Abstract
Phalloplasty has come a long way as Plastic Surgery has evolved over the years. The complication ridden multistage tube pedicles popularized by Gillis were, with the advent of microsurgery, replaced by radial forearm flaps. The composite osteo-cutaneous version of this flap promised ‘All for one and one for all’ assuring both a reliable urinary conduit and a phallus stiffener. Prelamination and prefabrication to make the neo-urethra came with the promise of reducing both fistula and strictures but that did not happen and flap failure rates increased. Penile stiffeners of various types have been introduced; the artificial ones were associated with high infection and failure rates and are best inserted after the neo-penis regains some sensitivity. With the introduction of perforator flaps the Anterolateral thigh flap in its sensate pedicled form has started replacing the Radial forearm free flap as the first choice flap because of a hidden donor area and lack of microsurgical expertise requirement. Being sensate it tolerates a stiffener better. It is now possible to reconstruct an aesthetically pleasing glans as well, thus meeting both the aesthetic and functional desires of the patient. Complications encountered in this reconstructive effort include flap failure, urethral fistula, urethral stricture and stiffener related problems.
KEY WORDS: Gender reassignment, penile reconstruction, phalloplasty
INTRODUCTION
One of the most enduring dreams in medicine has been the possibility to replicate or replace human organs lost to disease or trauma. The phallus, symbolic of manhood, has received much less attention than the kidney, liver the heart or the breast, possibly because it is presumed that one can live without a penis. Another possible reason for this could be that the function of this organ appears almost magical; responding as it does to changes in emotion and environment. Unlike other organs, cadaveric transplantation seems desecrating, to say the least. As the holy grail of plastic surgery has been ‘replacing like with like’ a real phalloplasty does appear to be a dream. This does not in any case undermine the importance of the goal to reconstruct as perfect a phallus as possible with the materials available to us, perfect not only in form but also in function.
In this article, we will review the various methods used for phalloplasty over the years with a view to determine the best choice. We will also discuss the common complications of these procedures and attempt to highlight the ways in which they can be avoided.
HISTORY OF PHALLOPLASTY
Bogoraz from Russia was the first to reconstruct a total penis using a tubed abdominal flap and autologous rib cartilage in 1936.[1] The First female to male gender reassignment procedure was performed by Sir Harold Gillies in 1946 on another British physician, Laurence Michael Dillon (born Laura Maude Dillon) who felt ‘not truly a woman’. This involved a series of 13 operations. This technique for female to male gender reassignment remained the standard technique for 40 years.[2] Other researchers like Orticochea[3] and Puckett et al.[4] introduced the first pedicled flap and first free flap phalloplasty techniques respectively in the 70s and early 80s. Despite further developments, the tubed lower abdominal phalloplasty remained quite popular until Chang and Hwang popularised the tube-within-a-tube design in the 1980s using the free radial forearm flap.[5] This concept of a single stage urethral-phalloplasty soon became the technique of choice and despite various issues associated with the use of forearm skin, remains the method with which others are compared.
INDICATIONS OF PHALLOPLASTY
Though many of the larger published series of phalloplasty consist predominantly of gender reassignment cases, the indications for this procedure are in no way limited to this group of patients. The main indications for phalloplasty are given in Table 1.
Table 1.
Indications for phalloplasty

GOALS
Hage and De Graaf addressed the ideal requirements for phalloplasty,[6] in 1993 as follows:
It should be a one-stage procedure that can be reproduced. There should be a competent neo-urethra to allow for voiding while standing. There should be the return of both tactile and erogenous sensibility. There should be enough bulk to tolerate the insertion of a stiffener. The result should be aesthetically acceptable to the patient. Finally, the procedure should cause minimal scarring with no functional loss in the donor area.
Patient's perception of normal form and function are also very relevant. Hage et al. looked into the patient's expectations after surgery.[7] According to their study 52% patients are looking for following: A scrotum (96%), a glans (92%), rigidity (86%) and an aesthetically appealing look either while wearing a tight swim suit (91%) or being nude (81%). All, but one patient wanted to be able to void in a standing position. A point to remember is that almost all of these were female to male transsexuals.
PHALLOPLASTY TECHNIQUES
Over the years, many different techniques have been used to reconstruct the penis in order to achieve the above goals. The bar for the expected goals kept on getting raised with the advances in plastic surgery, especially since the advent of microvascular surgery. In the following paragraphs, the various methods used for phalloplasty will be discussed.
Random pattern flaps
Tubed abdominal flaps were used by Bogoraz and Gillies for de novo fabrication of the penis. These flaps usually underwent multiple stages before being formally fashioned into a phallus. They were used for many years,[2,8] but were associated with prolonged hospital stays and high flap failure rates. In addition, the aesthetic and functional results were sub-optimal.
In a relatively recent publication, Bettocchi et al.[9] presented the results of 85 female-to-male transsexuals who underwent a phalloplasty using a tubed suprapubic abdominal flap incorporating the neo-urethra made from a pedicled tube of labial skin. They were able to create a penis with good cosmetic appearance in almost 2/3rd of their patients, but the complication rates were significantly high (70%).
As most of the complications with these flaps are related to urethroplasty, many surgeons like De Castro have attempted to perform phalloplasty and urethroplasty using an abdominal skin flap and a bladder/buccal mucosa graft. Although complications are common with this technique, it creates a phallus of reasonable size and shape.[10]
Pedicled flaps
Groin flap
The groin flap was first used for penile reconstruction by Puckett and Montie in 1978.[11] A study from Poland was published in 1999, in which 127 female-to-male transsexuals underwent one-stage phalloplasty using a lateral groin flap.[12] Necrosis of the distal part of the flap or other complications occurred in 20.5% of cases. Flap failure rate was <5%. Urethroplasty was done in only five cases and a stiffener was used in only 37% of cases. This procedure does not comply with the modern goals of phalloplasty but may have a role when other complex options are not possible.[13]
Various modifications of the groin flap have been described. To add rigidity to the neophallus, Sun and Huang proposed a composite flap, including the lateral groin skin flap (11 cm long and 10 cm wide) and the iliac crest bone in its entire length, based on the superficial circumflex pedicle.[14] Aköz et al.[15] reported the use of both the deep and the superficial circumflex iliac vessels to ensure well-vascularised extended skin and bone in the flap.
Anterolateral thigh flap
Descamps et al.[16] first reported using this flap for phalloplasty. He pointed out the advantage of phalloplasty without microvascular anastomsis but was able to complete urethroplasty in only one of his patients and that too in multiple stages. Later Rubino et al. described the use of ALTF as a sensate flap for phalloplasty.[17] Lateral cutaneous femoral nerve stump is sutured to the dorsal clitoris branch from the pudendal nerve for flap sensation. A two-point discrimination of up to 2.5 cm has been demonstrated after 6 months.[18] Lumene et al. reported the single stage phalloplasty using ALTF employing a tube in tube design for urethroplasty.[19] Rashid et al. published data of 14 patients with partial or complete penile loss reconstructed with a pedicled ALTF.[20] In all cases, the urethra was designed in a tube within a tube fashion. All the flaps survived completely. In more than half the cases, (57%) the urethral continuity was restored in a single stage with a fistula rate of 12.5% [Figure 1].
