Abstract
HER3 is a member of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases. In the present study, we investigated the capacity of the HER3 blocking antibody, U3-1287/AMG888, to modulate the in vitro and in vivo radiation response of human squamous cell carcinomas of the lung and head and neck. We screened a battery of cell lines from these tumors for HER3 expression and demonstrated that all cell lines screened exhibited expression of HER3. Importantly, U3-1287/AMG888 treatment could block both basal HER3 activity and radiation induced HER3 activation. Proliferation assays indicated that HER3 blockade could decrease the proliferation of both HNSCC cell line SCC6 and NSCLC cell line H226. Further, we demonstrated that U3-1287/AMG888 can sensitize cells to radiation in clonogenic survival assays, in addition to increasing DNA damage as detected via λ-H2AX immunofluo-rescence. To determine if U3-1287/AMG888 could enhance radiation sensitivity in vivo we performed tumor growth delay experiments using SCC6, SCC1483, and H226 xenografts. The results of these experiments indicated that the combination of U3-1287/AMG888 and radiation could decrease tumor growth in studies using single or fractionated doses of radiation. Analysis of HER3 expression in tumor samples indicated that radiation treatment activated HER3 in vivo and that U3-1287/AMG888 could abrogate this activation. Immunohistochemistry analysis of SCC6 tumors treated with both U3-1287/AMG888 and a single dose of radiation demonstrated that various cell survival and proliferation markers could be reduced. Collectively our findings suggest that U3-1287/AMG888 in combination with radiation has an impact on cell and tumor growth by increasing DNA damage and cell death. These findings suggest that HER3 may play an important role in response to radiation therapy and blocking its activity in combination with radiation may be of therapeutic benefit in human tumors.
Introduction
The HER family of receptor tyrosine kinases are key regulators of signaling pathways that regulate numerous cell functions. The HER/ErbB family consists of four members, the epidermal growth factor receptor (EGFR/ErbB1), HER2/Neu, HER3/ErbB3, and HER4/ErbB4. Upon ligand binding at the cell surface, HER family receptors are activated and form both homo- and hetero-dimer pairs with other HER family members (Yarden and Pines, 2012). Dimerization activates the intrinsic tyrosine kinase activity of each receptor in the pair leading to the phosphorylation of tyrosine residues on each partner’s C-terminal tail. Phosphorylated tyrosines serve to recruit numerous adaptor and effector molecules that signal through a multilayered network of proteins to ultimately influence cellular proliferation, survival, motility, differentiation, angiogenesis, and metabolism (Yarden and Pines, 2012).
The HER3 receptor is a unique HER family member in that it has a diminished tyrosine kinase activity (Guy et al., 1994; Sierke et al., 1997). However, HER3 has been shown to signal potently in heterodimeric partnerships, most notably with the HER2 receptor (Baselga and Swain, 2009). Upon binding to its cognate ligand, neuregulin-1, HER3 can heterodimerize with other HER family members leading to the direct recruitment of the p85 subunit of phosphoinositide 3-kinase (PI3K). The activation of PI3K leads to the conversion of phosphatidylinositol 4,5-biphosphosphate (PIP2) to phosphatidylinositol 2,4,5-triphosphate (PIP3) in the cell membrane (Campbell et al., 2010; Prigent and Gullick, 1994). The serine/threonine kinase AKT can associate with PIP3 through its high affinity Pleckstrin Homology (PH) domain, and subsequently become activated to induce a variety of cell survival signaling pathways. HER3 heterodimers also lead to the activation of cell proliferation signals through the recruitment of the adaptor protein GRB2 and the guanine nucleotide exchange factor SOS, which promotes the activation of the small GTPase RAS (Campbell et al., 2010). RAS can activate numerous cellular proliferation responses, most notably through the activation of MAPK. Overall, HER3 containing heterodimers signal through cell survival and proliferation pathways due to HER3’s ability to directly bind to PI3K and GRB2, enhancing the tumorigenic potential of HER3 expressing tumors.
The ability for HER3 to recruit PI3K has made it a critical dimerization partner to other HER family members since most tumors require PI3K/AKT signaling for enhanced survival. Thus, aggressive tumors often express HER3, including tumors of the breast (Bobrow et al., 1997), non-small cell lung (NSCLC) (Yi et al., 1997), metastatic colon (mCRC) (Kapitanovic et al., 2000), head and neck (HNSCC) (Takikita et al., 2011), pancreas (Lemoine et al., 1992), ovary (Rajkumar et al., 1993; Tanner et al., 2006), clear cell sarcomas (Schaefer et al., 2006), stomach (Rajkumar et al., 1993), and skin (Buac et al., 2009). In HNSCC and NSCLC, HER3 expression has been observed in approximately 77% of HNSCC and 30% of NSCLC tumor samples, where it was correlated with worse overall survival (Ocana et al., 2013; Takikita et al., 2011; Yi et al., 1997). HER3 overexpression is also commonly observed in tumors that have become resistant to various types of therapeutic agents, most well studied in resistance to both HER2 and EGFR targeting agents (Erjala et al., 2006; Garrett et al., 2011; Sergina et al., 2007; Wheeler et al., 2008). Currently, there are various anti-HER3 targeting agents being investigated for clinical use, one of which being the anti-HER3 antibody U3-1287/AMG888. U3-1287/AMG888 has shown promising anti-tumor effects in lung (Treder et al., 2008), head and neck (Freeman et al., 2011), and breast cancer (Garrett et al., 2011; 2013) in-vitro and invivo cancer models, where it effectively blocked HER3 phosphorylation, degraded total HER3 levels, and decreased tumor burden. A phase-I clinical study has deemed U3-1287/AMG888 safe for patient use, and is now being expanded to a phase-II study for treatment of various solid tumor types (LoRusso et al., 2013).
While anti-HER family monoclonal antibodies demonstrate success as monotherapy in certain types of tumors, various studies have demonstrated even greater response rates when these agents are combined with radiation therapy (Bonner et al., 2006; Jensen et al., 2011). In 2006, a pivotal study demonstrated that EGFR expressing HNSCC patients treated concurrently with cetuximab, an anti-EGFR monoclonal antibody (mAb), and radiation yielded a 10% overall survival advantage as compared to patients treated with radiotherapy alone. Preclinical data suggests that EGFR inhibition can abrogate radiation induced cell survival and DNA damage repair pathways leading to enhanced anti-tumor responses (Huang et al., 1999; Huang and Harari, 2000; Milas et al., 2000; Rodemann et al., 2007). While HER3 is expressed in HNSCC and NSCLC, the beneficial anti-tumor effect of radiation in combination with an anti-HER3 monoclonal antibody remains unexplored.
The objective of the current study was to analyze the efficacy of the anti-HER3 monoclonal antibody U3-1287/AMG888 in combination with radiation treatment. Examination of multiple HNSCC, NSCLC, and CRC cell lines indicated that numerous lines expressed both total and activated forms of HER3. Treatment of numerous cell lines with U3-1287/AMG888 effectively inhibited basal and radiation induced activation of HER3 and the downstream effector kinase AKT. Further, cell proliferation assays indicated that HER3 blockade could decrease the growth of the HNSCC cell line SCC6 and the NSCLC cell line H226, and increase sensitivity of these cell lines to radiation therapy as measured by clonogenic survival assays. The combinatorial treatment of U3-1287/AMG888 and radiation also lead to significant increases in DNA damage as detected via λ-H2AX immunofluorescence at different time points post radiation treatment. HNSCC and NSCLC mouse xenografts treated with U3- 1287/AMG888 in combination with radiation decreased tumor growth more potently than tumors treated with either therapy alone. Analysis of these tumors indicated that U3-1287/AMG888 could inhibit basal and radiation induced activation of HER3, AKT, rpS6, and MAPK. Overall, these data suggest that blocking HER3 activity can enhance the efficacy of radiation therapy, a combinatorial treatment that may benefit human tumors.
