Abstract
Introduction
This retrospective cohort study was designed to analyze factors associated with administration of chemotherapy and to examine the impact of chemotherapy on survival among elderly patients with small cell lung cancer (SCLC) in the community.
Methods
Elderly patients ages 65 and older with SCLC diagnosed between 1992 and 2001 were selected from the SEER-Medicare database. Logistic regression was used to evaluate which covariates influenced receipt of chemotherapy. Cox proportional hazards regression was employed to examine the influence of clinical and demographic variables on survival. Determining the independent effect of chemotherapy on survival was accomplished using propensity scores and quantile regression.
Results
In the final cohort of 10,428 patients, 67.1% received chemotherapy, 39.1% received radiation, 3.4% received surgery, and 21.8% received no treatment. The most common chemotherapy regimens included etoposide combined with either cisplatin or carboplatin. Patients ages 85 and older were significantly less likely to receive chemotherapy compared to patients ages 65 to 69 (OR 0.17, 95% CI 0.14-0.21). Median survival for all patients was 7 months. Factors associated with improved survival were being female, black race, having limited stage disease, receiving any treatment, and having a lower comorbidity score. Quantile regression demonstrated that chemotherapy provided a 6.5-month improvement in median survival (95% CI, 6.3–6.6, p<0.001).
Conclusions
Statistically significant differences in the receipt of chemotherapy exist among elderly patients with SCLC. Chemotherapy is associated with a greater than 6-month improvement in median survival among elderly patients with SCLC, even in patients over the age of 80.
Keywords: small cell, survival, chemotherapy
Introduction
The American Cancer Society estimates that there will be 228,190 new cases of lung cancer diagnosed in the United States in 2013.1 Lung cancer is associated with the highest mortality rate of any cancer in the United States with 159,480 deaths predicted in 2013. Lung cancer will be responsible for 27.5% of all cancer deaths in the U.S. this year. Indeed, it kills more people than colorectal cancer, breast cancer, pancreatic cancer, and prostate cancer combined, the second through fifth leading causes of cancer mortality in the U.S., respectively.1 Approximately 14% of lung cancer cases have small cell histology, which corresponds to more than 30,000 cases of small cell lung cancer (SCLC) annually.2,3
Five-year survival for lung cancer is 16.9%.4 Overall 5-year survival for SCLC is only 6.6%.5 For limited stage SCLC, 5-year survival is 12.1%; for extensive stage disease, 5-year survival is only 1.6%.5-7
The National Comprehensive Cancer Network (NCCN) recommends concurrent chemoradiation as the standard treatment for limited stage SCLC.8 For extensive stage disease, the recommended treatment is chemotherapy alone; radiation can be administered for symptomatic relief of metastatic sites. Surgery is less commonly used in the treatment of SCLC and is usually considered only for treatment of patients with T1N0 or T2N0 disease, followed by chemotherapy with or without radiation. Cisplatin and etoposide or carboplatin and etoposide are the two most commonly used regimens in the treatment of SCLC.6,7 Another treatment option is cyclophosphamide, doxorubicin, and vincristine (the CAV regimen). For relapsed disease, a number of chemotherapeutic options are available, including topotecan. Patients who have a complete response to chemotherapy should undergo prophylactic cranial irradiation.9,10
While numerous randomized clinical trials (RCTs) have demonstrated a benefit of chemotherapy for patients with SCLC, these trials have predominantly compared different chemotherapy regimens rather than comparing chemotherapy to best supportive care (BSC).6,7 Some of them included chest radiation or prophylactic cranial irradiation. Moreover, many RCTs excluded elderly patients or those with significant medical comorbidities.11 In a study published by Ramsey et al., non-small cell lung cancer (NSCLC) patients who received chemotherapy lived 2-4 months longer than those who did not.12 The purpose of this retrospective cohort study is to present factors associated with the receipt of chemotherapy and to quantify the benefit of chemotherapy on survival of elderly patients with SCLC in the community. This is the first large-scale analysis of chemotherapy use among patients with SCLC in the community setting.
Materials and Methods
Data Sources
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database contains information on nearly 5,000,000 cases of cancer in the United States diagnosed between 1973 and 2005. The catchment area includes 26.2% of the United States population and includes 18 geographical areas. While the database contains demographic information, primary site, histology, date of diagnosis, stage, treatment with surgery and radiation given in the first course of treatment, vital status, date and cause of death, and survival in months, it does not contain information about chemotherapy, smoking, comorbidities, or performance status.
To obtain chemotherapy and comorbidity information for these patients, the SEER-Medicare database was utilized. The Centers for Medicare and Medicaid Services Medicare database includes 97% of Americans ages 65 and older. The SEER database has been linked to the Medicare database using a unique registration number for each patient. The merged SEER-Medicare database contains 94% of cancer patients ages 65 and older in the SEER database.13 Information about chemotherapy, comorbidities, and census data is included in the SEER-Medicare database. The SEER-Medicare database contains information on 312,886 cases of lung cancer diagnosed between 1973 and 2002. We used the inpatient records, outpatient records, and physician billing claims. Follow-up data was available until December of 2004.
Patient Selection Criteria
Cases of SCLC were identified using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes 8041 to 8045. Of 312,886 cases of lung cancer, 43,013 were SCLC (13.7%). Patients who were diagnosed with SCLC between January of 1992 and December of 2001 were selected from the SEER-Medicare database (25,551 cases). Those younger than 65 (3,351 cases, 13.1%) or who had more than one primary were excluded. Patients enrolled in an HMO were excluded because chemotherapy information for them is not complete. Likewise, patients were required to have both Medicare parts A and B to have complete information about chemotherapy and comorbidities. Using these exclusion criteria ensures that all the patients in this study have the same insurance. Patients diagnosed by autopsy or death certificate could not be analyzed for survival and, therefore, were excluded. In the final analysis, 10,428 cases were included, or 40.8% of the total number of SCLC cases diagnosed between 1992 and 2001. Patients were followed until date of death, date of censoring, or December of 2004.