Figure 1.
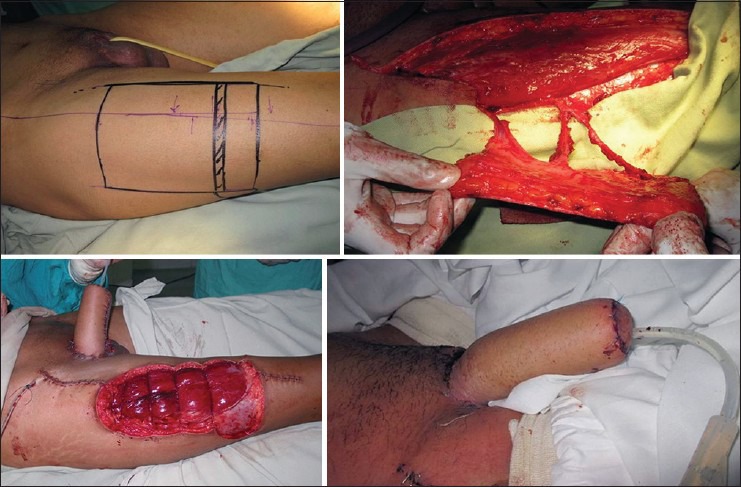
(a) Anterolateral thigh flap design for phalloplasty. (b) The flap being raised on multiple perforators. (c) The ALTF pedicle is passed underneath the rectus femoris and the sartorius to allow placement of the neo-phallus in the midline. (d) Well-healed flap seen at the follow-up
Island tensor fascia lata flap
Santanelli and Scuderi,[21] has demonstrated the use of island TFL flap for phalloplasties in female-to-male transsexuals with good success rates and recommends it as a safe and sensate flap that leaves a less conspicuous donor scar.
Free flaps
Radial forearm free flap
Since the introduction of the Chinese flap design for phalloplasty by Chang and Hwang using RFFF in 1984,[5] it has become the primary option for phalloplasty for most reconstructive surgeons all over the world. Presently, it is considered by many as the gold standard for phalloplasty with which other flaps are compared.[22–24] The ulnar hairless part of the forearm is used to reconstruct the urethra. The urethral part of the flap can be extended distally in a tongue shape for glans as well as proximally to give adequate neo-urethral length for anastomosis. The phallourethroplasty is performed by rolling the flap in tube-within-a-tube fashion [Figures 2 and 3]. An advantage of this flap is the potential for customising the flap to individual requirements especially in male patients who have suffered avulsion injuries.[25]
Figure 2.
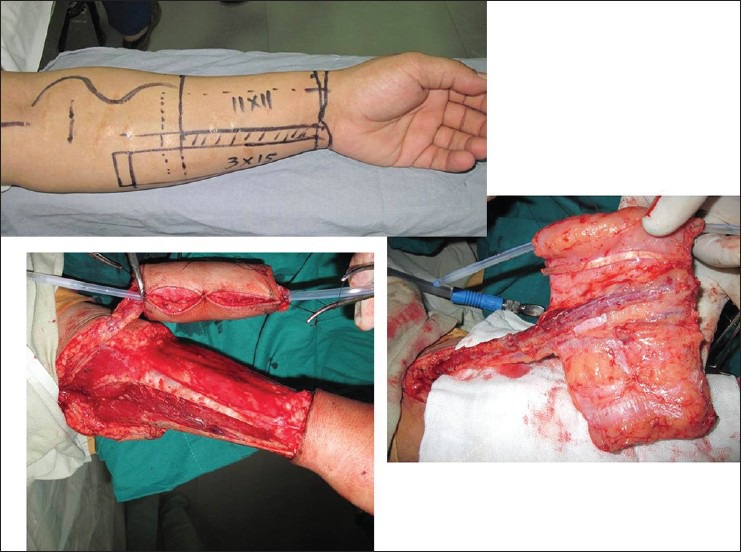
(a) Radial forearm free flap designed for phalloplasty. Note the long ulnar portion of the flap to allow single-stage restoration of urethral continuity. (b) The ulnar flap border being rolled over catheter to form urethra. (c) The radial portion of the flap rolled over the urethra to form the neo-phallus
Figure 3.
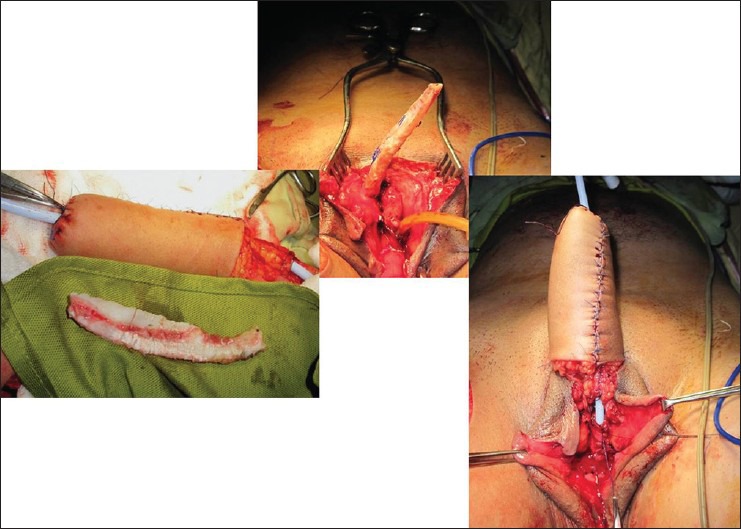
(a) Costal cartilage harvested for the use as stiffener. (b) The cartilage secured to the inferior margin of the pubic symphysis using non-absorbable suture material. (c) The neo-phallus being slid over the cartilage, keeping the cartilage away from the flap pedicle
The main problems include the forearm hair causing urethral obstruction, the high number of initial urinary fistulas, the need for a stiffener or prosthesis, the residual scar on the forearm donor site [Figure 4] and limitation in size of the available forearm skin. Some surgeons even feel that there is loss of phallic girth as a result of tissue atrophy, rendering a phallic contour cosmetically unsatisfactory.[26]
Figure 4.
