Abstract
The threat of lower limb loss is seen commonly in severe crush injury, cancer ablation, diabetes, peripheral vascular disease and neuropathy. The primary goal of limb salvage is to restore and maintain stability and ambulation. Reconstructive strategies differ in each condition such as: Meticulous debridement and early coverage in trauma, replacing lost functional units in cancer ablation, improving vascularity in ischaemic leg and providing stable walking surface for trophic ulcer. The decision to salvage the critically injured limb is multifactorial and should be individualised along with laid down definitive indications. Early cover remains the standard of care, delayed wound coverage not necessarily affect the final outcome. Limb salvage is more cost-effective than amputations in a long run. Limb salvage is the choice of procedure over amputation in 95% of limb sarcoma without affecting the survival. Compound flaps with different tissue components, skeletal reconstruction; tendon transfer/reconstruction helps to restore function. Adjuvant radiation alters tissue characters and calls for modification in reconstructive plan. Neuropathic ulcers are wide and deep often complicated by osteomyelitis. Free flap reconstruction aids in faster healing and provides superior surface for offloading. Diabetic wounds are primarily due to neuropathy and leads to six-fold increase in ulcerations. Control of infections, aggressive debridement and vascular cover are the mainstay of management. Endovascular procedures are gaining importance and have reduced extent of surgery and increased amputation free survival period. Though the standard approach remains utilising best option in the reconstruction ladder, the recent trend shows running down the ladder of reconstruction with newer reliable local flaps and negative wound pressure therapy.
KEY WORDS: Limb salvage, limb trauma, lower limb reconstruction, foot ulcers
INTRODUCTION
Lower extremities in human are evolutionally adapted for the bipedal gait and are exceptionally longer and powerful to bear the body weight and the locomotion. Loss of either or both profoundly affects the basic functions and results in manifold increase in energy expenditure with the prosthetic limb ambulation. Variety of conditions such as trauma, chronic infections, cancer ablation, diabetes, peripheral vascular diseases (PVDs) and so on afflicts the lower limb. Salvage of the limb remains a challenging task due to distinct anatomical factors such as close proximity of vital structures with limited local coverage options. The vascularity of the leg and foot is precarious and unlike in other regions the skin over the leg and foot is non-pliable even in elderly individuals.
The limb salvage has advanced since the time of the Civil War, when nearly all severely traumatised limbs were amputated. After World War I, Winett Orr and later Trueta, developed the technique of ‘closed plaster treatment’ of open fractures, based on two important principles, which are still remains the basis of current modern practice: Debridement and Stabilisation. The major breakthrough in limb salvage occurred with the introduction of microsurgery from the 1960 onwards. Not only did it allow replantation of amputated extremities, but also allowed the transfer of vascularised tissue to repair large soft-tissue defects.[1] With further advances in microvascular tissue transfer, advent of newer reconstructive options such as perforator and propeller flaps, negative pressure wound therapy (NPWT), endovascular procedures to improve limb perfusion, advances in skeletal fixation, Ilizarov bone lengthening for bony defects, growth factors for the osseous healing and improvised podiatric care with custom made footwear most limbs can now be salvaged.
The modern dilemma that reconstructive surgeons face is no longer how to salvage an extremity, but knowing whether or not attempting salvage is the best treatment for the patient. Achieving stable bony union and adequate soft-tissue cover are cardinal in restoring function to enable fitting normal footwear with protective plantar sensation. The protracted course of limb salvage procedures with its impact on psychosocial and economic status and the final functional outcome determines the decision making and should be individualised. Early amputations too are not necessarily cost-effective. Below knee stump salvage procedures should be considered to preserve mobile knee joint whenever the amputation becomes inevitable.
CONDITIONS OF LIMB SALVAGE
Various conditions can afflict limb survival such as:
Trauma
Stump salvage
Cancer ablation
Chronic infections
PVDs
Diabetic wound/non healing ulcers
Exposed prosthesis
Miscellaneous.
LIMB SALVAGE IN TRAUMA
Initial assessment
Trauma is most frequently encountered cause in the lower extremity salvage surgery. High velocity trauma is often associated potentially life-threatening injuries, which requires foremost attention. Quick and careful assessment of the limb for the vascularity, sensibility, soft-tissue and skeletal loss grades the severity. With routine radiographs, additional computed tomography angiogram or Doppler evaluation is useful to rule out suspected vascular compromise particularly in multilevel injuries. In a critically injured limb, the decision of salvage versus primary amputation continues to haunt the surgeons. Although various scoring systems like the mangled extremity severity score; the Limb Salvage Index; the Predictive Salvage Index; the nerve injury, ischaemia, soft-tissue injury, skeletal injury, shock and age of patient score; and the Hannover Fracture Scale-98[1–6] have been developed to assist the clinician in decision making, it is seen that they are not good predictors of limb amputation, salvage or the functional recovery when salvaged.[7–10] The criterion and the decision to amputate must be individualised for each patient.
The limb salvage versus amputation
The indications for limb salvage are mainly two; (1) Any limb injury in children and (2) adults with bony and soft-tissue loss with intact sensibility. Absolute indications for primary amputation as described by Lange[11] with open tibial fracture include (1) complete anatomical disruption of posterior tibial nerve in adults and (2) crush injuries with warm ischaemia time beyond six hours. The relative indications for amputation are: Associated life-threatening comorbidities, severe ipsilateral foot injury, multiple level injuries and anticipated protracted course of soft-tissue and skeletal reconstruction.
The high energy limb injuries requiring major reconstruction and salvage procedures are generally falls into type IIIB (exposed bone with periosteal stripping with extensive contamination) and type IIIC (IIIB with additional vascular injury) of the Gustilo-Anderson fracture classification.[12]
It should be noted that closed or open injuries around knee involving fractures of the tibia or femoral condyle frequently associated with neurovascular injuries in the popliteal region necessitating urgent exploration for skeletal stabilisation and vascular repair. Strong clinical suspicion, careful evaluation with expeditious imaging studies is cardinal to detect such innocuous appearing limb injuries.
