Abstract
This study investigated the age-dependent injury response of diffuse traumatic axonal injury (TAI) and regional subdural and subarachnoid intracranial hemorrhage (ICH) in two pediatric age groups using a porcine head injury model. Fifty-five 5-day-old and 40 four-week-old piglets—which developmentally correspond to infants and toddlers, respectively—underwent either a sham injury or a single rapid non-impact rotational injury in the sagittal plane and were grouped by post-TBI survival time (sham, 3–8 h, one day, 3–4 days, and 5–6 days). Both age groups exhibited similar initial levels of ICH and a significant reduction of ICH over time (p<0.0001). However, ICH took longer to resolve in the five-day-old age group. At 5–6 days post-injury, ICH in the cerebrum had returned to sham levels in the four-week-old piglets, while the five-day-olds still had significantly elevated cerebral ICH (p=0.012). Both ages also exhibited similar resolution of axonal injury with a peak in TAI at one day post-injury (p<0.03) and significantly elevated levels even at 5–6 days after the injury (p<0.008), which suggests a window of vulnerability to a second insult at one day post-injury that may extend for a prolonged period of time. However, five-day-old piglets had significantly more TAI than four-week-olds overall (p=0.016), which presents some evidence for an increased vulnerability to brain injury in this age group. These results provide insight into an optimal window for clinical intervention, the period of increased susceptibility to a second injury, and an age dependency in brain injury tolerance within the pediatric population.
Key words: : axonal injury; hemorrhage; pediatric traumatic brain injury; swine, time course
Introduction
In the United States, 1.7 million traumatic brain injuries (TBIs) occur each year, contributing to a third of all injury-related deaths, and making TBI a leading cause of death in children. Furthermore, children under the age of four have the highest rate of TBI-related emergency department visits, with over 1200 per 100,000 children.1,2
TBI is characterized by an initial mechanical trauma, during which inertial forces induce strains on the tissue, resulting in shearing of axons, tissue tears, and vascular disruption.3,4 This insult triggers a cascade of pathophysiological events, including metabolic disturbances, excitotoxicity, reactive oxygen species generation, and ischemia, which produce secondary necrosis and apoptosis in the already injured brain.4,5 These secondary responses emerge over the course of minutes to days after injury.5 Preventing deterioration due to this secondary cascade in patients with seemingly minor injuries results in the largest reduction in pediatric TBI mortality rates. 6,7 Thus, understanding the time course of secondary injury could be crucial in the development and delivery of interventions for pediatric TBI.
It is well known that children have a distinct response to TBI compared with adults, and that there is an age-dependent injury response within the pediatric population.3,8–19 During the first four years of life, numerous cerebral developmental changes occur, which may contribute to these differences within the pediatric population, including continued myelination, changes in cerebrovascular tone, and increases in both brain size and neurotransmitter density.20–22 Experimental studies of the age-dependent response to a scaled cortical impact found larger lesion volumes at seven days and one month post-TBI in four-week-old piglets, compared with five-day-old piglets. In addition, these animals displayed a differing evolution of secondary injury where five-day-old piglets had earlier peak lesion volumes that resolved more quickly than four-week-old piglets.18,19 These studies in focal brain injury demonstrate that the time course of secondary injury can be influenced by age at the time of injury.
The objective of this study was to complement and broaden our understanding by investigating the time course of diffuse traumatic axonal injury (TAI) and intracranial hemorrhage (ICH) in two pediatric age groups corresponding to human infants and toddlers using a porcine head injury model. This rapid non-impact rotational model previously has been shown to produce widespread TAI and ICH in piglets.9,17,23 The gyrencephalic piglet brain is similar to the human brain in growth patterns, gray and white matter distribution, and cerebrovascular anatomy and physiology, making it a good translational model.24–27 We sought to determine when TAI and ICH reach their peak post-injury, how long they remain elevated, and if their progression is age dependent; provide insight into the optimal window for clinical intervention and the period of increased vulnerability to a second injury; and glean further information about age-specific vulnerabilities that have been observed.17–19
Methods
We performed a retrospective study of piglet data from the Margulies laboratory animal database from June 2008 to September 2012. This date range was selected to keep immunohistochemistry methods consistent across all animals. We evaluated female five-day-old and four-week-old piglets, of which the brain development corresponds to the human infant and the human toddler, respectively.24,25 Animals met the inclusion criteria if they: 1) experienced no injury or a single rapid non-impact rotational injury (RNR) in the sagittal plane; 2) were not given any treatments; 3) were sacrificed for neuropathology; and 4) had post-TBI survival times of 3 h to six days. This criteria included 55 five-day-old piglets and 40 four-week-old piglets. Each piglet age group was further divided into five post-TBI survival time groups (sham, 3–8 h, one day, 3–4 days, and 5–6 days), yielding ten groups total with four to 15 animals per group (Table 1). The 3–8 h survival time group consisted entirely of animals on protocols with longer intended survival times but instead had to be euthanized early due to difficulty with recovery from the injury.
Table 1.
Measured Peak Angular Velocity and Mean Peak Angular Acceleration
| 5-day-old | 4-week-old | 4-week-old scaled | ||||||
|---|---|---|---|---|---|---|---|---|
| Survival Time | n | Peak velocity (rad/s) | Mean acceleration (krad/s2) | n | Peak velocity (rad/s) | Mean acceleration (krad/s2) | Peak velocity (rad/s) | Mean acceleration (krad/s2) |
| Sham | 14 | 0.0±0.0 | 0.0±0.0 | 8 | 0.0±0.0 | 0.0±0.0 | - | - |
| 3–8 h | 7 | 150.3±2.4 | 53.4±6.6 | 7 | 127.1±1.5b | 63.2±6.8 | 145.4±2.1 | 83.7±9.0c |
| 1 day | 14 | 150.4±0.7 | 55.3±1.7 | 15 | 127.5±0.8b | 48.5±1.7 | 146.8±0.9 | 64.3±2.3 |
| 3–4 days | 6 | 146.1±0.9 | 77.0±4.4a | 4 | 127.6±1.5b | 45.7±3.5b | 145.9±1.7 | 60.5±2.3 |
| 5–6 days | 14 | 147.3±2.2 | 59.4±4.2 | 6 | 123.9±1.9b | 42.9±2.6 | 141.6±2.2 | 56.8±3.4 |
| RNR average | 148.7±0.9 | 59.5±2.3 | 126.8±0.6b | 50.3±2.1b | 144.9±0.7b | 65.7±2.8 | ||
Significantly higher than infant 3–8 h and 1 day groups (p<0.05).