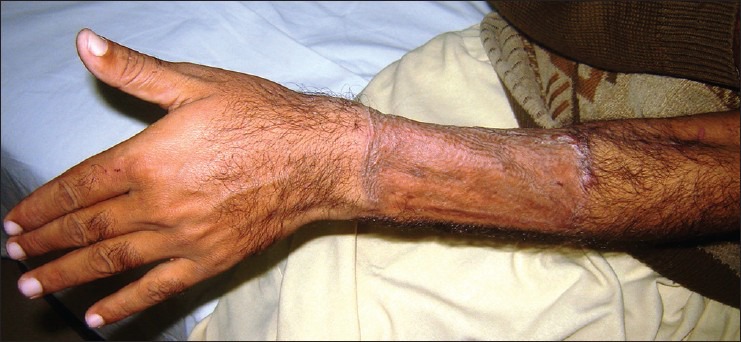
The donor site of the radial forearm free flap
Doornaert et al.[23] published the largest series of 316 radial forearm phalloplasties performed by a single surgical team. They describe this as a very reliable technique for the creation of a normal looking penis and scrotum. While full functionality is achieved through a minimum of two procedures, the patients are always able to void standing and in most cases to experience sexual satisfaction.[27] Long-term follow-up has shown good results in term of flap survival and patient satisfaction. Although most patients report physical and psychological satisfaction, they must be clearly informed that the procedure can seldom be completed in one stage, there are high rate of complications including up to 25% flap related complications and up to 64%,[28] urethroplasty related complications.
Various modifications of the radial forearm flap have been described over the years. Prefabrication of the flap with a tubed graft has been suggested as a technique with less complications and a lower fistula rate.[27] Prakash published a series of radial forearm phalloplasty with urethral prefabrication and did not see any urethral fistula or stricture.[29] Biemer,[30] in 1988 described a RFFF design incorporating urethra in the centre of the flap with a view to improve the vascularity of this segment, but it takes away the advantage of the hairless ulnar skin used for urethrolplasty in the Chinese flap design and there is also a propensity to develop meatal stenosis.[31] Gottlieb proposed a design, which incorporates a centrally located neourethra in continuity with neoglans. It eliminates the circumferential metal suture line and meatal stenosis, without sacrificing phallic length.[31]
Osteocutaneous radial forearm free flap
This technique was introduced to give the advantage of the radial forearm phalloplasty without the need for an additional stiffener.[32] Reported urethral fistula rates range from 40 to 50% and urethral stricture rates of up to 20%.[33,34] Significant donor forearm morbidity has been reported in 9% of cases including radius fracture. No ‘penile fracture’ was noted by Fang in a series of 22 phalloplasties with this technique.[34] More than 70% of cases report good to excellent stiffener function during the intercourse provided by the radial segment included in the flap.[35] A two-stage osteocutaneous radial forearm flap has also been described as a prefabricated sensate flap.[36]
Mutaf has described a pedicled osteocutaneous radial forearm flap designed in the classic ‘tube within a tube’ fashion.[37] The neo-penis is then transferred to the recipient site with its vascular pedicle attached to the forearm [Figure 5]. The pedicle is divided after 3 weeks. Although a multistage procedure, it provides all the advantages of the radial forearm flap, but does not require microsurgical facilities.
Figure 5.
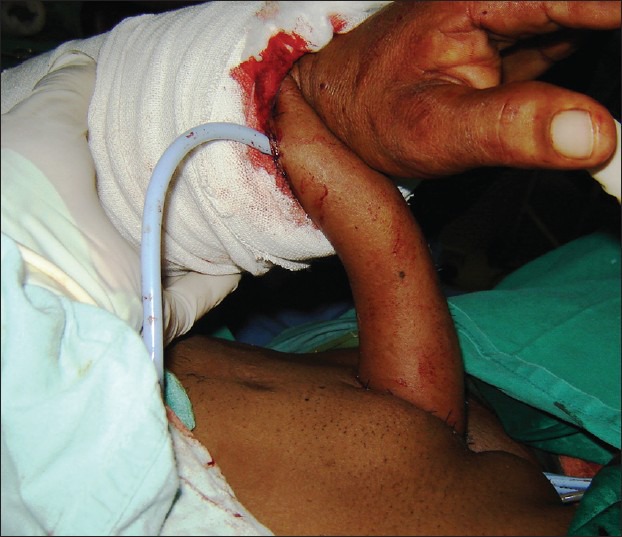
The pedicled radial foream flap, prefabricated and attached to the site of the neo-phallus
The ulnar forearm free flap,[38] has been described as an alternative to the RFFF in order to obtain more non-hair bearing skin and thus reducing hair growth problems in the neo-urethra.
Lateral arm free flap
The lateral arm flap phalloplasty was first described by Upton et al. in 1987,[39] giving the advantage of a relatively inconspicuous donor site without compromising the size of the neophallus. Similar to the concept used in two staged RFFF phalloplasty, a prefabricated neourethra within the lateral arm has also been described to permit the coexistence of an erectile prosthesis alongside a fully vascularised urethra.[40] Khouri et al. published impressive 5 year follow-up results with prefabricated LAF.[41] His patients required five procedures at an average to complete the phalloplasty. He reports complication-free course for all the cases beyond 1st year post-operatively. All neophalluses attained erogenous and tactile sensibility and the inflatable prosthesis allowed penetration during sexual intercourse.
Osteocutaneous free fibula
This flap was described for phalloplasty by Sadove et al. in 1993.[42] The main advantages of this flap lie in its rigidity without the need for an additional stiffener, its donor-site location which can be covered with socks and its long vascular pedicle allows end-to-side anastomosis of the flap to the femoral artery. Long-term follow-up has shown good results with this flap. The fibula has been shown to be viable even after 9 years of reconstruction neural integrity has been confirmed on the sensate flaps and most patients reported pleasurable sexual intercourse and orgasm.[43] The experience with urethral prelamination of this flap has not been very promising with high flap failure and urethral complication rate.[44,45]
Anterolateral thigh free flap
Felici and Felici[46] described the successful use of ALTFF in six cases with a consistent shape of the neo-phallus and high patient satisfaction. The flap can be sensate and an erectile prosthesis can easily be implanted.
Latissimus dorsi free flap
LDFF has been successfully used in both adults and children as a means for constructing a good-sized neo-phallus. Penile size, up to 14-18 cm long,[47] (13-16 cm in children),[48] and from 11 to 15 cm in circumference,[47] (10-12 cm in children),[48] has been achieved using this flap. This is a very reliable flap in terms of flap survival. It is possible to close the donor site primarily in most of the cases,[47] and the scar is at a hidden location. Urethroplasty is usually performed at a later stage using buccal mucosa.[47,48] Although an inflatable penile prosthesis can be used in this flap, one of the peculiar advantages of re-innervated LDFF (thoracodorsal nerve anastomosed to ilioinguinal nerve) is the ability to achieve a ‘paradox erection’ for sexual activity by voluntarily contracting the muscle, thereby avoiding the use of a stiffener.[49]
Free scapular flap
The free scapular flap for penile reconstruction was described by Yang et al. in 2003.[50] All the flaps in the study remained viable post-operatively over a follow-up period of up to 5 years. He did not report any case of urethral fistula, stenosis, prosthesis extrusion or infection. He recommended this flap as an ideal flap that achieves satisfactory function and appearance for penile reconstruction because of its adequate amount of tissue, acceptable donor-site morbidity and reliable blood supply.[51]
URETHROPLASTY
Earlier attempts at phalloplasty were directed only towards reconstruction of the male genital form and the urethral reconstruction was added as a secondary goal. Since constructing a neourethra has frequently led to fistulas and strictures, some authors abandoned their attempts. As the understanding of the patient's requirements improved,[52] urethroplasty was included in the primary goals of phalloplasty.