Reconstructive strategy
The objective of reconstruction is to achieve long-term functional stability with a minimal morbidity in a short time. This should result in skeletal union and stable soft-tissue coverage without persistence of sinus. The reconstructive strategy following the decision to salvage includes: Skeletal stabilisation, radical debridement, restoration of neurovascular continuity ensuring adequate limb perfusion, repair of soft-tissue and early wound coverage. Skeletal stabilisation with external fixation is preferred in wounds with extensive soft-tissue and bone loss. The direction of the fixator pin placement carefully planned to avoid access difficulties for flap cover. The debridement should include removal of all devitalised tissues and may be repeated several times until the healthy bed. All distal muscular attachment of the severed tendon is generally avascular and should be excised preserving tendons. Wound may be kept covered with collagen sheets while the definitive wound coverage is planned. At no point of time, the site of vascular repair is left exposed and providing an immediate flap cover is essential to prevent both limb and life-threatening blow outs. The radical debridement and early tissue coverage within 72 h emphasised by Godina in 1986 results in the best outcome in terms of reducing flap failure rate, wound infection, bone healing time, duration of hospital stay and number of surgical procedures. The advantages of early cover have been reemphasised in several subsequent studies.[13–16] However, a number of studies have reported minimal flap failure and a low infection rate even when wound coverage was provided at two weeks following the injury[14,16,17] In addition, a study by Kolker et al. has shown that the timing of reconstruction had no influence on the ultimate outcome and it is even safe to perform vascular anastomosis distal to the zone of injury.[18] Thus, the timing of definitive wound cover with free flaps should be individualised based on the condition of the wound and fitness of the patient.[18,19] Nevertheless, expeditious coverage of exposed vital neurovascular structures, tendon, bone and hardware remains a standard practice[20] [Figure 1a–d].
Figure 1.
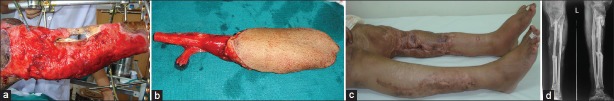
(a) A 24-year-old lady with Gustilo IIIB injury with circumferential soft-tissue loss and avascular tibial segment. Referred after failed attempts of both gastrocnemius flap. (b) Harvesting of fibula 12 cm with skin paddle. (c) Reconstructed tibial defect and soft-tissue following 1 year. (d) Osseous union of vascularised fibula
Choice of recipient vessels
The ‘zone of injury’ following limb trauma is known to exists beyond the limits of the wound and cited as a principle cause of flap failure.[21] Thus, the conventional practice is to select the recipient vessels away from this zone preferably in the proximal site. However, it is shown that the quality of the flow, pliability of the vessel wall and the vascular status of the limb are more important determinants in choosing the recipient vessel and eventual success[21,22] [Figure 2a–e]. This observation is further supported by the success of super microsurgery utilising perforators of this region for the anastomosis.[23]
Figure 2.
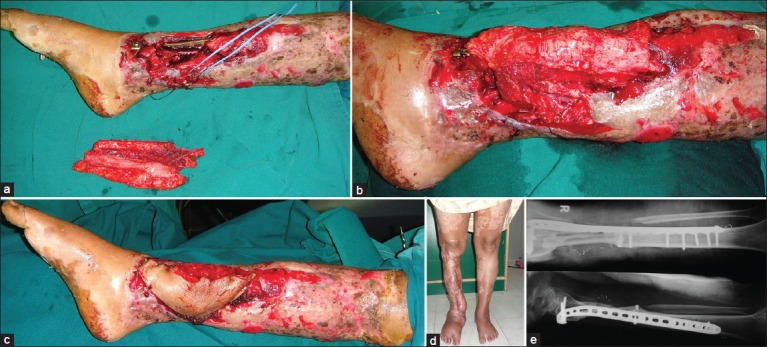
(a) Crush injury leg, Gustilo-IIIB type with circumferential skin loss and loss of lower third of tibia and fibula in a 55-year-old lady. Circumferential raw area was skin grafted and reconstruction planned with osteocutaneous free fibula flap. Posterior tibial artery was the only perfusing vessel. (b) Anastomosis end to side in the zone of injury to the posterior tibial vessels. (c) Anastomotic site covered with skin paddle. (d) Well healed flap with normal ambulation and weigh bearing without support at 22 months post surgery.(e) Stable osseous union at 38 months post reconstruction
Salvage of the stump
Whenever the amputation becomes inevitable the goal is to preserve as much length of the limb as may be possible, especially the functioning knee joint, which reduces energy expenditure as well as provides superior ambulation with below knee prosthesis.[24,25] Inadequate tissue for the stump cover can be addressed by utilising fillet flaps or microvascular free flaps.[26,27]
The outcome studies
The outcome of limb salvage versus amputation was systematically evaluated for type IIIB and C limb injuries.[28] In their review of 1947 articles and 28 observational studies, they found no evidence to support superior outcomes of either limb salvage or primary amputation for type IIIB and IIIC tibial fractures in the current literature. Length of hospital stay was 56.9 days for salvage patients versus 63.7 days for amputees. The most common complications after salvage attempt were osteomyelitis (17.9%), non-union (15.5%), secondary amputation (7.3%) and flap failure (5.8%). Rehabilitation time for salvaged patients was reported as time to union (10.2 months) and time to full weight-bearing (8.1 months). The proportion of patients who returned to work was 63.5% for salvage patients and 73% for amputees. They conclude that when outcomes are similar between two treatment strategies, economic analysis that incorporates cost and preference (utility) may define an optimal treatment strategy to guide physicians and patients.[28]
It is seen that following limb salvage over one-quarter of patients who were working before a severe lower extremity fracture do not returned to work by 12 months after injury and a minority of patients still report limitations at 30 months after injury, with one-fifth not returning to work.[29]
It is generally believed that in comparison to the complicated limb salvage procedures, early amputation and prosthetic rehabilitation offers a faster recovery at a lower cost. However, recent studies based on sickness impact profile and the lower extremity assessment project (study) have shown that there is no significant difference in the outcome at 2 years and amputation was more expensive and may be as high as three times the limb salvage.[30–33] While the overall cost of the limb salvage and amputation remains similar, additional cost of the prosthesis and its maintenance turns out to be more expensive for amputation.[31,32] These study suggests that limb salvage should be more aggressively pursued, especially where amputation is not clearly indicated. These studies however do not represent health care system of developing counties where the prosthetic rehabilitation is available at a subsided cost. Further, the team of expertise for expeditious treatment is not always available, which either prolongs the successful reconstruction or fails to achieve the goal. Amputation versus salvage needs to be individualised based on the given circumstances of available standard of care and affordability.
