Abstract
This review describes the biological problems faced by those managing primary flexor tendon injuries and explains why these problems still thwart attempts to achieve normal, or near normal, function after this injury, despite a century of surgical effort. It considers the historical background of the early 20th century attempts to improve the results and analyses the clinical usefulness of more recent research into tendon core and circumferential suture modification, including the authors’ work in this field, and changes in post-operative mobilisation over the last 50 years. More recent manipulation of the sheath to improve results and the future possibility of manipulation of adhesions are discussed. It also discusses other factors, e.g., the patient, the experience of the surgeon, the use of therapists, the timing of repair, complex injuries, injuries in zones other than zone 2, which can have a bearing on the results and considers how these can be modified to avoid an unfavourable outcome.
KEY WORDS: Avoidance unfavourable results, flexor tendon surgery, primary flexor tendon surgery, unfavourable results
INTRODUCTION
Repair of the divided flexor tendon to achieve normal, or near normal, function is an unsolved problem. Despite considerable research work and, to a lesser degree, changes of clinical practice over the last 20 years, we appear to have moved little from the situation of the 1990s when the best units in the world reported between 70 and 90% excellent or good results, a rupture rate of around 5% and a tenolysis rate of 5%, i.e., with 10 of every 100 patients coming to secondary surgery after primary flexor tendon repair of zone 2 tendon divisions. This remains the status of this surgery in the best units in the world.
THE BIOLOGICAL PROBLEMS
While all flexor tendon surgery is complicated, it is simplest in the newly injured and unscarred digit and the results of correctly rehabilitated strong primary repairs are likely to be the best attainable. Over and above the actual technical difficulties of repairing tendons, three problems have dogged this field and dominated thought on this subject for a century, viz.
Adherence of the flexor tendon repair to its surrounds
Rupture of the repair during healing
Tethering of the extensor tendons.
The first and third of these arise because the body creates a soup of fibrin-loaded oedema in any area of healing. The fibrin then converts to fibrous tissue to heal the injured tissues and achieve strength in any repairs. Watson-Jones called this fibrin-oedema ‘Physiological Glue’. While it mostly heals any broken structures, this ‘glueing’ process affects everything in the vicinity in discriminately to stick them together with scar adhesions, including the interstitial connective tissues and synovial sheaths surrounding tendons. This process allows little consideration of the particular need of these layers to allow gliding within them. Although the injury in these cases is on the palmar surface of the hand, movement of oedema onto the dorsum carries the fibrin glue with it and the movement of the digits into flexion is then restricted by fibrin tethering of the extensor tendons, preventing their distal movement to allow finger flexion.[1] The extensor tendons, moving between interstitial tissue layers and without synovial sheaths, are more susceptible to this problem after any oedema-inducing episode in the hand and are responsible for much of the failure of flexor tendon surgery to restore a full range of digital motion. This can be identified by loss of passive flexion of the fingers. Scar adhesions can also occur anywhere along the length of a flexor tendon, with the loss of active flexion. This is a particular problem in the fingers themselves, where the flexors are confined within the tendon sheath in a system as finely bored as the pistons in an engine. The amount of fibrin-oedema will increase with increasing magnitude of injury, and this will be compounded by our surgery, by post-operative haematoma or infection and by Chronic Regional Pain Syndrome Type I, should this occur. Although the degree to which extensor and/or flexor tethering by fibrin occurs may vary between individuals, and, arguably, between races, the possibility of either, or both, tendon systems becoming tethered requires that any digit with a flexor tendon repair, primary or secondary, be mobilised throughout the period immediately after repair.
Unfortunately, healing of a flexor tendon takes about 3 months, with tendon continuity during the first half of this time depending almost entirely on the strength of the sutures. This period is sometimes longer than that for which the hand can be kept free of activities, or accidents, liable to snap the repair. Rupture defeats the aim and is a total failure of the primary flexor tendon repair and early mobilisation strategy.
HISTORICAL BACKGROUND
Elaboration of the plan of management following this injury which is still in current use is first seen in the surgical literature towards the end of the First World War (1914-1918), the huge number of casualties of this event being, possibly, the stimulus to its evolution. In 1917, Dr. Harmer in Boston, USA, introduced a new tendon suture with the recommendation that ‘if a suture is not sufficiently strong to endure very early use, this connective tissue may seriously fix the tendon to the surrounding tissue’…. ‘no splint is used. Active motion is started as soon as the patient has recovered from the anaesthetic’.[2] In the same year, Kirchmayer, in Vienna, introduced his new, and special, tendon suture,[3] which we now know as the Kessler suture after its re-inventor 40 years ago.[4,5] We do not know how successful these early surgeons actually were because none of them reported their results. However, even Bunnell, writing in 1918, agreed that this approach could be taken in selected patients - ‘Movement should be instituted with care and judgement. In the first week, it will prevent the incision from healing and encourage infection. If begun late, adhesions will already have immobilized the tendon. Rough, extreme and continuous movements will cause fibrin and scar tissue to form and bind the tendons, and also cause the sutures to cut out. Rest favours a natural repair, with a minimum of inflammatory reaction, but, also allows adhesions to form to all raw surfaces. Movement encourages the formation of synovial membranes over the raw surfaces. It would seem that a moderate amount of intermittent movement, with as long an excursion as practical, interspersed by rest, will yield the best results’.[6] We then see a complete about turn by Bunnell. Presumably, his subsequent experience in the 1920s, possibly as a result of the effects of infection following primary repairs at a time before antibiotics were available, led to the dictate that flexor tendons divided in the tendon sheath of the fingers should not be repaired primarily but treated by delayed tendon grafting, which dominated the practice of flexor tendon surgery for 40 years. The last 50 years is notable for the reversal of this policy and recognition that results after primary, or delayed primary, that is within a few days of tendon division, flexor tendon repair are likely to be better than after delayed tendon grafting. This change was largely pioneered in the 1950s and 60s by Verdan, Young and Harman and Kleinert.[7–9] The availability of antibiotics in the treatment of hand injuries may have had a significant influence in the success of this advance. In our search for ever stronger repairs, it is sometimes forgotten that the Kleinert/Young-Harman/Verdan plan had TWO parts and that immediate repair alone is likely to lead to secondary surgery unless followed by immediate mobilisation. Although there is on-going debate about the details of technique, the central tenet of modern flexor tendon surgery is to avoid adhesion formation between the repaired tendon and the surrounding tissues by making a repair which is strong enough to move within a few days of injury. There follows an unproven assumption that, provided the sutures hold, and rupture does not occur, the results will be better with increasing early movement through the first five weeks, albeit within the protective environment of a dorsal splint.
SUTURE MODIFICATION – CORE SUTURE
Modification of the suturing of the divided tendon, in particular the core suturing, has been the main surgical drive in the last 30 years. A variety of materials have been used but no best suture material identified. Various core suture techniques have also been described over the years. Through the 1990s and the early years of this century, the Tajima and Strickland variations of the Kirchmayr/Kessler suture, in which the knot, or knots, are buried in the tendon, were probably the most commonly used core suture technique in Europe, while the Tsuge suture, or Tang's triple variation of it.[10–12] were more likely to be used in the Far East. Availability and historical factors, rather than measured strength, have been the main determinants of which suture material and configurations were used in individual units and countries. As most of the published series of two-strand core suture zone 2 repairs in civilian populations from all over the world had roughly the same results through the 1990s, it would seem that most materials and most core suture techniques in common use at that time worked equally well, with almost all having a rupture rate around 5% [Table 1].
Table 1.