Results
HER3 is expressed in NSCLC, HNSCC, and CRC tumor cell lines
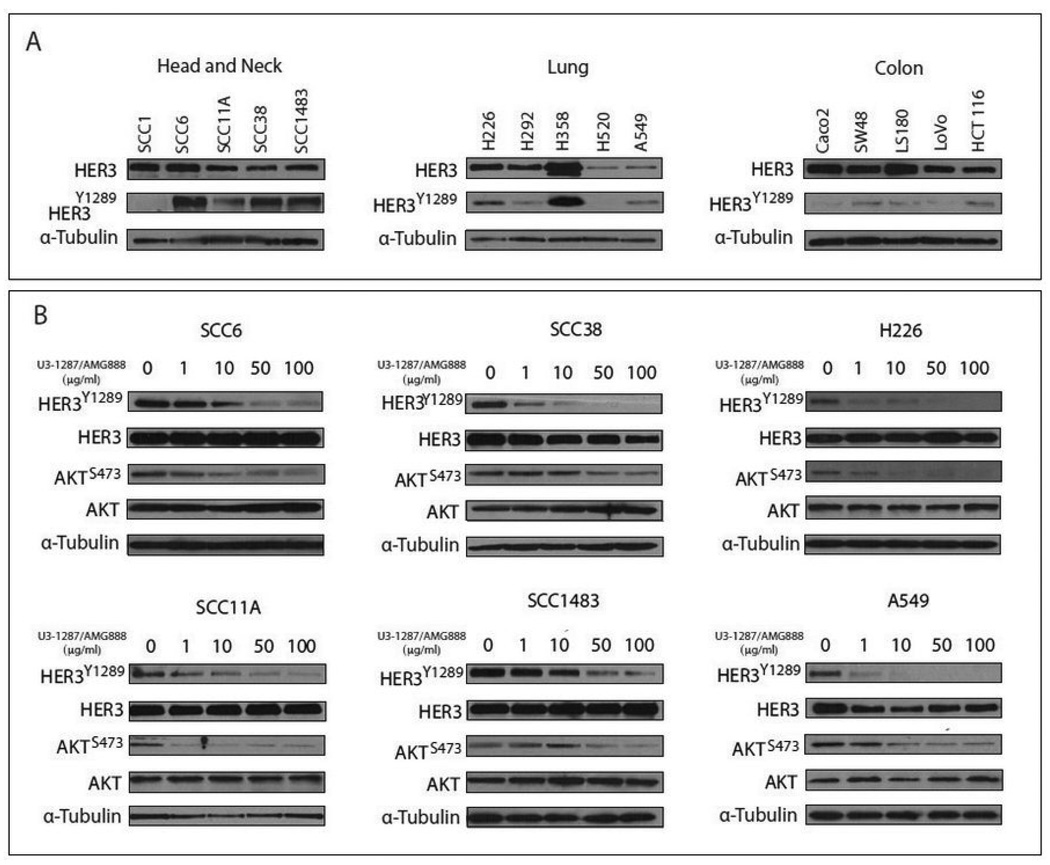
HER3 has been shown to play a crucial role in initiating oncogenic signaling pathways in numerous cancers (Campbell et al., 2010). While HER3 gene amplification is rare, HER3 expression and activation is commonly observed in numerous cancer types (Ocana et al., 2013). Examination of multiple HNSCC, NSCLC, and CRC tumor cell lines demonstrated that many express both total and activated HER3 (Figure 1A), suggesting that HER3 may play a role in enhancing the tumorigenic phenotype of these cell lines.
Figure 1.
(A) HER3 expression profile in multiple carcinoma cell lines. Five head and neck squamous carcinoma (HNSCC) cell lines (SCC1, SCC4, SCC6, SCC11A, and SCC1483), five human non-small cell lung cancer (NSCLC) cell lines (H226, H292, H358, H520, and A549), and five colorectal cancer (CRC) cell lines (Caco2, SW48, LS180, Lovo, and HCT116) were cultured in relevant mediums. Whole cell lysate was obtained and fractionated by SDS-PAGE gel electrophoresis followed by immunoblotting with the indicated antibodies. (B) U3-1287/AMG888 effectively targets HER3 and AKT activation. SCC6, SCC1483, SCC11A, SCC38, H226, and A549 cells were treated with the indicated doses of U3-1287/AMG888 for 24 hours. Whole cell lysate was obtained and fractionated by SDS-PAGE gel electrophoresis followed by immunoblotting with the indicated antibodies.
The anti-HER3 monoclonal antibody U3-1287/AMG888 can inhibit the activation of HER3 and downstream signaling pathways in vitro
Since HER3 was strongly activated in multiple cancer cell lines (Figure 1A), we examined the ability for the anti-HER3 monoclonal antibody U3-1287/AMG888 to inhibit both HER3 activation and downstream signaling pathways. We demonstrate that U3-1287/AMG888 can inhibit the activity of HER3 at tyrosine 1289 in multiple NSCLC and HNSCC tumor cell lines in a dose dependent manner (Figure 1B). We further show that the activation of the HER3 downstream signaling effector AKT can be inhibited in a dose dependent manner. Overall, these findings indicate that U3-1287/AMG888 can effectively target HER3 in a battery of NSCLC and HNSCC tumor cell lines.
U3-1287/AMG888 can prevent ionizing radiation induced activation of HER3 and AKT in vitro
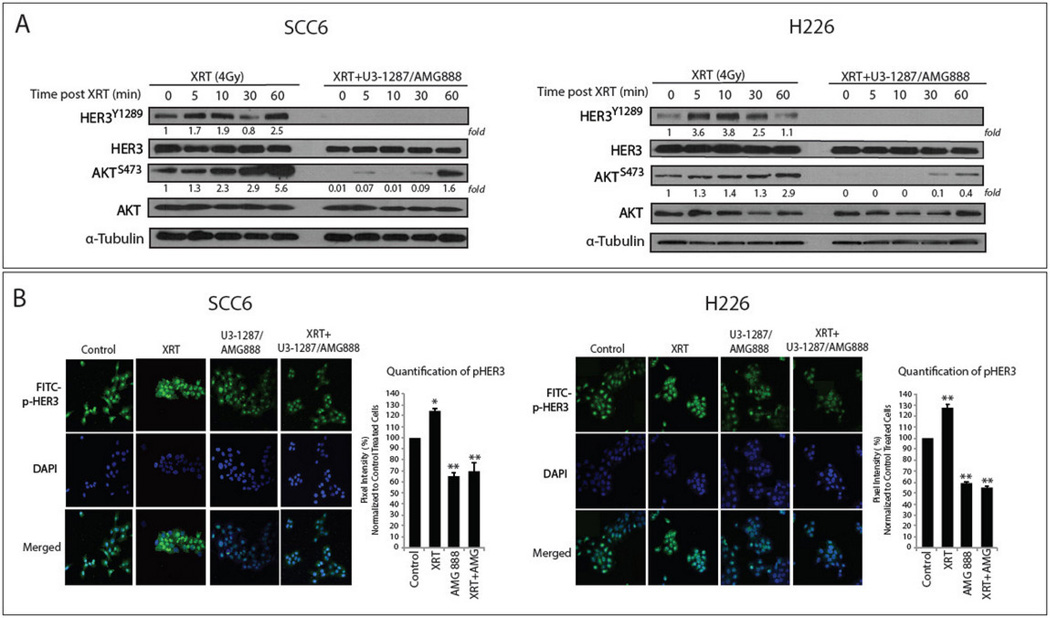
Ionizing radiation has been shown to enhance the activation of cell proliferation and survival pathways in numerous tumor types (Rodemann et al., 2007), including the compensatory activation of EGFR, HER3, and the downstream effector kinase AKT (Contessa et al., 2006; Dent et al., 2003; Schmidt-Ullrich, 2003). Treatment of both SCC6 and H226 cells with radiation lead to increases in HER3 and AKT activation between 5–60 minutes post 4 Gy radiation (Figure 2A).
