Abstract
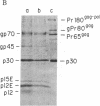
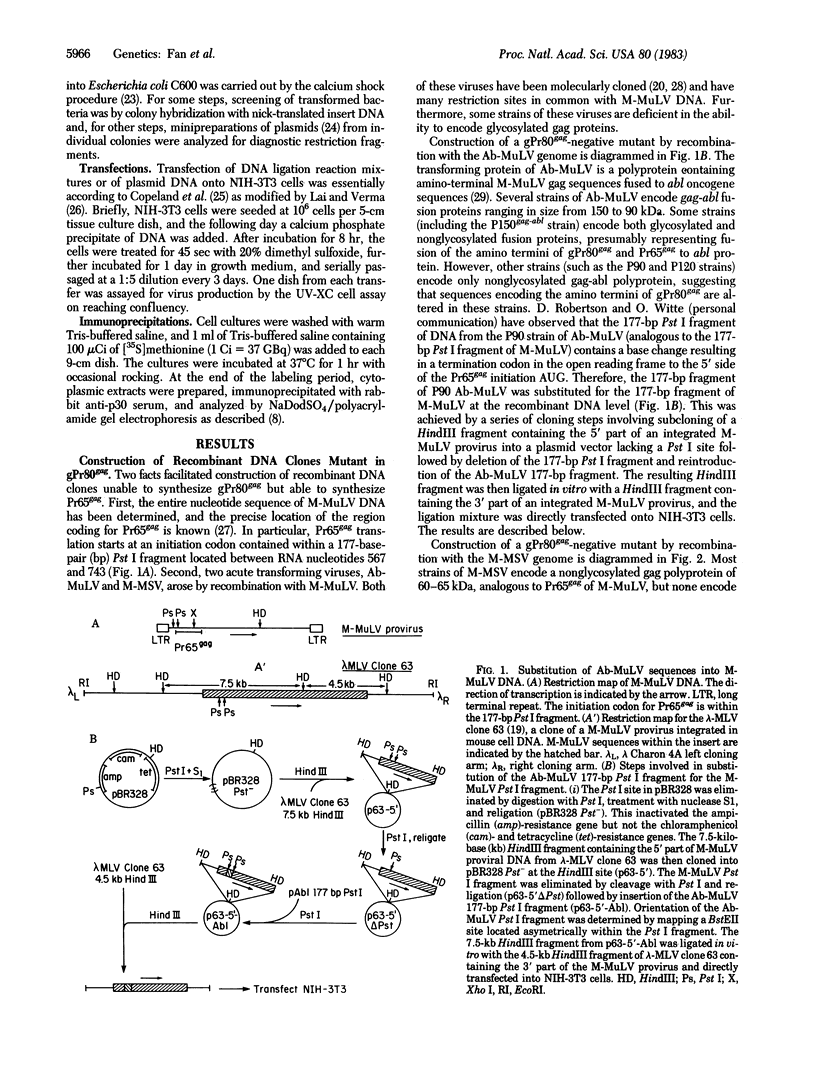
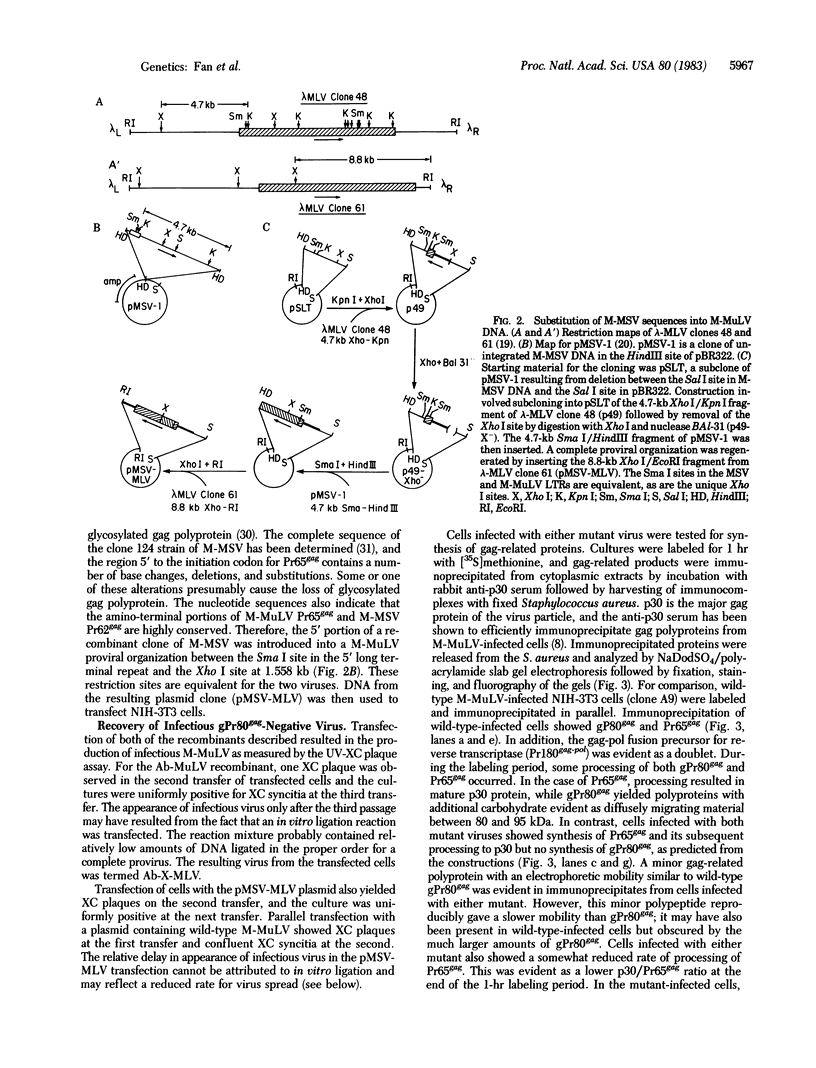
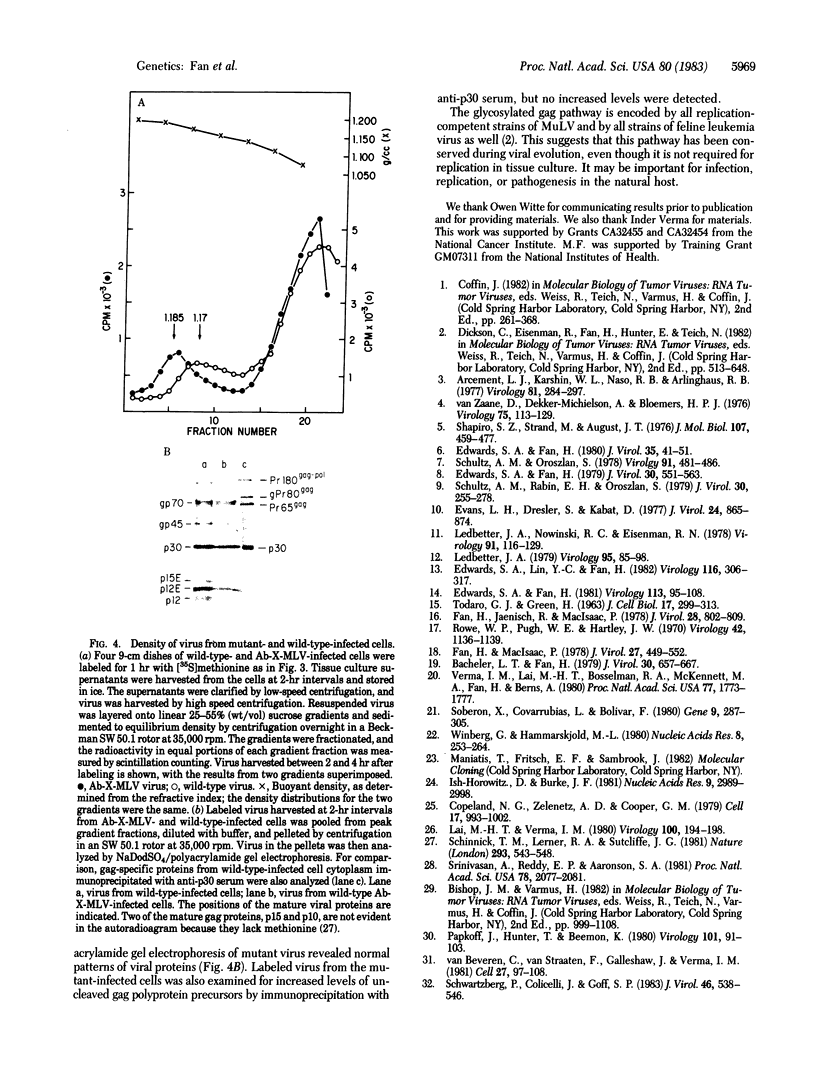
Murine leukemia virus (MuLV) encodes two independent pathways for expression of the gag gene. One pathway results in processing and cleavage of the precursor Pr65gag to yield the internal capsid proteins of the virion and is analogous to gag polyprotein precursors for all classes of retroviruses. The other pathway, which is not encoded by several other classes of retroviruses, begins with a glycosylated polyprotein gPr80gag . gPr80gag is synthesized independently of Pr65gag; it contains Pr65gag peptides and additional amino-terminal protein. It is modified by further addition of carbohydrate, exported to the cell surface, and released from the cell but does not appear in virus particles. To investigate the role of glycosylated gag in MuLV infection, two mutants of Moloney MuLV (M-MuLV) deficient for synthesis of gPr80gag but able to synthesize Pr65gag were constructed. The mutants were obtained by substitution into a molecular clone of M-MuLV DNA by DNA from two acutely transforming viruses, Ableson MuLV (Ab-MuLV) and Moloney murine sarcoma virus (M-MSV). Both Ab-MuLV and M-MSV are derived from M-MuLV and they express M-MuLV gag sequences, but some strains do not synthesize glycosylated gag protein. For Ab-MuLV, a 177-base-pair Pst I fragment from the P90 strain containing the initiation codon for Pr65gag was substituted for the equivalent fragment in M-MuLV DNA. For M-MSV, 1.5 kilobases at the 5' end of the genome was substituted. Transfection of the recombined DNAs onto NIH-3T3 cells produced infectious M-MuLV, although the infected cells did not produce gPr80gag. Therefore glycosylated gag is not absolutely required for MuLV replication. Deletion of