Major studies of primary repair of finger flexor tendon injuries in adults (1989-1999)
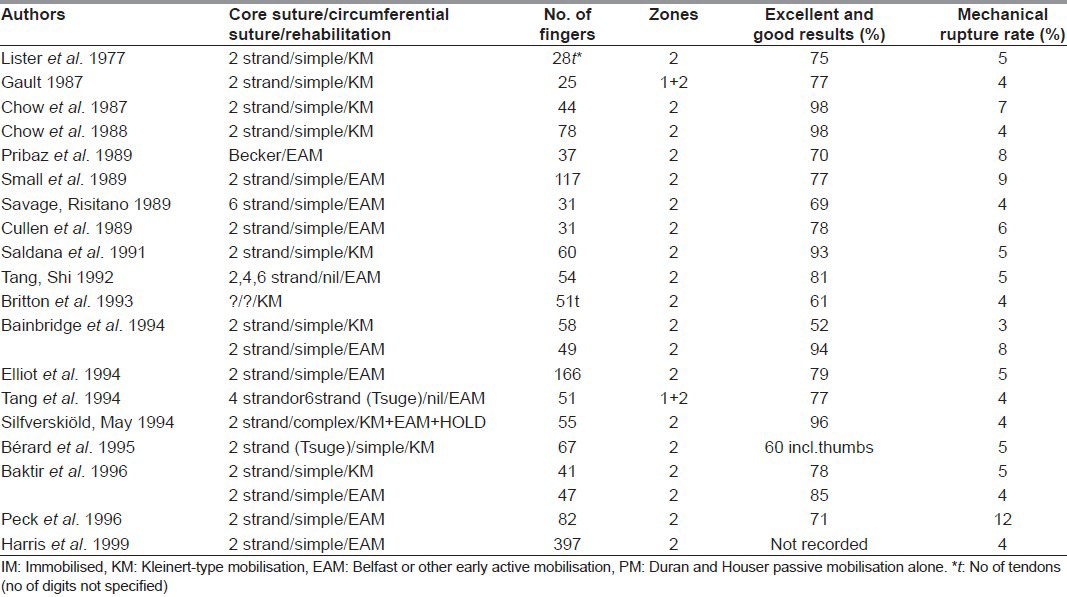
If one looks back to Urbaniak's classic paper on two strand core sutures in dog flexor tendon repairs, one sees part of the answer.[13] On day 1, the various Kessler sutures had quite varied strengths. By day 5, the tendons start softening and suture snapping changed to suture pull-out as the mechanism of failure. Consequently, they all become equal, with the repair now reliant on the suture hold on the tendon. While the Kessler types of suture then outmatched the Bunnell suture, which does not grip the tendon, the Tsuge types of suture were equally good as they also grip the tendon. Although no one had actually defined what was meant by mild, moderate, and maximum resistance, it was evident that a strength of about 9 to 15 Newtons was needed to allow the use of early mobilisation, whether using the Kleinert or Belfast technique. However, to prevent rupture in patients who use the hand early after repair, the sutures might need to resist 50 Newtons, or more. In 1992, Schuind examining intact human flexor tendons at the wrist, measured forces of 120 Newtons being transmitted through the flexor tendons during strong pinch.[14]
In 1989, Savage increased the core suture strength substantially using a Kessler type of core suture with six strands between the tendon ends.[15,16] This stimulated a great deal of laboratory work, and a lesser number of clinical studies. These have continued unremittingly since that time, leaving us with a very confusing multitude of core suture options and no clear ‘winner’. Savage's suture has seldom been surpassed for strength, but it is difficult to use. For this reason, it is widely avoided in clinical practice. Research since might be seen as attempting to devise a multistrand core suture technique with the strength advantage of the Savage suture but being more practical for clinical use. The array of options is well documented in a recent book chapter by Professor Tang.[17]
In this chapter, Tang's review identifies nine other factors which are of importance. While modification of the number of strands of the core suture, and the various ways of achieving this, has attracted most attention, another factor is discussed rarely, but appears simpler, viz. use of a larger calibre of core suture. The benefits of this approach have been shown fairly convincingly in the laboratory.[18,19] However, thicker sutures become cumbersome to tie and the knot is bulky within the tendon with sutures thicker than a 3/0 guage. This is avoided if the knot is taken out of the tendon and onto the tip of the digit, as in the Mantero technique,[20–23] probably based on a technique of distal tendon suture fixation described by Brunelli 20 years earlier.[24] This technique originally used a two-strand core suture of 2/0 guage with the suture attached to the proximal tendon using half of a Kessler suture and to the distal tendon over a button at the tip of the digit, avoiding a knot within the flexor tendon itself. The technique and this suture size are still used routinely in many units in South Europe, including Mantero's own unit in Northern Italy, although some now use a smaller suture.[25–29] Although we have no personal experience of the Mantero technique, it would seem most suited for use in cases in which the flexor tendons are cut in the fingers beyond the A2 pulley, i.e., in Zone 1[30] and in Tang's zones 2A and 2B,[31] and in Flexor Pollicis Longus (FPL) division within the thumb, as the needles have to be passed within the distal tendon from the site of division to the tip of the digit. This probably becomes more difficult as the length of distal tendon increases.
SUTURE MODIFICATION – CIRCUMFERENTIAL SUTURE
The circumferential suture, which is never strictly ‘epitendinous’, even in its simplest form, was originally introduced as an attempt to smooth down loose ends and improve gliding of the repair.[32] In 1986, Wade realised that it had considerable strength in itself,[33] leading to description of about five or six variants of the circumferential suture in the 1990s and several trials in the laboratory of the various alternatives. These, broadly, showed that some of the new circumferential sutures are very strong, and as strong as the core sutures: the multiple gripping bites of the newer circumferential sutures are not unlike core sutures in principle and there may be 8, 10, or more of them. In 1996, Manske's team looked at tendons repaired with circumferential sutures only and recorded breaking strengths of up to 63 Newtons.[34] Initially, we perceived these new circumferential sutures as a possible alternative to elaborate core suture, rather than a way of augmenting the latter. However, the Kubota study also showed that, the more material there is on the surface of the tendon, the more friction there is on mobilisation, identifying an upper limit to how much we can elaborate the circumferential suture. Although this work identified that the simple running suture is the weakest of these sutures, we mostly all still use it, whether for historical reasons, speed and simplicity, or for fear of this friction factor.
THE ST. ANDREW'S FPL EXPERIMENTAL WORK
The extensive literature of an earlier era[35,36] and our early experience with FPL repair[37] made us realise that the higher rupture rate following repair of this tendon might make the FPL a good clinical model to test new sutures and suture techniques. Using this model, we were able to examine some of the new suture configurations, mostly previously described in laboratory experiments, in a series of clinical studies.[37–40] Although this trial, carried out over a number of years, elaborated an increasingly safe technique for dealing with division of the FPL tendon, the research was undertaken primarily to examine possible ways forward for all primary flexor tendon surgery, whatever the digit. Ultimately, these clinical experiments with the divided FPL achieved zero rupture rates using two different suture techniques. This clinical work identified that both the core and the circumferential suture could have a place in eliminating rupture. However, during the same period as this work was being done, two other units reported no mechanical ruptures of FPL repairs using various conventional two-strand core sutures and simple circumferential repairs, albeit in small numbers of patients,[41,42] raising doubt as to whether increasing suture mass and complexity are the only answer to this problem. A very interesting laboratory study from Professor McGrouther's laboratory in Manchester showed that even a single suture passed through a tendon significantly affects the cell population of the tendon around it: The suture foreign body causes the tenocytes to move away.[43] So, perhaps, we are, unwittingly, making tendon repair breakdown more likely as we put more foreign suture material into the tendon!