Figure 2.
U3-1287/AMG888 can inhibit radiation-induced activation of HER3 and AKT. (A) HER3 was transiently activated by radiation and blocked by U3-1287/AMG888. SCC6 and H226 cells were pre-incubated with/without U3-1287/AMG888 (20 µg/ml) for 24 hours. After exposure to 4 Gy radiation, whole cell lysates were isolated at the indicated times, fractionated by SDS-PAGE gel electrophoresis followed by immunoblotting with p-HER3, HER3, p-AKT, and AKT antibodies. (B) Immunofluorescence (IF) staining of pHER3-Y1289 demonstrates that radiation can activate HER3, and U3-1287/AMG888 can block this activation. SCC6 and H226 cells were pre-incubated with/without U3-1287/AMG888 (20 µg/ml) for 24 hours. 30 minutes post 4 Gy radiation, cells were fixed for IF. Graphs indicate relative pixel intensity differences between treatment groups. Data presented are the mean±SD; *, P≤0.05 and normalized to control. Western blot quantitation of pHER3 and pAKT was performed via ImageJ, and all values were normalized to lane 1 (untreated cells).
Importantly, we demonstrated that cells pre-incubated with 20 µg/mL of U3-1287/AMG888 for 24 hours prior to radiation exhibited a pronounced decrease in activated HER3 and AKT. In cells pre-treated with U3-1287/AMG888, HER3 activation remained inhibited 60 minutes post radiation, while AKT activation was severely diminished as compared to cells treated with radiation only. We further show via confocal immuno-fluorescent microscopy (IF) that radiation (4 Gy) can effectively activate HER3 (approximately 24% and 27% higher than vehicle treated SCC6 and H226 cells), and that U3-1287/AMG888 treatment either alone or in combination with radiation can effectively inhibit HER3 activation (approximately 35% and 41% lower than vehicle treated SCC6 and H226 cells) (Figure 2B). Overall, these data suggest that U3-1287/AMG888 can effectively inhibit baseline HER3 activity and radiation-induced activation of HER3 and AKT.
U3-1287/AMG888 in combination with radiation can impact cell proliferation and DNA damage repair in vitro
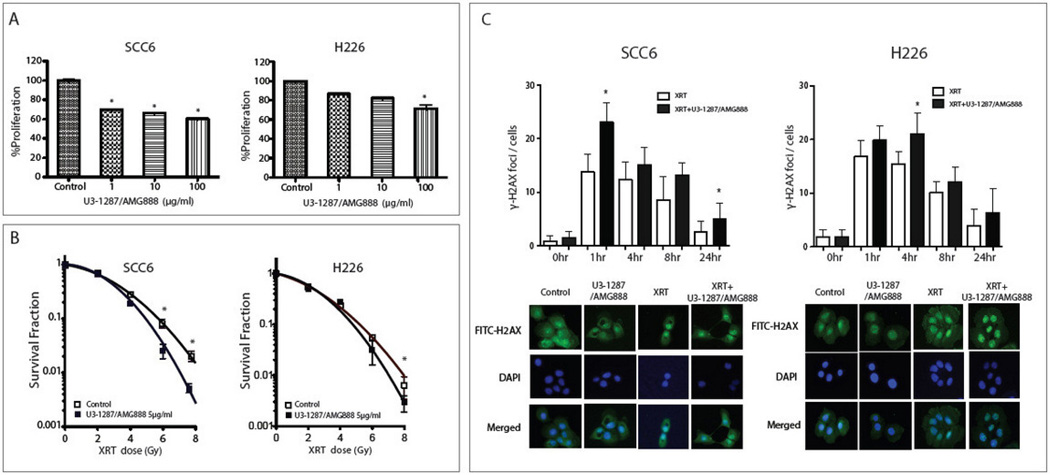
Based on our previous findings that U3-1287/AMG888 treatment could effectively prevent radiation-induced activation of both HER3 and AKT, we hypothesized that U3-1287/AMG888 could enhance the sensitivity of both HNSCC and NSCLC cell lines to radiation. We demonstrate that increasing concentrations of U3-1287/AMG888 can significantly inhibit the growth of both SCC6 and H226 cell lines; 100 µg/ml of U3-1287/AMG888 treatment inhibited cell growth approximately 40% in SCC6 cells, and 30% in H226 cells (Figure 3A). To determine the effect of U3-1287/AMG888 on radiation response, we pre-incubated both cell lines with U3-1287/AMG888 for 4 hours prior to radiation treatment, and subsequently measured their ability to survive in clonogenic survival assays (Figure 3B). U3-1287/AMG888 pre-treated SCC6 and H226 cells exhibited increased sensitivity to radiation, demonstrated by an increase in the slope of the radiation survival curves.
Figure 3.
(A) U3-1287/AMG888 can inhibit cell proliferation in a dose-dependent manner. SCC6 and H226 cells were seeded in 96-well plates and incubated with the indicated doses of U3-1287/AMG888 for 72 hours. Cell proliferation was detected using CCK8 assays. (B) U3-1287/AMG888 can sensitize cells to radiation. SCC6 and H226 cells were incubated with the indicated doses of U3-1287/AMG888 for 4 hours, and radiated with indicated doses. Clonogenic assays were performed as described. Control curves were exposed to radiation without U3-1287/AMG888 treatment. (C) Combination of U3-1287/AMG888 with radiation can increase DNA damage. SCC6 and H226 cells were incubated with U3-1287/AMG888 for 24 hours prior to 4 Gy radiation treatment. Cells were fixed and stained for γ-H2AX foci 1, 4, 8, and 24 hours post radiation. Confocal microscopy images represent 4 hours post radiation therapy. Data presented are the mean±SD; *, P≤0.05 and normalized to control in (A), and radiation alone in (B), and (C).
Finally, we analyzed the rate of DNA damage repair post U3-1287/AMG888 and radiation treatment (Figure 3C). Cells were pre-treated with either U3-1287/AMG888 or vehicle 24 hours prior to radiation treatment. Cells were fixed and stained for γ-H2AX foci at 0, 1, 4, 8, and 24 hours post radiation treatment. These data demonstrated that SCC6 and H226 cells pre-treated with U3-1287/AMG888 had increased γ-H2AX foci at 1, 4, 8, and 24 hours post radiation, with statistical significance seen at 1 hour and 24 hours in SCC6 and 1 hour in H226 cells when compared to cells treated with radiation only. Overall, both SCC6 and H226 cells exhibit significant enhancements of γ-H2AX staining upon dual treatment demonstrating that this combination can induce a higher level of DNA damage than radiation therapy alone.
