Introduction
One of the most common cancers in women worldwide, breast cancer is classically an endocrine-dependent cancer.1 It has been known for over a century that development, progression and metastasis of breast cancer are strongly influenced by hormonal factors.2 Indeed about two-thirds of breast cancers express the estrogen receptor α (ERα) protein, a key predictor of prognosis and response to endocrine therapy. These cancers are frequently amenable to therapies that target estrogen signaling pathways, including selective estrogen receptor modulators like tamoxifen, selective estrogen receptor downregulators like fulvestrant; and agents that reduce estrogen ligand like aromatase inhibitors and ovarian suppression through luteinizing hormone-releasing hormone (LHRH) agonists (Fig.1). It is likely that these approaches, especially adjuvant tamoxifen, have contributed to the reduction in breast cancer mortality that has been observed in recent years.3 However, data from clinical studies have suggested that only about 60% of ERα-positive breast cancers respond to hormonal therapy.4,5 Further, those tumors that lack expression of ERα and the estrogen-regulated progesterone receptor (PgR) are unresponsive to hormone therapy. Thus the problem of acquired or de novo endocrine resistance is a substantial one. Recent molecular and biological advances have contributed to our understanding about potential underlying mechanisms. Here we will focus especially on silencing the expression of ERα as one such endocrine-resistance mechanism and how it might be exploited clinically.
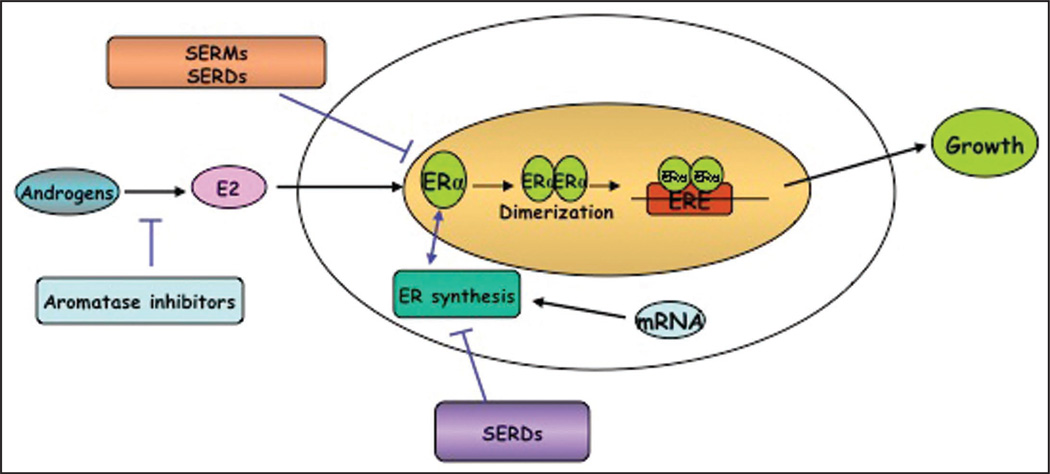
Figure 1.
Therapeutic strategies to block estrogen receptor function: Aromatase inhibitors like letrozole, anastrozole and exemestane inhibit the enzyme leading to decreased E2 levels. Selective ER downregulators (SERDs) like fulvestrant bind to ER and sequester it, thereby inhibiting ER-mediated gene transcripton and disrupting ER nuclear localization through the ubiquitin-proteosomal pathway.
A key contributor to our understanding of estrogen as a critical regulator of growth and differentiation in normal breast tissues and malignant progression has been the elucidation of estrogen signaling pathways. Estrogen functions by binding to two distinct estrogen receptors (ERα and ERβ ) to modulate their transcriptional activity.6 ERα and ERβ exhibit differential transcriptional properties and can form hetero- and homo-dimers.7 While both isoforms share similar binding affinities for estrogen (E2), they play different roles in regulation of gene expression. This review will focus entirely on ERα.
ERα mediates both genomic and non-genomic signaling pathways.7 It has two distinct transactivation function (AF) domains, AF1 and AF2, that mediate transcriptional activation. Depending on the cell type, AF1 and AF2 may activate transcription independently and/or synergistically. The classic mechanism of action of ERα involves binding of estrogen to ERα, formation of dimers, and localization to estrogen response elements (ERE) on regulatory promoter regions of estrogen responsive genes in association with either coactivators or corepressors to alter transcription of the responsive gene 2 In addition, ERα can also mediate transcription of other target genes through protein-protein interactions with other transcriptional factors9 like activator protein-1 (AP-1)10.