Different techniques have been described for the reconstruction of the urethra. Prelamination, prefabrication, tube-in-tube or even separate flaps have been used for the reconstruction of the pendulous urethra. For the fixed urethra, local vaginal or labial flaps, extra-long urethral component of the phalloplasty flap, separate flaps and free skin or mucosal grafts have been described. Improved outcome has been seen by the use of prelamination techniques, but these increase the stages to complete the procedure.
Single stage procedures, in which a single flap is rolled upon itself to from the urethra are impressive, but increase the chances of urethral complications. Although the concept of a ‘tube within a tube’ was propagated by Borogaz and Gillies using tubed flaps, the present technique was popularised by Chang and Hwang,[5] using a radial forearm flap. A part of the flap is tubed to construct the urethra and the rest of the flap is rolled to cover the urethra as well as to give a phallic form. This design has been later used in other free as well as pedicled flap phalloplasties.[20,39]
Prelamination and prefabrication
A two stage phalloplasty with urethral prelamination using a full thickness graft has been used with all the commonly used free flaps including the RFFF,[53,54] osteocutaneous radial forearm flap,[36] OCFF,[44] ALTF.[55] Prelaminated OCFF phalloplasty has fistula rates ranging from 15 to 22,[44,45] and up to 32% urethral stricture rates.[54] At the same time, prelamination has been associated with increased flap failure rates in OCFF flaps.[45]
True prefabrication has also been used in phalloplasty in the form of incorporating a RFFF-prefabricated-neourethra within the lateral arm to permit the coexistence of an erectile prosthesis alongside a fully vascularised urethra.[40]
Local tissue flaps
Anteriorly based vaginal flap, labia minoral flaps and urethral plate has been used separately[56] or in combination,[57] for the construction of the fixed part of the neourethra or the bulbar urethra. These flaps can be used in conjunction with a formal flap for a single stage phalloplasty. A very high fistula and/or stricture rate has been reported by the use of these flaps, but it has also been suggested that these complications can be readily corrected by modern urethral surgery techniques.[56]
GLANSPLASTY
The reconstruction of glans has now become an important part of the patient's wishes for an aesthetic and normal looking penis. Horton described a procedure, which involves raising a circumferentially deepithelializing skin flap at the level of the proposed coronal ridge, which is then rolled-up, suturing the free edge of the flap to its own base; thus, forming a ridge. The raw area is then covered with a graft.[58] Split-thickness skin graft produces a more normal-looking coronal sulcus than full-thickness skin graft.[59] It has been regarded as a technique of coronal ridge and sulcus construction that has the best results[60,59] [Figure 6].
Figure 6.
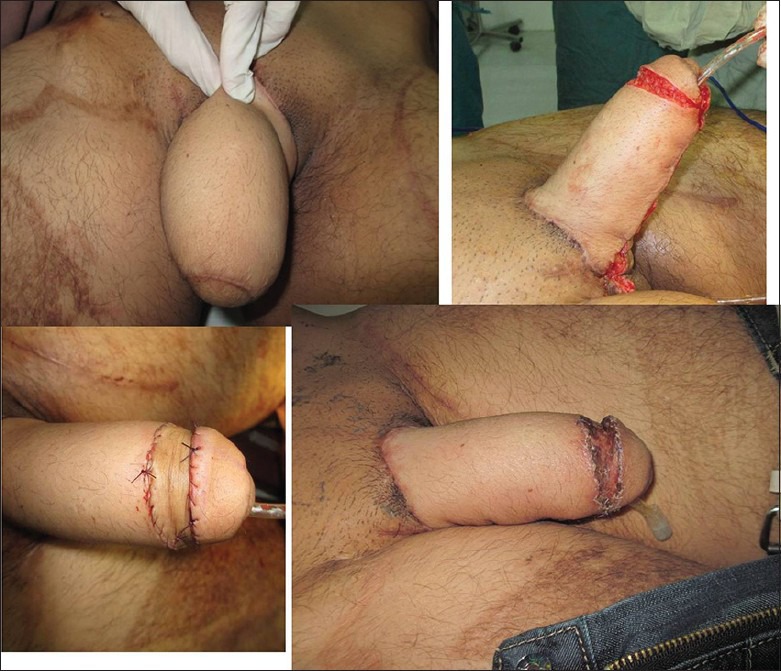
Glansplasty using modified Horton technique (a) Neophallus without glans. (b) Circumferential distally-based- dermal flap raised and stitched onto itself. (c) Raw surface skin grafted. (d) Completed glansplasty
COMPLICATIONS
Total phalloplasty is one of the most complex reconstructions that plastic surgeons are called upon to perform as it involves replicating a form and function that is truly unique. Add to this the fact that the materials available are sub-optimal and the emotional overlay associated with this reconstruction is very significant, the task assumes nearly Herculean dimensions. It is no wonder that it is fraught with a plethora of complications. Flap survival and appearance is of course the first requisite to a successful reconstruction, but it is the function, urinary and sexual, which finally determine success or failure.
The importance of counselling and making the patient understand what to expect and what not to, from a phalloplasty result, cannot be overemphasised. If a phalloplasty fails, most of the time, patient's local tissues are much more scarred than ever before, limiting the scope for any worthwhile future attempt. Alternative procedures are merely lifeboats and can never give the same result as expected from the procedure of first choice. This too is a point to be emphasised and discussed with patient before the doctor agrees to perform a phalloplasty.
Flap failure
Free flap survival rates have gradually increased to 98% in many large series while pedicled flaps will rarely show total flap loss. Nevertheless, flap survival is of paramount importance as even partial loss can lead to exposure of implant, urethral fistulae, infection and thrombosis of the pedicle leading to total flap loss. The radial forearm, lateral arm and the Latissimus dorsi flap have the highest reported rates of survival among the most commonly performed free flaps.[23,41,47] Total and partial flap failure rates are more commonly seen in OCFF as compared with other flaps.[27,28,44,61,62]
Among the pedicled flaps, the ALTF has impressive survival rates with the added benefit of a hidden donor site and obviating the need for a microsurgical setup.
Urethral fistula
Suprapubic abdominal flaps have a high fistula rate of 55%.[9] RFFF phalloplasty has reported fistula rates ranging from 22 to 68%,[25,27] [Figure 7]. These fistulas are more common where the urethral anastomosis is located more proximally.[25] Reduced fistula rates are reported when local flaps are used for urethroplasty in addition to the RFFF, in gender reassignment cases.[57] Similar statistics also hold true for OCRFFF phalloplasties.[34,57] Prelaminated OCFF phalloplasty has fistula rates ranging from 15 to 22.[44,45] Surprisingly, the pedicled flaps namely the ALTF and the extended pedicle groin flap have the lowest reported fistula rates of <10%.[20,62]
Figure 7.