U3-1287/AMG888 in combination with radiation inhibits the growth of HNSCC and NSCLC tumor xenograft models
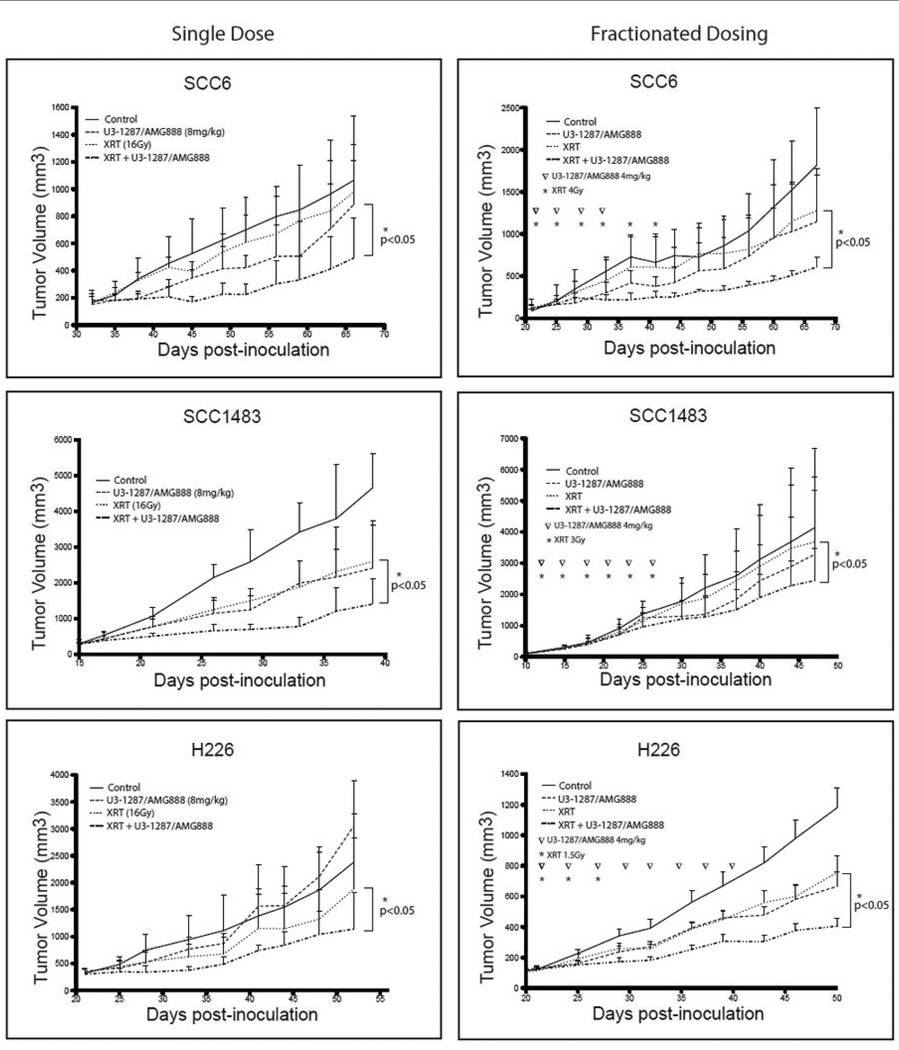
To further evaluate the efficacy of U3-1287/AMG888 combined with radiation therapy, we inoculated SCC6, SCC1483, and H226 cell lines into athymic mice. Once tumors reached 100–200 mm3, mice were divided into two groups to receive either a single or fractionated dose of radiation (Figure 4). In the single radiation dose group, xenograft mice (12 mice/group) were administered with IgG, U3-1287/AMG888 (8 mg/kg), radiation (at indicated doses), or the combination (Figure 4). In the fractionated dose treatment group (Figure 4), xenograft mice were administered with IgG, U3-1287/AMG888 (4 mg/kg), radiation (twice a week at indicated doses), or a combination of both U3-1287/AMG888 and radiation (Figure 4). All three xenograft tumor models demonstrated statistically significant reductions in tumor volumes when treated with U3-1287/AMG888 in combination with either a single or fractionated dose of radiation as compared to single therapy treated mice or IgG control treated mice. Collectively, these data demonstrate that U3-1287/AMG888 can enhance the sensitivity of HNSCC and NSCLC xenograft tumor models to radiation therapy.
Figure 4.
U3-1287/AMG888 in combination with radiation can delay the growth of HNSCC and NSCLC xenograft tumors. SCC6, SCC1483, and H226 xenografts were established as described in Materials and Methods. Mice were treated with vehicle control, single dose or fractionated dosing of radiation, U3-1287/AMG888 (at doses indicated in figure), or U3-1287/AMG888 in combination with radiation. Tumor volumes were presented as the mean tumor size±SE. *, P≤0.05.
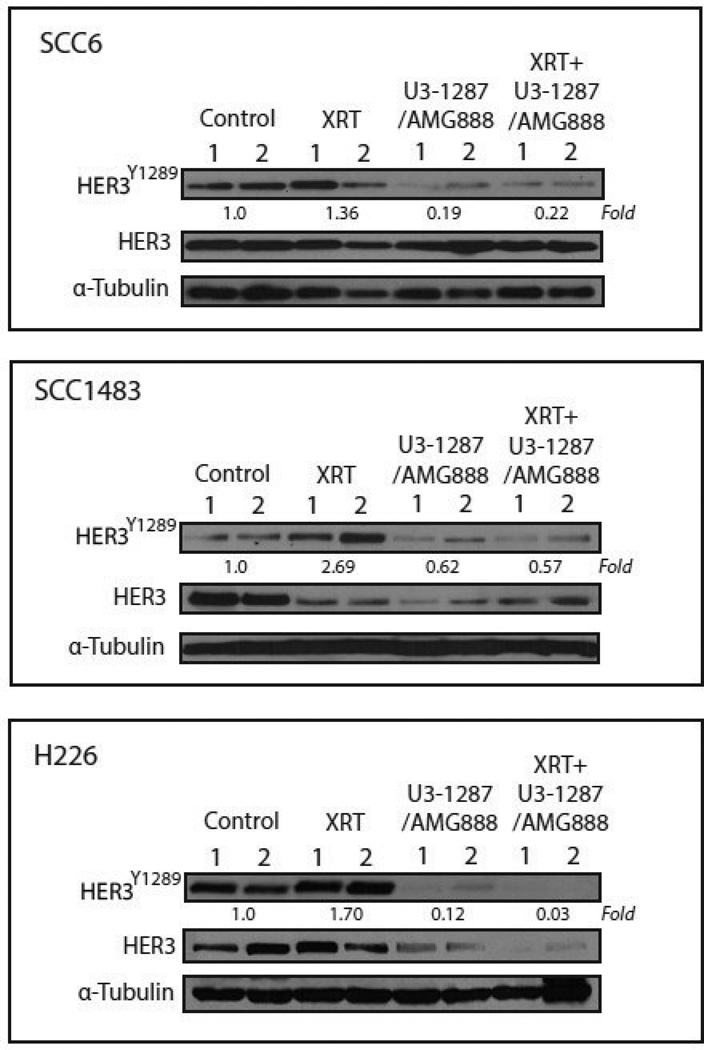
To validate that U3-1287/AMG888 effectively inhibited the activation of HER3 in vivo, we isolated protein from tumors treated with a single dose of radiation. Xenograft tumors treated with radiation only (XRT) demonstrated activated levels of HER3 (most notably in SCC1483 and H226 cells), while tumors treated with U3-1287/AMG888 in combination with radiation expressed approximately 78%, 43%, and 97% less HER3 activity in SCC6, SCC1483, and H226 tumors, respectively (quantification was performed using ImageJ analysis of the average band intensities between two tumors per treatment group) (Figure 5). Interestingly, total levels of HER3 were also decreased in SCC1483 and H226 xenograft tumors treated with U3-1287/AMG888, a phenomenon that was not observed in vitro. Overall, these data suggest that inhibition of HER3 activity may play a role in enhancing HNSCC and NSCLC tumor xenograft sensitivity to radiation treatment.
Figure 5.
U3-1287/AMG888 can inhibit basal and radiation-induced activation of HER3 in xenograft tumors. SCC6 xenograft tumors treated with single dose of radiation (16 Gy), U3-1287/AMG888 (8 mg/kg) or combination of both. Tumors were harvested and protein isolated 24 hours after the last treatment, fractionated by SDS-PAGE gel electrophoresis and followed by immunoblotting with HER3 and p-HER3 Y1289. Western blot quantitation of pHER3 was averaged between two tumors of the same treatment group via ImageJ, and all values were normalized to the average of the control (vehicle treated) tumors.
