DIEP flaps: Investigating the effect on venous congestion and flap survival. Microsurgery. 2010;30:185–91. doi: 10.1002/micr.20712. [DOI] [PubMed] [Google Scholar]
- 31.Loos MS, Freeman BG, McClellan WT. Free muscle flap reconstructions using interpositional vein grafts vs. local anastomosis: A 5-year experience at a rural tertiary care center. W V Med J. 2010;106:19–23. [PubMed] [Google Scholar]
- 32.Calhoun KH, Tan L, Seikaly H. An integrated theory of the no-reflow phenomenon and the beneficial effect of vascular washout on no-reflow. Laryngoscope. 1999;109:528–35. doi: 10.1097/00005537-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Salgado CJ, Moran SL, Mardini S. Flap monitoring and patient management. Plast Reconstr Surg. 2009;124:e295–302. doi: 10.1097/PRS.0b013e3181bcf07b. [DOI] [PubMed] [Google Scholar]
- 34.Disa JJ, Cordeiro PG, Hidalgo DA. Efficacy of conventional monitoring techniques in free tissue transfer: An 11-year experience in 750 consecutive cases. Plast Reconstr Surg. 1999;104:97–101. [PubMed] [Google Scholar]
- 35.Nguyen MQ, Crosby MA, Skoracki RJ, Hanasono MM. Outcomes of flap salvage with medicinal leech therapy. Microsurgery. 2012;32:351–7. doi: 10.1002/micr.21960. [DOI] [PubMed] [Google Scholar]
- 36.Pantazi G, Knight KR, Romeo R, Hurley JV, Hennessy O, Willemart G, et al. The beneficial effect of heparin in preischemic perfusion solutions for cold-stored skin flaps. Ann Plast Surg. 2000;44:304–10. doi: 10.1097/00000637-200044030-00009. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Cooley BC, Fowler JD, Gould JS. Intravascular heparin protects muscle flaps from ischemia/reperfusion injury. Microsurgery. 1995;16:90–3. doi: 10.1002/micr.1920160209. [DOI] [PubMed] [Google Scholar]
- 38.Kato H, Takada T, Yukawa Y, Yanase M, Torii S. External venous shunt as a solution to venous thrombosis in microvascular surgery. Br J Plast Surg. 2001;54:164–6. doi: 10.1054/bjps.2000.3511. [DOI] [PubMed] [Google Scholar]
- 39.Shridharani SM, Folstein MK, Chung TL, Silverman RP. Prevention of microsurgical thrombosis. In: Agullo F, editor. Current Concepts in Plastic Surgery. Europe: InTech; 2012. pp. 257–64. [Google Scholar]
- 40.Boca R, Kuo YR, Hsieh CH, Huang EY, Jeng SF. A reliable parameter for primary closure of the free anterolateral thigh flap donor site. Plast Reconstr Surg. 2010;126:1558–62. doi: 10.1097/PRS.0b013e3181ef8cb7. [DOI] [PubMed] [Google Scholar]