CLINICAL INTERPRETATION OF CORE SUTURE RESEARCH
However, the general body of opinion at this point in time would favour at least a four-strand core suture, despite many of these having only been tested in the laboratory and not clinically in patients. The plethora of choice is our current problem: Which option to choose? We believe that various facts should make us wary of simply relying on ever increasing strands of core suture, or more elaborate circumferential sutures, or both. The first is the difficulty in interpreting the data presented, particularly when interpreting the laboratory data of experiments on different animal tendons. Even clinical studies need very careful scrutiny. For example, in 1989, Savage reported one rupture in 30 fingers and 3 thumbs with zone 2 complete flexor divisions repaired with a six strand suture, a rupture rate in the fingers of 5%, or a rupture rate of 3% overall.[16] In 1999, Harris et al., reporting results from our own unit from June 1989 to December 1996 (the same era), recorded 17 ruptures in a series of 397 fingers (4%) with zone 2 complete flexor divisions using a two-strand modified Kessler core suture.[44] In a study as small as 30 fingers, one additional rupture changes the rupture rate by 3%. Comparison of these two studies, with this fact in mind, would suggest no difference in rupture rate between them! These figures also highlight a further problem of the literature in this field. It is difficult for individual units to collect substantial numbers of patients with this injury. Many published results, particularly when the rupture rate is presented as a percentage of the total, might present a different picture if the number of cases were more than 30 or 50.
Table 2 summarises the literature of this century. There appears to be a trend towards early active (Belfast) mobilisation and away from rubber-band (Kleinert) mobilisation. Surprisingly, most units reporting on this topic are still using two-strand core sutures. Although some have moved on to four or six strand core sutures in clinical practice, albeit with the circumferential suture having returned to its original and simple form, this Table would suggest that, despite 30 years of research and clinical endeavour, we have not yet eliminated rupture of primary repairs or are routinely achieving 100% excellent results. In fact, the improvements reported with the four and six-strand core sutures are marginal.
Table 2.
Major studies of primary repair of finger flexor tendon injuries in adults (2000-2011)
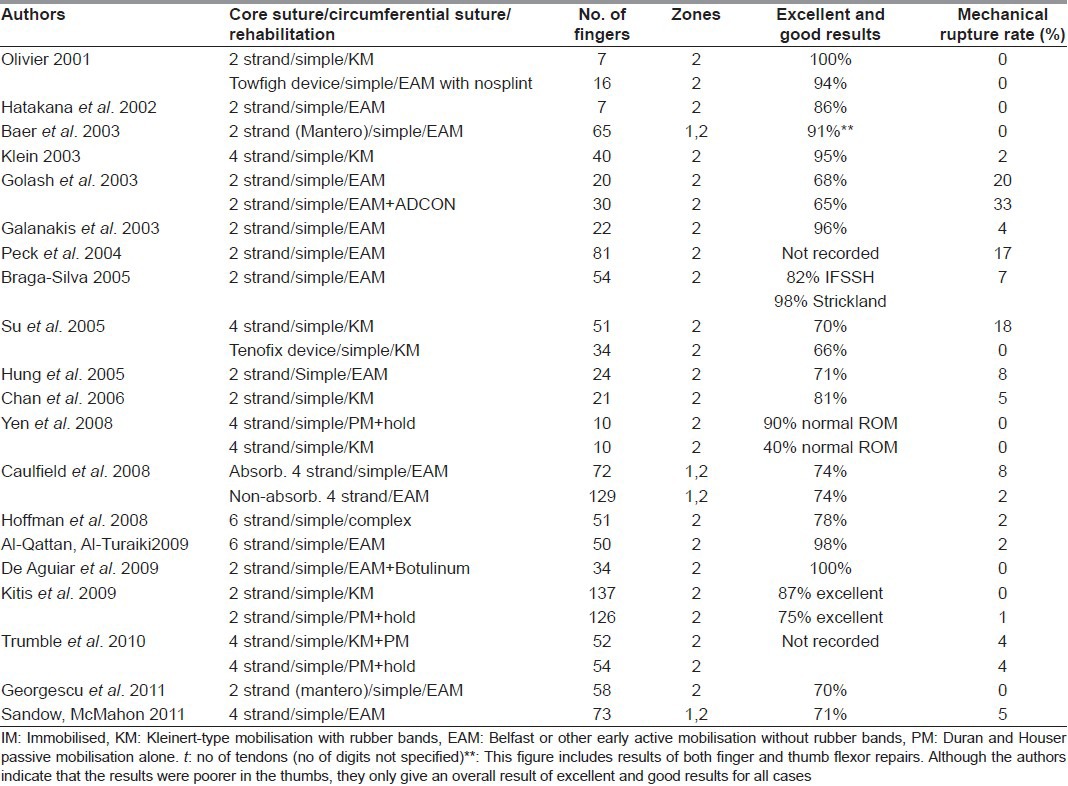
Given the complexity of the sutures currently being advocated and the fact that most flexor tendons, at best, will be repaired by trainee hand surgeons and, at worst, by surgeons with no particular expertise in this surgery, we believe these cases either merit surgery by senior hand surgeons or the techniques of repair being advocated need to remain simple, not complicated. Of the stronger sutures of which we have experience, the Tang triple Tsuge suture technique, using a looped nylon suture is the simplest and quickest means of achieving a six strand core suture, at the same time obviating the need for a circumferential suture entirely.[12] The technique described by Smith and Evans in which two two-strand Kessler sutures are inserted into the tendon in planes at 90° to each other is probably the simplest of the four strand repairs to execute.[45]
REHABILITATION BY EARLY MOBILISATION
The other main drive in the last 30 year has been to earlier and increasingly enthusiastic mobilisation of primary flexor tendon repairs, albeit protected in a dorsal splint for the first 5 week period, which is the time during which rupture is most likely. The latter part of the senior author's training coincided with the publishing of a paper by the hand surgeons in Belfast, in which they described mobilisation after routine zone 2 flexor tendon repairs in a Kleinert traction splinting system, but without the elastic bands, i.e. actively moving the fingers when flexing as well as when extending.[46] This was not actually new, as many before had either never used rubber bands or had tried to get rid of them,[26,47–50] although always stressing the use of some variant of suture technique to make the repair stronger, presuming this would be necessary to withstand early active movement. What the Belfast surgeons identified was the fact that the sutures did not need to be stronger to allow early active mobilisation in both directions. The desire to be free of the rubber bands had been prevalent for years, largely because of the problems arising from the flexed resting position of the proximal interphalangeal joints in Kleinert traction and, also, because of the difficulties in managing Kleinert traction. It was also realised that many patients never actually used the rubber bands to passively flex, but simply flexed their fingers actively, even when the bands were correctly tensioned, which was often only for 5 minutes after leaving the therapy department. This stimulated the senior author to repeat the Belfast experiment.[51] This study, performed between 1986 and late 1987 (although only published much later), and the paper from Sheffield, UK, reporting the results of their repeat of the Belfast experiment,[52] convinced the senior author that this was the way forward for rehabilitation in respect of simplifying it to a level commensurate with the availability of therapy in our own unit and likely to be available worldwide. The results of these, and subsequent experience throughout the 1990s,[37,53,54] including the analysis of the large number of cases mentioned previously between June 1989 and December 1996 in our unit,[44] confirmed a rupture rate of around 5% when using variants of the ‘Belfast’ regimen which was similar to that reported at the time by units worldwide using the ‘Kleinert’ regimen. We were no longer concerned about the safety of the Belfast regimen, although this concern still persists elsewhere, particularly in the United States of America. At the time, we wrote that we believed the Belfast type of rehabilitation was both simpler and less expensive to maintain than the Kleinert type of rehabilitation regimes. However, we had also reached the conclusion that argument over which was the best of the two active regimes was probably unproductive and that we had not solved the problem of rupture.