SOFT-TISSUE SARCOMA
Sarcomas, a malignant component of the wide array of soft-tissue tumours are classified based on their mesoderm origin and their resemblance to the differentiated adult tissue. They present with a wide spectrum of aggressiveness from low- to high-grade and from local destruction to distant metastasis. Lower limbs are most commonly affected than the upper limb (74 vs. 26%) and they represent 45% of overall sarcoma afflicting the body.[34] Surgical excision remains the mainstay of the treatment supported by the adjuvant radiation and chemotherapy. The oncological clearance demands wide excision including one or more compartments, which were managed earlier with limb amputation. It is established that limb amputation against limb salvage surgery has no better survival advantage over the latter.[35] When the consideration for the limb salvage from oncological point of view began in post-World War II, the reconstructive options were limited to the pedicled, tube pedicled flaps and cross leg flap. Following the integration of microsurgical techniques in reconstruction, the limb salvage has become the standard approach of the sarcoma management with better patient acceptance, improved quality-of-life without compromising the survival.[34–36] Primary amputations as the treatment choice in lower extremity sarcomas are now reduced to <5% of all patients and <15% among recurrences while demonstrating a comparable long-term survival.[34,35,36] Primary amputation is however investable in extensive circumferential limb sarcoma, multi component proximal thigh tumours, extensive skeletal involvement, compromised general condition for a longer procedure, local unfavourable conditions like PVD or technically difficult reconstructions.
The aim of limb salvage is thus to provide stability, weight bearing capability and ambulation. A multidisciplinary team approach with input from Oncologists, Pathologists and resective and reconstructive surgeons is essential to achieve such a goal.[37] The reconstruction however is challenging in lower limbs due to the vicinity of vital structures running close together, which are often invaded by a relatively smaller tumour. Poor vascularity of lower extremity, wound healing problems, prone for infections, high venous pressure and poor nerve repair or grafting results further hinders in achieving the goal.
Wide resection of the tumour entails sacrifice of considerable skin, soft-tissue, skeletal and neurovascular structures leaving composite defects. In addition, pre-operative and adjuvant radiation along with chemotherapy alters the conventional reconstructive options of a successful coverage. While prior radiation deprives availability of local tissue for the cover, adjuvant radiation demands supple vascularised tissue to fill the defects and cover and protect exposed neurovascular structures and skeleton. The objectives of soft-tissue reconstruction include obliteration of dead space, tension free skin closure, supporting the preserved skin with underneath muscle flaps and to provide adequate cushion for exposed bony prominences or amputation stumps. Irradiated skin precludes usage of local flaps or local perforator flaps due to fibrosis and inelasticity of the surrounding skin. Similarly, when radiation is planned following surgery, skin grafts or muscle flap covered with skin grafts are avoided due to their poor tolerance for radiation. Perforator or fasciocutaneous flaps are superior to skin grafts, but inadequate to cover large defects and donor site skin graft in the immediate vicinity is a disadvantage. As most sarcoma are located in the proximal limb, pedicled flap based on deep inferior epigastric artery system either vertical [Figure 3a–d] or transverse myocutaneous flap offers an excellent choice. Superior or inferior gluteal artery flap from the buttock is another option available for the proximal limb defects. All these flaps can also be converted into free flaps if pedicle length limits adequate coverage. The microsurgical reconstruction offers a superior tailored flap to the defects from various donor sites. It is essential to ensure patency of recipient vessels preferably chosen away from the irradiated areas.
Figure 3.
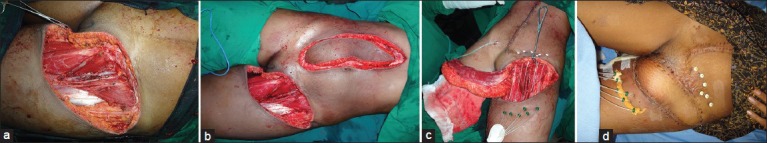
(a) A young lady with soft-tissue sarcoma resection defect of left groin. Tumour infiltrating the femoral artery was resected. Femoral artery reconstructed with saphenous vein graft. (b) Reconstruction planned with vertical rectus abdominus flap based on inferior epigastric artery. (c) Prior to inset of flap, brachytherapy ports inserted for radiotherapy. (d) Early post-operative with well settled flap
Immediate reconstruction though is a preferred choice, but not always obligatory unless neurovascular cover or reconstruction is involved. All major vascular defects are reconstructed simultaneously to ensure limb viability with a mandatory soft-tissue cover. Vascular continuity can be achieved with interpositional vein grafts or alloplastic material. It should be noted that autologous vein grafts have higher chances of thrombosis in oncosurgical reconstruction than in for traumatic conditions possibly due to longer length.