the glycosylated gag pathway did not significantly reduce the level of virus production, although a minor difference in XC plaque morphology was observed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Arlinghaus R. B. "gag" polyprotein precursors of Rauscher murine leukemia virus. Virology. 1977 Sep;81(2):284–297. doi: 10.1016/0042-6822(77)90145-3. [DOI] [PubMed] [Google Scholar]
- Bacheler L. T., Fan H. Multiple integration sites for Moloney murine leukemia virus in productively infected mouse fibroblasts. J Virol. 1979 Jun;30(3):657–667. doi: 10.1128/jvi.30.3.657-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Zelenetz A. D., Cooper G. M. Transformation of NIH/3T3 mouse cells by DNA of Rous sarcoma virus. Cell. 1979 Aug;17(4):993–1002. doi: 10.1016/0092-8674(79)90338-6. [DOI] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. Immunoselection and characterization of Moloney murine leukemia virus-infected cell lines deficient in surface gag antigen expression. Virology. 1981 Aug;113(1):95–108. doi: 10.1016/0042-6822(81)90139-2. [DOI] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. Sequence relationship of glycosylated and unglycosylated gag polyproteins of Moloney murine leukemia virus. J Virol. 1980 Jul;35(1):41–51. doi: 10.1128/jvi.35.1.41-51.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. gag-Related polyproteins of Moloney murine leukemia virus: evidence for independent synthesis of glycosylated and unglycosylated forms. J Virol. 1979 May;30(2):551–563. doi: 10.1128/jvi.30.2.551-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. A., Lin Y. C., Fan H. Association of murine leukemia virus gag antigen with extracellular matrices in productively infected mouse cells. Virology. 1982 Jan 15;116(1):306–317. doi: 10.1016/0042-6822(82)90422-6. [DOI] [PubMed] [Google Scholar]