Significantly lower than corresponding 5-day-old group (p<0.01).
Significantly higher than scaled toddler 1 day and 5–6 day groups (p<0.05) and significantly higher than corresponding 5-day-old group (p=0.002).
All values are mean±standard error of mean.
RNR, rapid non-impact rotational injury.
Animal preparation
All protocols from the collected studies were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Induction of anesthesia was performed with inhaled 4% isoflurane via snout mask. Four-week-old piglets also were given an intramuscular injection of ketamine (20 mg/kg IM) and xylazine (2 mg/kg IM) prior to inhalation of isoflurane. Anesthesia was maintained with 1.25–3.25% isoflurane. Using a heating pad, core body temperature was kept constant between 36 and 38°C and monitored via rectal probe. Vital signs, oxygen saturation, heart rate, respiratory rate, and end tidal carbon dioxide (CO2) were recorded every 15 min throughout the experiment (Surgivet v9240, Smiths Medical, Dublin, OH [five-day-old]; VetCap model 2050081, SDI, Waukesha, WI [four-week-old]). After the proper depth of surgical anesthesia was reached as determined by a lack of foot withdrawal to a mild toe pinch, the piglets were orally intubated with a 3.5-mm (five-day-old) or 4.5-mm (four-week-old) endotracheal tube. Mechanical ventilation was utilized to maintain normoxia and normocarbia in the piglets as needed. Buprenorphine (0.02 mg/kg) was delivered intramuscularly for analgesia within an hour prior to RNR.
Rapid non-impact rotational injury
TBI was induced using an established diffuse TBI piglet model.9,17,28 Prior to RNR, the piglets were secured to a bite plate by a snout strap and isoflurane was withdrawn. Injury groups were subject to a single RNR in the sagittal plane with the center of rotation at the cervical spine via a pneumatic actuator. During the injury, the head rapidly rotates in a ventral-to-dorsal direction through a 58° arc. The rotational velocity of the injury device was measured by an angular rate sensor (Applied Technology Associates, Albuquerque, NM) and sampled at 10,000 Hz using a data acquisition system (National Instruments, Austin, TX). Angular acceleration was calculated by differentiating the angular velocity trace. Mean peak acceleration was obtained by averaging the peak acceleration and peak deceleration for each animal. Uninjured sham animals were secured to the injury apparatus, but the apparatus was not activated.
Immediately after the RNR or sham, animals were removed from the injury device and recovered from anesthesia. The endotracheal tube was removed when animals met the following criteria: return of pinch reflex; spontaneously breathing and able to maintain oxygenation and ventilation; normotensive; and stable heart and respiratory rates. After extubation, animals were returned to the animal housing facility when they met the following criteria: vocalization without squealing, steady ambulation, no aggression or avoidance behavior, no piloerection, and proper feeding and drinking behaviors. Piglets who did not meet the criteria for either extubation or return to the animal care facility were euthanized on the day of injury. These animals that were sacrificed early due to adverse response to TBI made up the entire 3–8 hour survival time group. Piglets with 1–6 day survival times were housed in the animal husbandry facility until the targeted post-TBI survival time point.
Euthanasia
A subset of RNR five-day-old piglets (n=23) had microdialysis and thermal diffusion intracranial monitoring probes placed in the right and left frontal white matter during the time immediately before sacrifice for other outcome measures of interest in those experimental protocols.29 This subset received an additional injection of buprenorphine (0.02 mg/kg) on the day of sacrifice.
Animals included in this study were on one of two different post-TBI sacrifice day anesthetic protocols. On the designated post-TBI survival time point, animals were anesthetized with 1% isoflurane (n=74) or a continuous intravenous infusion of ketamine (16mg/kg/hr) and midazolam (0.8mg/kg/h; n=21). Piglets infused with ketamine and midazolam had peripheral intravenous lines placed while on isoflurane and then given a bolus dose of ketamine (10 mg/kg) and midazolam (0.25 mg/kg) intravenously. Animals maintained with ketamine and midazolam infusions were weaned off isoflurane over a 15 min period. Ketamine and midazolam infusions were titrated by increasing the dose by 2 mg/kg/h and 0.1 mg/kg/h, respectively, until the ideal level of anesthesia was reached. All piglets were sacrificed by pentobarbital overdose. The brains were perfusion-fixed and carefully removed from the cranium. Digital photographs were taken of the left, right, superior, and inferior surfaces of the cerebrum, and brains were weighed.
Intracranial hemorrhage
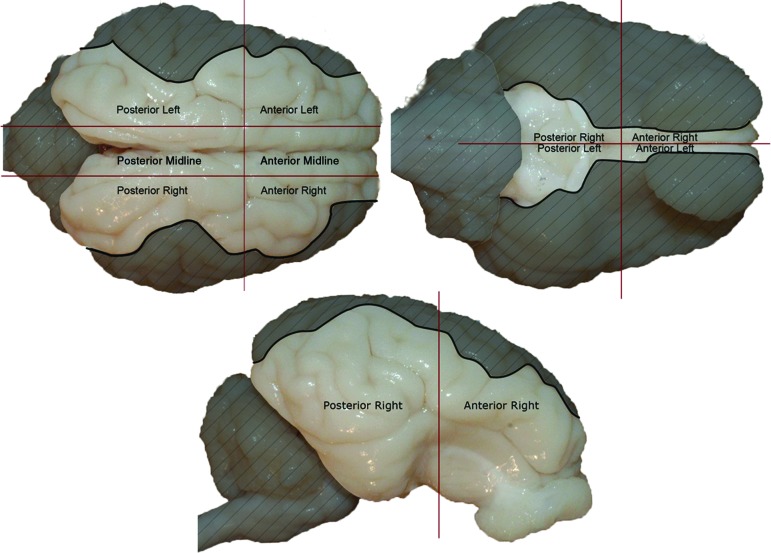
Regional ICH was quantified using a previously developed protocol.23 Briefly, the digital photographs of the brain surface were imported into Adobe Photoshop (Adobe Systems, San Jose, CA) and the cerebrum was divided into six regions (anterior left, anterior right, posterior left, posterior right, anterior midline, posterior midline) based on anatomical markers (Fig. 1). The percentage of ICH was calculated for each of these regions by dividing the number of pixels containing blood (as classified by pixel color) by the number of total pixels in a region. The areas scored in each view of the brain were carefully selected so that a particular area was not counted more than once if represented in multiple views. The overall ICH percentage for the whole cerebrum also was calculated for each animal. All percentages were rounded to the nearest whole number. These ICH values account for subdural and subarachnoid hemorrhage. Because we used a non-impact TBI model, we did not expect epidural hemorrhage to form after injury.30
FIG. 1.