In addition to nuclear ERα, plasma membrane and mitochondrial ERα mediate non-genomic signaling.11–5 Here, ERα may be activated by a ligand-independent mechanism through the phosphorylation of specific sites in AF-1 by kinases and peptide growth factors.16 ERα in mitochondria bind to ERE-like sequences in the DNA of the mitochondrial (mt) genome and drive the levels of mRNA. mtERα signaling inhibits apoptosis through mt-superoxide dismutase (mtSOD) 17
Potential Mechanisms for ERα-Negative Breast Cancer
About one-quarter of breast cancers lack ERα expression and such tumors are associated with poorer clinical outcome and are unresponsive to endocrine treatments.4,5,18 Molecular classification has allowed us to identify at least two types of ERα-negative breast cancer, the human epidermal growth factor (HER)-2 overexpressing subtype as well as the “triple-negative” subtype that lacks expression of ERα, PgR and HER-2. Currently risk factors and pathways for the development or evolution of such cancers are poorly understood.
Breast cancer, like other cancers, results from an accumulation of genetic and epigenetic events. Such changes contribute to the establishment of ERα negative cancer, a hormone resistant phenotype which could result from a variety of mechanisms. These include the classic genetic mechanisms of homozygous deletion of the ERα gene or loss of heterozygosity along with mutation in the remaining allele (reviewed in ref. 19). However, genetic alterations within the ERα gene like insertions, deletions, rearrangements and point mutations have not been historically reported as a major cause of loss of ERα function.20,21 Instead, ERα-negative cancers are frequently associated with the absence of ERα mRNA expression and it has been suggested that epigenetic alterations at the ERα promoter or alterations in other growth factor and signal transduction pathways may be responsible for downregulation of transcription (Fig. 2).22 Because these changes are potentially reversible, the use of strategies to modulate these alterations has been proposed.
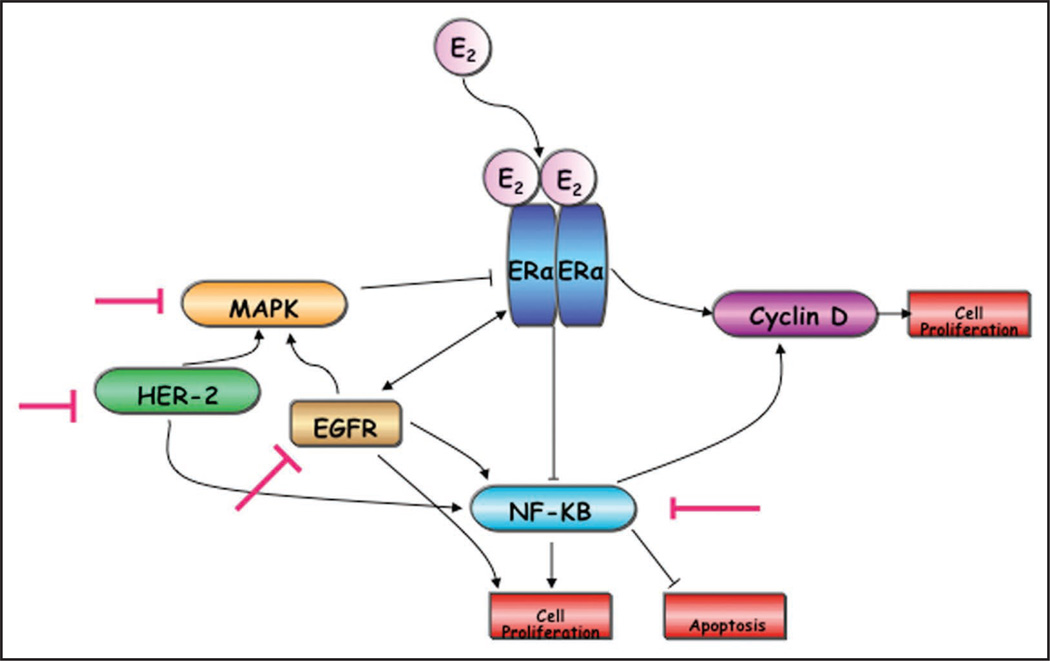
Figure 2.
Crosstalk between estrogen receptor-α (ERα) and growth factor signaling: overexpression of growth factors is a proposed cause for suppression of ERα expression. E2 bound ER modulates expression of estrogen response genes like cyclin D while its non-genomic targets include inhibition of nuclear factor-κB (NFκB). Activation of epidermal growth factor receptors like EGFR and HER2 (as a consequence of ERα downregualtion) triggers activation of mitogen activated protein kinases (MAPKs) and NFκB. MAPKs are implicated in repression of ERα expression and in enhanced ERα independent cell proliferation. Red T symbol indicates potential targets for inhibition that may result in ERα re-expression.