The other alternative to the Kleinert technique of mobilisation, introduced in the United States by Duran and Houser (1975) and supported by Strickland and Glogovac (1980), in which the fingers were only mobilised passively by a therapist, or the patient's other hand was never popular in the UK as it was very therapy intensive, with no seeming advantage.[55,56] A common debate at the time which, to my knowledge, has never been settled was whether the tendons actually moved significantly with this regimen, or simply bunched up as the fingers were passively flexed. Another factor which made it unattractive was the fact that the first paper reported a 14% rupture rate, while the second had only 56% good and excellent results, both unacceptable when compared with published results at the time using the Kleinert regimen and, subsequently, the Belfast regimen. The commonest use of the Duran Houser idea now is in helping Kleinert and Belfast regimes to push for better results at the extremes of movement.
MANAGEMENT OF THE SHEATH ANDVENTING THE PULLEYS
In retrospect, a factor in achieving our own results in the 1990s which received no attention at the time but was, possibly, of significance was that, from the earliest of the studies, it had been routine to ‘vent’, i.e. divide, pulleys as necessary to allow repairs to travel through a full range of excursion on passive movement of the finger after repair without impinging on the A2 or A4 pulleys. For the senior author, the conviction that this ‘made sense’ followed a private conversation as a trainee in the mid-1980s at the Derby Hand Course with Dr. Strickland. At the time, Dr. Lister and others were quite adamant that all pulleys should remain entirely intact. Dr. Strickland seemed less certain. Knowing the problems experienced in the emergency theatre, it seemed logical that venting was correct and necessary in many cases. Venting the sheath was not new: in the 1950s, adhesions were thought necessary to achieve tendon healing and surgeons cut windows in the sheath. Surgeons then were aware of the need to compensate for the tendon repair increasing the bulk of the tendon within a sheath of constant dimensions and cut the windows to allow a full passive range of motion of the repairs.[57,58] In the 1970s, when synovial fluid was believed to be the most important healer of the tendons,[59] surgeons moved to obsessive closure of the sheath. However, at that time and before, various authors had pointed out that repaired and thickened flexor tendons might not move freely in a closed sheath and subsequent research work supported this view.[60] Others could find no evidence of any benefit from sheath closure, while many among the best results in the world were being achieved in series in which the sheath had not been sutured. So, the enthusiasm for complete closure eventually diminished and most of us now simply lay the sheath back in place without suturing it.
Catching of repairs on the edges of the pulleys was another practical problem which went largely unmentioned through the era of complete sheath closure. In 1975, Duran and Houser had suggested partially releasing one side of any pulley on which any repair was catching[55] and Strickland elaborated the technique and, probably, introduced the term ‘venting’ the pulley, meaning cutting the side of it.[61] There was a reluctance to admit to a need to vent pulleys as, in practice, this usually entailed partial division of the A2 or the A4 pulley, the complete integrity of which had been believed to be of over-riding importance to the mechanical efficiency of the flexor system.[62–66] This notion has its origin in a curious twist of logic: it had been recognised for a long time that the minimum one needed to preserve, or reconstruct, when doing secondary flexor surgery for the reconstructed flexor to achieve its mechanical intention of flexing the finger with power was an A2 and an A4 pulley. This was carried over into primary flexor surgery as a mandate to preserve these two pulleys in their entirety at all costs. More recently, several research papers have shown that there is no absolute need to preserve the A2 or the A4 pulley so completely, or even at all, when most of the remainder of the sheath is intact.[67–70] A study of our own confirmed the clinical need for venting of both the A2 and the A4 pulley to achieve a full passive range of motion after repair of tendons in Tang's zones 2A and 2B, between Zone 1 and the distal edge of the A2 pulley under certain common circumstances.[71] With the onset of post-operative oedema, it is likely that the need for venting would be accentuated.[72] This problem also becomes more likely if we use more complex repairs, as the repairs will be of even greater volume than the original tendon. The discussion of ‘venting’ was taken to its logical conclusion in two review articles.[73,74] Analysing the sites along the tendon sheath where tendon injury commonly occurs, Dr. Tang has described appropriate pulley releases for each injury. This opinion is updated in more recent book chapters and both authors accord this process of pulley ‘venting’ equal importance to the use of stronger repairs in increasing the margin of safety of early active mobilisation.[54,75,76] We believe the results of Zone 1 primary flexor tendon surgery are equally dependent on judicious venting of the A4 pulley.[30]
MANIPULATION OF ADHESIONS
An alternative, and possibly more scientific, approach to the problem of adhesions would be to minimise their formation and this has been attempted with a variety of drugs, probably the most well-known of which in Europe was Adcon. Until Adcon, this idea had largely remained in the laboratory as surgeons were nervous of the effect of these chemicals on the healing of the tendon repair and of the overlying tissues, which we presume to be healing by the same process as occurs in formation of adhesions. The senior author was asked to research Adcon prior to discussion at the FESSH meeting in Barcelona (2000), as it was being marketed for clinical use much more seriously than chemicals previously considered for this purpose. At that time, a reasonable number of published papers seemed to show that Adcon does reduce adhesions around the nerve roots following disk surgery and might be helpful here, although its use clinically in this field has now been abandoned. However, despite anecdotal support for its use in hand surgery, there was virtually no objective evidence ever produced in tendon surgery and absolutely no objective evidence in peripheral nerve surgery to support its use.[77–79] Since Adcon, further products have been introduced for this purpose but research is sporadic and unconvincing. One, hyaloglide, was researched diligently by the Italian Hand Society but discussion of this has ‘gone quiet’.[80]
To date, adhesion prevention has been approached in terms of a chemical, i.e. a liquid in which to bathe the sticking structures, or a more solid barrier wrapped around the repaired tendon. A time may be approaching in which we must consider adhesion limitation as other than experimental, as other modalities of treatment are on the horizon. Recent research has greatly advanced our knowledge of the complex biochemical pathways that take place after a tendon is injured and it has become clear that growth factors play a critical role.[81,82] With this realisation comes the possibility that healing may be manipulated through these growth factors. There would appear to be two main ways in which growth factors could be delivered to the injured tendon: firstly, by direct application and, secondly, through the use of gene transfer techniques. The latter would involve delivering the gene that encodes the growth factor, not the growth factor itself, to the injured tendon. The gene would then be incorporated into cells at the injury site and these cells would take over production of the growth factor. However, for the meantime, we have to consider adhesion limitation as experimental and continue to think mechanically, using sutures strong enough to allow early movement, albeit protected from the full forces of normal activity by some system of protective splinting and limited movement during early rehabilitation.
OTHER FACTORS
This field also needs a more multifactorial approach to treatment, with more consideration not only of our sutures and suture configurations and our rehabilitation methods, but of unresolved problems such as the ‘uncooperative’ patient, complicated injuries, delayed presentation and the immediate failures of our primary surgical efforts.
OTHER FACTORS – THE PATIENT
The patient, whether compliant but unfortunate, or uncompliant, is another factor likely to affect our results. Our research over 20 years was largely aimed at liberating rehabilitation from unnecessary constraints while, coincidently, reducing the rupture rate after primary repair. However, we also looked at the patient. In 1999,[44] we examined the 23 patients who ruptured tendon repairs from the series of 440 patients who had undergone primary surgery and postoperative mobilisation in a controlled or early active motion between 1989 and 1996. Around 50% of the ruptures occurred in patients who had seemingly been compliant and as a result of factors beyond their control. Over and above these accidental causes of rupture, there were other unwitting patient causes of both rupture and bad results. These included some children, patients incapable of comprehending what was required of them by the therapists, excessive scar formers, patients with social circumstances which precluded therapy attendance, patients with low pain thresholds and those presenting later than ideal for surgery. These failures can be considered ‘Acts of God’ and, so, immutable, or factors which require us to modify our behaviour to achieve success. We can, often, help these individuals more, given thought and/or adequate resources.