U3-1287/AMG888 in combination with radiation can inhibit signaling through cell proliferation and survival pathways in vivo
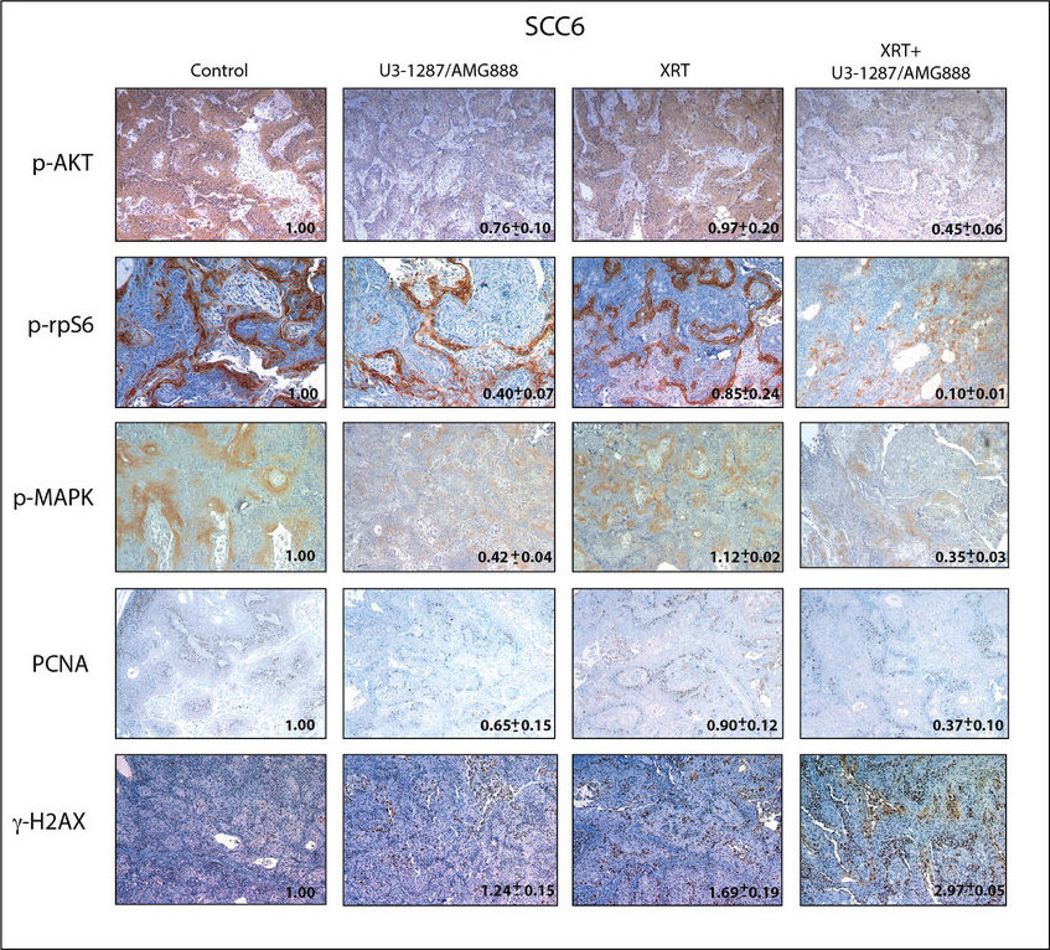
To further analyze the radiosensitizing effect of U3-1287/AMG888, we performed immunohistochemical analysis for various cell proliferation and survival markers from SCC6 tumors treated with a single dose of radiation (Figure 6). The cell markers stained for include phospho AKT (serine 473) and phospho rpS6 (serine 235/236) as survival markers, phospho p44/42 MAPK (threonine 202 and tyrosine 204) and PCNA as proliferation markers, and γ-H2AX as a DNA damage marker. Tumors treated with a single dose of radiation in combination with U3-1287/AMG888 demonstrated prominent decreases in activity of all proliferation and survival markers analyzed, with phospho-rpS6 inhibition being the most prominent (quantified via ImageJ analysis of the average staining intensity measured from 3 random fields, two tumors per treatment group) (Figure 6). Additionally, SCC6 tumors treated with combination therapy contained a 2.97 fold increase in γ-H2AX staining, a significantly higher level of DNA damage as compared to tumors treated with either therapy alone. These data suggest that U3-1287/AMG888 treatment in combination with radiation can inhibit cell proliferation and survival signals in vivo, providing a rationale for the use of this combination in a clinical setting.
Figure 6.
U3-1287/AMG888 in combination with radiation can reduce cell proliferation and survival signals, and enhance γ-H2AX foci in xenograft tumors. SCC6 xenograft tumors treated with single dose of radiation (16 Gy), U3-1287/AMG888 (8 mg/kg), or combination of both were harvested 24 hours post treatment. IHC staining was performed as described in Materials and Methods. Images depict the expression of pAKT (Ser473), p-rpS6 (Ser235/236), p-p44/42 MAPK (Thr202/Tyr204), PCNA, and γ-H2AX in xenograft tumors. Images were quantified via taking the average staining intensity measured from two tumors per treatment group (3 images/tumor).
Discussion
Many human cancers are characterized by altered signaling of HER family receptors including HNSCC, NSCLC, CRC, and breast cancer (Yarden and Pines, 2012). While the development of therapeutics targeting both the EGFR and HER2 receptors have been ongoing for decades, the limited kinase activity of the HER3 receptor has led to the delayed development of cancer therapeutics targeting its activation. However, it is now clear that HER3 not only exhibits low-level kinase activity (Shi et al., 2010), but also forms potent dimers with other HER family members to drive tumorigenesis and resistance to other HER family targeting agents (Erjala et al., 2006; Garrett et al., 2011; Sergina et al., 2007; Wheeler et al., 2008). Thus, HER3 is now a promising molecular target in several human cancers.
In the present study we demonstrate that HER3 is expressed and active in several human cancer cell lines originating from NSCLC, HNSCC, and CRC origin. HER3 inhibition, achieved through treatment with the monoclonal antibody U3-1287/AMG888 in combination with radiation therapy, inhibited both basal and radiation induced activation of HER3 and AKT. Further, we demonstrate that U3-1287/AMG888 can sensitize cells to radiation in clonogenic survival assays, in addition to enhancing DNA damage. Importantly, U3-1287/AMG888 in combination with both single and fractionated doses of radiation diminished growth of in vivo xenograft tumor models, which was accompanied by a decrease in HER3, AKT, MAPK, and rpS6 activation. Overall, our results reveal that HER3 blockade in combination with radiation may prove to be a strong anti-cancer treatment regime.
Radiation therapy in combination with anti-HER family targeting agents has proven to be beneficial in various tumor settings (Bonner et al., 2006; Jensen et al., 2011). One of the most influential examples includes EGFR blockade in combination with radiation in HNSCC. Early preclinical studies with the use of cetuximab demonstrated potent radiosensitizing potential (Huang et al., 1999; Huang and Harari, 2000). Xenograft studies in HNSCC, in addition to other solid tumor types, yielded even more potent growth inhibitory effects than observed in vitro (Huang and Harari, 2000; Milas et al., 2000; Raben et al., 2005), similar to the results we present in the current study. Following preclinical modeling, a phase III multinational clinical study in locoregionally advanced HNSCC patients demonstrated that cetuximab given simultaneously with radiation could improve both local control and overall survival as compared to radiation treatment alone (Bonner et al., 2006). In a recent phase II study, cetuximab in combination with radiation in stage III NSCLC patients proved to be safe and feasible even in elderly patients (Jensen et al., 2011). Small molecule TKI’s targeting the EGFR and HER2 have also demonstrated substantial survival advantages when combined with radiation treatment in various cancers (Chinnaiyan et al., 2005; Sambade et al., 2010). While the effects of EGFR and HER2 blockade in combination with radiation therapy have demonstrated substantial clinical benefits, HER3 blockade in combination with radiation has remained unexplored.