Staging
SCLC has traditionally been classified as either limited stage or extensive stage.8 Limited stage disease is confined to the ipsilateral hemithorax and can be encompassed in a tolerable radiation field. Extensive stage disease extends beyond the ipsilateral hemithorax and may include metastases. The SEER database classifies cancers as localized, regional, distant, or unstaged. Localized and regional stage disease is similar to limited stage, and distant stage corresponds to extensive stage.
Identification of Treatments
In this study, treatment was defined as surgery and/or radiation within the first course of treatment and chemotherapy within the first 6 months following a diagnosis of SCLC. Six months was chosen because chemotherapy is usually administered over a period of 3 to 5 months and begins a short time after diagnosis. Therapeutic and prophylactic cranial irradiation were not assessed because of a lack of data indicating which patients had brain metastases. Whether radiation is given for curative intent or for palliation is not available in the SEER-Medicare database. Chemotherapy data were divided into 4 categories: no chemotherapy; etoposide with carboplatin or cisplatin with or without other agents (EP); cyclophosphamide, doxorubicin, and vincristine with other agents (CAV); and other combinations. The last two groups did not include cisplatin or etoposide.
Comorbidity Data
For outpatient and physician billing claims files, comorbidities appearing in at least 2 different months in the 12 months before diagnosis were included.12 To make the criteria for inclusion of comorbidities more stringent, comorbidities had to appear in both the outpatient and physician billing claims files to be included. All inpatient comorbidities in the 12 months prior to the diagnosis of SCLC were included. A rule out algorithm was used to exclude comorbidity codes accompanying billing codes for procedures, laboratory tests, radiologic tests, or durable medical equipment because they may not have been entered by physicians and may not reflect true diagnoses.14,15
Using the Deyo adaptation, ICD-9-CM codes were converted into comorbidity variables.16 A comorbidity score was calculated for each patient using Cox proportional hazards regression modeling with non-cancer mortality as the outcome.17 Weighting of comorbidities was accomplished by taking the hazard ratio (HR) for each comorbidity and rounding to the nearest integer. The comorbidity score was calculated by adding the comorbid conditions multiplied by their respective weights.15
Statistical Analysis
SCLC cases diagnosed between January of 1992 and December of 2001 were extracted from SEER-Medicare using Statistical Analysis Software (SAS) version 9.1 (SAS Institute, Cary, NC). Selected files in SEER-Medicare were converted from SAS into Stata version 8.2 (StataCorp, College Station, TX) using Stat/Transfer version 9. All analyses were conducted in Stata 8.2.
Patients were stratified according to sex, age at diagnosis, race and ethnicity, stage, comorbidities, treatment with surgery, radiation, or chemotherapy, and specific chemotherapy categories. The probability of receiving chemotherapy was analyzed according to demographic variables, stage, and treatment. The Pearson χ2 test was used for comparisons of proportions.
Logistic regression was used to analyze factors associated with the administration of chemotherapy. Univariate analyses were performed to determine which confounders were statistically significant. Clinically or statistically significant variables were added to the model in a stepwise fashion. Covariates included in the model were sex, age, race, stage, treatment, comorbidity score, SEER registry, and median income by census tract.
Overall survival was the primary endpoint of the study and was calculated from date of diagnosis to date of death or last known date that the patient remained alive. Patients who were not deceased were censored at the date they were last known to be alive based on the date of their most recent contact.
Median survival was calculated for the entire cohort while stratifying on sex, age, race, stage, and treatment. Kaplan-Meier survival curves were generated to demonstrate differences in survival. Evaluating the effects of covariates on survival was performed using Cox proportional hazards regression modeling. Variables that were significant in the univariate analysis or of clinical significance were included in the model. Covariates included were sex, age, race, stage, treatment, comorbidity score, SEER registry, and income. Patients were followed for a minimum of 1 month and a maximum of 156 months. Two-year survival was examined according to year of diagnosis.
Because this is an observational study rather than an RCT, a propensity score was used to approximate a randomized trial using observational data. Propensity scores attempt to equalize confounding factors when evaluating a particular outcome. Patients are matched or stratified according to their likelihood of receiving chemotherapy.18,19 In this study, logistic regression was used to generate a propensity score for each patient, which is the probability of receiving chemotherapy based on the following 8 variables: sex, age, race, stage, treatment with surgery or radiation, comorbidity score, and geographic area. The propensity score was divided into 20 quantiles, from the lowest to the highest, to minimize confounding. Median survival for patients receiving or not receiving each treatment was assessed for each propensity score quantile. The median survivals were then averaged, and the differences between patients receiving and not receiving chemotherapy were examined.
Quantile regression was used to predict median survival based on sex, age, stage, treatment, comorbidity score, and college education. The model provided the survival benefit in months for surgery, radiation, and chemotherapy.
Results
Patient Characteristics and Treatment
The total number of patients who fulfilled the eligibility criteria was 10,428 (Table 1). There were slightly more men (5,337, 51.2%) than women (5,091, 48.8%) with SCLC. Median age for both men and women was 73 years. The average follow-up time for the 10,428 patients in this study was 11 months, and the total number of person-years was 9,592 years. At the time of this study, 97.8% of the patients had died. Of the patients in this study, 25.3% had at least one comorbidity; and the comorbidity score ranged from 0 to 21 points (mean, 0.53 points). Women had a higher comorbidity score than men (p=0.002).