Most ruptures in our study occurred with the splint in place. These tendon repairs might have been protected from rupture by better mechanical obstruction of the palm. Rubber bands across the palm have a definite obstructing action which was not present in the original technique of early active motion.[46] However, all of our patients at that time wore a modified Belfast splint which included wide thermoplastic bars across the open side of the splint, running from the distal edge back to the volar aspect of the wrist and known locally as ‘beer-can bars’.[37] Attempts to increase this feature of splinting and/or attempts to make splints impossible to remove would be likely to interfere with rehabilitation and would probably still fail in a proportion of patients. They would certainly have little effect in those who remove the splint to use the hand for grasping (see below).
Just under 50% of the total in the above study, ruptured the repairs while using the hand in, or out, of their splint, for a variety of tasks contrary to therapy advice. They highlight the role of patient non-compliance in the aetiology of tendon rupture. This injury occurs mainly at an age, and in a social group, in which improving compliance is likely to be difficult and this figure may be an underestimate of the problem of patient non-compliance in this population worldwide. The group labelled ‘uncooperative patients’ includes adults and children who do not cooperate and small children who cannot. As adults constitute by far the greater proportion of patients who sustain flexor tendon injuries, they are the major concern. Psychological manipulation and more time spent are the only direct means we have of improving the results of these patients and it is debateable whether we can change this cause of poor results more than a little.
A question which arises in respect of uncooperative patients is whether we need to go through the difficulties of primary flexor surgery for any of them, as failure is expensive for the patient, the surgical unit and the State. Would they be better and, possibly, easier treated, by reversion to grafting, single or two-stage, after a period of therapy training? Unfortunately, the nature of uncooperative patients is usually only identified after the primary (emergency) surgery. For most of us, whatever problems we may have doing primary repairs, they are still easier than tendon grafting. The reported results of secondary surgery are also generally worse than those of primary surgery and it is generally more difficult to get good results using the techniques of secondary flexor surgery. It has also generally been recognised over the last 50 years that limbs repaired immediately and moved early do better because they do not stiffen in scar tissue and do not develop contractures during healing as a result of inactivation of one, or more, of the normal parts of the locomotor system. This truism applies to the hand also. Surgery in scarred tissues is less likely to be followed by good movement, even if mobilisation is immediate. It is also more difficult, with higher risk of intraoperative complications such as inadvertent division of intact structures embedded in scar. So, unfortunately, we have to persevere with primary surgery in all patients – including the uncooperative.
OTHER FACTORS – TIMING OF THE REPAIR AND LATE REPAIR
Primary repair of the flexor tendons should be as early as possible after the injury. However, there is a body of evidence that delay of 24 to 72 h is not followed by poorer results and it is likely that delayed primary repair by an experienced surgeon will achieve a better result than immediate surgery by an inexperienced surgeon. Transfer of patients to specialist units and delay to investigate, or even treat, more pressing problems has become acceptable practice. Although primary treatment is necessary within 72 h, this surgery need not be considered an emergency, or treated as such. Largely as a result of discovering a paper written back in the 1960s, the significance of which was probably not appreciated at that time, we are now much more enthusiastic in our policy with respect to delayed primary repair than previously. This paper identified the fact that delayed primary repair is possible far more often than thought and far longer after the index injury.[83] At that time, everyone in North America was trying to get started with the Kleinert/Verdan/Young-Harman philosophy of immediate repair and immediate mobilisation. However, the hand units were still receiving patients at quite long times after the initial injury, as the casualty units were expecting them to be treated by secondary grafting in the conventional manner of that time. McFarlane and his co-workers tried to do primary repairs in 100 patients sent slowly to them, whatever the delay. A number of these patients arrived more than 12 months after the initial injury. That the flexors in 36% of 100 fingers could be repaired directly, even months after the injury, negates the assumption that delayed presentation routinely necessitates tendon grafting. Now, if a patient comes later than 72 h and the finger is not infected and is mobile passively, we explore the finger immediately and try to repair the tendons. With the possibility of slight tendon lengthening in the muscles without slowing the early mobilisation programme,[84] this figure might now be even higher. If the tendon ends will not quite come together, we perform a Le Viet tendon lengthening within the muscle in the forearm. Although the tendon is cut, the muscle has not been, and the muscle maintains the continuity needed to allow immediate mobilisation. A single cut gives about half a centimetre in extra tendon length distally. If one repeats this cut again, about 1-2 centimetres from the first cut, but still within the muscle, the second cut will give another quarter centimetre of lengthening. If repair still proves impossible, then a graft can be done with no loss of time, or a silicone rod can be put in as the first stage of a two stage tendon grafting procedure.
OTHER FACTORS – COMPLEX INJURIES
Our knowledge of the effectiveness of our current techniques of primary flexor tendon repair and rehabilitation is restricted to that gained from examination of the results of treatment of simple injuries. It has been necessary to restrict analysis to these cases in this way to reduce the variables and allow comparison of techniques of suturing and of rehabilitation between units worldwide. However, this leaves us in a position in which we do not know whether the techniques suitable for simple cases should be applied to more severe injuries. An example in which this has not been true has been the policy of repairing both flexor tendons under all, or nearly all, circumstances which has remained standard practice since advocated by Verdan and by Kleinert 40-50 years ago.[85] In the 1970s, Boyes had pointed out the problem of repairs sticking under the A2 pulley, which is the tightest part of the sheath, and, more recently, Professor Tang re-examined this problem and showed better results when only the profundus was repaired for injuries under the A2 pulley.[86] Except in the hands of very experienced tendon surgeons, we have come to believe that this policy should be applied to simple flexor tendon divisions under the A2 pulley. This single tendon repair is even more necessary after more complex injuries of the distal palm and proximal part of the fingers, such as crush injuries of the distal palm and proximal part of the fingers, distally based flaps on the distal palm, replantations and revascularisations, and multifinger injuries. In these cases, particularly, the two repaired flexor tendons can become so oedematous that they may completely stick under the A2 pulley, with no possibility of mobilisation after the first procedure. Although severe injuries in zone 4, in the carpal tunnel, are rarer, the same problem arises. If multiple tendons are divided in zone 4, we also only repair the profundus tendons.
OTHER FACTORS – THE SURGEON
This concentration of effort on analysing simpler cases and ignoring the complicated injuries in this literature has had another consequence. These bad injuries are often added to the list of emergencies to be done by trainee surgeons, often with little expertise in this field. The consequence is inevitable and too frequently excused as a bad result from a bad injury. Emergency referral of bad hand injuries which include flexor tendon divisions is inevitable for us all: we cannot redirect these, but we can modify our response to them. These cases need the same level of senior attention as amputated fingers for replantation or high pressure injection injuries. Given our failure to achieve normal hands after primary flexor tendon surgery routinely in even simple cases, and the passing of the earlier Louisville philosophy that the sun should never set on a cut flexor tendon, there is a case for all flexor tendon divisions being considered difficult surgery and for their being repaired by more experienced hand surgeons on elective surgery lists. This applies even more to complex injuries.
OTHER FACTORS - THERAPISTS
Training of these patients is necessary to achieving good results for even simple flexor tendon divisions but is expensive of medical time. Complicated patients and injuries are particularly likely to do better after primary surgery if they are moved up this training curve. This, in our experience, is more often achieved by our therapists than by us and more therapy unit time is required than usually available for these patients. Surgeons can help in this by supporting the position and skill of their therapists. For many years, on the morning after surgery, our surgeons, therapists and nurses have routinely done a combined ward round. Patients are confronted verbally with the hard facts of the chances of achieving a good return of hand function without re-operation and told that their salvation is by doing exactly as the therapists say. However, as well as verbal support for the therapists to a, not always, receptive audience, we need to address the politics of this vital factor for success. This field needs more therapists and more time for patients in therapy. This is currently being given little of our attention, partly because of the surgical concentration on stronger sutures, and, partly, because few of us are expert in the field of medical politics. At a time of economic downturn, many hospitals with therapy services find it convenient to reduce rehabilitative services, which administrators mostly do not understand as vital. In situations in which there are no therapists, justifying them economically to the same people is difficult. More surgical voices fighting the therapists’ cause are needed if results in this field of surgery are to improve.