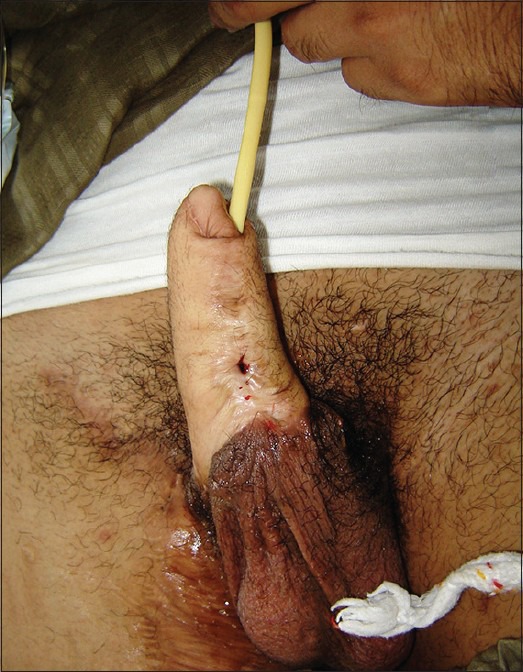
Urethral fistula following single stage-urethral-phalloplasty. More commonly the fistula occurs at the junction of the neo-urethra and the native urethra
Urethral stricture
Phalloplasty with suprapubic abdominal flap has the highest urethral stricture rates of 64%.[9] RFFF or pre-laminated OCFF stricture rates vary from 17% to 31%, with those associated with OCFF falling towards the higher range.[24,25,44] Similar to the urethral fistula rates, the urethral stricture rates are much lower in cases of RFFF urethroplasty only or extended pedicle groin flap phalloplasty (2.56%[61] and 4.15%[62] respectively). Mean stricture length is usually around 3.5 cm.[63] Stricture locations include the meatus, phallic urethra, anastomosis (most common), fixed part and multiple sites. Different types of urethroplasty used for the treatment of urethral strictures after phallic reconstructions include: Meatotomy, Heineke-Mikulicz principle, excision and primary anastomosis, free graft urethroplasty, pedicled flap urethroplasty, two-stage urethroplasty and perineotomy followed by urethral reconstruction.[63] Endoscopic incision has been recommended for short (<3 cm) urethral strictures.[64] Stricture recurrence rate after various treatments is up to 61.9%.[63]
Stiffener related complications
Combining a fully patent neourethra and trying to achieve a sufficiently rigid neophallus to allow sexual intercourse has always been a daunting task. Rigidity in the neophallus can be achieved by using external stiffeners, by transplanting autologous tissue as stiffeners or by implanting semi-rigid or inflatable prosthesis. Autologous tissue include rib and cartilage grafts, which in addition to permanently keeping the phallus in a sometimes embarrassingly semi-rigid state, are complicated by resorption, fracture and even extrusion and infection. The prosthesis is also associated with a high extrusion and infection rate [Figure 8]. Nearly, 30% of neophalluses inserted with implants have implant related complications, mostly in the form of infection or device failure, leading to high implant removal rates.[24,28]
Figure 8.
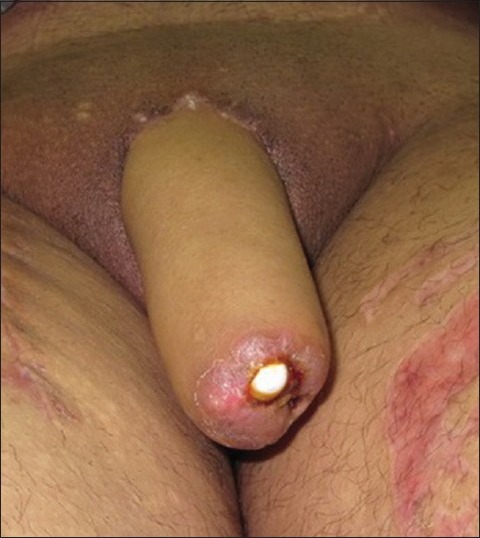
Exposed artificial stiffener which almost invariable results in the need removal of the implant
The autologous stiffeners are usually placed at the time of phalloplasty. The senior author has been using 9-11 cm long costal cartilage graft as a stiffener since 1995 and has not found any complications directly related to the immediate placement of an autogenous stiffener,[25] [Figure 9]. With regard to the osteocutaneous flaps OCFF phalloplasty patients experience a superior intercourse experience than OCRFFF phalloplasty patients.[54] Implantable prostheses are recommended to be placed as a secondary procedure after the neophallus has gained sensitivity.[65]
Figure 9.
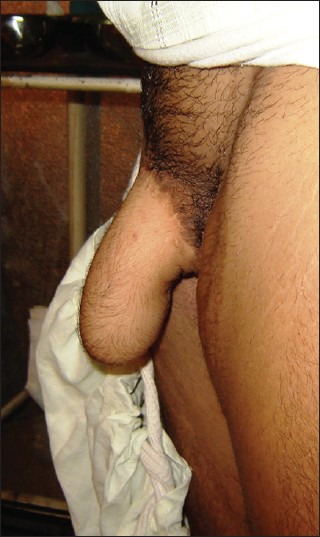
Semi-rigid state of penis with rib cartilage used as a stiffener
Avoidance of major complications
A phalloplasty is not a procedure with clear cut indications and a homogenous patient population. Even the decision to use a particular flap cannot be based on flap survival rates and functional outcomes alone. The indication, whether for gender reassignment or for loss can influence flap selection as the visible forearm donor site can stigmatize transgender individuals. For the surgeon starting out with these challenging reconstructions, it is advisable to train with an expert and then pick up a particular technique, which can be used in most patients. The radial forearm flap, with its reliable anatomy, long pedicle with good calibre vessels and pliable sensate skin should be considered as the first choice for most indications. It has been the gold standard for phalloplasty and its various modifications can prove useful in particular situations. Flap survival rates are very high with this technique, but due to its hair bearing nature fistulae and strictures are common. The visible donor site is a serious drawback. The other option which can find wide usage is the pedicled ALTF. It provides a large mount of skin, the donor site is hidden and as it's a pedicled flap, total flap loss is unlikely. It is usually very thick, but can be successfully thinned using liposuction. The sensory recovery is poorer as compared with the radial forearm flap.
Persistently high rates of urethral fistulas and strictures are related to multiple factors. Firstly, the urethral segment of the flap may be lying away from the pedicle thus being relatively less vascularised. Secondly, the length of the urethral segment may be limited due to the overall dimensions of the flap and thus may be inadequate for tension free urethral anastomosis. Lastly, as most of these flaps are from the hair-bearing parts of body, hair growth within the urethra soon causes obstruction to the urinary flow with its resultant complications. How these can be reduced will depend to a large extent on the technique being used. Although it is difficult to generalise, two stage procedures generally have a lower rate of complications than single stage procedures. In selected cases, pre-lamination with a full thickness graft can reduce the complication rate significantly.