HER3 overexpression has proven to play a large role in the promotion of various cancers via initiating strong signaling through the PI3K/AKT signaling network. In the current study we demonstrate that we can effectively reduce signaling through the PI3K/AKT signaling network with U3-1287/AMG888 treatment both in vitro (Figure 2) and in vivo (Figure 6). Additionally, we demonstrate that U3-1287/AMG888 can prevent radiation-induced compensatory activation of HER3. Compensatory activation of receptor tyrosine kinases is often observed post radiation therapy (Coffer et al., 1995; Devary et al., 1992; Dittmann et al., 2009; Knebel et al., 1996; Singh et al., 2009; Sturla et al., 2005; Xu et al., 2006). Various studies have indicated that radiation and UV exposure can activate the EGFR via protein phosphatase inhibition (Knebel et al., 1996; Sturla et al., 2005; Xu et al., 2006), induction of EGF ligand shedding (Singh et al., 2009), and enhancement of membrane lipid peroxidation through the release of reactive oxygen species (Dittmann et al., 2009). Whether radiation can activate HER3 via one of these mechanisms remains unexplored but highly possible. Additionally, it is well accepted that treating cancer cells with anti-EGFR agents prior to radiation can prevent compensatory upregulation of cell survival and proliferation pathways, leading to more prolonged antitumor responses (Chinnaiyan et al., 2005; Huang and Harari, 2000; Santiago et al., 2010). Recent data suggests that the dual inhibition of EGFR and HER3 yield potent radio-sensitizing effects (Huang et al., 2013). Our data indicate that treatment with U3-1287/AMG888 in combination with radiation can potently inhibit AKT, MAPK, and rpS6 (Figure 6), and thus this combination may provide benefit in the treatment of tumors resistant to EGFR targeting agents.
In the current study we observed strong antitumor effects upon combined U3-1287/AMG888 and radiation treatment in several tumor xenografts (Figure 4). The reduced tumor burden of mice treated with both U3-1287/AMG888 and radiation treatment may be due to microenvironmental factors that cannot be measured in in vitro settings, including the impact on angiogenesis and tumor targeting by the immune system via antibody dependent cellular cytotoxicity. There have been various reports identifying the role of HER2:HER3 heterodimers in the regulation of tumor angiogenesis, and one recent report attributed HER3 to playing a specific role through knockdown with MiR-148a leading to a large reduction of tumor angiogenesis in vivo (Kumar and Yarmand-Bagheri, 2001; Yu et al., 2011).
U3-1287/AMG-888 has been previously tested for efficacy in various cell models of HNSCC (Freeman et al., 2011), NSCLC (Treder et al., 2008), and breast cancer (Garrett et al., 2011; 2013). In both HNSCC and NSCLC preclinical models, U3-1287/AMG888 demonstrated strong anti-tumor effects as a single agent, which was further enhanced by dual treatment with anti-EGFR agents (Freeman et al., 2011; Treder et al., 2008). In preclinical breast cancer models, U3-1287/AMG888 demonstrated anti-tumor activity; several investigators hypothesized that U3-1287/AMG888 may act in synergy with both trastuzumab or lapatinib in HER2 positive breast cancer patients (Abramson and Arteaga, 2011; Garrett et al., 2011; 2013). Additionally, original studies using U3-1287/AMG888 demonstrated that it could inhibit the activation of HER3 in addition to promoting HER3 internalization and degradation (Hettmann, 2010). In the current study we observed a substantial loss of total HER3 protein in SCC1483 and H226 xenograft tumors treated with U3-1287/AMG888 (Figure 5); the degradation of total HER3 may be another reason for why an enhanced anti-tumor effect was observed with dual U3-1287/AMG888 and radiation treatment in vivo. Currently, U3-1287/AMG888 has undergone phase I clinical testing, where it was deemed well tolerated by patients with solid tumors (LoRusso et al., 2013). The study presented herein is the first to demonstrate preclinical success with U3-1287/AMG888 in combination with radiation therapy, suggesting that this combination may provide further clinical benefit.
In conclusion, treatment with U3-1287/AMG888 in combination with radiation therapy holds promise for tumors that express HER3. HNSCC and NSCLC often express HER3, and radiation therapy is a common treatment regime for these tumor types. Additionally, HNSCC and NSCLC tumors are generally treated with anti-EGFR therapies, many of which contain intrinsic or acquired resistance to these therapeutic agents. Since HER3 expression is often observed in anti-EGFR therapy resistant tumors, HER3 blockade may prove advantageous in this setting. We demonstrate that blockade of HER3 activity with U3-1287/AMG888 can prevent signaling through proliferation and survival pathways, which remain inhibited upon radiation treatment. Importantly, we observe a prominent anti-tumor response in vivo as compared to either agent alone, suggesting the importance for further investigation of combined therapy for clinical use in the future.
Materials and Methods
Cell culture and therapeutics
Five NSCLC cell lines (NCI-H226, H292, H358, H520, and A549) and 5 colorectal cancer cell lines (Caco2, SW48, LS180, Lovo, and HCT116) were purchased from ATCC (Manassas, VA, USA) and maintained in 10% fetal bovine serum (FBS) in RPMI1640 or DMEM (Mediatech Inc., Manassas, VA, USA) with 1% penicillin and streptomycin. Five HNSCC lines (UM-SCC1, UM-SCC4, UM-SCC6, UM-SCC11A, and UM-SCC1483 cells) were kindly supplied by Dr. T. Carey (University of Michigan, MI, USA) (Brenner et al., 2010) and maintained in 10% FBS (Invitrogen, Carlsbad, CA, USA) in DMEM supplemented with 1% hydrocortisone and 1% penicillin and streptomycin. U3-1287/AMG888 was provided by U3 Pharma (Martinsried, Germany).
Cell proliferation assay
Cells were seeded in 96-well plate and exposed to doses of U3-1287/AMG888 for 72 hours. Cell proliferation was tested by Cell Counting Kit-8 (Dojindo Molecular Technologies, Rockville, MD, USA).
Clonogenic assay
An equal number of cells were seeded into six-well tissue culture plates. After allowing cells time to attach (6 hours), U3-1287/AMG888 or the vehicle control (IgG) was added at specified concentrations. The plates were irradiated 4 hours later at the doses of 2, 4, 6, and 8 Gy. Ten to 14 days after seeding, colonies were stained with crystal violet, the number of colonies containing at least 50 cells was determined and the surviving fractions were calculated. Survival curves were generated after normalizing for cytotoxicity generated by U3-1287/AMG888 alone. Data presented are the mean ± SE from at least three independent experiments.
Immunoblotting analysis
Following treatment, cells were lysed with buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 0.1% Tween-20, 10% glycerol, 2.5 mM EGTA, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, and 10 µg/ml of leupeptin and aprotinin). Protein was quantitated using a standard Bradford absorbance assay. Equal amounts of protein were fractionated by SDS-PAGE. Thereafter, proteins were transferred to PVDF membrane and analyzed by incubation with the appropriate primary antibody. Proteins were detected via incubation with HRP-conjugated secondary antibodies and ECL chemiluminescence detection system. NIH ImageJ was used to evaluate densitometry of western blots. The antibodies used in this study were as follows. HER3, AKT, MAPK, horseradish peroxidase-conjugated goat-anti-rabbit IgG, and goat-anti-mouse IgG were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). p-HER3 (Tyr1289), p-AKT (Ser473), p-rpS6 (Ser235/236), p-p44/42 MAPK (Thr202/Tyr204), and PCNA were purchased from Cell Signaling Technology (Beverly, MA, USA). α-tubulin was purchased from Calbiochem (San Diego, CA, USA).