OTHER FACTORS – ZONES 1, 3, 4 AND 5
Because Zone 2 was perceived by surgeons fifty years ago to be the most complicated part of the flexor tendon to repair, almost all clinical research was, and remains, directed at this zone. While true to a degree, zone 2 injuries are not unique in their technical difficulties. Zone 1 injuries can be equally difficult to repair. It is also debateable whether Zone 2 injuries result in the worst functional problems in the long-term for the patient. This is because of the associated structures likely to be injured in zone 3, 4 and 5 injuries. The literature on the other zones is small or, in the case of zones 3 and 4, entirely absent. This led us to add contributions to the literature on zones 1 and 5,[30,87] but this literature remains lamentably small, with few more recent contributions, and mostly fails to identify useful techniques which might be applied specifically to the individual zones to achieve better results.
CONCLUSION
We believe the way forward in avoiding unfavourable results after primary flexor tendon surgery is by use of strengthened but simpler sutures, venting of the pulley system appropriately and maintaining early rehabilitation. However, there needs also be consideration of patient, and other factors discussed above. Our research needs to continue both in the laboratory and in the clinical environment to these ends and until we find ways of modifying adhesion formation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kulkarni M, Harris SB, Elliot D. The significance of extensor tendon tethering and dorsal joint capsule tightening after injury to the hand. J Hand Surg. 2006;31:52–60. doi: 10.1016/j.jhsb.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Harmer TW. Tendon Suture. Boston Med Surg J. 1917;177:808–10. [Google Scholar]
- 3.Kirchmayr L. Zurtechnik der Sehnenaht. Zentralbl Chir. 1917;44:27–52. [Google Scholar]
- 4.Kessler I, Nissim F. Primary repair without immobilisation of flexor tendon division within the digital sheath. Acta Orthop Scand. 1969;40:587–601. doi: 10.3109/17453676908989524. [DOI] [PubMed] [Google Scholar]
- 5.Kessler I. The “grasping” technique for tendon repair. Hand. 1973;5:253–5. doi: 10.1016/0072-968x(73)90038-7. [DOI] [PubMed] [Google Scholar]
- 6.Bunnell S. Repair of tendons in the fingers and description of two new instruments. Surg Gynecol Obstet. 1918;126:103–10. [Google Scholar]
- 7.Verdan C. Primary repair of flexor tendons. J Bone Joint Surg Am. 1960;42:647–57. [PubMed] [Google Scholar]
- 8.Kleinert HE, Kutz JE, Ashbell TS, Martinez E. Primary repair of flexor tendons in “no man's land”. Proceedings, American Society for Surgery of the Hand. J Bone Joint Surg Am. 1967;49:577. [Google Scholar]
- 9.Young RE, Harmon JM. Repair of tendon injuries of the hand. Ann Surg. 1960;151:562–6. doi: 10.1097/00000658-196004000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuge K, Ikuta Y, Matsuishi Y. Intra-tendinous tendon suture in the hand: A new technique. Hand. 1975;7:250–5. doi: 10.1016/0072-968x(75)90062-5. [DOI] [PubMed] [Google Scholar]
- 11.Tsuge K, Ikuta Y, Matsuishi Y. Repair of flexor tendons by intratendinous suture. J Hand Surg Am. 1977;2:436–40. doi: 10.1016/s0363-5023(77)80024-5. [DOI] [PubMed] [Google Scholar]
- 12.Tang JB, Shi D, Gu YQ, Chen JC, Zhou B. Double and multiple looped suture tendon repair. J Hand Surg Br. 1994;17:699–703. doi: 10.1016/0266-7681(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 13.Urbaniak JR, Cahill JD, Mortenson RA. Tendon suturing methods: Analysis of tendon strengths, in American Academy of Orthopaedic Surgeons Symposium on Tendon Surgery in the Hand. In: Hunter JM, Schneider LH, editors. St. Louis: C.V. Mosby; 1975. pp. 70–80. [Google Scholar]
- 14.Schuind F, Garcia-Elias M, Cooney WP, 3rd, An KN. Flexor tendon forces: In vivo measurements. J Hand Surg Am. 1992;17:291–8. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]
- 15.Savage R. In vitro studies of a new method of flexor tendon repair. J Hand Surg Br. 1985;10:135–41. doi: 10.1016/0266-7681(85)90001-4. [DOI] [PubMed] [Google Scholar]
- 16.Savage R, Risitano G. Flexor tendon repair using a “six strand” method of repair and early active mobilisation. J Hand Surg Br. 1989;14:396–9. doi: 10.1016/0266-7681_89_90154-x. [DOI] [PubMed] [Google Scholar]
- 17.Tang JB, Xie RG. Biomechanics of core and peripheral tendon repairs. In: Tang JB, Amadio PC, Guimberteau JC, Chang J, editors. Tendon Surgery of the Hand. Philadelphia: Elsevier Saunders; 2012. pp. 35–48. [Google Scholar]
- 18.Barrie KA, Tomak SL, Cholewicki J, Merrell GA, Wolfe SW. Effect of suture locking and suture caliber on fatigue strength of flexor tendon repairs. J Hand Surg. 2001;26:340–6. doi: 10.1053/jhsu.2001.22926. [DOI] [PubMed] [Google Scholar]
- 19.Taras JS, Raphael JS, Marczyk SC, Bauerle WB. Evaluation of suture caliber in flexor tendon repair. J Hand Surg Am. 2001;26:1100–4. doi: 10.1053/jhsu.2001.28946. [DOI] [PubMed] [Google Scholar]
- 20.Mantero R, Bertolotti P, Badoini C. Il pull-out in “no man's land et al canale digitale nelle lesioni dei flessori (metodo personale) Rivista di Chirurgiadella Mano. 1973/4;11:119–30. [Google Scholar]
- 21.Mantero R, Bertolotti P, Badoino C, Ferrari GL. Revisione critica di 20 anni di esperienza sul trattamento delle lesioni dei flessori. Rivista di Chirurgiadella Mano. 1974/5;12:87–110. [Google Scholar]
- 22.Mantero R, Bertolotti P. La mobilisation précoce dans le traitement des lésions des tendons fléchisseurs au canal digital. Ann Chir Main. 1976;30:889–96. [PubMed] [Google Scholar]
- 23.Emery FE. Immediate mobilization following flexor tendon repair. J Trauma. 1977;17:1–7. [PubMed] [Google Scholar]
- 24.Brunelli G. Tenorrafia semplificata con material estraibile. Minerva Ortop. 1954;5:321–3. [PubMed] [Google Scholar]
- 25.Brunelli G, Monini L. Technique personelle de suture des tendons fléchisseurs des doigts avec mobilisation immédiate. Ann Chir Main. 1982;1:92–6. doi: 10.1016/s0753-9053(82)80050-1. [DOI] [PubMed] [Google Scholar]
- 26.Brunelli G, Vigasio A, Brunelli F. Slip-knot flexor tendon suture in zone 2 allowing immediate mobilisation. Hand. 1983;3:352–8. [PubMed] [Google Scholar]
- 27.Grandis C, Rossello MI. Diecianni di esperienza con il pull-out intertendineo nella chirurgia dei tendini flessorial canale digitale (zona 1-2). Ten years experience with intertendinous pull-out in flexor tendon surgery at the digital canal (zones 1-2) Rivista di Chirurgiadella Mano. 1988;25:43–9. [Google Scholar]