Using anatomical markers, digital photographs of piglet brains were divided into six regions for quantification of intracranial hemorrhage (ICH) percentage scores. The brain regions (anterior left, anterior right, posterior left, posterior right, anterior midline, posterior midline) and the whole cerebrum for each animal were examined. Grey areas in the image were not included in the quantification of ICH for the particular view shown to prevent an overlap in regional calculations.
To account for any potential ICH that may have been caused by insertion of the intracranial monitoring probes, we acquired the ICH percentages of four sham five-day-old piglets that also had microdialysis and thermal diffusion probes inserted into the brain. The average sham percentages for each region and the whole cerebrum were subtracted from the corresponding percentages of the RNR piglets with intracranial monitoring. These four sham animals were not included in the overall sham group for ICH comparisons.
Immunohistochemistry
After the digital photographs were taken, the brains were placed in 10% formalin for one week and then stored in 1×phosphate buffered saline. The fixed brains were sectioned into 3-mm thick coronal slices (15–18 slices total), photographed, and embedded in paraffin wax. Subsequently, 6-μm slices were cut from each section and immunostained with β-amyloid precursor protein (β-APP; Chemicon 22C11 used at dilution of 1:5000), and lightly counterstained with Mayer's hematoxylin. A single blinded neuropathologist examined the slices for TAI, which was defined as an accumulation in β-APP. Areas of injury were marked on the photographs of the coronal sections. Using Adobe Photoshop, each coronal slice was traced and the total tissue area measured, then each marked region of TAI was traced and measured. The total percentage of TAI was calculated as the area of TAI divided by the total brain area. TAI data for a subset of the five-day-old piglets were previously published by Kilbaugh and colleagues (n=10) and Sullivan and colleagues (n=15).29,31
The presence of intracranial monitoring probes in the aforementioned group of four sham five-day-old piglets was not associated with positive β-APP staining. Therefore, unlike ICH, there was no need to adjust the percentage of injury for RNR animals that had probes inserted. These sham animals were included in all subsequent analyses of axonal injury.
Scaled kinematic loading conditions
In order to compare injury outcomes between ages and post-TBI sacrifice time groups, we needed to evaluate whether each group received comparable head rotation kinematics. For velocity, the brain mass scaling principle proposes that similar injury patterns will result between two animals whose angular velocity is related by the one-third power of the inverse ratio of their brain masses.17,32 Thus, higher rotational velocities applied to the smaller five-day-old piglet brain should result in a similar injury as lower rotational velocities on the larger four-week-old brain. Accordingly, we utilized the brain mass scaling equation (Eqn. 1) to scale the four-week-old velocity values to the corresponding five-day-old velocity level, assuming geometry and material properties of the brains are the same:
 |
where ω5d and M5d are the angular velocity and brain mass, respectively, of the 5-day-old piglet, and where ω4wk and M4wk are the angular velocity and brain mass, respectively, of the 4-week-old piglet.
We similarly scaled the acceleration loading conditions utilizing the angular acceleration brain mass-scaling equation (Eq. 2), using the inverse ratio of the brain masses raised to the two-thirds power. As with velocity, this relationship implies that higher angular accelerations are needed in the 5-day-old to achieve the same injury severity as a 4-week-old.
 |
where θ5d and θ4wk are the angular acceleration of the 5-day-old piglet and the 4-week-old piglet respectively. For both scaling equations, the average brain mass values used for the two age groups were M5d = 41.6 g (n = 7) and M4wk = 62.1 g (n = 7). These values were taken from the 3–8 hour survival groups to achieve the brain mass values at the time of head rotation.
Statistical analysis
Piglets were divided by age and then into 3–8 h, one day, 3–4 day, and 5–6 day survival time groups, and a two-way analysis of variance (ANOVA) was performed to assess how the velocity and acceleration loading conditions varied with animal age and survival time. Additional two-way ANOVAs were then performed to evaluate the effects of survival time, animal age, and their interaction on regional ICH and TAI. Post hoc group comparisons were examined using the Tukey-Kramer method. Regional ICH and TAI values for each survival time group also were compared to age-matched sham groups using a Student's t-test. Significance was defined as p<0.05, and all results are reported as mean±standard error of the mean.
Results
For the purposes of comparing across ages and survival times, it was important for each group in this dataset to have experienced comparable initial injury severity. The mass scaling principle proposes that a smaller brain will require a larger angular velocity and acceleration to result in the same injury severity as a larger brain. In this retrospective dataset, an ANOVA shows that, as desired, angular velocities and accelerations were significantly higher in the five-day-old than the four-week-old (p=0.0005 and p<0.0001, respectively). The average peak angular velocity was 148.7 rad/s for five-day-olds, compared with 126.8 rad/s for four-week-olds, and the average mean angular acceleration decreased from 59.5 krad/s2 for five-day-olds to 50.3 krad/s2 for four-week-olds (Table 1).