Immunofluorescence assay
Approximately 2 × 103 cells were seeded in a four-well glass chamber slides (Nalgene Nunc, Naperville, IL, USA). After the treatment, cells were washed 3 times with PBS and fixed with 2% formaldehyde for 15 minutes at room temperature. Cells were incubated in icecold 100% methanol for 10 minutes at −20°C and blocked with 5% normal serum in PBS with 0.3% Triton X100 solution for 1 hour at room temperature and incubated with p-HER3 or λ-H2AX (Cell Signaling Technology) antibodies overnight at 4°C. Next, cells were incubated with FITC-conjugated appropriate secondary antibody in PBS containing 0.3% Triton X100 and 1% BSA for 2 hour. Slides were mounted using ProLong gold with DAPI (Invitrogen). Photographs were captured by a Nikon confocal microscope and pixel intensity was quantitated via ImageJ.
Mouse xenograft models
Athymic nude mice (4- to 6-week old; male) were obtained from the Harlan Laboratories (Indianapolis, IN, USA). All animal procedures and maintenance were conducted in accordance with the institutional guidelines of the University of Wisconsin. Cells were injected bilaterally in the dorsal flanks of the mice at day 0 (2 × 106 cells). Once tumors reached expected volumes (200–300 mm3) mice were treated with 1) IgG, 2) U3-1287/AMG888, 3) radiation (single or fractionated dosing), or 4) the combination. Measurements were evaluated by digital calipers and calculated by the formula (π)/6 × (large diameter) × (small diameter)2.
Mouse tumor collection and protein isolation
Tumors were collected 24 hours after the treatments. Mice were sedated using isofluorane mixed with oxygen until unconscious. Mice were euthanized by cervical dislocation and tumors were promptly collected, washed in PBS, and frozen on dry ice. Tumors were crushed using a mortar and pestle until the tumor was the consistency of a powder. Whole cell protein lysate was obtained with lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 0.1% Tween-20, 10% glycerol, 2.5 mM EGTA, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, and 10 µg/ml of leupeptin and aprotinin), sonicated, and quantified. Protein quantitation and immunoblotting analysis were performed as stated above.
Immunohistochemistry
Xenograft tumors were fixed in 10% neutral buffered formalin, paraffin embedded, and serially cut onto slides. Immunohistochemical staining was performed for PCNA, p-MAPK, p-AKT, p-rpS6, and γ-H2AX. In brief, specimens were deparaffinized and rehydrated routinely, following antigen retrieval by citrate buffer (pH=6.0) for 15 minutes at 98°C in water bath, incubation in 3% hydrogen peroxide for 10 minutes, and 3% BSA blocking for 30 minutes. Staining was performed as below: incubation in primary antibody diluted at 4°C overnight, incubation in biotinylated secondary antibody for 30 minutes at room temperature, and peroxidase visualization using Dako Cytomation Liquid DAB+Substrate Chromogen System (Dako North America, Inc., Carpinteria, CA). Quantitation of staining intensity was performed with ImageJ.
Statistical analysis
A two-way ANOVA analysis was performed and P≤0.05 was considered significant at the 95% confidence level as shown by the asterisks (*). Statistical analysis comparing differences in fluorescent and staining intensities were evaluated via Student t-test. Differences were considered statistically significant if P≤0.05.
Acknowledgments
The project described was supported, in part, by grant UL1TR000427 from the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant RSG-10-193-01-TBG from the American Cancer Society (DLW), grant 2010523665 from Amgen Inc. (D.L.W.), and NIH grant T32 GM08.1061-01A2 from Graduate Training in Cellular and Molecular Pathogenesis of Human Diseases (T.M.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosure
D.L.W. holds a laboratory research agreement with Amgen Inc. Other authors do not have conflicts of interest to disclose.
References
- Abramson V, Arteaga CL. New strategies in HER2-overex-pressing breast cancer: many combinations of targeted drugs available. Clin Cancer Res. 2011;17(5):952–958. doi: 10.1158/1078-0432.CCR-09-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- Bobrow LG, Millis RR, Happerfield LC, Gullick WJ. c-erbB-3 protein expression in ductal carcinoma in situ of the breast. Eur J Cancer. 1997;33(11):1846–1850. doi: 10.1016/s0959-8049(97)00244-x. [DOI] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, Bradford CR, Carey TE. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32(4):417–426. doi: 10.1002/hed.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buac K, Xu M, Cronin J, Weeraratna AT, Hewitt SM, Pavan WJ. NRG1 / ERBB3 signaling in melanocyte development and melanoma: inhibition of differentiation and promotion of proliferation. Pigment Cell Melanoma Res. 2009;22(6):773–784. doi: 10.1111/j.1755-148X.2009.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res. 2010;16(5):1373–1383. doi: 10.1158/1078-0432.CCR-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan P, Huang S, Vallabhaneni G, Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM, Harari PM. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva) Cancer Res. 2005;65(8):3328–3335. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Burgering BM, Peppelenbosch MP, Bos JL, Kruijer W. UV activation of receptor tyrosine kinase activity. Oncogene. 1995;11(3):561–569. [PubMed] [Google Scholar]
- Contessa JN, Abell A, Mikkelsen RB, Valerie K, Schmidt-Ullrich RK. Compensatory ErbB3/c-Src signaling enhances carcinoma cell survival to ionizing radiation. Breast Cancer Res Tr. 2006;95(1):17–27. doi: 10.1007/s10549-005-9023-9. [DOI] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159(3):283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Devary Y, Gottlieb RA, Smeal T, Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Dittmann K, Mayer C, Kehlbach R, Rothmund MC, Peter Rodemann H. Radiation-induced lipid peroxidation activates src kinase and triggers nuclear EGFR transport. Radiother Oncol. 2009;92(3):379–382. doi: 10.1016/j.radonc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Erjala K, Sundvall M, Junttila TT, Zhang N, Savisalo M, Mali P, Kulmaia J, Pulkkinen J, Grenman R, Elenius K. Signaling via ErbB2 and ErbB3 associates with resistance and Epidermal Growth Factor Receptor (EGFR) amplification with sensitivity to EGFR inhibitor gefitinib in head and neck squamous cell carcinoma cells. Clin Cancer Res. 2006;12(13):4103–4111. doi: 10.1158/1078-0432.CCR-05-2404. [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Ogbagabriel S, James B, Sun J, Radinsky R, Hettmann T. U3-1287 (AMG 888), a fully human anti-HER3 mAB, demonstrates in vitro and in vivo efficacy in the FaDu model of human squamous cell carcinoma of the head and neck. AACR-Molecular Target and Cancer Therapeutics Meeting Proceedings, abstr #A182. 2011 [Google Scholar]
- Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, Dave B, Cook RS, Pao W, Mckinely E, Manning HC, Chang J, Arteaga CL. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108(12):5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JT, Sutton CR, Kuba MG, Cook RS, Arteaga CL. Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function. Clin Cancer Res. 2013;19(3):610–619. doi: 10.1158/1078-0432.CCR-12-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., 3rd Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci U S A. 1994;91(17):8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettmann T. U3-1287 (AMG 888), a fully human anti-HER3 mAB, inhibits HER3 activation and induces HER3 internal-ization and degradation. American Association for Cancer Research Annual Meeting, Abstract #LB-306. 2010 [Google Scholar]