Table 1.
Characteristics and Treatment of Patients with Small Cell Lung Cancer
| Variable | All Patients N (%) | Ages 65 to 74 | Ages 75 and older | p-value |
|---|---|---|---|---|
| Total patients | 10,428 | 6,243 | 4,185 | |
| Median age in years (range) | 73 (65-97) | |||
| Males | 5,337 (51.2%) | 3,204 (51.3%) | 2,133 (51.0%) | 0.72 |
| Females | 5,091 (48.8%) | 3,039 (48.7%) | 2,052 (49.0%) | |
| Race | ||||
| White non-Hispanic | 9,067 (87.0%) | 5,383 (86.3%) | 3,684 (88.0%) | 0.004 |
| White Hispanic | 304 (2.9%) | 188 (3.0%) | 116 (2.8%) | |
| Black | 698 (6.7%) | 463 (7.4%) | 235 (5.6%) | |
| Asian | 321 (3.1%) | 182 (2.9%) | 139 (3.3%) | |
| Native American | 33 (0.3%) | 22 (0.4%) | 11 (0.3%) | |
| SEER Stage | ||||
| Limited | 4,241 (40.7%) | 2,498 (40.0%) | 1,743 (41.7%) | <0.001 |
| Extensive | 5,500 (52.7%) | 3,371 (54.0%) | 2,129 (50.9%) | |
| Unstaged | 687 (6.6%) | 374 (6.0%) | 313 (7.5%) | |
| Comorbidity score | ||||
| 0 | 7,790 (74.7%) | 4,762 (76.3%) | 3,028 (72.4%) | <0.001 |
| 1 | 1,203 (11.5%) | 713 (11.4%) | 490 (11.7%) | |
| 2 | 659 (6.3%) | 361 (5.8%) | 298 (7.1%) | |
| 3 | 381 (3.7%) | 197 (3.2%) | 184 (4.4%) | |
| ≥ 4 | 395 (3.8%) | 210 (3.4%) | 185 (4.4%) | |
| Treatment | ||||
| Any Surgery | 351 (3.4%) | 235 (3.8%) | 116 (2.8%) | 0.02 |
| Any Radiation | 4,077 (39.1%) | 2,739 (43.9%) | 1,338 (32.0%) | <0.001 |
| Any Chemotherapy | 6,994 (67.1%) | 4,613 (73.9%) | 2,381 (56.9%) | <0.001 |
| Treatment | ||||
| No treatment | 2,275 (21.8%) | 1,023 (16.4%) | 1,252 (29.9%) | <0.001 |
| Surgery only | 83 (0.8%) | 44 (0.7%) | 39 (0.9%) | 0.2 |
| Radiation only | 907 (8.7%) | 483 (7.7%) | 424 (10.1%) | <0.001 |
| Chemotherapy only | 3,475 (33.3%) | 2,106 (33.7%) | 1,369 (32.7%) | 0.3 |
| Surgery and Radiation | 29 (0.3%) | 15 (0.2%) | 14 (0.3%) | 0.4 |
| Surgery and Chemotherapy | 136 (1.3%) | 97 (1.6%) | 39 (0.9%) | 0.006 |
| Radiation and Chemotherapy | 3,021 (29.0%) | 2,153 (34.5%) | 868 (20.7%) | <0.001 |
| Surgery, Radiation, and Chemotherapy | 90 (0.9%) | 69 (1.1%) | 21 (0.5%) | 0.001 |
| Unknown if Surgery or Radiation | 412 (4.0%) | 253 (4.1%) | 159 (4.0%) | 0.5 |
| Type of Chemotherapy * | ||||
| No chemotherapy | 3,434 (32.9%) | 1,630 (26.1%) | 1,804 (43.1%) | <0.001 |
| Etoposide with carboplatin or cisplatin (with or without other agents) | 4,337 (41.6%) | 2,898 (46.4%) | 1,439 (34.4%) | <0.001 |
| Cyclophosphamide, doxorubicin, and vincristine (with other agents) | 202 (1.9%) | 134 (2.2%) | 68 (1.6%) | 0.06 |
| Other combinations | 2,455 (23.5%) | 1,581 (25.3%) | 874 (20.9%) | <0.001 |
These are chemotherapeutic agents given at any time in the first 6 months after a diagnosis of SCLC.
Among all patients in the study, 3.4% received surgery, 39.1% were treated with radiation, and 67.1% received chemotherapy. Treatments were divided into 9 categories. The three most common treatment categories were chemotherapy alone (33.3%), radiation and chemotherapy (29.0%), and no treatment (21.8%). This data does not distinguish between chemotherapy and radiation given sequentially versus concurrently. Most chemotherapy regimens included etoposide with cisplatin or carboplatin (EP regimen, 41.6%). The CAV regimen was given less frequently (1.9%).
Univariate Analysis of Chemotherapy
Statistically significant differences in receipt of chemotherapy were seen when patients were stratified by age, race, stage, radiation, comorbidities, and geographic area (Table 2). In this univariate analysis, patients ages 85 and older were less than half as likely to receive chemotherapy as patients ages 65 to 69 (34.7% vs. 76.1%, p<0.001). Blacks were 9% less likely to receive chemotherapy than white non-Hispanics (58.9% vs. 67.8%, p<0.001). Extensive stage patients received chemotherapy less often than limited stage patients (63.2% vs. 72.8%, p<0.001). While there was a difference in the probability of receiving chemotherapy for patients who received radiation versus those who did not receive radiation (76.9 vs. 60.5%, p<0.001), there was no difference between patients who were and were not treated with surgery. Significant differences in receipt of chemotherapy were seen by SEER registry with patients in Seattle, Iowa, and rural Georgia being more likely to receive chemotherapy than patients in San Francisco.