A contentious issue is whether ERα negative tumors arise de novo or represent an acquired phenotype. Certainly, acquired alterations in multiple signaling pathways in normal or ERα positive cells resulting in clonal selection and evolution into ERα-negative breast cancer cells are one potential mechanism. However, the possibility that self-renewing stem-like cells within breast tumors may contribute to the emergence of the ERα negative phenotype has also been raised. Putative breast cancer stem cells (BCSC) of the CD44+/CD24(−/low) antigenic phenotype that is hypothesized to be stem cell specific were isolated from malignant pleural effusions and ascites samples from breast cancer patients. These were marked by the absence of ERα, PgR and HER2 expression raising the possibility that they play a role in the origin of ERα negative tumors.23–30 However, this is an area of great controversy as it is also possible that these BCSC may also be clonally selected from a highly heterogeneous tumor with the pressure of time and/or therapy. A lack of availability of good markers for identification of BCSC and the rarity of these cells, if they do indeed exist, represent two limitations in this field at the present time.31
In sum, although several theories exist for the origin of ERα negative hormone-resistant breast cancers, there is no consensus about their relative contributions at present. But two compelling lines of evidence attribute loss of ERα expression in breast cancer to crosstalk between ERα and growth factor signaling pathways and epigenetic silencing of ERα and these areas are reviewed below.
Crosstalk between ERα and growth factor signaling pathways
Epidermal growth factor receptors (EGFR)
The human epidermal growth factor receptor family is comprised of four members: EGFR/ c-erbB1/HER-1, c-erbB2/HER2/neu, c-erbB3/HER3 and c-erbB4/ HER4. Upon ligand binding these transmembrane receptor tyrosine kinases form homo- and heterodimers, resulting in their activation and transduction of signaling into the cell.32 A role for EGFR family members has been implicated in the development and progression of many human cancers including breast cancer.33,34 Indeed, one proposed mechanism for the suppression of ERα expression is through the hyperactivation of mitogen activated protein kinases (MAPK) due to EGFR and HER2.35–45
EGFR is believed to be a marker of poor prognosis in breast cancer patients.39,46 A significant inverse relationship between ERα and EGFR expression has been observed in human breast cancer specimens and human breast cancer cell lines.39,47 It appears that EGFR does not alter ERα gene expression but EGFR mRNA expression is negatively regulated by ERα.48 ERα-negative breast cancer cells that overexpress EGFR activate the EGF growth signaling pathway and bypass the requirement of E2 for proliferation. Hence, activation of EGFR may play a role in cell growth in ERα-negative breast cancers and targeting EGFR could be a therapeutic strategy.31,49
Overexpression of a second member of the HER family, HER2, is observed in 20–25% of breast cancers, usually as a consequence of HER-2 gene amplification; such tumors are divided between ERα-positive and ERα-negative but ERα positive breast cancers with amplified HER2 possess low ERα and PgR content in comparison to ER positive breast cancers that are HER2 negative.50,51 Thus, reduced ERα and PgR content is a proposed mechanism of hormonal resistance in HER2 overexpressing tumors, and the possibility that anti-HER-2 targeted therapy might help to restore endocrine sensitivity has been raised.33,43,51–53
MAPK
One proposed mechanism for the emergence of ERα-negative breast cancers from an ERα-positive background is hyperactivation of the MAPK pathway; possible triggers for MAPK hyperactivation can include overexpression of EGFR and/or HER2, estrogen withdrawal, or hypoxia. Using engineered cell line models of upregulated growth factor signaling, it has been demonstrated that hyperactivation of MAPK represses ERα expression.35 However, inhibition of ERα through MAPK is dynamic and reversible, and inhibition of the MAPK pathway in MAPK-overexpressing MCF-7 cells reverses the effects and allows the re-expression of ERα.35,54 Importantly, either abrogation of the MAPK pathway using MAPK inhibitors or inhibition of upstream signaling from overexpressed growth factor