The next step in ensuring that groups received a similar injury load is to compare the five-day-old kinematics to four-week-old kinematics that have been scaled to the five-day-old level using the mass scaling principle (Table 1). For peak angular velocities, the two-way ANOVA showed that there was an overall age effect with the five-day-old angular velocities (148.7±0.9 rad/s) significantly higher than the four-week-old values (144.9±0.7 rad/s) even after scaling (p=0.0047). However, the post hoc test showed no statistical difference between five-day-olds and four-week-olds at each survival time point. For mean peak angular accelerations, the ANOVA showed no significant age effect, and the post hoc testing revealed that four-week-old scaled angular accelerations were equivalent to the applied five-day-old angular accelerations with the exception of the four-week-old 3–8 h survival time group. This four-week-old group had significantly higher angular accelerations than the corresponding five-day-old survival group (p=0.002). Overall, these results demonstrate that both age groups received comparable kinematic loading conditions by the brain mass scaling principle.
Lastly, because we made comparisons within age groups to evaluate the influence of survival time on injury severity, we used the post hoc testing to evaluate if there were loading condition differences between survival time groups within each age. There were no differences in peak angular velocity by survival time for both ages. However, for mean peak angular acceleration, there was one survival time group for each age that had significantly higher accelerations than two other survival time groups in that age. For five-day-olds, the 3–4 day survival time group had significantly higher mean angular accelerations than the 3–8 h (p=0.0347) and one day (p=0.0224) survival time groups. For four-week-olds, the 3–8 h survival time group had significantly higher mean angular accelerations than the groups that survived one day (p=0.0394) and 5–6 days (p=0.0109). Overall, kinematic loading conditions were similar across survival time within each age group, but care should be exercised when interpreting results involving the five-day-old 3–4 day survival group and the four-week-old 3–8 h survival group, as these may have experienced slightly higher injury levels.
Intracranial hemorrhage
ICH was not observed in 18 of the 22 sham animals; the exceptions were the four 5-day-old piglets with intracranial monitoring probes. In these four invasively-instrumented animals, ICH was present in the anterior and posterior midline, anterior right and left, and posterior left regions, and averaged 3±2%, 6±6%, 1±1%, 2±1%, and 1±1%, respectively. As a fraction of the total cerebrum, the average ICH attributable to the monitoring probes was 1±1%. These shams were used to subtract the influence of the probes from the ICH values obtained in the 23 injured five-day-old piglets that had invasive instrumentation and were not included in any further analysis.
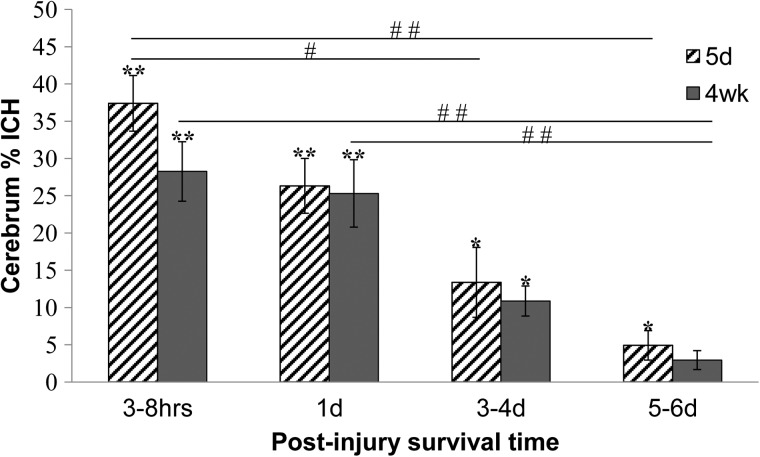
When the two animal age groups were combined, the two-way ANOVA revealed that each region and the whole cerebrum demonstrated a significant survival time effect (p<0.0001). ICH is significantly decreased at 3–4 days and 5–6 days post-TBI, compared with the 3–8 h and one day survival time points for each region. Whole cerebrum ICH percentages dramatically decreased from an average of 28%-37% at 3–8 h to 3%-5% at 5–6 days for four-week-olds and five-day-olds respectively (Fig. 2). Although the 3–4 day and 5–6 day post-TBI survival time groups are not significantly different from each other for any region, a trend of decreasing hemorrhage exists across increasing post-TBI survival time.
FIG. 2.
Whole cerebrum intracranial hemorrhage percentages for five-day-old and four-week-old piglet brains. For both ages, percentages at the 5–6 day post-injury survival time points were significantly lower than at the age-matched 3–8 h time points, and percentages for four-week-olds at 5–6 days were significantly lower than those of four-week-olds at one day (##p<0.01). The percentages for five-day-olds at 3–4 days were significantly lower than at 3–8 h (#p<0.05). Percentages for all groups except the four-week-olds at 5–6 days were significantly higher than sham levels (*p<0.05; **p<0.01). All values are mean±standard error of mean.
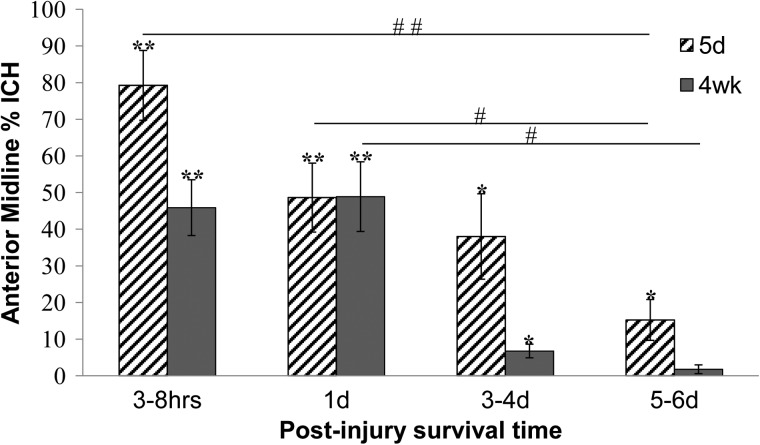
The two-way ANOVA showed that the anterior midline is the only region with an overall age effect, with the five-day-old group significantly higher than the four-week-old group (p=0.0099; Fig. 3). However, post hoc analysis did not demonstrate a significant difference between the age groups at any specific post-injury survival time point.