Table 2.
Univariate Analysis and Logistic Regression Model of Factors Associated with Receiving Chemotherapy for Small Cell Lung Cancer
| Factor | Percentage who received chemotherapy, univariate analysis | Odds Ratio, logistic regression | 95% Confidence Interval | p-value |
|---|---|---|---|---|
| Males | 67.0% | reference | ||
| Females | 67.1% | 0.97 | 0.89-1.06 | 0.52 |
| Age group | ||||
| 65 to 69 years | 76.1% | reference | ||
| 70 to 74 years | 71.9% | 0.80 | 0.71-0.90 | <0.001 |
| 75 to 79 years | 63.7% | 0.57 | 0.50-0.64 | <0.001 |
| 80 to 84 years | 50.9% | 0.33 | 0.28-0.38 | <0.001 |
| 85 years and older | 34.7% | 0.17 | 0.14-0.21 | <0.001 |
| Race | ||||
| White non-Hispanic | 67.8% | reference | ||
| White Hispanic | 65.8% | 1.01 | 0.78-1.31 | 0.95 |
| Black | 58.9% | 0.67 | 0.56-0.81 | <0.001 |
| Asian | 64.8% | 1.21 | 0.90-1.63 | 0.20 |
| Native American | 75.8% | 1.45 | 0.64-3.29 | 0.37 |
| Unknown | 60.0% | 0.37 | 0.06-2.45 | 0.30 |
| SEER Stage | ||||
| Limited | 72.8% | reference | ||
| Extensive | 63.2% | 0.61 | 0.55-0.67 | <0.001 |
| Unstaged | 62.3% | 0.68 | 0.49-0.81 | <0.001 |
| Lung cancer surgery | 66.7% | 0.81 | 0.64-1.03 | 0.08 |
| Radiation | 76.9% | 1.88 | 1.71-2.06 | <0.001 |
| Comorbidity score | ||||
| 0 | 69.1% | reference | ||
| 1 | 66.2% | 0.92 | 0.81-1.06 | 0.25 |
| 2 | 61.5% | 0.77 | 0.65-0.91 | 0.003 |
| 3 | 53.0% | 0.56 | 0.45-0.70 | <0.001 |
| ≥ 4 | 53.7% | 0.57 | 0.46-0.70 | <0.001 |
| SEER Registry | ||||
| San Francisco | 59.0% | reference | ||
| Connecticut | 67.3% | 1.44 | 1.15-1.79 | 0.001 |
| Detroit | 66.3% | 1.44 | 1.17-1.78 | 0.001 |
| Hawaii | 59.9% | 1.01 | 0.69-1.48 | 0.97 |
| Iowa | 70.7% | 1.89 | 1.50-2.37 | <0.001 |
| New Mexico | 63.6% | 1.19 | 0.89-1.60 | 0.23 |
| Seattle | 71.9% | 1.87 | 1.48-2.37 | <0.001 |
| Utah | 62.2% | 1.23 | 0.86-1.77 | 0.26 |
| Atlanta | 67.7% | 1.54 | 1.18-2.00 | 0.001 |
| San Jose | 69.2% | 1.57 | 1.16-2.13 | 0.004 |
| Los Angeles | 65.4% | 1.39 | 1.11-1.74 | 0.005 |
| Rural Georgia | 71.4% | 2.02 | 1.01-4.02 | 0.05 |
| Greater California | 66.5% | 1.59 | 1.21-2.08 | 0.001 |
| Kentucky | 67.1% | 1.44 | 1.06-1.95 | 0.02 |
| Louisiana | 67.7% | 1.60 | 1.12-2.27 | 0.01 |
| New Jersey | 66.2% | 1.57 | 1.19-2.08 | 0.001 |
| Median Income by Census Tract in 1990 | ||||
| 1st quartile, < $25,802 | 65.3% | reference | ||
| 2nd quartile, $25,803-$33,881 | 67.0% | 1.03 | 0.91-1.17 | 0.65 |
| 3rd quartile, $33,882-$43,291 | 67.6% | 1.14 | 0.99-1.31 | 0.06 |
| 4th quartile, ≥ $43,292 | 68.8% | 1.24 | 1.07-1.43 | 0.003 |
Multivariate Analysis of Chemotherapy
A multivariate logistic regression model was used to control for confounding variables in the evaluation of which patients received chemotherapy for SCLC (Table 2). Older patients were significantly less likely than younger patients to receive chemotherapy (OR 0.17, 95% CI 0.14-0.21). Black patients were also less likely to receive chemotherapy (OR 0.67, 95% CI 0.56-0.81), as were patients with extensive stage disease (OR 0.61, 95% CI 0.55-0.67). Patients with a higher comorbidity score had a lower odds of receiving chemotherapy. There were no differences between men and women and between patients receiving and not receiving surgery in the likelihood of receiving chemotherapy. Radiation was positively associated with receipt of chemotherapy (OR 1.88, 95% CI 1.71-2.06). Patients in the highest quartile of median household income had a greater odds of receiving chemotherapy than patients in the lowest quartile (OR 1.24, 95% CI 1.07-1.43). An interaction term between age and stage and an interaction term between race and site were not significant.