CONCLUSION
As Hage et al. pointed out ‘the development of technique of phalloplasty has paralleled the evolution of plastic surgery’.[66] In terms of practicability and popularity the tubed pedicled flaps were followed by RFFF as a standard method for phalloplasty [Figure 10]. Osteocutaneous version of the forearm flap and OCFF held many promises, mainly an ‘all for one and one for all’ functions being provided by the same single flap. Pre-laminations and prefabrications were introduced to reduce the urethral complication rates. One after another stiffener prostheses were introduced for erection. Nevertheless, the fact remains; the RFFF design has by far stood the test of time, still offering a potential single stage method of total penile reconstruction.
Figure 10.
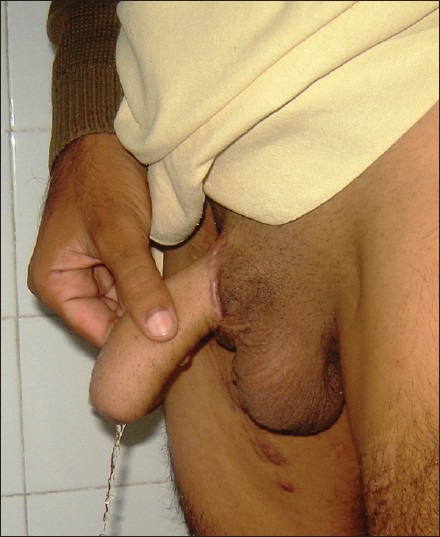
Final outcome after single stage radial forearm free flap phalloplasty
With the advent of perforator flaps, the uncontested place of many conventional free flaps has been challenged in reconstructive surgery. Likewise, the introduction of ALTF in its sensate pedicled form has suddenly started replacing the RFFF as the first choice for penile reconstruction. The trump card being the donor site which is hidden in everyday clothing and the lack of microvascular expertise requirement [Figure 11]. Hage argued that ‘because no sensibility in the phallus is to be expected; however, no stiffeners can be used in any pedicled flap’ consequently regarding these to be ‘techniques with few indications for phalloplasty’.[67] But things are changing and innervated pedicled ALTF has been shown to be a workable solution, thus solving the problem of stiffener retention.[17,18,20]
Figure 11.
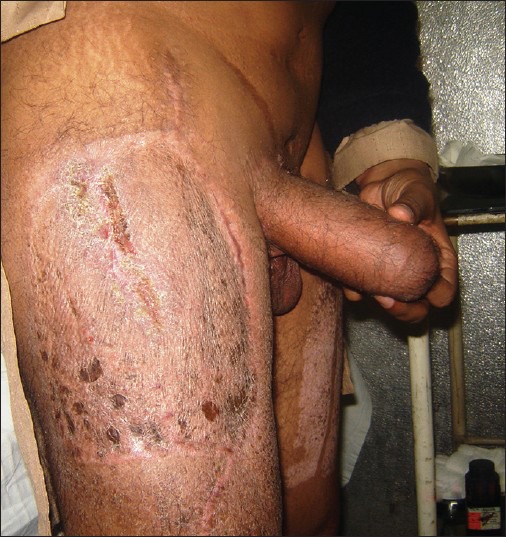
A well-healed anterolateral thigh flap donor-site which can be easily concealed in everyday clothing
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bogoraz NA. On complete plastic reconstruction of a penis sufficient for coitus [in Russian] Sov Surg. 1936;8:303–9. [Google Scholar]
- 2.Nair R, Sriprasad S. 1129 Sir Harold Gillies: Pioneer of phalloplasty and the birth of uroplastic surgery. J Urol. 2010;183(4):e437. [Google Scholar]
- 3.Orticochea M. A new method of total reconstruction of the penis. Br J Plast Surg. 1972;25:347–66. doi: 10.1016/s0007-1226(72)80077-8. [DOI] [PubMed] [Google Scholar]
- 4.Puckett CL, Reinisch JF, Montie JE. Free flap phalloplasty. J Urol. 1982;128:294–7. doi: 10.1016/s0022-5347(17)52893-1. [DOI] [PubMed] [Google Scholar]
- 5.Chang TS, Hwang WY. Forearm flap in one-stage reconstruction of the penis. Plast Reconstr Surg. 1984;74:251–8. doi: 10.1097/00006534-198408000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Hage JJ, De Graaf FH. Addressing the ideal requirements by free flap phalloplasty: Some reflections on refinements of technique. Microsurgery. 1993;14:592–8. doi: 10.1002/micr.1920140910. [DOI] [PubMed] [Google Scholar]
- 7.Hage JJ, Bout CA, Bloem JJ, Megens JA. Phalloplasty in female-to-male transsexuals: What do our patients ask for? Ann Plast Surg. 1993;30:323–6. doi: 10.1097/00000637-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Nikolova-Vateva A, Katsarov M, Panchev P, Evstatiev D. The 1st steps in the surgical treatment of transsexualism in Bulgaria. Khirurgiia (Sofiia) 1996;49:40–1. [PubMed] [Google Scholar]
- 9.Bettocchi C, Ralph DJ, Pryor JP. Pedicled pubic phalloplasty in females with gender dysphoria. BJU Int. 2005;95:120–4. doi: 10.1111/j.1464-410X.2004.05262.x. [DOI] [PubMed] [Google Scholar]
- 10.Willihnganz-Lawson KH, Malaeb BS, Shukla AR. De castro technique used to create neophallus: A Case of aphallia. Urology. 2012;79(5):1149–51. doi: 10.1016/j.urology.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Puckett CL, Montie JE. Construction of male genitalia in the transsexual, using a tubed groin flap for the penis and a hydraulic inflation device. Plast Reconstr Surg. 1978;61:523–30. doi: 10.1097/00006534-197804000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Zieliński T. Phalloplasty using a lateral groin flap in female-to-male transsexuals. Acta Chir Plast. 1999;41:15–9. [PubMed] [Google Scholar]
- 13.Sridhar R, Jayaraman V. A challenging case of total phalloplasty. Indian J Plast Surg. 2012;45:148–50. doi: 10.4103/0970-0358.96618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun GC, Huang JJ. One-stage reconstruction of the penis with composite iliac crest and lateral groin skin flap. Ann Plast Surg. 1985;15:519–28. doi: 10.1097/00000637-198512000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Aköz T, Erdoğan B, Görgü M, Kapucu MR, Kargi E. Penile reconstruction in children using a double vascular pedicle composite groin flap. Scand J Urol Nephrol. 1998;32:225–30. doi: 10.1080/003655998750015647. [DOI] [PubMed] [Google Scholar]
- 16.Descamps MJ, Hayes PM, Hudson DA. Phalloplasty in complete aphallia: Pedicled anterolateral thigh flap. J Plast Reconstr Aesthet Surg. 2009;62:e51–4. doi: 10.1016/j.bjps.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Rubino C, Figus A, Dessy LA, Alei G, Mazzocchi M, Trignano E, et al. Innervated island pedicled anterolateral thigh flap for neo-phallic reconstruction in female-to-male transsexuals. J Plast Reconstr Aesthet Surg. 2009;62:e45–9. doi: 10.1016/j.bjps.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 18.Holzbach T, Giunta RE, Machens HG, Müller D. Phalloplasty with pedicled anterolateral thigh flap (“ALT-Flap”) Handchir Mikrochir Plast Chir. 2011;43:227–31. doi: 10.1055/s-0030-1269908. [DOI] [PubMed] [Google Scholar]