receptors like EGFR or HER-2 using targeted agents in ERα negative breast cancers cell will, in some cases, result in ERα re-expression, restoration of estrogen dependency and sensitivity to hormonal therapy.54 These effects can be amplified by the concomitant use of a MAPK inhibitor and an epigenetic modifier. For example, treatment of ERα negative breast cancer cell lines with the classic DNMT inhibitor, 5-aza-2’-deoxycytidine (5-azaDc) for three days (which reduces MAPK activity 9.4 fold), followed by inhibition of MAPK signaling (leading to a further 4.4. decrease in MAPK activity) synergistically increased re-expression of ERα protein (1.5-fold in comparison to azaDc alone) at eight hours.35
Nuclear factor κB (NFκB)
The NFκB complex is a ubiquitously expressed family of inducible transcription factors.55–57 Activation of NFκB has been implicated in promotion of proliferation, motility and invasion and inhibition of apoptosis during mammary gland development. Normally NFκB is inactive and is sequestered in cytoplasm by IκB inhibitory proteins. However, once activated, NFκB translocates to the nucleus and binds to specific DNA sequences to induce transcription of target genes.58
Variable NFκB activity has been observed in human breast cancer cell models. NFκB is constitutively active in most ERα negative human breast cancer cell lines (MDA-MB-231 and SKBR3) and shows minimal activity in ERα positive/HER2 negative breast cancer cells (MCF-7 and T47D). Intermediate NFκB activity has been seen in ERα positive/HER2 positive breast cancer cell lines including tamoxifen-resistant BT474 and MCF7/HER2 cells.58–60 A study in tumors from 81 breast cancer patients with ERα-positive breast cancers treated with tamoxifen showed that higher NFκB activity as measured by higher DNA binding is significantly correlated with lower ERα content. Further, AP-1-DNA binding activity as well as uPA and HER2 protein content showed a strong correlation with NFκB activity and disease-free survival.58 Although the mechanism is not well understood, inhibition of NFκB activity by estrogen bound ERα is widely proposed.61,62 Indeed, in human breast cancer cell lines and tissues from malignant tumors, NFκB was predominantly activated in ERα negative tumors (mostly in ERα negative and HER2 positive).63 It is possible that NFκB activation in ERα negative breast cancer cells is a consequence of enhanced growth factor signaling as both EGFR and HER2 activate NFκB64 via the PI3-kinase/Akt pathway.65
In aggregate, these data suggest that activation of signaling through one or more factors including EGFR, HER2, MAPK and/ or NFκB plays a key role in ERα expression. These factors may act independently or collectively, resulting in ERα loss and altered clinical course. Therefore inhibitors that target MAPKs, EGFR, HER2 and NFκB alone or in combination with standard chemotherapies are rational agents to pursue in a quest to restore the reactivation of hormone sensitivity in ERα-negative breast cancers.
Epigenetic silencing of ERα
Epigenetic events are defined as heritable changes in gene expression without alterations in DNA sequence. Epigenetic silencing involves the interplay of multiple processes including chromatin modifications, alterations in nucleosomal positioning/remodeling, and DNA methylation, resulting in the transcriptional downregulation of target genes. Gene silencing via such epigenetic changes is necessary for regulating many normal biological processes. Dysregulation of these processes that results in aberrant epigenetic control is a common occurrence in many cancers.66 We, and others, have hypothesized that epigenetic alterations in the promoter of ERα may play an important role in ERα inactivation and hence hormone resistance in breast cancer. Particular areas of focus include the role of methylation of the ERα promoter and the contributions of modifications of histone proteins in the expression and silencing of ERα.
Methylation of ERα promoter