Referral Patterns
Overall, 83.4% of patients were referred to a hematologist/oncologist. Among those referred, 80.4% received chemotherapy. Older patients, extensive stage patients, and unstaged patients were less likely to be referred to a hematologist/oncologist and less likely to receive chemotherapy if they were seen.
Survival in Univariate Analysis
To examine survival, unadjusted Kaplan-Meier survival curves were generated, stratifying for various covariates (Figure 1). The unadjusted median survival was 7 months for all patients, 7 months for males, and 8 months for females. Median survival decreased with increasing age. For example, patients who were 65-69 had a median survival of 9 months compared to a median survival of 3 months for patients ≥85. Median survival for whites, blacks, and Native Americans was 7 months; while survival for Asians was 8 months. Median survival for limited stage disease was 10 months compared to 5 months for extensive stage disease. In the unadjusted analysis, patients who received chemotherapy and radiation had a longer median survival (11 months) than patients who received chemotherapy alone (9 months) or radiation alone (3 months). Treatment with EP was associated with a longer median survival (11 months) than treatment with other combinations (8 months) or no chemotherapy (2 months).
Figure 1.
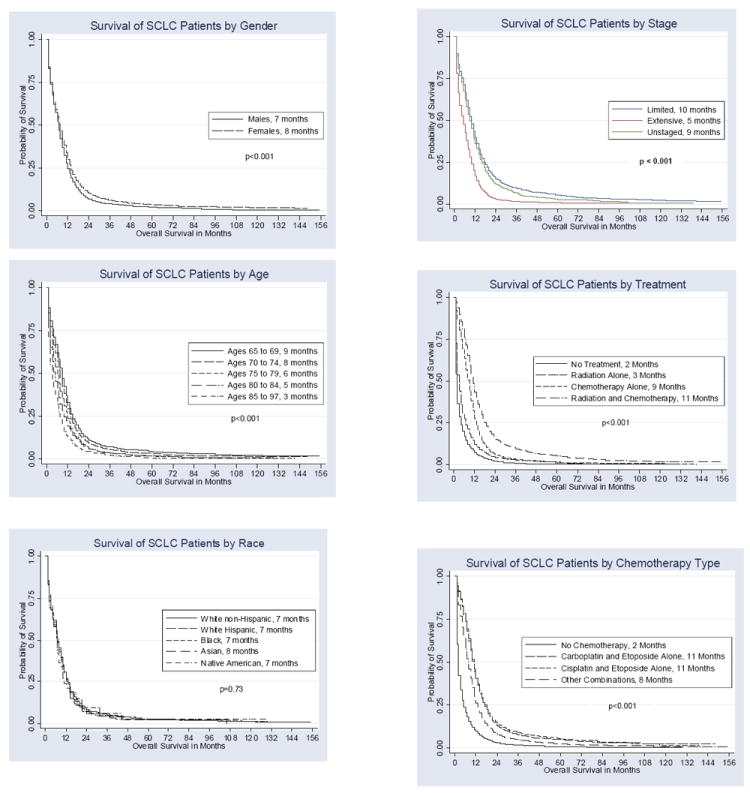
Kaplan-Meier Survival Curves of Overall Survival of Patients with SCLC
Survival in Multivariate Analysis
To adjust for confounding in the assessment of survival, multivariate Cox proportional hazards regression was used. Survival was significantly correlated with sex, age, race, stage, treatment, comorbidity score, and SEER registry (Table 3). Females had a significantly better survival compared to males (HR 0.91, p<0.001). There was a consistent increase in mortality with increasing age with patients ≥85 having a HR of 1.30 (p<0.001) compared with patients ages 65-69. Black patients had a statistically significant decreased risk of dying compared to white non-Hispanic patients (HR 0.88, p=0.001) when controlling for chemotherapy. When chemotherapy was removed from the model (data not shown), survival in blacks and white non-Hispanics was similar (HR for black patients 1.01 [p=0.89]).
Table 3.
Multivariate Cox Proportional Hazards Regression Model of Patients with Small Cell Lung Cancer
| Factor | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Males | reference | ||
| Females | 0.91 | 0.87-0.94 | <0.001 |
| Age group | |||
| 65 to 69 years | reference | ||
| 70 to 74 years | 1.09 | 1.04-1.15 | 0.001 |
| 75 to 79 years | 1.21 | 1.14-1.28 | <0.001 |
| 80 to 84 years | 1.23 | 1.15-1.32 | <0.001 |
| 85 years and older | 1.30 | 1.17-1.44 | <0.001 |
| Race | |||
| White non-Hispanic | reference | ||
| White Hispanic | 1.00 | 0.88-1.12 | 0.96 |
| Black | 0.88 | 0.81-0.96 | 0.003 |
| Asian | 0.99 | 0.87-1.14 | 0.93 |
| Native American | 1.03 | 0.73-1.47 | 0.85 |
| Unknown | 0.89 | 0.33-2.37 | 0.81 |
| SEER Stage | |||
| Limited | reference | ||
| Extensive | 1.78 | 1.70-1.85 | <0.001 |
| Unstaged | 0.93 | 0.86-1.02 | 0.11 |
| Lung cancer surgery | 0.50 | 0.44-0.56 | <0.001 |
| Radiation | 0.78 | 0.75-0.81 | <0.001 |
| Chemotherapy | |||
| No chemotherapy | reference | ||
| Etoposide with carboplatin or cisplatin (with or without other agents) | 0.37 | 0.36-0.39 | <0.001 |
| Cyclophosphamide, doxorubicin, vincristine (with other agents) | 0.46 | 0.40-0.54 | <0.001 |
| Other combinations | 0.47 | 0.44-0.50 | <0.001 |
| Comorbidity score | |||
| 0 | reference | ||
| 1 | 1.10 | 1.04-1.17 | 0.002 |
| 2 | 1.08 | 0.99-1.17 | 0.07 |
| 3 | 1.24 | 1.12-1.38 | <0.001 |
| ≥ 4 | 1.26 | 1.14-1.40 | <0.001 |
| SEER Registry | |||
| San Francisco | reference | ||
| Connecticut | 1.04 | 0.94-1.15 | 0.47 |
| Detroit | 1.12 | 1.01-1.23 | 0.03 |
| Hawaii | 0.91 | 0.76-1.09 | 0.32 |
| Iowa | 1.02 | 0.92-1.14 | 0.67 |
| New Mexico | 1.02 | 0.89-1.17 | 0.76 |
| Seattle | 1.03 | 0.93-1.15 | 0.54 |
| Utah | 1.28 | 1.08-1.51 | 0.005 |
| Atlanta | 1.05 | 0.93-1.18 | 0.47 |
| San Jose | 1.02 | 0.89-1.18 | 0.74 |
| Los Angeles | 1.12 | 1.01-1.24 | 0.04 |
| Rural Georgia | 1.11 | 0.82-1.50 | 0.51 |