FIG. 3.
Anterior midline intracranial hemorrhage in five-day-old and four-week-old piglet brains. For both ages, percentages at the 5–6 day post-injury survival time points were significantly lower than at the age-matched one day time point, and the percentages for five-day-olds at 5–6 days were significantly lower than at 3–8 h (#p<0.05; ##p<0.01). Percentages for all groups except the four-week-olds at 5–6 days were significantly higher than sham levels (*p<0.05; **p<0.01). All values are mean±standard error of mean.
The two-way ANOVA did not show a significant interaction between age and the time post-TBI in ICH for any region (p>0.2 for each region). However, when comparing each age group and time point to sham levels via a Student's t-test, the four-week-old brain had returned to sham levels by 5–6 days post-injury for a majority of regions and the whole cerebrum value, while the five-day-old brain still had elevated levels of ICH in many regions. Specifically, for each age group and region studied, the Student's t-test revealed a significant presence of ICH in the two early time points (3–8 h and one day; p<0.01). However, by 5–6 days post-TBI, all but one region in the four-week-old had returned to sham levels; for the five-day-old, the ICH in half of the regions, including the whole cerebrum and anterior midline, remained elevated (Fig. 4, Table 2). Taken together, these results reveal that ICH takes longer to resolve in the younger brain.
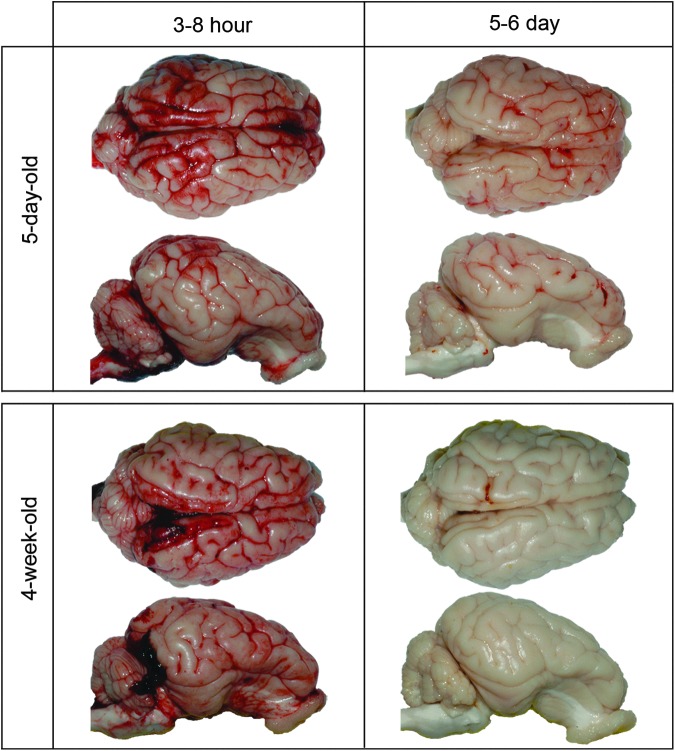
FIG. 4.
Representative whole brain images showing intracranial hemorrhage (ICH) in five-day-old and four-week-old piglet brains at 3–8 h post-injury and 5–6 days post-injury. At 5–6 days, whole brain ICH percentages were elevated above sham levels in the five-day-old brain (p=0.02) but returned to sham levels in the four-week-old brain (p=0.06).
Table 2.
Summary of Intracranial Hemorrhage Percentages by Region and for Whole Cerebrum
| Piglet age at RNR | Post-TBI survival time | Anterior midline (%) | Posterior midline (%) | Anterior right (%) | Anterior left (%) | Posterior right (%) | Posterior left (%) | Whole cerebrum (%) |
|---|---|---|---|---|---|---|---|---|
| 5-day-old | Sham | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 |
| 3–8 h | 79±10** | 74±10** | 32±5** | 35±4** | 36±4** | 35±5** | 37±4** | |
| 1 day | 49±9** | 48±8** | 17±3** | 27±6** | 27±4** | 24±3** | 26±4** | |
| 3–4 days | 38±12* | 28±10*a | 14±6 | 9±3*a | 12±5a | 13±4*a | 13±5*a | |
| 5–6 days | 15±6*ab | 5±2ab | 8±3*a | 6±3a | 3±1*bc | 4±2a | 5±2*a | |
| 4-week-old | Sham | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 |
| 3–8 h | 46±8** | 57±8** | 25±5** | 26±5** | 28±3** | 27±5** | 28±4** | |
| 1 day | 49±10** | 53±9** | 20±5** | 22±5** | 25±5** | 24±4** | 25±8** | |
| 3–4 days | 7±2* | 24±8 | 6±1* | 8±2 | 14±3* | 13±4 | 11±2* | |
| 5–6 days | 2±1a | 8±2*ab | 1±0a | 2±1 | 3±1ab | 4±2ab | 3±1ab |
Significantly higher than sham; *p<0.05; ** p<0.01
Significantly lower than 3–8 h survival time group in same age/region; p<0.05.
Significantly lower than 1 day survival time group in same age/region; p<0.05.
Significantly lower than 3–4 day survival time group in same age/region; p<0.05.
All values are mean±standard error of mean.
RNR, rapid non-impact rotational injury; TBI, traumatic brain injury.
Immunohistochemistry
A significant presence of TAI was found in every animal that experienced a rotational injury (p<0.002), and a trace amount (0.0003%) was detected in one four-week-old sham. All other sham animals had no TAI, including the four 5-day-old shams with invasive instrumentation.