DNA methylation involves a covalent modification of the C5 position of the pyrimidine ring of cytosine residues occurring in CpG dinucleotides. These CpG dinucleotides are primarily found clustered in, and near, the promoter regions of genes. Human cancer cells typically exhibit global hypomethylation and promoter specific hypermethylation, resulting in genomic instability.67 Aberrant promoter methylation is most commonly implicated in transcriptional repression of tumor suppressor genes in cancer cells. In mammalian cells, DNMT1 is a maintenance methyltransferase while DNMT3a and 3b are de novo methyltransferases.68–70 Together DNMT1 and DNMT3b account for 95% of genomic methylation.71 Such promoter specific methylation could serve to block gene transcription directly or indirectly by recruitment of repressor proteins or interference with recruitment of transcription factors. Reports on aberrant methylation of ERα gene in different cancers date back two decades.72–74 Indeed, the downregulation of ERα in multiple tissues including breast, colon, lung, ovary, prostate and hematopoietic neoplasms is associated with promoter methylation.75–77
Elevated DNMT1 protein levels have been reported in breast cancer tissues and MCF-7 breast cancer cells relative to normal human mammary epithelial cells (HMECs).78 Initial cell culture studies also suggested that DNMT1 mRNA and protein levels are typically higher in ERα negative than ERα positive breast cancer cell lines.76,79 DNMT1 protein expression is cell cycle-dependent in normal and ERα positive breast cancer cells, while its expression was felt to be elevated and cell cycle independent in ERα negative breast cancer cells.80,81 Dysregulation of DNMT1 in breast cancer appears to reflect increased DNMT1 protein stability, perhaps due to the destruction of the N-terminal region of DNMT1 that is required for its proper ubiquitination and degradation.78,82 A role for DNMT3b is suggested by a study in tissue samples from breast cancer patients. Here, overexpression of DNMT3b mRNA (30%) predominated over DNMT1 (5.4%) and DNMT3a (3.1%) and DNMT3b overexpression was significantly related to ERα negativity and poor relapse free survival in the patient samples.83
The ERα gene is comprised of eight exons and spans 300 kb of the chromosome 6q25.1 locus.84,85 Like many nuclear receptor genes, the ERα gene has multiple promoters (A–F) that can give rise to isoforms with differentially spliced 5’ UTR regions.86 Examination of normal and malignant breast cell lines and tissue identified three ERα gene transcripts arising from distinct promoters, including the proximal promoter and two distal promoters located approx imately 2 kb and 21 kb upstream (promoter B and promoter C respectively).87–90 The 5’ upstream region of ERα has areas that are heavily CG-enriched,91,92 and aberrant methylation has been observed here in multiple human cancers.21,22,76,93–97 Methylation of CpGs at both the proximal promoter and distal promoter B has been associated with altered ERα expression in human breast cell lines as well as in primary breast cancer specimens. In ERα-positive breast cancer cell lines the ERα promoter is predominately unmethy-lated at both the proximal promoter and distal promoter B, whereas methylation of both these regions is observed in ERα-negative breast cancer cell lines (Table 1).98–101 This is also observed in human breast cancer specimens where ERα CpG island methylation of both the proximal promoter and distal promoter B is far more common in primary ER-negative human breast cancers than in ERα-positive breast cancer specimens.101–104
Table 1.
Epigenetic Regulation of ERα in Normal and Malignant Human Breast Cell Lines
| Cell line (ref) | ER expression |
Methylation proximal promoter |
Methylation distal (B) promoter |
Cell line origin |
Epigenetic factors associated with specific ER promoter regions |
|---|---|---|---|---|---|
| MCF10A (98, 99) | − | − | NE | F | NE |
| MCF12A (98) | +/− | − | NE | F | NE |
| MCF7 (100, 119, 120, 133) | ++ | − | +/− | IDC |  |
| T47D (101) | ++ | − | − | IDC | NE |
| ZR-75-1 (101) | + | − | + | IDC | NE |
| SKBR3 (102) | − | + | NE | IDC | NE |
| Hs578t (19) | − | + | NE | IDC | NE |
| MDA-MB-468 (19) | − | + | NE | IDC | NE |
| MDA-MB-231 (19, 101, 119, 133) | − | + | +/− | IDC |  |
NE, not examined; F, fibrocystic disease; IDC, invasive ductal carcinoma. Epigenetic factors categorized by color; DNA Methyltransferases (red), Methyl-CpG-binding proteins (blue), histone modifying enzymes (green) and other transcriptional repressors (purple). <1>,<2>,<3> and <4> designate specific promoter regions (as identified by the black arrows in Fig. 3) found to be associated with unique sets of epigenetic factors.