| Greater California | 1.09 | 0.95-1.23 | 0.21 |
| Kentucky | 1.17 | 1.02-1.34 | 0.03 |
| Louisiana | 1.35 | 1.15-1.59 | <0.001 |
| New Jersey | 1.09 | 0.95-1.23 | 0.21 |
| Median Income by Census Tract in 1990 | |||
| 1st quartile, < $25,802 | reference | ||
| 2nd quartile, $25,803-$33,881 | 1.02 | 0.97-1.08 | 0.44 |
| 3rd quartile, $33,882-$43,291 | 0.99 | 0.93-1.05 | 0.74 |
| 4th quartile, ≥ $43,292 | 1.04 | 0.97-1.11 | 0.25 |
Extensive stage patients had an inferior survival compared to patients with limited disease (HR 1.78, p<0.001). Patients who underwent surgical resection had improved survival (HR 0.50, p<0.001) as did patients who received radiation (HR 0.78, p<0.001). Chemotherapy with EP conferred a significantly improved survival compared to no chemotherapy (HR 0.37, p<0.001). Chemotherapy with CAV was associated with a HR of 0.47 (p<0.001). The 95% confidence intervals for EP and CAV did not overlap, suggesting that EP is a superior regimen. When all chemotherapy categories were combined, the HR in the multivariate model was 0.40 (p<0.001, data not shown). Small differences in survival were seen among various SEER regions, but not by income. Not surprisingly, having a higher comorbidity score was associated with a higher mortality. A sensitivity analysis employing less stringent criteria to assess comorbidities (such that comorbid conditions were counted if they appeared twice anywhere in the billing records) did not significantly change the model (data not shown). Two-year survival for SCLC did not improve from 1992 to 2001 and ranged from 7.1% to 9.6%.
Propensity Score
To determine the benefit of chemotherapy administration while controlling for confounding variables, a propensity score was used. This was derived from a logistic regression model with chemotherapy versus no chemotherapy as the outcome. The median survival for patients receiving no chemotherapy ranged from 2 to 8 months across the 20 quantiles. The median survival for patients who did receive chemotherapy ranged from 6 to 15 months across 20 quantiles. The unweighted averages of the median survivals for patients not receiving chemotherapy were compared to the unweighted averages of the median survivals for the chemotherapy patients (Table 4).
Table 4.
Differences in Median Survival in Months for Patients Receiving Chemotherapy Versus No Chemotherapy Using Propensity Scores
| No Chemotherapy | Chemotherapy | Difference in Survival | |
|---|---|---|---|
| All Patients | 2.6 | 9.5 | 6.9 |
| Males | 2.8 | 9.1 | 6.3 |
| Females | 2.4 | 10.5 | 8.1 |
| Age Group | |||
| 65 to 69 years | 2.8 | 9.9 | 7.1 |
| 70 to 74 years | 2.6 | 9.9 | 7.3 |
| 75 to 79 years | 3.0 | 9.4 | 6.4 |
| 80 to 84 years | 4.8 | 10.3 | 5.5 |
| 85 years and older | 2.9 | 9.3 | 6.4 |
| Race | |||
| White non-Hispanic | 2.5 | 9.5 | 7.0 |
| White Hispanic | 3.5 | 9.0 | 5.5 |
| Black | 3.8 | 10.1 | 6.2 |
| Asian | 4.1 | 9.7 | 5.6 |
| SEER Stage | |||
| Limited | 3.9 | 10.7 | 6.9 |
| Extensive | 3.2 | 10.4 | 7.2 |
| Unstaged | 6.2 | 12 | 5.8 |
Median survival for patients not receiving chemotherapy was 2.6 months compared to 9.5 months for those receiving chemotherapy. Accordingly, receiving chemotherapy improved median survival by an average of 6.9 months after controlling for known confounders. For males, the benefit of chemotherapy was 6.3 months, while for females, it was 8.1 months. Chemotherapy conferred a benefit in survival for all age groups, races, and stages, with an improvement in median survival ranging from 5.5 to 8.1 months. Even patients ages 85 and older derived a similar benefit from chemotherapy compared to patients 65 to 69 years old.
Quantile Regression
Multivariate quantile regression was used to develop a model for the prediction of median survival in elderly patients with SCLC (Table 5). Factors associated with an improved median survival included being female (+0.37 months, 95% CI, +0.26 to +0.49), receiving treatment of any kind, and living in an area where a relatively high proportion of people have a college degree. Undergoing surgery conferred a survival benefit of 5.40 months (95% CI, +5.07 to +5.72), radiation 1.47 months (95% CI, +1.30 to +1.64), and chemotherapy 6.45 months (95% CI, +6.32 to +6.58).