Skeletal defects following resection of primary osseous sarcoma or bony invasion of STS calls for a multidisciplinary approach involving orthopaedic and plastic surgeons.[38] Modern orthopaedic techniques include expandable and non-expandable tumour prostheses, resection arthroplasties, distraction osteogenesis, segment transport or total joint replacements. In combination with such procedures, vascularised bone and soft-tissue cover is mandatory for long lasting desired outcomes. Free bone grafts are not useful whenever irradiation is planned are limited to the defects <4 cm with adequate soft-tissue envelop. Ipsilateral pedicled fibula transfer, free latissimuss dorsi muscle with scapular bone up to 11 cm or parascapular flap have been utilised for augmenting the prosthetic skeletal reconstruction and obliteration of the dead space. For large bony defects, vascularised fibula and Iliac crest offers an excellent choice though discrepancy in bony diameter exists. They are dynamic, hypertrophies with walking stress, relatively infection and radiation resistant.[39]
Prognosis of patients with extremity STS, has not changed over the past 20 years, indicating that current therapy has reached the limits of efficacy.[34] Limb salvage with microvascular reconstruction is safe and reliable in lower extremity STS reconstruction, which has avoided amputation in most patients.[40,41]
TROPHIC ULCERS
Trophic ulcers (Neuropathic or plantar ulcers) on the sole have been described as the scourge to the insensitive foot.[42] These ulcers initiate a vicious cycle of progressive infection and deformity associated with crippling morbidity. They bear a multi-factorial aetiology that ranges from diabetes, Hansen's disease to spinal injury and spina bifida. Chronicity and recalcitrance to local and systemic treatment are the hallmarks of these lesions. Many of these patients if left untreated develop complications and eventually undergo amputation.
An understanding of the contributory factors and addressing them is essential to ensure successful limb salvage. The natural history of the disease is initiated by the high biomechanical stresses that develop on walking with an insensate foot. However, the onset of peripheral neuropathy removes the protective mechanism against excessive pressure and pain and initiates the breakdown of plantar skin, which proceeds to alter the biomechanics of the foot with regard to ankle kinematics, gait distribution and gait kinetics.[43] A complex interplay of neurologic, autonomic and vascular damage advances the disease over time.
The main treatment principles are (1) radical debridement, particularly the osteomyelitic bones; (2) wound coverage with adequate bulk and (3) podiatric support for offloading. Additional measures include endovascular procedures to improve foot perfusion, neural decompression for sensory recovery and optimisation of physical condition. Most challenging aspect of treatment however would be reconstruction of the plantar defect. The heel and the forefoot represent the weight bearing zones and contribute to 95% of locations of these ulcers.
Satisfactory functional reconstruction of these ulcers need a flap with adequate bulk, provide shock absorption and remain stable and durable against shearing forces of ambulation. In addition, the transferred tissue should deliver enough vascularity to the bed to clear the infection as well as retain normal foot contour to fit footwear. Perry has calculated the shearing and pressure forces during walking to be around 9.3 kg/cm2 in an average individual, which would translate to forces around 60 tons/foot when walking a mile.[44] Thus, the most superior mode of reconstruction needs to be used to ensure optimum rehabilitation of the patient.
Local flaps undeniably satisfy the physical properties of the weight bearing plantar region as the skin is glabrous, sensate (in otherwise normal foot) and has qualities superior to distant tissue transfer thus replacing ‘like with like’. However, the local skin conditions of a neuropathic foot are vastly different. Peripheral neuropathy renders the skin insensate and brittle, which breaks down easily even with normal shearing force thus losing the advantage over the transferred tissue. A local flap, when chosen, is mostly from a non-weight bearing area and leaves the donor defect skin grafted and this skin grafted area is not ideal for weight bearing during offloading.
A smaller looking ulcer generally has a wide base often with an exposed bone in the floor surrounded by callous skin edges resulting from both chronic inflammation and the neuropathy. Radical debridement invariably necessary until a healthy fat pad margin is obtained. This results in a much larger defect than apparently visible. Concomitant osteomyelitis farther complicates the wound and demands a good vascular tissue cover to obliterate the dead space and to promote healing. Thus, a larger sized defect, insensate local tissue, associated osteomyelitis and need of preserving the non-weight bearing areas for the purpose of offloading favours free tissue transfer for the better and long-term outcome of these ulcers. The prerequisite however is adequate vascularity of the affected limb, which is assessed clinically by palpable distal pulsations. Commonly used free flaps for sole reconstruction are fasciocutaneous flaps such as the anterolateral thigh (ALT) flap, radial forearm flap [Figure 4a–d], lateral arm flap, parascapular flap. Among this ALT flap offers an ideal choice as defect can be obliterated with desired thickness, additional muscle can be included and flap harvesting possible in the same position. Muscle flaps like gracilis or latissimus dorsi with split skin grafts are good vascular tissue fitting to the defect, but provide inferior surface for the walking.
Figure 4.
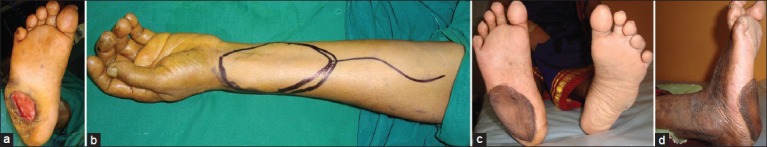
(a) A 58-year-old lady with insensate foot with trophic ulcer of 8 years duration due to Hansen's disease. (b) Reconstruction planned with radial artery forearm free flap with posterior tibial as end to side recipient vessel. (c) 2 years post-reconstruction with well-settled flap. (d) Flap without excessive bulk, contoured to the foot
Although free vascularised tissues are arguably superior form of reconstruction, the decision needs to be individualised as it is technically demanding and is subject to local conditions and physical fitness of the patient. Debilitating illness, poor cardiac status, associated PVD and uncooperative or noncompliant patients should be excluded. Local conditions like extensive subcutaneous fibrosis due to repeated cellulitis and chronic suppurative arthritis generally results in unfavourable outcomes. Infected charcoit (neuropathic) joints with unstable and insensate foot should be evaluated cautiously and realistic decision should be taken between the salvage and the prosthesis in conjunction with the patient.