Increased prevalence of aberrant methylation of ERα 5’CpG islands has been reported as human breast cancer progresses from ductal carcinoma in situ (34%) to invasive ductal carcinoma (52%) to metastatic/locally advanced disease (61%) in a total of 111 human breast tumor samples.93,105 In addition, the ERα repression typically observed in BRCA1-linked breast cancers is associated with methylation of critical CpG sites within the ERα promoter, and patients with methylated ERα are three times more likely to be BRCA1-methylated than patients with unmethylated ERα.102,106,107 However, no clear correlation has been observed between ERα methylation and ERα gene expression in PgR+ve breast tumors. This discrepancy may reflect that exon 1A of the ERα promoter rather than the promoter region itself was selected for this study.108 DNA methylation is also an important mechanism for ER-β gene silencing in breast cancer and treatment with DNMT inhibitors restores ER-β expression in ER-β-negative breast cancer cell lines.103,109
Although methylation appears to play an important role in ERα downregulation it is not the only mechanism involved in ERα silencing. Increasing evidence suggests that methylation is closely associated with histone deacetylation.13 Alteration in steps that precede methylation including loss of transcriptional coactivators and factors that block methyltransferases are other proposed causes.110
Histone modifications
DNA is wrapped around structural proteins called histones to form nucleosomes. These histones may be modified in a variety of ways to alter the accessibility of the associated DNA. Direct modifications can include acetylation, methylation, phosphorylation and ubiquitination of residues in the aminoterminal tail of core histones. A variety of proteins are responsible for the addition of these groups, includingx histone methyltransferases, demethylases, deacetylases and acetyltransferases. Additionally, histone/DNA associations may be regulated by ATP-dependent chromatin remodeling factors which adjust nucleosome positioning. The resulting histone code can alter chromatin structure, regulate the accessibility of the packaged DNA to transcriptional activator proteins, and thereby modulate gene expression.111
Histone methylation, which is regulated by histone methyltransferases (HMTs) and demethylases, occurs on the lysine and arginine residues of the histone tails. Lysine residues can be mono-, di- or tri-methylated. Histone methylation on different residues appears to provide binding sites for specific transcriptional regulators thereby positively or negatively regulating gene expression. Methylation of lysine 4 and 6 of histone H3 (H3K4 and H3K6) is associated with transcriptional activation while methylation of lysine 9 of histone H3 (H3K9) is associated with transcriptional silencing.112–118 SUV39H1, which methylates H3K9, has been identified at the ERα promoter in several breast cancer cell lines (Fig. 3 and Table 1).119 Additionally, the silenced ERα promoter in ERα negative human breast cancer cells is associated with methylated H3K9 while the active promoter in ERα positive cell lines is associated with methylated H3K4 by chromatin immunoprecipita-tion assay (Table 1).120
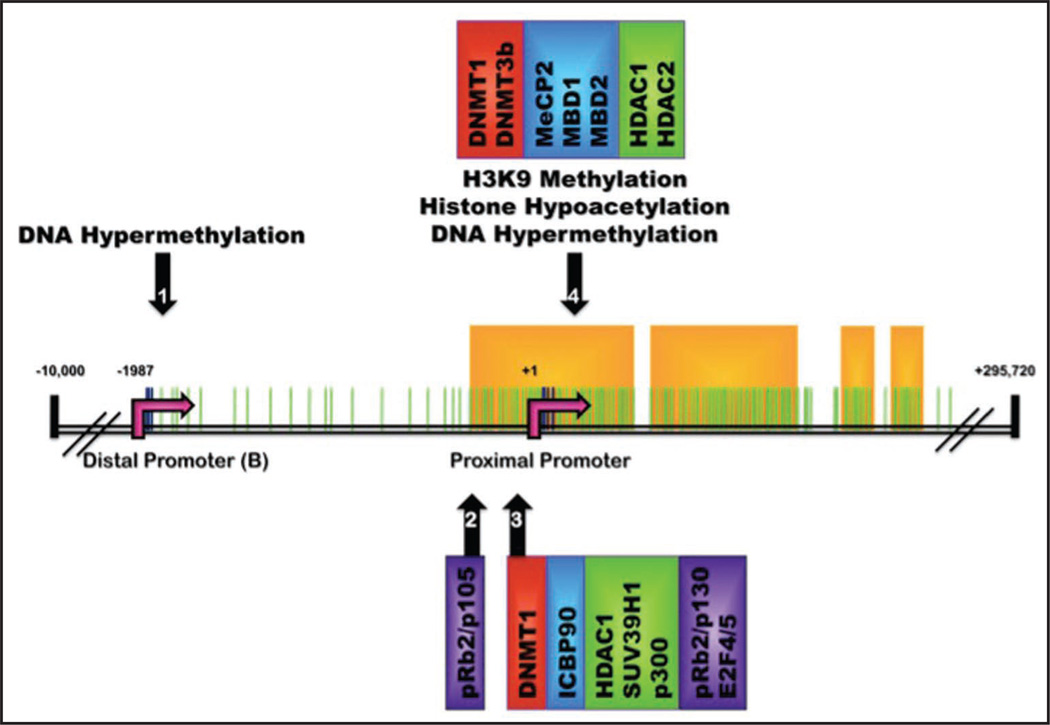
Figure 3.
Methylation sites and epigenetic complexes identified at the estrogen receptor α promoter: Schematic of the ERα gene (ESR1) on chromosome 6q25.1. CpG dinucleotides located on ERα promoter region from −2000 to +2000 are depicted by green lines. Specific CpG residues demonstrated to be sites of potential CpG methylation are marked by dark blue lines and are situated at the distal (B) and proximal promoters (identified by pink arrows at −1987 and +1 respectively). Yellow boxes designate predicted CpG islands (as defined by www.urogene.org/methprimer) which identify four regions of dense CpG population located at the proximal promoter (nucleotides −280 to +359) and just upstream of the proximal promoter (residing at nucleotides +443 to +1140; +1380 to +1427; and +1463 to +1563). Promoter regions determined to contain specific DNA and histone modifications are marked by numbered black arrows. Epigenetic regulators associated with each specific promoter region include DNA Methyltransferases (red boxes), Methyl-CpG-Binding Proteins (blue boxes), histone modifying enzymes (green boxes) and other transcriptional repressors (purple boxes).