- 19.Lumen N, Monstrey S, Ceulemans P, van Laecke E, Hoebeke P. Reconstructive surgery for severe penile inadequacy: Phalloplasty with a free radial forearm flap or a pedicled anterolateral thigh flap. Advances in Urology. 2008 doi: 10.1155/2008/704343. 704343. doi: 10.1155/2008/704343. Epub 2008 Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashid M, Aslam A, Malik S, Tamimy MS, Ehtesham-ul-Haq, Aman S, et al. Clinical applications of the pedicled anterolateral thigh flap in penile reconstruction. J Plast Reconstr Aesthet Surg. 2011;64:1075–81. doi: 10.1016/j.bjps.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Santanelli F, Scuderi N. Neophalloplasty in female-to-male transsexuals with the island tensor fasciae latae flap. Plast Reconstr Surg. 2000;105:1990–6. doi: 10.1097/00006534-200005000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Hage JJ, Winters HA, Van Lieshout J. Fibula free flap phalloplasty: Modifications and recommendations. Microsurgery. 1996;17:358–65. doi: 10.1002/(SICI)1098-2752(1996)17:7<358::AID-MICR3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Doornaert M, Hoebeke P, Ceulemans P, T’sjoen G, Heylens G, Monstrey S. Penile reconstruction with the radial forearm flap: An update. Handchir Mikrochir Plast Chir. 2011;43:208–14. doi: 10.1055/s-0030-1267215. [DOI] [PubMed] [Google Scholar]
- 24.Garaffa G, Raheem AA, Christopher NA, Ralph DJ. Total phallic reconstruction after penile amputation for carcinoma. BJU Int. 2009;104:852–6. doi: 10.1111/j.1464-410X.2009.08424.x. [DOI] [PubMed] [Google Scholar]
- 25.Rashid M, Sarwar SU. Avulsion injuries of the male external genitalia: Classification and reconstruction with the customised radial forearm free flap. Br J Plast Surg. 2005;58:585–92. doi: 10.1016/j.bjps.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki K, Nozaki M, Morioka K, Huang TT. Penile reconstruction: Combined use of an innervated forearm osteocutaneous flap and big toe pulp. Plast Reconstr Surg. 1999;104:1054–8. doi: 10.1097/00006534-199909040-00024. [DOI] [PubMed] [Google Scholar]
- 27.Leriche A, Timsit MO, Morel-Journel N, Bouillot A, Dembele D, Ruffion A. Long-term outcome of forearm flee-flap phalloplasty in the treatment of transsexualism. BJU Int. 2008;101:1297–300. doi: 10.1111/j.1464-410X.2007.07362.x. [DOI] [PubMed] [Google Scholar]
- 28.Fang RH, Lin JT, Ma S. Phalloplasty for female transsexuals with sensate free forearm flap. Microsurgery. 1994;15:349–52. doi: 10.1002/micr.1920150512. [DOI] [PubMed] [Google Scholar]
- 29.Prakash V. Amputation of the penis due to electrical burn – Role of prefabricated urethra in penile reconstruction. Burns. 2008;34:119–21. doi: 10.1016/j.burns.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Biemer E. Penile construction by the radial arm flap. Clin Plast Surg. 1988;15:425–30. [PubMed] [Google Scholar]
- 31.Gottlieb LJ, Levine LA. A new design for the radial forearm free-flap phallic construction. Plast Reconstr Surg. 1993;92:276–83. [PubMed] [Google Scholar]
- 32.Koshima I, Tai T, Yamasaki M. One-stage reconstruction of the penis using an innervated radial forearm osteocutaneous flap. J Reconstr Microsurg. 1986;3:19–26. doi: 10.1055/s-2007-1007033. [DOI] [PubMed] [Google Scholar]
- 33.Byun JS, Cho BC, Baik BS. Results of one-stage penile reconstruction using an innervated radial osteocutaneous flap. J Reconstr Microsurg. 1994;10:321–31. doi: 10.1055/s-2007-1006601. [DOI] [PubMed] [Google Scholar]
- 34.Fang RH, Kao YS, Ma S, Lin JT. Phalloplasty in female-to-male transsexuals using free radial osteocutaneous flap: A series of 22 cases. Br J Plast Surg. 1999;52:217–22. doi: 10.1054/bjps.1998.3027. [DOI] [PubMed] [Google Scholar]
- 35.Kim SK, Lee KC, Kwon YS, Cha BH. Phalloplasty using radial forearm osteocutaneous free flaps in female-to-male transsexuals. J Plast Reconstr Aesthet Surg. 2009;62:309–17. doi: 10.1016/j.bjps.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Ramesh S, Serjius A, Wong TB, Jagjeet S, John R. Two stage penile reconstruction with free prefabricated sensate radial forearm osteocutaneous flap. Med J Malaysia. 2008;63:343–5. [PubMed] [Google Scholar]
- 37.Mutaf M. Nonmicrosurgical use of the radial forearm flap for penile reconstruction. Plast Reconstr Surg. 2001;107:80–6. doi: 10.1097/00006534-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Glasson DW, Lovie MJ, Duncan GM. The ulnar forearm free flap in penile reconstruction. Aust N Z J Surg. 1986;56:477–9. doi: 10.1111/j.1445-2197.1986.tb02358.x. [DOI] [PubMed] [Google Scholar]
- 39.Upton J, Mutimer KL, Loughlin K, Ritchie J. Penile reconstruction using the lateral arm flap. J R Coll Surg Edinb. 1987;32:97–101. [PubMed] [Google Scholar]
- 40.Young VL, Khouri RK, Lee GW, Nemecek JA. Advances in total phalloplasty and urethroplasty with microvascular free flaps. Clin Plast Surg. 1992;19:927–38. [PubMed] [Google Scholar]
- 41.Khouri RK, Young VL, Casoli VM. Long-term results of total penile reconstruction with a prefabricated lateral arm free flap. J Urol. 1998;160:383–8. [PubMed] [Google Scholar]
- 42.Sadove RC, Sengezer M, McRoberts JW, Wells MD. One-stage total penile reconstruction with a free sensate osteocutaneous fibula flap. Plast Reconstr Surg. 1993;92:1314–23. [PubMed] [Google Scholar]
- 43.Sengezer M, Oztürk S, Deveci M, Odabaşi Z. Long-term follow-up of total penile reconstruction with sensate osteocutaneous free fibula flap in 18 biological male patients. Plast Reconstr Surg. 2004;114:439–50. doi: 10.1097/01.prs.0000131883.27191.86. [DOI] [PubMed] [Google Scholar]