DIABETIC FOOT
Diabetic patients have multitude of problems such as microvascular angiopathy, poor metabolic control, neuropathy and nephropathy leading to chronic bacterial colonisation, ischaemic wounds, osteomyelitis with recurrent cellulitis and bony deformity.[45–48] About 15% of them during their lifetime develop leg ulceration and they constitute two-third of all amputation performed.[49,50] Diabetic neuropathy is a single most major cause for foot ulceration and more than 80% of diabetic foot ulcers have some form of neuropathy. About 80-85% of amputations are preceded by non-healing ulcers in patients with neuropathy.[51,52] Involvement of medium sized vessels is well-known in the infrapopliteal region,[53] however the peripheral neuropathy is the primary cause of foot ulceration leads to 6 times higher chances of ulceration.[52]
A multidisciplinary team is essential for a holistic approach and success of reconstructive procedures. Treatment strategies include optimising the patient, correction of vascular insufficiency, aggressive debridement and providing a well-vascularised tissue cover.
The risk factors for lower extremity amputation (LEA) in diabetic patients are peripheral sensory neuropathy, PVD, foot ulcers (particularly if they appear on the same side as the eventual LEA), former amputation and treatment with insulin.[54] Half of them undergo reamputation leading to eventual death within 3 years especially patients presenting with gangrene of the foot in insulin-dependent diabetes mellitus.[55]
Diabetic foot reconstruction using free flaps reported to have more than 91% success which significantly increases the 5-year limb salvage rate (86%). Risk factors such as peripheral arterial diseases, history of angioplasties in the extremity and using immunosuppressive agents after transplant likely to increase the flap loss.[56]
LIMB ISCHAEMIA WITH INTRACTABLE LEG ULCERS
Ischaemic ulcers present as painful non healing wounds with extreme threat to the limb and disproportionally cause severe rest pain. Unless limb perfusion is improved, proximal limb amputation is inevitable. The most common cause is atherosclerosis and diabetes followed by Buerger's disease, vasculitis and thromboembolic disease. Doppler evaluation showing a monophasic flow warrants treatment, which should be initiated prior to any surgical management. Unless a wet gangrene, necrotising fasciitis or ascending cellulitis exists, the initial surgical debridement is delayed to avoid any loss of potentially salvageable tissue. To improve perfusion both bypass-surgery and endovascular procedures have been employed. The results of Endovascular procedures such as balloon angioplasty and stenting are comparable to the invasive bypass surgery in terms of amputation free survival.[57,58] Endovascular procedures in combination with surgical bypass reduces the extent of surgery and the length of grafts required particularly in surgically high risk individuals. With such procedures the limb salvage rate of 87% at 2 years has been reported.[59]
EXPOSED PROSTHESIS
Espoused hardware and prosthesis poses risk of not only the implant loss, but also the limb causing considerable setback to the patient in terms of suffering, disability and financial burden. Basic principles of management include irrigation, debridement, antibiotics and removal of hardware itself. However, several factors should be taken into account before considering removal such as location of the hardware, microbiological flora, duration of infection, duration of exposure of hardware and hardware loosening. In a retrospective review by Viol et al., they concluded that if hardware is clinically stable, time of exposure is <2 weeks, infection is controlled and the location of the hardware is for bony consolidation; then, it may increase the likelihood of salvage of hardware using the surgical soft-tissue coverage.[60]
Exposed vascular grafts present life- and limb-threatening complications. It requires urgent intervention and early debridement and muscle flap coverage to salvage the graft. Synthetic grafts a source of bacterial colonisation preferably are replaced with an autologous graft. Local muscle flaps such as gracilis, sartorius or tensor fascia lata are invaluable in achieving cover and obliteration of the dead space. Exposure of vein graft should also be managed in the similar manner after ensuring the graft patency.
EVOLVING CONCEPTS FROM COMPLEX TO SIMPLER APPROACHES IN RECONSTRUCTION
The successful limb salvage over the past five decades has evolved in parallel with the advances in soft-tissue and skeletal reconstructions. From a limited option of tube pedicled flaps of then to the super-microsurgery of today the newer concepts continue to emerge. The extensive, complex defects are being reconstructed from a single flap option to a more versatile compound flaps with superior functional and aesthetic results. Exploiting multiple perforators from a single vascular source, various flap designs with different tissue components to suit the anatomical defect has been successful. These flaps include composite flaps (multiple tissue components served by single vascular source), conjoined flap (multiple flap territory with physical interconnections each retaining independent vascular supply) and chimeric flap (multiple flap territory without interconnections, receiving independent blood supply). Sequential flaps are two different flaps linked together with their pedicles and anastomosed to a single recipient vessel. A flow through flap acts as a conduit to re-establish vascular continuity and provides simultaneous soft-tissue cover. All these flap modifications and in combination with available conventional flaps have enormously increased the microsurgical coverage options of composite defects.
While reconstructing such defects with microsurgery has been undisputedly practiced as a preferred option, there is an evolving trend to explore newer local coverage options for the same purpose based on improved understanding of leg vascularity and angiosomes. Such local flaps successfully employed include Keystone flap, propeller perforator and free style perforator flaps/free flaps.[61–64]
Keystone/perforator flaps
Described by Behan in 2003 the keystone flap is based on fasciocutaneous perforators, is reliable and expeditious to execute by local rearrangement of the tissues.[61] A high success rate of 97% in large lower extremity and trunk defect reconstruction is reported. Several advantages include robust vascularity based on the axial or bunch of perforators, ease of harvest, primary closure of the defect, and superior aesthetic appearance by utilising adjacent skin. It provides robust vascularity of a perforator flap without technical difficulties of a perforator flap and it also obviate need of free flaps in select cases.[62]
Although technically more challenging to perform, free style perforator free flaps have endless donor site and recipient vessel option without disturbing the vascularity of the foot. Further advantages include minimal donor-site morbidity, reliable coverage and improved contour. This represents a paradigm shift in flap selection however demands a surgical proficiency in flap dissection technique and anstomosis.