- Evans L. H., Dresler S., Kabat D. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J Virol. 1977 Dec;24(3):865–874. doi: 10.1128/jvi.24.3.865-874.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Jaenisch R., MacIsaac P. Low-multiplicity infection of Moloney murine leukemia virus in mouse cells: effect on number of viral DNA copies and virus production in producer cells. J Virol. 1978 Dec;28(3):802–809. doi: 10.1128/jvi.28.3.802-809.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., MacIsaac P. Virus-specific RNA synthesis in interferon-treated mouse cells productively infected with Moloney murine leukemia virus. J Virol. 1978 Aug;27(2):449–452. doi: 10.1128/jvi.27.2.449-452.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. H., Verma I. M. Genome organization of retroviruses. VII. Infection by double-stranded DNA synthesized in vitro from Moloney murine leukemia virus generates a virus indistinguishable from the original virus used in reverse transcription. Virology. 1980 Jan 15;100(1):194–198. doi: 10.1016/0042-6822(80)90567-x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Nowinski R. C., Eisenman R. N. Biosynthesis and metabolism of viral proteins expressed on the surface of murine leukemia virus-infected cells. Virology. 1978 Nov;91(1):116–129. doi: 10.1016/0042-6822(78)90360-4. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A. Two-dimensional analysis of murine leukemia virus gag-gene polyproteins. Virology. 1979 May;95(1):85–98. doi: 10.1016/0042-6822(79)90403-3. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Hunter T., Beemon K. In vitro translation of virion RNA from Moloney murine sarcoma virus. Virology. 1980 Feb;101(1):91–103. doi: 10.1016/0042-6822(80)90486-9. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. Murine leukemia virus gag polyproteins: the peptide chain unique to Pr80 is located at the amino terminus. Virology. 