- 44.Papadopulos NA, Schaff J, Biemer E. The use of free prelaminated and sensate osteofasciocutaneous fibular flap in phalloplasty. Injury. 2008;39(Suppl 3):S62–7. doi: 10.1016/j.injury.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Dabernig J, Schumacher O, Lenz C, Rickard R, Turner A, Dabernig W, et al. Modern concept for treatment of the female-to-male transsexual. Urologe A. 2007;46:656–61. doi: 10.1007/s00120-007-1346-1. [DOI] [PubMed] [Google Scholar]
- 46.Felici N, Felici A. A new phalloplasty technique: The free anterolateral thigh flap phalloplasty. J Plast Reconstr Aesthet Surg. 2006;59:153–7. doi: 10.1016/j.bjps.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 47.Perovic SV, Djinovic R, Bumbasirevic M, Djordjevic M, Vukovic P. Total phalloplasty using a musculocutaneous latissimus dorsi flap. BJU Int. 2007;100:899–905. doi: 10.1111/j.1464-410X.2007.07084.x. [DOI] [PubMed] [Google Scholar]
- 48.Djordjevic ML, Bumbasirevic MZ, Vukovic PM, Sansalone S, Perovic SV. Musculocutaneous latissimus dorsi free transfer flap for total phalloplasty in children. J Pediatr Urol. 2006;2:333–9. doi: 10.1016/j.jpurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Ranno R, Hýza P, Veselý J, Dessy LA, Kadanka Z. An objective evaluation of contraction power of neo-phallus reconstructed with free re-innervated LD in female-to-male transsexuals. Acta Chir Plast. 2007;49:8–12. [PubMed] [Google Scholar]
- 50.Yang MY, Li SK, Li YQ, Li Q, Huang WQ, Zhou CD, et al. Penile reconstruction by using a scapular free flap. Zhonghua Zheng Xing Wai Ke Za Zhi. 2003;19:88–90. [PubMed] [Google Scholar]
- 51.Yang M, Zhao M, Li S, Li Y. Penile reconstruction by the free scapular flap and malleable penis prosthesis. Ann Plast Surg. 2007;59:95–101. doi: 10.1097/01.sap.0000253745.07940.da. [DOI] [PubMed] [Google Scholar]
- 52.Hage JJ, Bloem JJ. Review of the literature on construction of a neourethra in female-to-male transsexuals. Ann Plast Surg. 1993;30:278–86. doi: 10.1097/00000637-199303000-00015. [DOI] [PubMed] [Google Scholar]
- 53.Song C, Wong M, Wong CH, Ong YS. Modifications of the radial forearm flap phalloplasty for female-to-male gender reassignment. J Reconstr Microsurg. 2011;27:115–20. doi: 10.1055/s-0030-1268210. [DOI] [PubMed] [Google Scholar]
- 54.Schaff J, Papadopulos NA. A new protocol for complete phalloplasty with free sensate and prelaminated osteofasciocutaneous flaps: Experience in 37 patients. Microsurgery. 2009;29:413–9. doi: 10.1002/micr.20647. [DOI] [PubMed] [Google Scholar]
- 55.Ozkan O, Ozkan O. The prefabricated pedicled anterolateral thigh flap for reconstruction of a full-thickness defect of the urethra. J Plast Reconstr Aesthet Surg. 2009;62:380–4. doi: 10.1016/j.bjps.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 56.Rohrmann D, Jakse G. Urethroplasty in female-to-male transsexuals. Eur Urol. 2003;44:611–4. doi: 10.1016/s0302-2838(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 57.Kim SK, Moon JB, Heo J, Kwon YS, Lee KC. A new method of urethroplasty for prevention of fistula in female-to-male gender reassignment surgery. Ann Plast Surg. 2010;64:759–64. doi: 10.1097/SAP.0b013e3181a5b719. [DOI] [PubMed] [Google Scholar]
- 58.Horton CE, Dean JA. Reconstruction of traumatically acquired defects of the phallus. World J Surg. 1990;14:757–62. doi: 10.1007/BF01670522. [DOI] [PubMed] [Google Scholar]
- 59.Fang RH, Kao YS, Ma S, Lin JT. Glans sculpting in phalloplasty: Experiences in female-to-male transsexuals. Br J Plast Surg. 1998;51:376–9. doi: 10.1054/bjps.1997.0220. [DOI] [PubMed] [Google Scholar]
- 60.Hage JJ, de Graaf FH, Bouman FG, Bloem JJ. Sculpturing the glans in phalloplasty. Plast Reconstr Surg. 1993;92:157–61. [PubMed] [Google Scholar]
- 61.Dabernig J, Shelley OP, Cuccia G, Schaff J. Urethral reconstruction using the radial forearm free flap: Experience in oncologic cases and gender reassignment. Eur Urol. 2007;52:547–53. doi: 10.1016/j.eururo.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Perović S. Phalloplasty in children and adolescents using the extended pedicle island groin flap. J Urol. 1995;154:848–53. doi: 10.1097/00005392-199508000-00142. [DOI] [PubMed] [Google Scholar]
- 63.Lumen N, Monstrey S, Goessaert AS, Oosterlinck W, Hoebeke P. Urethroplasty for strictures after phallic reconstruction: A single-institution experience. Eur Urol. 2011;60:150–8. doi: 10.1016/j.eururo.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Lumen N, Oosterlinck W, Decaestecker K, Monstrey S, Hoebeke P. Endoscopic incision of short (<3 cm) urethral strictures after phallic reconstruction. J Endourol. 2009;23:1329–32. doi: 10.1089/end.2008.0666. [DOI] [PubMed] [Google Scholar]
- 65.Hage JJ, Bloem JJ, Bouman FG. Obtaining rigidity in the neophallus of female-to-male transsexuals: A review of the literature. Ann Plast Surg. 1993;30:327–33. doi: 10.1097/00000637-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Hage JJ, Bloem JJ, Suliman HM. Review of the literature on techniques for phalloplasty with emphasis on the applicability in female-to-male transsexuals. J Urol. 1993;150:1093–8. doi: 10.1016/s0022-5347(17)35695-1. [DOI] [PubMed] [Google Scholar]
- 67.Hage JJ. Reconstruction of the penis. In: Thorne CH, editor. Grabb and Smith's Plastic Surgery. 6th ed. Philladelphia: Wolters Kluwer, Lippincott Williams and Wilkins; 2007. pp. 730–4. [Google Scholar]