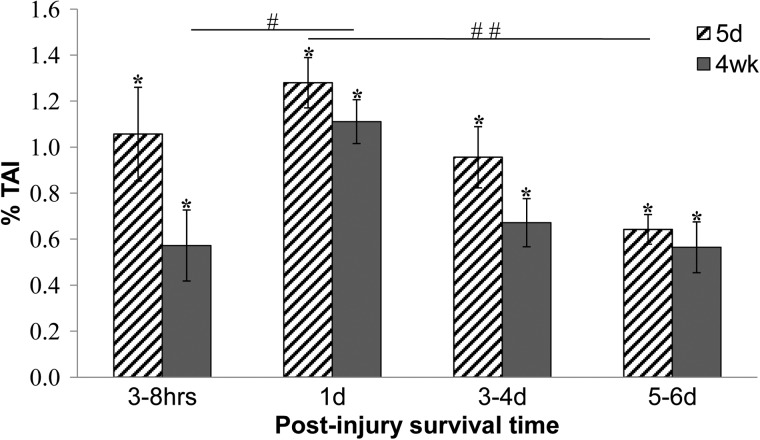
The two-way ANOVA demonstrated an overall survival time effect (p<0.0001), with the one day post-TBI time point significantly higher than all other time points (p<0.03; Fig. 5). There was also a significant age effect with more TAI in five-day-olds than four-week-olds overall (p=0.016). However, the post hoc analysis showed no differences between four-week-olds and five-day-olds at specific survival times.
FIG. 5.
Percent traumatic axonal injury (TAI) for five-day-old and four-week-old piglet brains. TAI in the four-week-old at one day post-injury was significantly higher than at 3–8 h post injury (#p=0.03). TAI in the five-day-old at one day was significantly higher than at 5–6 days (##p=0.003). Percentages for all groups were significantly higher than sham levels (*p<0.008). All values are mean±standard error of mean.
The two-way ANOVA did not demonstrate a significant interaction between age and survival time in TAI (p=0.28) but the post hoc test did show some significant differences between time points within each age. Specifically, within the four-week-old age group, the one day survival time group had significantly more TAI than the 3–8 h time point (p=0.0331; Fig. 5). Within the five-day-old age group, the one day survival time point had significantly more TAI than the 5–6 day time point (p=0.003). Our post hoc analysis revealed no other significant comparisons between survival time groups within age.
In summary, TAI in the five-day-old decreased significantly from peak levels only by 5–6 days post-injury. In contrast, in the four-week-old TAI increased significantly at one day after injury and was not significantly decreased from these peak levels at 5–6 days post-injury. Importantly, TAI in both ages reached a peak at one day after injury and remained significantly above sham levels at 5–6 days post-injury, indicating persistent white matter injury.
Discussion
In this study, we demonstrated a time-dependent and age-dependent response to a rapid rotational head injury in infant and toddler piglets. Five-day-old piglets required longer to resolve ICH than four-week-old piglets, with the overall percent ICH elevated above sham levels at 5–6 days post-injury in the five-day-olds, while it returned to sham levels in the four-week-olds. In addition, there were larger areas of combined subdural and subarachnoid hemorrhage in the anterior midline region of five-day-old piglets. TAI showed the same time course for each age group with peak TAI occurring at one day post-injury and still elevated at 5–6 days post-injury. While the progression of TAI was the same for both ages, five-day-olds showed overall higher levels of TAI than four-week-old piglets.
Both age groups demonstrated a significant decrease in ICH over time for each region studied. However, our data suggest that ICH may resolve more rapidly in the toddler piglet than the infant piglet. By 5–6 days post-injury, ICH had resolved completely in five regions in the four-week-old, compared with only three regions in the five-day-old. By 5–6 days post-TBI, the percentage of hemorrhage in the whole cerebrum also returned to sham levels for the four-week-old, whereas the five-day-old whole cerebrum value remained elevated. This suggests that the four-week-old piglets may be reabsorbing the ICH faster than the five-day-old piglets or the five-day-old piglets still may have some degree of persistent intracranial bleeding combined with reabsorption.
For TAI, both age groups reached their peak at one day post-TBI and continued to be significantly greater than sham values at 5–6 days post-injury. In fact, the percent TAI at 5–6 days post-injury is unchanged from 3–8 h post-injury in both ages, indicating that minimal recovery has occurred over this time frame. Although the presence of TAI is known to be related to brain tissue strain, the rise in TAI at one day post-injury may be attributed to the cascade of events following this initial strain.33 A similar peak in axonal pathology at one day post-injury also has been observed in studies of adult rats using immunohistochemistry and electrophysiology. Immunostaining of the rat corpus callosum showed peaks in both impaired axoplasmic transport and neurofilament compaction at 28 h post-injury.34 Likewise, an electrophysiology study of the rat corpus callosum showed the largest decreases in compound action potentials at one day post-injury for both fast myelinated and slow unmyelinated axons. The unmyelinated axons continued to have significantly suppressed compound action potentials until seven days post-injury.35 In addition to peaks in TAI at one day post-TBI, previous studies also have demonstrated a period of decreased cerebral glucose metabolism that has been shown to peak at one day post-injury in both adult and juvenile rats.11,13,36,37
These findings highlight a critical time point at one day post-injury where a window of vulnerability to a second injury may exist. This is supported by previous studies in rats and piglets that investigated repeated brain injuries. Friess and colleagues showed that two injuries delivered one day apart were associated with significantly higher mortality rates than either a single injury or two injuries delivered seven days apart in neonatal piglets.38 Similarly, Prins and colleagues demonstrated that two injuries separated by 24 h were associated with a greater and more persistent decrease in cerebral metabolism than either a single injury or two injuries separated by three days in rats.39
The evolution of secondary injury also likely depends upon the brain injury mechanism with different injury models yielding varying time dependencies. Duhaime and colleagues used a porcine controlled cortical impact model and although they also found peak lesion volumes in five-day-old piglets at one day post-injury, four-week-old piglets had peak lesion volumes at seven days post-injury.18 Therefore, while the time course for our diffuse model of brain injury did not vary with age, a focal brain injury model exhibited age-dependencies in the time course of recovery. This suggests that different injury types resolve differently and have unique age dependencies.