- 28.Wulle C. Flexor tendon suture in zone 1 and distal zone 2 by the Mantero technique. Ann Chir Main MembSuer. 1992;11:200–6. doi: 10.1016/s0753-9053(05)80370-9. [DOI] [PubMed] [Google Scholar]
- 29.Guinard D, Montanier D, Thomas D, Corcella D, Moutet F. The Mantero flexor tendon repair in zone 1. J Hand Surg Br. 1999;24:148–51. doi: 10.1054/jhsb.1998.0173. [DOI] [PubMed] [Google Scholar]
- 30.Moiemen NS, Elliot D. Early active mobilization of primary flexor tendon repairs in zone 1. J Hand Surg Br. 2000;25:78–84. doi: 10.1054/jhsb.1999.0319. [DOI] [PubMed] [Google Scholar]
- 31.Tang JB, Shi D. Subdivision of flexor tendon “no man's land and different treatment methods in each sub-zone. A preliminary report. Chir Med J (Engl) 1992;105:60–8. [PubMed] [Google Scholar]
- 32.Lister G, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilisation. J Hand Surg Am. 1977;2:441–51. doi: 10.1016/s0363-5023(77)80025-7. [DOI] [PubMed] [Google Scholar]
- 33.Wade PJF, Muir IFK, Hutcheon LL. Primary flexor tendon repair: The mechanical limitations of the modified Kessler technique. J Hand Surg Br. 1986;11:71–6. doi: 10.1016/0266-7681(86)90018-5. [DOI] [PubMed] [Google Scholar]
- 34.Kubota H, Aoki M, Pruitt DL, Manske PR. Mechanical properties of various circumferential tendon suture techniques. J Hand Surg Br. 1996;21:474–80. doi: 10.1016/s0266-7681(96)80049-0. [DOI] [PubMed] [Google Scholar]
- 35.Murphy FG. Repair of laceration of flexor pollicis longus tendon. J Bone Joint Surg. 1937;19:1121–3. [Google Scholar]
- 36.Urbaniak JR. Repair of the flexor pollicis longus. Hand Clin. 1985;1:69–75. [PubMed] [Google Scholar]
- 37.Elliot D, Moiemen NS, Flemming AF, Harris SB, Foster AJ. The rupture rate of acute flexor tendon repairs mobilized by the controlled active motion regimen. J Hand Surg Br. 1994;19B:607–12. doi: 10.1016/0266-7681(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 38.Sirotakova M, Elliot D. Early active mobilization of primary repairs of the flexor pollicis longus tendon. J Hand Surg Br. 1999;24:647–53. doi: 10.1054/jhsb.1999.0230. [DOI] [PubMed] [Google Scholar]
- 39.Sirotakova M, Elliot D. Early active mobilization of primary repairs of the flexor pollicislongus tendon with two Kessler two strand core sutures and a strengthened circumferential suture. J Hand Surg Br. 2004;29:531–5. doi: 10.1016/j.jhsb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Giesen T, Sirotakova M, Elliot D. Flexor pollicic longus primary repair: Further experience with the Tang technique and controlled active mobilisation. J Hand Surg Eur Vol. 2009;34:758–61. doi: 10.1177/1753193408096025. [DOI] [PubMed] [Google Scholar]
- 41.Kasashima T, Kato H, Minami A. Factors influencing prognosis after direct repair of the flexor pollicis longus tendon: Multivariate regression model analysis. Hand Surg. 2002;7:171–6. doi: 10.1142/s0218810402001126. [DOI] [PubMed] [Google Scholar]
- 42.Baer W, Jungwirth N, Wulle C, Schaller P. Die Beugesehnennaht nach Mantero eine Alternative. Handchir Mikrochir Plast Chir. 2003;35:363–7. doi: 10.1055/s-2003-44680. [DOI] [PubMed] [Google Scholar]
- 43.Wong JK, Cerovac S, Ferguson MW, McGrouther DA. The cellular effect of a single interrupted suture on tendon. J Hand Surg Br. 2006;31:358–67. doi: 10.1016/j.jhsb.2006.03.162. [DOI] [PubMed] [Google Scholar]
- 44.Harris SB, Harris D, Foster AJ, Elliot D. The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 of the fingers during early mobilization. J Hand Surg Br. 1999;24:275–80. doi: 10.1054/jhsb.1998.0212. [DOI] [PubMed] [Google Scholar]
- 45.Smith AM, Evans DM. Biomechanical assessment of a new type of flexor tendon repair. J Hand Surg Br. 2001;26:217–9. doi: 10.1054/jhsb.2000.0542. [DOI] [PubMed] [Google Scholar]
- 46.Small JO, Brennen MD, Colville J. Early active mobilisation following flexor tendon repair in zone 2. J Hand Surg Br. 1989;14:383–91. doi: 10.1016/0266-7681_89_90152-6. [DOI] [PubMed] [Google Scholar]
- 47.Hernandez A, Velasco F, Rivas A, Preciado A. Preliminary report on early mobilization for the rehabilitation of flexor tendons. Plast Reconstr Surg. 1967;40:354–8. doi: 10.1097/00006534-196710000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Mangus DJ, Brown F, Byrnes W, Habal A. Tendon repairs with nylon and a modified pullout technique. Plast Reconstr Surg. 1971;48:32–5. doi: 10.1097/00006534-197107000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Nigst H. Chirurgie der Beugesehnen. Handchir. 1976;8:225–36. [PubMed] [Google Scholar]
- 50.Becker H, Orak F, Duponselle E. Early active motion following a bevelled technique of flexor tendon repair: Report on fifty cases. J Hand Surg Br. 1979;4:454–60. doi: 10.1016/s0363-5023(79)80043-x. [DOI] [PubMed] [Google Scholar]
- 51.Bainbridge LC, Robertson C, Gillies D, Elliot D. A comparison of post-operative mobilization of flexor tendon repairs with “passive flexion-active extension” and “controlled active motion” techniques. J Hand Surg. 1994;19B:517–21. doi: 10.1016/0266-7681(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 52.Cullen KW, Tolhurst P, Lang D, Page RE. Flexor tendon repair in zone 2 followed by controlled active mobilisation. J Hand Surg Br. 1989;14:392–5. doi: 10.1016/0266-7681_89_90153-8. [DOI] [PubMed] [Google Scholar]
- 53.Baktir A, Türk CY, Kabak S, Sahin V, Kardas Y. Flexor tendon repair in zone 2 followed by early active mobilization. J Hand Surg Br. 1996;21:624–8. doi: 10.1016/s0266-7681(96)80145-8. [DOI] [PubMed] [Google Scholar]
- 54.Elliot D. Clinical primary flexor tendon repair and rehabilitation. The Chelmsford experience. In: Tang JB, Amadio PC, Guimberteau JC, Chang J, editors. Tendon Surgery of the Hand. 13B. Philadelphia: Elsevier Saunders; 2012. pp. 125–32. [Google Scholar]
- 55.Duran RH, Houser RG. Controlled passive motion following flexor tendon repairs in zones II and III. In: Hunter JM, Schneider LH, editors. American Academy of Orthopaedic Surgeons Symposium on Flexor Tendon Surgery in the Hand. St. Louis: C.V. Mosby; 1975. pp. 105–14. [Google Scholar]
- 56.Strickland JW. Flexor tendon injuries. Part 2. Flexor tendon repair. Orthop Rev. 1986;15:701–21. [PubMed] [Google Scholar]
- 57.Mason ML. Primary and secondary tendon suture. A discussion of significance in tendon surgery. Surg Gynecol Obstet. 1940;70:392–404. [Google Scholar]