Acetylation of histones (primarily H3 and H4) on the lysine residues of the N-terminal tails is maintained by a dynamic balance in the activity of the histone acetyltransferases (HATs) and the histone deacetylases (HDACs). It is proposed that decetylation represses gene expression prior to long-term silencing by DNA methylation.121 HDACs fall into several classes including class I (HDAC 1, 2, 3 and 8), class II (HDAC 4, 5, 6, 7, 9 and 10) and class III (sirtuins or SIRT1–7).122 Because multiple HDACs exist, there has been much interest in which HDACs might play a role in the epigenetic silencing of ERα. This has been only partially studied. HDAC1 has been shown to be associated with the silenced ERα promoter in ERα-negative breast cancer cell lines; other HDACs were not evaluated in these studies.123,124 Indeed, overexpression of HDAC1 in MCF-7 cells induces loss of ERα and increases cell proliferation and colony formation, all of which could be reversed by trichostatin-A (TSA).125,126 That HDAC2 is more relevant than HDAC1 or HDAC6 in regulation of ERα and PR expression and potentiation of the effects of tamoxifen in both ERα negative and ERα positive human breast cancer cell lines was reported by Bicaku et al.127 In sum, though, a comprehensive analysis of the role of individual HDACs in ERα expression and silencing has not been completed.
It has recently been reported that HDACs also regulate the functions of non-histone proteins including transcription factors and regulators, signal transduction mediators, DNA repair enzymes, nuclear import regulators, chaperone proteins, structural proteins, inflammation mediators and viral proteins in a pattern similar to histone proteins.128 Alteration in acetylation status of these proteins affects their DNA-binding affinity, stability and/or subcellular organization, leading to alterations in signal transduction.122,129 Another unexpected target is DNMT1. HDAC inhibition results in DNMT1 degradation through the ubiquitin-proteosomal pathway. HDAC inhibition results in hyperacetylation of the molecular chaperone heat shock protein 90 (Hsp90) that forms a chaperone complex with DNMT1. This leads to its dissociation from DNMT1 followed by ubiquitination and degradation of DNMT1.82 Therefore it appears that HDAC inhibition not only results in chromatin modifications that favor an active chromatin but also potentially promotes gene reactivation through degradation of DNMT1.
Components of corepressor complexes
Apart from the engagement of DNMT and HDAC in epigenetic silencing, a variety of other DNA binding proteins and co-repressors have roles in the regulation of chromatin structure and transcription including the methyl CpG binding domain proteins (MBDs) and the metastasis associated proteins (MTAs).130,131 These proteins appear to serve as epigenetic regulators that read or interpret methylation rather than directly modifying DNA methylation; they are of interest as they might serve as promoter specific components of corepressor complexes.130,131 Chromatin immunoprecipitation (ChIP) assay was used to carry out comparative mapping of the ERα promoter for localization of MBD family proteins in MCF-7 and MDA-MB-231 cells. MBD1, MBD2 and MeCP2 but not MBD3 were easily detected at the ERα promoter in ERα-negative MDA-MB-231 cells but not in the ERα-positive MCF-7 (Fig. 3 and Table 1). The pattern of MBD localization at the ERα promoter paralleled that of DNMTs and HDACs, indicating that MBDs may also be involved in epigenetic silencing of ERα. Indeed treatment with either DNMT or HDAC inhibitors simultaneously released these MBDs, DNMTs and HDACs from the ERα promoter as shown through ChIP in conjunction with ERα reexpression. The findings that DNMTs, HDACs and corepressor complex elements like MBDs may be acting individually and in unison is a current model under investigation for epigenetic silencing of ERα.120
The role of other components of corepressor complexes like MTA family proteins on epigenetic silencing of ERα is also under investigation. The three main members of this family; MTA1, MTA2 and MTA3, are reported to play a role in breast cancer invasion and metastasis. MTA1s, an isoform of MTA1, is cytoplasm-specific and sequesters ERα in the cytoplasm and has been implicated in hormone resistance in breast cancers.132
Other factors
It has also been suggested that the transcription and methylation pattern of ERα could be determined by protein-protein and protein-DNA interactions around specific sites of the promoter. Studies by Macaluso and colleagues have identified specific regions of the ERα promoter that are differentially bound by members of the retinoblastoma family including pRb1/p105, p107 and pRb/p130 and suggest that these associations may induce modifications of local chromatin structure possibly leading to increased DNA methylation. This group has additionally identified regionspecific binding of pRB2/p130-E2F4/5-HDAC1-SUV39H1-p300 and pRB2/p130-E2F4/5-HDAC1-SUV39H1-DNMT1 complexes in ERα positive and ERα negative breast cancer cells respectively (Fig. 3 and Table 1).119,133
Potential Strategies to Reactivate ER α Expression and Activity
Preclinical data suggesting that ERα expression can be modulated by the upregulation of growth factor signaling pathways and/or epigenetic silencing naturally lead to the hypothesis that abrogation of growth factor signaling pathways with targeted agents and/or reactivation of epigenetically silenced ERα with epigenetic modifiers can re-express functional ERα and perhaps restore hormone sensitivity. The rationale and experience for both of these approaches are reviewed below.