Our data lend some support to previous studies that have suggested infants are more vulnerable to brain injury than other pediatric age groups.9,17,40,41 In particular, Ibrahim and colleagues used the same RNR injury model but with rotations in the axial plane, and found that when five-day-old and four-week-old piglets experienced comparable mass-scaled angular accelerations the five-day-olds sustained significantly more TAI than the four-week-old piglets at the 6 h post-injury time point.17 Although our dataset exhibited significantly higher TAI in five-day-olds overall (p=0.016), this did not reach significance at the 3–8 h time point and is somewhat confounded by the overall lower scaled angular velocities in the four-week-old age group. Further, Ibrahim and colleagues found that five-day-old piglets had approximately four times more TAI than four-week-old piglets on average, while our data showed only 1.8 times more TAI in five-day-olds at 3–8 h post-injury. However, the four-week-old 3–8 h survival time group experienced significantly higher mass-scaled angular accelerations than the corresponding five-day-old group. If five-day-old and four-week-old groups with the same mass-scaled angular accelerations had been available, we speculate that the difference in TAI between them would have been larger.
The retrospective nature of the study leads to some limitations. First, the scaled loading conditions were not precisely the same for all age/time groups. Because the four-week-old 3–8 h survival group had significantly higher angular accelerations than the corresponding five-day-old group, caution should be exercised when directly comparing these groups. Similarly, while there was no difference in scaled angular velocity across time for each age or between ages at the same time point, the four-week-old group overall had lower scaled angular velocities. Second, our 3–8 h survival piglets all were acutely sacrificed due to adverse complications at the time of study; thus, this group may represent an atypically vulnerable group and have artificially inflated percent injuries at that time point. In this case, we would be underestimating the increase in axonal injury and hemorrhage between the 3–8 h and one day survival time points. Third, different anesthetic protocols were utilized on the day of sacrifice for most of the five-day-old animals due to alternative study designs. Importantly, we did not note any departures from our acceptable range of clinical oxygen saturation or end-tidal CO2 values in any sedation group. Although we do not think the terminal day anesthetic affects our study measures, its influence is unknown. Lastly, with a sample size of 4–14/group, it is difficult to truly assess for normality. Use of an ANOVA on data that are not truly normal can increase Type I errors.
In summary, we have evaluated the influence of age and post-injury survival time on an immature closed head injury model. We found similar initial levels of ICH in both age groups and a significant resolution of hemorrhage over time. However, ICH took longer to resolve in the infant piglet age group. Both ages also exhibited a similar time course of axonal injury with a peak in TAI at one day post-injury and significantly elevated levels even at 5–6 days after the injury. This suggests a particularly vulnerable window to a second injury within one day of initial injury, which may extend for a prolonged period of time. In addition, the presence of ICH, especially traumatic subarachnoid hemorrhage, may compound the evolution of secondary injury due to vasospasm and ongoing ischemia. We also present some evidence for an increased vulnerability to brain injury in the infant piglet age group, although the magnitude is less than previously reported and is confounded by differences in the scaled loading conditions experienced by the two ages. The combination of TBI and traumatic subarachnoid hemorrhage may complicate intensive care unit management and underscore the importance of optimal cerebral perfusion pressure, surgical intervention, hemostatic pharmacology and multi-modal neuromonitoring in children.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Michelle Skettini, Melissa Byro, Jill Ralston, Shreya Reddy, and Sharon Martinez. Studies were supported by NIH R01 NS039679 and NIH U01 NS069545.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Available at: www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf Accessed October29, 2013 [Google Scholar]
- 2.Langlois J.A., Rutland-Brown W., and Thomas K.E. (2005). The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 20, 229–238 [DOI] [PubMed] [Google Scholar]
- 3.Ommaya A.K., Goldsmith W., and Thibault L. (2002). Biomechanics and neuropathology of adult and paediatric head injury. Br. J. Neurosurg. 16, 220–242 [DOI] [PubMed] [Google Scholar]
- 4.Werner C. and Engelhard K. (2007). Pathophysiology of traumatic brain injury. Br. J. Anaesth. 99, 4–9 [DOI] [PubMed] [Google Scholar]
- 5.Giza C. and Hovda D. (2001). The neuroetabolic cascade of concussion. J. Athl. Train. 46, 228–235 [PMC free article] [PubMed] [Google Scholar]
- 6.Schutzman S.A. and Greenes D.S. (2001). Pediatric minor head trauma. Ann. Emerg. Med. 37, 65–74 [DOI] [PubMed] [Google Scholar]
- 7.Klauber M.R., Marshall L.F., Luerssen T.G., Frankowski R., Tabaddor K., and Eisenberg H.M. (1989). Determinants of head injury mortality: importance of the low risk patient. Neurosurgery 24, 31–36 [DOI] [PubMed] [Google Scholar]
- 8.Giza C.C., Mink R.B., and Madikians A. (2007). Pediatric traumatic brain injury: not just little adults. Curr. Opin. Crit. Care 13, 143–152 [DOI] [PubMed] [Google Scholar]
- 9.Raghupathi R. and Margulies S.S. (2002). Traumatic axonal injury after closed head injury in the neonatal pig. J. Neurotrauma 19, 843–853 [DOI] [PubMed] [Google Scholar]
- 10.Bruce D.A. (1990). Head injuries in the pediatric population. Curr. Prob. Pediatr. 20, 61–107 [DOI] [PubMed] [Google Scholar]
- 11.Thomas S., Prins M.L., Samii M., and Hovda D.A. (2000). Cerebral metabolic response to traumatic brain injury sustained early in development: a 2-deoxy-D-glucose autoradiographic study. J. Neurotrauma 17, 649–665 [DOI] [PubMed] [Google Scholar]
- 12.Osteen C.L., Moore A.H., Prins M.L., and Hovda D.A. (2001). Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J. Neurotrauma 18, 141–162 [DOI] [PubMed] [Google Scholar]