- 58.Verdan C. La réparation immédiate des tendons fléchisseurs dans le canal digital. Acta Orthop Belg. 1958;24(Suppl 3):15–23. [Google Scholar]
- 59.Lundborg G, Rank F. Experimental intrinsic healing of flexor tendons based upon synovial fluid nutrition. J Hand Surg. 1978;3:21–31. doi: 10.1016/s0363-5023(78)80114-2. [DOI] [PubMed] [Google Scholar]
- 60.Tang JB, Shi D, Zhang QG. Biomechanical and histological evaluation of tendon sheath management. J Hand Surg Am. 1996;21:900–8. doi: 10.1016/S0363-5023(96)80212-7. [DOI] [PubMed] [Google Scholar]
- 61.Strickland JW. Flexor tendon injuries. Part 2. Flexor tendon repair. Orthop Rev. 1986;15:701–21. [PubMed] [Google Scholar]
- 62.Barton NJ. Experimental study of optimal location of flexor tendon pulleys. Plast Reconstr Surg. 1969;43:125–9. doi: 10.1097/00006534-196902000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Doyle JR, Blythe W. AAOS symposium on tendon surgery in the hand. St Louis: Mosby; 1975. The finger flexor tendon sheath and pulleys; anatomy and reconstruction; pp. 81–8. [Google Scholar]
- 64.Hunter JM, Cook JF, Ochiai N. The pulley system. Proceedings of the American Society for Surgery of the Hand. Orthop Trans. 1980;4:4. [Google Scholar]
- 65.Idler RS. Anatomy and biomechanics of the digital flexor tendons. Hand Clin. 1985;1:3–11. [PubMed] [Google Scholar]
- 66.Idler RS, Strickland JW. The effects of pulley resection on the biomechanics of the proximal interphalangeal joint. Univ Pennsylvania Orthop J. 1986;2:20–3. [Google Scholar]
- 67.Savage R. The mechanical effect of partial resection of the digital fibrous flexor sheath. J Hand Surg Br. 1990;15:435–42. doi: 10.1016/0266-7681(90)90086-j. [DOI] [PubMed] [Google Scholar]
- 68.Tomaino M, Mitsionis G, Basitidas J, Grewal R, Pfaeffle J. The effect of partial excision of the A2 and A4 pulleys on the biomechanics of finger flexion. J Hand Surg Br. 1998;23:50–2. doi: 10.1016/s0266-7681(98)80218-0. [DOI] [PubMed] [Google Scholar]
- 69.Mitsionis G, Bastidas JA, Grewal R, Pfaeffle HJ, Fischer KJ, Tomaino MM. Feasibility of partial A2 and A4 pulley excision: Effect on finger flexor tendon biomechanics. J Hand Surg Am. 1999;24:310–4. doi: 10.1053/jhsu.1999.0310. [DOI] [PubMed] [Google Scholar]
- 70.Franko OI, Lee NM, Finneran JJ, Shillito MC, Meunier MJ, Abrams RA, et al. Quantification of partial or complete A4 pulley release with FDP repair in cadaveric tendons. J Hand Surg Am. 2011;36:439–45. doi: 10.1016/j.jhsa.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwai Ben I, Elliot D. ‘Venting’ or partial lateral release of the A2 and A4 pulleys after repair of zone 2 flexor tendon injuries. J Hand Surg Br. 1998;23:649–54. doi: 10.1016/s0266-7681(98)80020-x. [DOI] [PubMed] [Google Scholar]
- 72.Wu YF, Zhou YL, Tang JB. Relative contribution of tissue oedema and the presence of an A2 pulley to resistance to flexor tendon movement: An in vitro and in vivo study. J Hand Surg Eur. 2012;37:310–5. doi: 10.1177/1753193411425329. [DOI] [PubMed] [Google Scholar]
- 73.Elliot D. Invited Personal view. Primary flexor tendon repair-Operative repair, pulley management and rehabilitation. J Hand Surg Br. 2002;27:507–14. doi: 10.1054/jhsb.2002.0800. [DOI] [PubMed] [Google Scholar]
- 74.Tang JB. Indications, methods, postoperative motion and outcome evaluation of primary flexor tendon repairs in zone 2. J Hand Surg Eur Vol. 2007;32:118–29. doi: 10.1016/J.JHSB.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Tang JB. Treatment of the flexor tendon sheath and pulleys. In: Tang JB, Amadio PC, Guimberteau JC, Chang J, editors. Tendon Surgery of the Hand. Vol. 9. Philadelphia: Elsevier Saunders; 2012. pp. 88–97. [Google Scholar]
- 76.Elliot D. Venting of the major pulleys. In: Tang JB, Amadio PC, Guimberteau JC, Chang J, editors. Tendon Surgery of the Hand. Vol. 10. Philadelphia: Elsevier Saunders; 2012. pp. 98–103. [Google Scholar]
- 77.Liew SH, Potokar T, Bantick GL, Morgan I, Ford C, Murison MS. The use of ADCON-T/N after repair of zone II flexor tendons. Chir Main. 2001;20:384–7. doi: 10.1016/s1297-3203(01)00062-2. [DOI] [PubMed] [Google Scholar]
- 78.Mentzel M, Hoss H, Keppler P, Ebinger T, Kinzl L, Wachter NJ. The effectiveness of ADCON-T/N, a new anti-adhesion barrier gel, in fresh divisions of the flexor tendons in Zone II. J Hand Surg Br. 2000;25:590–2. doi: 10.1054/jhsb.2000.0385. [DOI] [PubMed] [Google Scholar]
- 79.Golash A, Kay A, Warner JG, Peck F, Watson JS, Lees VC. Efficacy of Adcon-T/N after primary flexor tendon repair in zone II: A controlled clinical trial. J Hand Surg Br. 2003;28:113–5. doi: 10.1016/s0266-7681(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 80.Riccio M, Battiston B, Pajardi G, Corradi M, Passaretti U, Atzei A, et al. Study Group on Tendon Adhesion of Italian Society of Hand Surgery. Efficiency of Hyaloglide in the prevention of the recurrence of adhesions after tenolysis of flexor tendons in zone II: A randomized, controlled, multicentre clinical trial. J Hand Surg Eur. 2010;35:130–8. doi: 10.1177/1753193409342044. [DOI] [PubMed] [Google Scholar]
- 81.Amadio P, An KN, Ejeskar A, Guimberteau JC, Harris S, Savage R, et al. IFSSH Flexor Tendon Committee report. J Hand Surg Br. 2005;30:100–16. doi: 10.1016/j.jhsb.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang JB, Wu YF, Cao Y, Chen CH, Wang XT, Liu PY. Gene Therapy for tendon healing. In: Tang JB, Amadio PC, Guimberteau JC, Chang J, editors. Tendon Surgery of the Hand. Vol. 6. Philadelphia: Elsevier Saunders; 2012. pp. 59–70. [Google Scholar]
- 83.McFarlane RM, Lamon R, Jarvis G. Flexor tendon injuries within the finger. A study of the results of tendon suture and tendon graft. J Trauma. 1968;8:987–1003. [PubMed] [Google Scholar]
- 84.Le Viet D. Flexor tendon lengthening by tenotomy at the musculotendinous junction. Ann Plast Surg. 1986;17:239–46. doi: 10.1097/00000637-198609000-00010. [DOI] [PubMed] [Google Scholar]
- 85.Kleinert HE, Weiland AJ. Primary repair of flexor tendon lacerations in zone II. In: Verdan C, editor. Tendon surgery of the hand. Edinburgh: Churchill Livingstone; 1979. pp. 71–5. [Google Scholar]
- 86.Tang JB. Flexor tendon repair in zone 2C. J Hand Surg Br. 1994;19:72–5. doi: 10.1016/0266-7681(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 87.Yii NW, Urban M, Elliot D. A prospective study of flexor tendon repair in zone 5. J Hand Surg Br. 1998;23:642–8. doi: 10.1016/s0266-7681(98)80019-3. [DOI] [PubMed] [Google Scholar]