NPWT
Further down in the reconstruction ladder from super microsurgery to local flaps, the downward trend continues to ‘downsize’ the defect with increasing acceptance of the role of NPWT. Reduction of oedema and wound surface area, rapid granulation growth and lesser infection rate has prompted usage of NPWT in more complex wounds such as exposed tendon, bone or fractures sites. Successful coverage has been achieved even in Gustilo type III wounds with delayed closure, skin grafting or local flap cover. This is cost-effective, avoids complex surgical procedures and its complications.[65–67]
CONCLUSIONS
Limb salvage should be aimed at providing stability and function. While the objective remains common, strategies of reconstruction differ such as: Early coverage in trauma, replacing lost functional units in cancer ablation, improving vascularity in ischaemic leg and providing stable walking surface for trophic ulcers. The decision to salvage the critically injured limb is multifactorial and should be individualised along with laid down definitive indications. Though the limb salvage is claimed cost-effective than amputation in a long run, no data available from developing counties. The advent of microsurgery has salvaged critically injured limbs, with early and versatile reconstructive options including combined flaps and perforator free flaps. Recent trend also shows running down the ladder of reconstruction with newer reliable local flaps and NPWT. Endovascular procedures have given scope for further reconstructive attempts in ischaemic limbs thus prolonging the amputation free survival.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ong YS, Levin LS. Lower limb salvage in trauma. Plast Reconstr Surg. 2010;125:582–8. doi: 10.1097/PRS.0b013e3181c82ed1. [DOI] [PubMed] [Google Scholar]
- 2.Helfet DL, Howey T, Sanders R, Johansen K. Limb salvage versus amputation. Preliminary results of the mangled extremity severity score. Clin Orthop Relat Res. 1990;256:80–6. [PubMed] [Google Scholar]
- 3.Howe HR, Jr, Poole GV, Jr, Hansen KJ, Clark T, Plonk GW, Koman LA, et al. Salvage of lower extremities following combined orthopedic and vascular trauma. A predictive salvage index. Am Surg. 1987;53:205–8. [PubMed] [Google Scholar]
- 4.Johansen K, Daines M, Howey T, Helfet D, Hansen ST., Jr Objective criteria accurately predict amputation following lower extremity trauma. J Trauma. 1990;30:568–72. doi: 10.1097/00005373-199005000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Russell WL, Sailors DM, Whittle TB, Fisher DF, Jr, Burns RP. Limb salvage versus traumatic amputation. A decision based on a seven-part predictive index. Ann Surg. 1991;213:473–80. doi: 10.1097/00000658-199105000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krettek C, Seekamp A, Köntopp H, Tscherne H. Hannover fracture scale ‘98: Re-evaluation and new perspectives of an established extremity salvage score. Injury. 2001;32:317–28. doi: 10.1016/s0020-1383(00)00201-1. [DOI] [PubMed] [Google Scholar]
- 7.Bonanni F, Rhodes M, Lucke JF. The futility of predictive scoring of mangled lower extremities. J Trauma. 1993;34:99–104. doi: 10.1097/00005373-199301000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Bosse MJ, MacKenzie EJ, Kellam JF, Burgess AR, Webb LX, Swiontkowski MF, et al. A prospective evaluation of the clinical utility of the lower-extremity injury-severity scores. J Bone Joint Surg Am. 2001;83-A:3–14. doi: 10.2106/00004623-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Durham RM, Mistry BM, Mazuski JE, Shapiro M, Jacobs D. Outcome and utility of scoring systems in the management of the mangled extremity. Am J Surg. 1996;172:569–73. doi: 10.1016/S0002-9610(96)00245-0. [DOI] [PubMed] [Google Scholar]
- 10.Ly TV, Travison TG, Castillo RC, Bosse MJ, MacKenzie EJ LEAP Study Group. Ability of lower-extremity injury severity scores to predict functional outcome after limb salvage. J Bone Joint Surg Am. 2008;90:1738–43. doi: 10.2106/JBJS.G.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange RH. Limb reconstruction versus amputation decision making in massive lower extremity trauma. Clin Orthop Relat Res. 1989;243:92–9. [PubMed] [Google Scholar]
- 12.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: A new classification of type III open fractures. J Trauma. 1984;24:742–6. doi: 10.1097/00005373-198408000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78:285–92. doi: 10.1097/00006534-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Francel TJ, Vander Kolk CA, Hoopes JE, Manson PN, Yaremchuk MJ. Microvascular soft-tissue transplantation for reconstruction of acute open tibial fractures: Timing of coverage and long-term functional results. Plast Reconstr Surg. 1992;89:478–87. [PubMed] [Google Scholar]
- 15.Organek AJ, Klebuc MJ, Zuker RM. Indications and outcomes of free tissue transfer to the lower extremity in children: Review. J Reconstr Microsurg. 2006;22:173–81. doi: 10.1055/s-2006-939963. [DOI] [PubMed] [Google Scholar]
- 16.Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80:1–14. doi: 10.1097/00006534-198707000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Heller L, Levin LS. Lower extremity microsurgical reconstruction. Plast Reconstr Surg. 2001;108:1029–41. doi: 10.1097/00006534-200109150-00036. [DOI] [PubMed] [Google Scholar]