- 13.Yoshino A., Hovda D.A., Kawamata T., Katayama Y., and Becker D.P. (1991). Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 561, 106–119 [DOI] [PubMed] [Google Scholar]
- 14.Agran P.F., Anderson C., Winn D., Trent R., Walton-Haynes L., and Thayer S. (2003). Rates of pediatric injuries by 3-month intervals for children 0 to 3 years of age. Pediatrics 111, e683–e692 [DOI] [PubMed] [Google Scholar]
- 15.Levin H.S., Aldrich E.F., Saydjari C., Eisenberg H.M., Foulkes M.A., Bellefleur M., Luerssen T.G., Jane J.A., Marmarou A., Marshall L.F., et al. (1992). Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery 31, 435–443 [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim N.G., Wood J., Margulies S.S., and Christian C.W. (2012). Influence of age and fall type on head injuries in infants and toddlers. Int. J. Dev. Neurosci. 30, 201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim N.G., Ralston J., Smith C. and Margulies S.S.Physiological and pathological responses to head rotations in toddler piglets. J. Neurotrauma 27, 1021–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duhaime A.C., Hunter J.V., Grate L.L., Kim A., Golden J., Demidenko E., and Harris C. (2003). Magnetic resonance imaging studies of age-dependent responses to scaled focal brain injury in the piglet. J. Neurosurg. 99, 542–548 [DOI] [PubMed] [Google Scholar]
- 19.Duhaime A.C., Margulies S.S., Durham S.R., O'Rourke M.M., Golden J.A., Marwaha S., and Raghupathi R. (2000). Maturation-dependent response of the piglet brain to scaled cortical impact. J. Neurosurg. 93, 455–462 [DOI] [PubMed] [Google Scholar]
- 20.Prange M. and Margulies S. (2002). Regional, directional, and age-dependent properties of brain undergoing large deformation. J. Biomech. Eng. 124, 244–252 [DOI] [PubMed] [Google Scholar]
- 21.Dobbing J. (1968). The development of the blood-brain barrier. Prog. Brain Res. 29, 417–427 [DOI] [PubMed] [Google Scholar]
- 22.Snyder R.C., Schneider L.W., Reynolds Owings C.L., Golomb D.H., and Schork M.A. (1977). Anthropometry of infants, children and youth to age 18 for product safety design. UM-HSRI-77 Final Report Contract CPSC-C-75-0068. Prepared for Consumer Product Safety Commission; Bethesda, MD [Google Scholar]
- 23.Coats B., Eucker S.A., Sullivan S., and Margulies S.S. (2012). Finite element model predictions of intracranial hemorrhage from non-impact, rapid head rotations in the piglet. Int. J. Dev. Neurosci. 30, 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstead W.M. (2005). Age and cerebral circulation. Pathophysiology 12, 5–15 [DOI] [PubMed] [Google Scholar]
- 25.Duhaime A.C. (2006). Large animal models of traumatic injury to the immature brain. Dev. Neurosci. 28, 380–387 [DOI] [PubMed] [Google Scholar]
- 26.Conrad M.S., Dilger R.N. and Johnson R.W. (2012). Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev. Neurosci. 34, 291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagberg H., Ichord R., Palmer C., Yager J.Y. and Vannucci S.J. (2002). Animal models of developmental brain injury: relevance to human disease. A summary of the panel discussion from the Third Hershey Conference on Developmental Cerebral Blood Flow and Metabolism. Dev. Neurosci. 24, 364–366 [DOI] [PubMed] [Google Scholar]
- 28.Eucker S.A., Smith C., Ralston J., Friess S.H., and Margulies S.S. (2011). Physiological and histopathological responses following closed rotational head injury depend on direction of head motion. Exp. Neurol. 227, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilbaugh T.J., Bhandare S., Lorom D.H., Saraswati M., Robertson C.L., and Margulies S.S.Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J. Neurotrauma 28, 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto P.S., Meoded A., Poretti A., Tekes A., and Huisman T.A. (2012). The unique features of traumatic brain injury in children. review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications, and their imaging findings—part 2. J. Neuroimaging 22, e18–e41 [DOI] [PubMed] [Google Scholar]
- 31.Sullivan S., Friess S.H., Ralston J., Smith C., Propert K.J., Rapp P.E. and Margulies S.S. (2013). Behavioral deficits and axonal injury persistence following rotational head injury are direction dependent. J. Neurotrauma 30, 538–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ommaya A.K. and Hirsch A.E. (1971). Tolerances for cerebral concussion from head impact and whiplash in primates. J. Biomech. 4, 13–21 [DOI] [PubMed] [Google Scholar]
- 33.Bain A.C. and Meaney D.F. (2000). Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J. Biomech. Eng. 122, 615–622 [DOI] [PubMed] [Google Scholar]
- 34.Zakaria N., Kallakuri S., Bandaru S., and Cavanaugh J.M. (2012). Temporal assessment of traumatic axonal injury in the rat corpus callosum and optic chiasm. Brain Res. 1467, 81–90 [DOI] [PubMed] [Google Scholar]
- 35.Reeves T.M., Phillips L.L., and Povlishock J.T. (2005). Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp. Neurol. 196, 126–137 [DOI] [PubMed] [Google Scholar]
- 36.Sutton R.L., Hovda D.A., Adelson P.D., Benzel E.C., and Becker D.P. (1994). Metabolic changes following cortical contusion: relationships to edema and morphological changes. Acta Neurochir. Suppl. (Wien) 60, 446–448 [DOI] [PubMed] [Google Scholar]
- 37.Prins M.L. and Hovda D.A. (2009). The effects of age and ketogenic diet on local cerebral metabolic rates of glucose after controlled cortical impact injury in rats. J. Neurotrauma 26, 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friess S.H., Ichord R.N., Ralston J., Ryall K., Helfaer M.A., Smith C., and Margulies S.S. (2009). Repeated traumatic brain injury affects composite cognitive function in piglets. J. Neurotrauma 26, 1111–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prins M.L., Alexander D., Giza C.C., and Hovda D.A. (2013). Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J. Neurotrauma 30, 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson V., Catroppa C., Morse S., Haritou F., and Rosenfeld J. (2005). Functional plasticity or vulnerability after early brain injury? Pediatrics 116, 1374–1382 [DOI] [PubMed] [Google Scholar]
- 41.Raghupathi R. and Huh J.W. (2007). Diffuse brain injury in the immature rat: evidence for an age-at-injury effect on cognitive function and histopathologic damage. J Neurotrauma 24, 1596–1608 [DOI] [PubMed] [Google Scholar]