Table 5.
Quantile Regression of Median Survival in Months for SCLC
| Variable | Median Survival in Months | 95% Confidence Interval | p-value |
|---|---|---|---|
| Constant | +4.44 | +3.67 to +5.22 | <0.001 |
| Males | reference | ||
| Females | +0.37 | +0.26 to +0.49 | <0.001 |
| Age Group | |||
| 65 to 69 years | reference | ||
| 70 to 74 years | -0.53 | -0.70 to -0.37 | <0.001 |
| 75 to 79 years | -0.73 | -0.95 to -0.52 | <0.001 |
| 80 to 84 years | -0.76 | -1.07 to -0.46 | <0.001 |
| 85 years and older | -1.40 | -1.85 to -0.94 | <0.001 |
| SEER Stage | |||
| Limited | reference | ||
| Extensive | -2.65 | -2.80 to -2.50 | <0.001 |
| Unstaged | +0.37 | +0.11 to +0.63 | 0.005 |
| Surgery | +5.40 | +5.07 to +5.72 | <0.001 |
| Radiation | +1.47 | +1.30 to +1.64 | <0.001 |
| Chemotherapy | +6.45 | +6.32 to +6.58 | <0.001 |
| Comorbidity Score | |||
| 0 | reference | ||
| 1 | -0.17 | -0.36 to +0.01 | 0.07 |
| 2 | -0.35 | -0.60 to -0.11 | 0.005 |
| 3 | -0.84 | -1.16 to -0.51 | <0.001 |
| 4 or greater | -0.88 | -1.21 to -0.55 | <0.001 |
| College education* | +0.29 | +0.13 to +0.45 | <0.001 |
| Propensity score | -0.14 | -1.10 to +0.82 | 0.78 |
At least 29% of people with a college degree by census tract
Variables associated with a worse survival included having extensive stage disease (-2.65 months, 95% CI, -2.80 to -2.50) and a higher comorbidity score. Unlike the results of our Cox regression model, in the quantile regression model, race was not significantly associated with survival and was dropped from the model. Separate quantile regression models for males and females showed that chemotherapy improved survival by 6 months for males and 7.5 months for females, confirming the results of our propensity score analysis. When an interaction term for sex and chemotherapy was added to the quantile regression model, it was significant (p<0.001), indicating an enhanced benefit of chemotherapy for women compared to men (data not shown).
This quantile regression model can be used to estimate survival after diagnosis. For example, a 65-year-old male with extensive stage SCLC has a comorbidity score of 0 and receives chemotherapy alone. His predicted survival according to this model is 8.2 months. Using the propensity score and quantile regression techniques yielded similar results with chemotherapy benefiting males and females and all ages, races, and stages.
Discussion
This retrospective cohort study was designed to examine factors associated with chemotherapy administration among elderly patients with SCLC in the community and to quantify the survival benefit. To our knowledge, this is the largest published study of SCLC. Among 10,428 patients ages 65 and older who were diagnosed with SCLC between 1992 and 2001 in this study, 67.1% received chemotherapy, most of whom received EP (41.6%).
Receipt of chemotherapy was inversely correlated with age; patients in the oldest age group were less than half as likely to receive chemotherapy as patients in the youngest age group. Earle et al. demonstrated that with each incremental decade of life, the OR for receiving chemotherapy for metastatic NSCLC was 0.46 (95% CI 0.41-0.51).20 Black patients in this study were less likely to be treated with chemotherapy compared to non-Hispanic whites in the univariate analysis and the multivariate analysis. A racial difference in chemotherapy was also seen in the study by Earle et al., where the OR for receiving chemotherapy was 0.70 (95% CI 0.55-0.88) for black patients with NSCLC compared to non-Hispanic white patients. In our study, black patients were less likely to be referred to a hematologist/oncologist, but were equally likely to receive chemotherapy if referred.
A higher comorbidity score was associated with a decreased likelihood of receiving chemotherapy. A higher comorbidity score may indicate worse performance status; however, information about performance status was not available. Even income was positively correlated with chemotherapy administration, despite all patients in this study having the same insurance. Note, however, that the income provided is by census tract and not for individual patients.
As illustrated in the multivariate Cox regression model, chemotherapy had a significant impact on survival with HR’s of 0.37 to 0.47. Chemotherapy with EP offered the most dramatic risk reduction (HR 0.37). The CAV regimen and other combinations were associated with a HR of 0.47. These HR’s are far superior to HR’s reported for metastatic NSCLC patients who receive chemotherapy (0.78 to 0.85).21 This reflects the high responsiveness that SCLC has to chemotherapy. Nonetheless, the vast majority of patients relapse, as reflected by the poor median survival in this cohort.
In our multivariate survival model, women had better survival than men after controlling for other factors. This trend has also been seen in NSCLC with HR’s ranging from 0.78 to 0.91 in one study and in lung cancer with all histologies in another SEER study (relative 5-year survival 17.3% for females vs. 13.8% for males).22,23 Improved survival for women may reflect genetic and hormonal differences in women.24
The unadjusted median survival for patients in this study was only 7 months compared to 17 months for limited stage patients and 8.9 months for extensive stage patients in the control arms of RCTs.6,7 Inferior survival in our study likely reflects older age and greater comorbidities of this cohort compared to patients included in RCTs.