- 18.Kolker AR, Kasabian AK, Karp NS, Gottlieb JJ. Fate of free flap microanastomosis distal to the zone of injury in lower extremity trauma. Plast Reconstr Surg. 1997;99:1068–73. doi: 10.1097/00006534-199704000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Karanas YL, Nigriny J, Chang J. The timing of microsurgical reconstruction in lower extremity trauma. Microsurgery. 2008;28:632–4. doi: 10.1002/micr.20551. [DOI] [PubMed] [Google Scholar]
- 20.Arnez ZM. Immediate reconstruction of the lower extremity: An update. Clin Plast Surg. 1991;18:449–57. [PubMed] [Google Scholar]
- 21.Isenberg JS, Sherman R. Zone of injury: A valid concept in microvascular reconstruction of the traumatized lower limb? Ann Plast Surg. 1996;36:270–2. doi: 10.1097/00000637-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Park S, Han SH, Lee TJ. Algorithm for recipient vessel selection in free tissue transfer to the lower extremity. Plast Reconstr Surg. 1999;103:1937–48. doi: 10.1097/00006534-199906000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Hong JP. The use of supermicrosurgery in lower extremity reconstruction: The next step in evolution. Plast Reconstr Surg. 2009;123:230–5. doi: 10.1097/PRS.0b013e3181904dc4. [DOI] [PubMed] [Google Scholar]
- 24.Dormandy J, Heeck L, Vig S. Major amputations: Clinical patterns and predictors. Semin Vasc Surg. 1999;12:154–61. [PubMed] [Google Scholar]
- 25.Gonzalez EG, Corcoran PJ, Reyes RL. Energy expenditure in below-knee amputees: Correlation with stump length. Arch Phys Med Rehabil. 1974;55:111–9. [PubMed] [Google Scholar]
- 26.Kasabian AK, Colen SR, Shaw WW, Pachter HL. The role of microvascular free flaps in salvaging below-knee amputation stumps: A review of 22 cases. J Trauma. 1991;31:495–500. doi: 10.1097/00005373-199104000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Kadam D. Secondary reconstruction of below knee amputation stump with free anterolateral thigh flap. Indian J Plast Surg. 2010;43:108–10. doi: 10.4103/0970-0358.63964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saddawi-Konefka D, Kim HM, Chung KC. A systematic review of outcomes and complications of reconstruction and amputation for type IIIB and IIIC fractures of the tibia. Plast Reconstr Surg. 2008;122:1796–805. doi: 10.1097/PRS.0b013e31818d69c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butcher JL, MacKenzie EJ, Cushing B, Jurkovich G, Morris J, Burgess A, et al. Long-term outcomes after lower extremity trauma. J Trauma. 1996;41:4–9. doi: 10.1097/00005373-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Bosse MJ, MacKenzie EJ, Kellam JF, Burgess AR, Webb LX, Swiontkowski MF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N Engl J Med. 2002;347:1924–31. doi: 10.1056/NEJMoa012604. [DOI] [PubMed] [Google Scholar]
- 31.Chung KC, Saddawi-Konefka D, Haase SC, Kaul G. A cost-utility analysis of amputation versus salvage for Gustilo type IIIB and IIIC open tibial fractures. Plast Reconstr Surg. 2009;124:1965–73. doi: 10.1097/PRS.0b013e3181bcf156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKenzie EJ, Jones AS, Bosse MJ, Castillo RC, Pollak AN, Webb LX, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am. 2007;89:1685–92. doi: 10.2106/JBJS.F.01350. [DOI] [PubMed] [Google Scholar]
- 33.Williams MO. Long-term cost comparison of major limb salvage using the Ilizarov method versus amputation. Clin Orthop Relat Res. 1994;301:156–8. [PubMed] [Google Scholar]
- 34.Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: Improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21:2719–25. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Papagelopoulos PJ, Mavrogenis AF, Mastorakos DP, Patapis P, Soucacos PN. Current concepts for management of soft tissue sarcomas of the extremities. J Surg Orthop Adv. 2008;17:204–15. [PubMed] [Google Scholar]
- 36.Daigeler A, Lehnhardt M, Khadra A, Hauser J, Steinstraesser L, Langer S, et al. Proximal major limb amputations: A retrospective analysis of 45 oncological cases. World J Surg Oncol. 2009;7:15. doi: 10.1186/1477-7819-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heller L, Kronowitz SJ. Lower extremity reconstruction. J Surg Oncol. 2006;94:479–89. doi: 10.1002/jso.20485. [DOI] [PubMed] [Google Scholar]
- 38.Chang DW, Robb GL. Recent advances in reconstructive surgery for soft-tissue sarcomas. Curr Oncol Rep. 2000;2:495–501. doi: 10.1007/s11912-000-0102-0. [DOI] [PubMed] [Google Scholar]
- 39.Chen CM, Disa JJ, Lee HY, Mehrara BJ, Hu QY, Nathan S, et al. Reconstruction of extremity long bone defects after sarcoma resection with vascularized fibula flaps: A 10-year review. Plast Reconstr Surg. 2007;119:915–24. doi: 10.1097/01.prs.0000252306.72483.9b. [DOI] [PubMed] [Google Scholar]
- 40.Barner-Rasmussen I, Popov P, Böhling T, Tarkkanen M, Sampo M, Tukiainen E. Microvascular reconstruction after resection of soft tissue sarcoma of the leg. Br J Surg. 2009;96:482–9. doi: 10.1002/bjs.6581. [DOI] [PubMed] [Google Scholar]
- 41.Topham NS. Reconstruction for lower extremity limb salvage in soft tissue carcinoma. Curr Treat Options Oncol. 2003;4:465–75. doi: 10.1007/s11864-003-0047-2. [DOI] [PubMed] [Google Scholar]
- 42.Lang-Stevenson AI, Sharrard WJ, Betts RP, Duckworth T. Neuropathic ulcers of the foot. J Bone Joint Surg Br. 1985;67:438–42. doi: 10.1302/0301-620X.67B3.2987271. [DOI] [PubMed] [Google Scholar]