- Huang S, Li CR, Armstrong EA, Peet CR, Saker J, Amler LC, Sliwkowski MX, Harari PM. Dual Targeting of EGFR and HER3 with MEHD7945A Overcomes Acquired Resistance to EGFR Inhibitors and Radiation. Cancer Res. 2013;73(2):824–833. doi: 10.1158/0008-5472.CAN-12-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apop-tosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59(8):1935–1940. [PubMed] [Google Scholar]
- Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6(6):2166–2174. [PubMed] [Google Scholar]
- Jensen AD, Munter MW, Bischoff HG, Haselmann R, Haberkorn U, Huber PE, Thomas M, Debus J, Herfarth KK. Combined treatment of nonsmall cell lung cancer NSCLC stage III with intensity-modulated RT radiotherapy and cetuximab: the NEAR trial. Cancer. 2011;117(13):2986–2994. doi: 10.1002/cncr.25888. [DOI] [PubMed] [Google Scholar]
- Kapitanovic S, Radosevic S, Slade N, Kapitanovic M, Andelinovic S, Ferencic Z, Tavassoli M, Spaventi S, Pavelic K, Spaventi R. Expression of erbB-3 protein in colorectal adenocarcinoma: correlation with poor survival. J Cancer Res Clin. 2000;126(4):205–211. doi: 10.1007/s004320050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebel A, Rahmsdorf HJ, Ullrich A, Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996;15(19):5314–5325. [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Yarmand-Bagheri R. The role of HER2 in angio-genesis. Semin Oncol. 2001;28(5 Suppl 16):27–32. doi: 10.1016/s0093-7754(01)90279-9. [DOI] [PubMed] [Google Scholar]
- Lemoine NR, Lobresco M, Leung H, Barton C, Hughes CM, Prigent SA, Gullick WJ, Kloppel G. The erbB-3 gene in human pancreatic cancer. J Pathol. 1992;168(3):269–273. doi: 10.1002/path.1711680305. [DOI] [PubMed] [Google Scholar]
- Lorusso P, Janne PA, Oliveira M, Rizvi N, Malburg L, Keedy V, Yee L, Copigneaux C, Hettmann T, Wu CY, Ang A, Halim AB, Beckman RA, Beaupre D, Berlin J. Phase I Study of U3-1287, a Fully Human Anti-HER3 Monoclonal Antibody, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2013;19(11):3078–3087. doi: 10.1158/1078-0432.CCR-12-3051. [DOI] [PubMed] [Google Scholar]
- Milas L, Mason K, Hunter N, Petersen S, Yamakawa M, Ang K, Mendelsohn J, Fan Z. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6(2):701–708. [PubMed] [Google Scholar]
- Ocana A, Vera-Badillo F, Seruga B, Templeton A, Pandiella A, Amir E. HER3 Overexpression and Survival in Solid Tumors: A Meta-analysis. J Natl Cancer Institute. 2013;105(4):266–273. doi: 10.1093/jnci/djs501. [DOI] [PubMed] [Google Scholar]
- Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3′-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13(12):2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben D, Helfrich B, Chan DC, Ciardiello F, Zhao L, Franklin W, Baron AE, Zeng C, Johnson TK, Bunn PA., Jr The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res. 2005;11(2 Pt 1):795–805. [PubMed] [Google Scholar]
- Rajkumar T, Gooden CS, Lemoine NR, Gullick WJ, Goden CS. Expression of the c-erbB-3 protein in gastrointestinal tract tumours determined by monoclonal antibody RTJ1. J Pathol. 1993;170(3):271–278. doi: 10.1002/path.1711700309. [DOI] [PubMed] [Google Scholar]
- Rodemann HP, Dittmann K, Toulany M. Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol. 2007;83(11–12):781–791. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]
- Sambade MJ, Kimple RJ, Camp JT, Peters E, Livasy CA, Sartor CI, Shields JM. Lapatinib in combination with radiation diminishes tumor regrowth in HER2+ and basal-like/EGFR+ breast tumor xenografts. Int J Radiat Oncol Biol Phys. 2010;77(2):575–581. doi: 10.1016/j.ijrobp.2009.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago A, Eicheler W, Bussink J, Rijken P, Yaromina A, Beuthien-Baumann B, Van Der Kogel AJ, Baumann M, Krause M. Effect of cetuximab and fractionated irradiation on tumour micro-environment. Radiother Oncol. 2010;97(2):322–329. doi: 10.1016/j.radonc.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Schaefer KL, Brachwitz K, Braun Y, Diallo R, Wai DH, Zahn S, Schneider DT, Kuhnen C, Vollmann A, Brockhoff G, Gabbert HE, Poremba C. Constitutive activation of neureg-ulin/ERBB3 signaling pathway in clear cell sarcoma of soft tissue. Neoplasia. 2006;8(7):613–622. doi: 10.1593/neo.06238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK. Molecular targets in radiation oncology. Oncogene. 2003;22(37):5730–5733. doi: 10.1038/sj.onc.1206662. [DOI] [PubMed] [Google Scholar]
- Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445(7126):437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A. 2010;107(17):7692–7697. doi: 10.1073/pnas.1002753107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierke SL, Cheng K, Kim HH, Koland JG. Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptor protein. Biochem J. 1997;322(Pt 3):757–763. doi: 10.1042/bj3220757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Schneider M, Knyazev P, Ullrich A. UV-induced EGFR signal transactivation is dependent on proligand shedding by activated metalloproteases in skin cancer cell lines. Int J Cancer. 2009;124(3):531–539. doi: 10.1002/ijc.23974. [DOI] [PubMed] [Google Scholar]
- Sturla LM, Amorino G, Alexander MS, Mikkelsen RB, Valerie K, Schmidt-Ullrichr RK. Requirement of Tyr-992 and Tyr-1173 in phosphorylation of the epidermal growth factor receptor by ionizing radiation and modulation by SHP2. J Biol Chem. 2005;280(15):14597–14604. doi: 10.1074/jbc.M413287200. [DOI] [PubMed] [Google Scholar]
- Takikita M, Xie R, Chung JY, Cho H, Ylaya K, Hong SM, Moskaluk CA, Hewitt SM. Membranous expression of Her3 is associated with a decreased survival in head and neck squamous cell carcinoma. J Transl Med. 2011;9:126. doi: 10.1186/1479-5876-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner B, Hasenclever D, Stern K, Schormann W, Bezler M, Hermes M, Brulport M, Bauer A, Schiffer IB, Gebhard S, Schmidt M, Steiner E, Sehouli J, Edelmann J, Lauter J, Lessig R, Krishnamurthi K, Ullrich A, Hengstler JG. ErbB-3 predicts survival in ovarian cancer. J Clin Oncol. 2006;24(26):4317–4323. doi: 10.1200/JCO.2005.04.8397. [DOI] [PubMed] [Google Scholar]
- Treder M, Ogbagabriel S, Moor R, Schulze-Horsel U, Hettmann T, Rothe M, Radinsky R, Freeman D. Fully human anti-HER3 mAb U3-1287 (AMG 888) demonstrates unique in vitro and in vivo activities versus other HER family inhibitors in NSCLC models. EJC Suppl. 2008;6(12):99–99. [Google Scholar]
- Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27(28):3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Shao Y, Voorhees JJ, Fisher GJ. Oxidative inhibition of receptor-type protein-tyrosine phosphatase kappa by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. J Biol Chem. 2006;281(37):27389–27397. doi: 10.1074/jbc.M602355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nature reviews. Cancer. 2012;12(8):553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- Yi ES, Harclerode D, Gondo M, Stephenson M, Brown RW, Younes M, Cagle PT. High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol. 1997;10(2):142–148. [PubMed] [Google Scholar]
- Yu J, Li Q, Xu Q, Liu L, Jiang B. MiR-148a inhibits angiogensis by targeting ERBB3. J Biomed Res. 2011;25(3):170–177. doi: 10.1016/S1674-8301(11)60022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]