Many RCTs in SCLC compare different chemotherapy regimens, rather than comparing chemotherapy to BSC. The first data supporting a role for chemotherapy in SCLC was based upon a 1969 report of an RCT conducted by the Veterans Administration Lung Cancer Study Group.25 Among those receiving cyclophosphamide, median survival was >4 months compared to <2 months among placebo treated patients (p=0.0005). A systematic review of RCTs comparing chemotherapy to BSC in extensive SCLC concluded that chemotherapy conferred a survival advantage of 2.6 months.26 Despite limited data from randomized trials that chemotherapy is superior to BSC in SCLC, combination chemotherapy has been recognized as having a pivotal role in SCLC management since the early 1970’s.6,7
Chemotherapy was demonstrated to improve survival for all age groups, races, and stages. Females derived a greater survival benefit from chemotherapy than males. While older patients, black patients, and patients with extensive stage disease were less likely to be treated with chemotherapy, they all derived a significant benefit from the administration of chemotherapy. Improvement in survival associated with chemotherapy was 6.9 months using the propensity score analysis and 6.5 months using quantile regression. Quantile regression can be used to predict prognosis for elderly patients with SCLC. This model has been shown to have internal validity but has not been tested utilizing an independent data source.
The main limitation of this study is that it is an observational rather than a randomized trial. Despite the use of rigorous statistical methods to control for confounders, residual confounding may still remain. Other limitations include the lack of information about smoking and brain metastases, which precluded an analysis of prophylactic cranial irradiation. The results of this study are generalizable for patients ages 65 and older with SCLC because the data are taken from geographical areas representing 26% of the U.S. population and because Medicare captures 97% of elderly patients.
In conclusion, our study demonstrates that treatment with chemotherapy is associated with a statistically significant greater than 6-month improvement in median survival among elderly patients with SCLC. One third of elderly patients with SCLC never receive chemotherapy, and one sixth are never referred to a medical oncologist, suggesting that chemotherapy is underutilized. This large retrospective cohort study is the first study to quantify the survival benefit of chemotherapy for SCLC in the community. Despite methodological limitations, our study provides strong evidence that chemotherapy improves survival in elderly patients with SCLC.
Acknowledgments
We thank Thomas A. Trikalinos, MD, PhD, at the Tufts Medical Center Institute for Clinical Research and Health Policy Studies for his excellent statistical support during this project.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2. [2008];Surveillance, Epidemiology, and End Results (SEER) Program, November 2006 submission (1973-2004), SEER*Stat 6.3.5 program. Available from: http://www.seer.cancer.gov.
- 3.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results database. [March 5, 2013];SEER Cancer Statistics Review. 1975-2009 Available from: http://seer.cancer.gov/csr/1975_2009_pops09/results_single/sect_15_table.12.pdf.
- 5.Surveillance, Epidemiology, and End Results database. [March 5, 2013];SEER Cancer Statistics Review. 1975-2009 Available from: http://seer.cancer.gov/csr/1975_2009_pops09/results_single/sect_15_table.13.pdf.
- 6.Jänne PA, Freidlin B, Saxman S, et al. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer. 2002;95:1528–1538. doi: 10.1002/cncr.10841. [DOI] [PubMed] [Google Scholar]
- 7.Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol. 1999;17:1794–1801. doi: 10.1200/JCO.1999.17.6.1794. [DOI] [PubMed] [Google Scholar]
- 8.Johnson BE, Crawford J, Downey RJ, et al. Small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:602–622. doi: 10.6004/jnccn.2006.0050. [DOI] [PubMed] [Google Scholar]
- 9.Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 10.Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 11.Gross CP, Herrin J, Wong N, Krumholz HM. Enrolling older persons in cancer trials: The effect of sociodemographic, protocol, and recruitment center characteristics. J Clin Oncol. 2005;23:4755–4763. doi: 10.1200/JCO.2005.14.365. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey SD, Howlander N, Etzioni RD, et al. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from Surveillance, Epidemiology, and End Results-Medicare. J Clin Oncol. 2004;22:4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 13.SEER-Medicare Linked Database. [2008]; Available at: http://healthservices.cancer.gov/seermedicare/
- 14.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 19.D’Agostino RB. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statist Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Earle CC, Vendetti LN, Newmann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117:1239–1246. doi: 10.1378/chest.117.5.1239. [DOI] [PubMed] [Google Scholar]
- 21.Earle CC, Tsai JS, Gelbert RD, et al. Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol. 2001;19:1064–1070. doi: 10.1200/JCO.2001.19.4.1064. [DOI] [PubMed] [Google Scholar]
- 22.Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol. 2007;25:1705–1712. doi: 10.1200/JCO.2006.08.1455. [DOI] [PubMed] [Google Scholar]
- 23.Fu JB, Kau TY, Severson RK, et al. Lung cancer in women: analysis of the national Surveillance, Epidemiology, and End Results database. Chest. 2005;127:768–777. doi: 10.1378/chest.127.3.768. [DOI] [PubMed] [Google Scholar]
- 24.Patel JD. Lung cancer in women. J Clin Oncol. 2005;23:3212–3218. doi: 10.1200/JCO.2005.11.486. [DOI] [PubMed] [Google Scholar]
- 25.Green RA, Humphrey E, Close H, Patno ME. Alkylating agents in bronchogenic carcinoma. American Journal of Medicine. 1969;46:516–525. doi: 10.1016/0002-9343(69)90071-0. [DOI] [PubMed] [Google Scholar]
- 26.Agra Y, Pelayo M, Sacristan M, et al. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Sys Rev. 2003 doi: 10.1002/14651858.CD001990. CD001990. [DOI] [PubMed] [Google Scholar]