1978 Dec;91(2):481–486. doi: 10.1016/0042-6822(78)90395-1. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Rabin E. H., Oroszlan S. Post-translational modification of Rauscher leukemia virus precursor polyproteins encoded by the gag gene. J Virol. 1979 Apr;30(1):255–266. doi: 10.1128/jvi.30.1.255-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Goff S. P. Deletion mutants of Moloney murine leukemia virus which lack glycosylated gag protein are replication competent. J Virol. 1983 May;46(2):538–546. doi: 10.1128/jvi.46.2.538-546.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., Strand M., August J. T. High molecular weight precursor polypeptides to structural proteins of Rauscher murine leukemia virus. J Mol Biol. 1976 Nov 15;107(4):459–477. doi: 10.1016/s0022-2836(76)80078-2. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Reddy E. P., Aaronson S. A. Abelson murine leukemia virus: molecular cloning of infectious integrated proviral DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2077–2081. doi: 10.1073/pnas.78.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Galleshaw J. A., Verma I. M. Nucleotide sequence of the genome of a murine sarcoma virus. Cell. 1981 Nov;27(1 Pt 2):97–108. doi: 10.1016/0092-8674(81)90364-0. [DOI] [PubMed] [Google Scholar]
- Van Zaane D., Dekker-Michielsen J. A., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus: synthesis, identification, and processing. Virology. 1976 Nov;75(1):113–129. doi: 10.1016/0042-6822(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Lai M. H., Bosselman R. A., McKennett M. A., Fan H., Berns A. Molecular cloning of unintegrated Moloney mouse sarcoma virus DNA in bacteriophage lambda. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1773–1777. doi: 10.1073/pnas.77.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]