- 43.Bacarin TA, Sacco IC, Hennig EM. Plantar pressure distribution patterns during gait in diabetic neuropathy patients with a history of foot ulcers. Clinics (Sao Paulo) 2009;64:113–20. doi: 10.1590/S1807-59322009000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry J. Anatomy and biomechanics of the hindfoot. Clin Orthop Relat Res. 1983;177:9–15. [PubMed] [Google Scholar]
- 45.Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Plast Reconstr Surg. 2006;117:212S–38. doi: 10.1097/01.prs.0000222737.09322.77. [DOI] [PubMed] [Google Scholar]
- 46.Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331:854–60. doi: 10.1056/NEJM199409293311307. [DOI] [PubMed] [Google Scholar]
- 47.Dargis V, Pantelejeva O, Jonushaite A, Vileikyte L, Boulton AJ. Benefits of a multidisciplinary approach in the management of recurrent diabetic foot ulceration in Lithuania: A prospective study. Diabetes Care. 1999;22:1428–31. doi: 10.2337/diacare.22.9.1428. [DOI] [PubMed] [Google Scholar]
- 48.Jolly GP, Zgonis T, Blume P. Soft tissue reconstruction of the diabetic foot. Clin Podiatr Med Surg. 2003;20:757–81. doi: 10.1016/S0891-8422(03)00072-7. [DOI] [PubMed] [Google Scholar]
- 49.Block P. The diabetic foot ulcer: A complex problem with a simple treatment approach. Mil Med. 1981;146:644–6. [PubMed] [Google Scholar]
- 50.Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower-extremity major amputation in individuals with and without diabetes in the medicare population. Diabetes Care. 2001;24:860–4. doi: 10.2337/diacare.24.5.860. [DOI] [PubMed] [Google Scholar]
- 51.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 52.Ollendorf DA, Kotsanos JG, Wishner WJ, Friedman M, Cooper T, Bittoni M, et al. Potential economic benefits of lower-extremity amputation prevention strategies in diabetes. Diabetes Care. 1998;21:1240–5. doi: 10.2337/diacare.21.8.1240. [DOI] [PubMed] [Google Scholar]
- 53.LoGerfo FW, Coffman JD. Current concepts. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med. 1984;311:1615–9. doi: 10.1056/NEJM198412203112506. [DOI] [PubMed] [Google Scholar]
- 54.Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22:1029–35. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- 55.Kono Y, Muder RR. Identifying the incidence of and risk factors for reamputation among patients who underwent foot amputation. Ann Vasc Surg. 2012;26:1120–6. doi: 10.1016/j.avsg.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Oh TS, Lee HS, Hong JP. Diabetic foot reconstruction using free flaps increases 5-year-survival rate. J Plast Reconstr Aesthet Surg. 2013;66:243–50. doi: 10.1016/j.bjps.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 57.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 58.Ino K, Kiyokawa K, Akaiwa K, Ishida M, Furuyama T, Onohara T. A team approach to the management of intractable leg ulcers. Ann Vasc Dis. 2013;6:39–45. doi: 10.3400/avd.oa.13.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masaki H, Tabuchi A, Yunoki Y, Kubo H, Nishikawa K, Yakiuchi H, et al. Collective therapy and therapeutic strategy for critical limb ischemia. Ann Vasc Dis. 2013;6:27–32. doi: 10.3400/avd.oa.12.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viol A, Pradka SP, Baumeister SP, Wang D, Moyer KE, Zura RD, et al. Soft-tissue defects and exposed hardware: A review of indications for soft-tissue reconstruction and hardware preservation. Plast Reconstr Surg. 2009;123:1256–63. doi: 10.1097/PRS.0b013e31819f2b5e. [DOI] [PubMed] [Google Scholar]
- 61.Behan FC. The keystone design perforator island flap in reconstructive surgery. ANZ J Surg. 2003;73:112–20. doi: 10.1046/j.1445-2197.2003.02638.x. [DOI] [PubMed] [Google Scholar]
- 62.Khouri JS, Egeland BM, Daily SD, Harake MS, Kwon S, Neligan PC, et al. The keystone island flap: Use in large defects of the trunk and extremities in soft-tissue reconstruction. Plast Reconstr Surg. 2011;127:1212–21. doi: 10.1097/PRS.0b013e318205f36f. [DOI] [PubMed] [Google Scholar]
- 63.Georgescu AV, Matei IR, Capota IM. Diabetic Foot & Ankle. North America: 2012. [Last accessed on 21 Apr 2013]. The use of propeller perforator flaps for diabetic limb salvage: A retrospective review of 25 cases; p. 3. Available from: http://diabeticfootandankle.net/index.php/dfa/article/view/18978 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rinaldi S, Piperno A, Toscani M, Tarallo M, Cigna E, Fino P, et al. Free-style perforator flaps in the reconstruction of the lower limb. Ann Ital Chir. 2013. [Last accessed on 21 Apr 2013]. p. 84. Available from: http://www.annaliitalianidichirurgia.it/PDF/ONLINE/10_04_2013.pdf . [PubMed]
- 65.Nazerali RS, Pu LL. Free tissue transfer to the lower extremity: A paradigm shift in flap selection for soft tissue reconstruction. Ann Plast Surg. 2013 doi: 10.1097/SAP.0b013e31828a0c3c. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Parrett BM, Talbot SG, Pribaz JJ, Lee BT. A review of local and regional flaps for distal leg reconstruction. J Reconstr Microsurg. 2009;25:445–55. doi: 10.1055/s-0029-1223847. [DOI] [PubMed] [Google Scholar]
- 67.DeFranzo AJ, Argenta LC, Marks MW, Molnar JA, David LR, Webb LX, et al. The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast Reconstr Surg. 2001;108:1184–91. doi: 10.1097/00006534-200110000-00013. [DOI] [PubMed] [Google Scholar]