Growth Factor Signaling Inhibitors
The utility of inhibitors of the HER family or MAPK to abrogate ERα silencing has been explored in human breast cancer cell lines and transient human tumor explant cultures. Inhibition of growth factor signaling directly with the MAPK/ERK inhibitor, U0126 or indirectly by upstream inhibition of EGFR by gefitinib or HER2 through trastuzumab resulted in functional ERα expression in several ERα negative breast cancer cell lines with varying levels of EGFR and HER2 expression including SUM 149 (high levels of EGFR), SUM 229 (EGFR overexpressor) and SUM 190 (EGFR and HER2 overexpressor). Treatment with these compounds sensitized all the breast cancer cells studied (except SUM 149 that has high NFκB activity) to 4-hydroxytamoxifen. These studies were extended to show that short term ex vivo treatment of ERα-negative human breast cancer explants with the MAPK inhibitor, U0126, led to enhanced ERα mRNA expression in six of ten specimens. Further, U0126 treatment of a cell line established from one of these ERα-negative tumors led to re-expression of ERα and sensitization of the cells to tamoxifen or fulvestrant.54
However, treatment of two ERα negative breast cancer cell lines with a basal phenotype, SUM 102 and SUM 159, that are hypermethylated at the ERα promoter with the MAPK inhibitor did not lead to re-expression of ERα, even though MAPK was downregulated. These data suggest that MAPK inhibition alone may not be sufficient to restore ERα expression in some cell lines with hypermethylation of the ERα promoter.54
Because NFκB potentiates tumorigenesis in ERα negative breast cancer cells, the possibility that NFκB inhibitors might also be exploited to reverse ERα silencing is being explored.134 Agents that target upstream NFκB signaling and downstream IκB degradation mechanisms, including antioxidants like pyrrolidine dithiocarbamate, proteasomal inhibitors like MG-132 and PS-341; and sesquiterpene lactones like parthenolide (PA) that specifically inhibit IKK are under evaluation.135–138 Natural compounds such as betullinic acid, sauchinone, rocaglamide, panepoxydone, helenalin, octylcaffeate and CAPE that also inhibit NFκB activity are worthy of study.
Epigenetic Therapeutics
Given the wealth of evidence that implicates a role for epigenetic silencing of ERα as well as the myriad of epigenetic modifiers that are available or under development, the strategy of using epigenetic modifying agents to re-express ERα with therapeutic intent has received much attention. Epigenetically targeted therapies that are available for human use include the DNMT inhibitors, 5’azacyti-dine (azaC) and 5-aza-2’-deoxycytidine also known as decitabine (5-azaDc), and the HDAC inhibitor, vorinostat or suberoylanilide hydroxamic acid (SAHA); they are approved by the US Food and Drug Administration for the treatment of myelodysplastic syndrome and refractory cutaneous T cell lymphoma respectively.
DNMT Inhibitors
Several investigators have demonstrated that treatment of ERα-negative human breast cancer cells with azaC or 5-azaDc leads to ERα mRNA and protein expression.35,120,123,139–141 There remains uncertainty about the exact mechanism.
Direct evidence demonstrating the role of CpG island methyla-tion of the ERα promoter in the epigenetic silencing of ERα gene expression in ERα negative breast cancer cells was presented by Ferguson et al.139 Treatment of MDA-MB-231 or Hs578t cells with azaC or 5-azaDc was associated with demethylation of the ERα CpG island and ERα mRNA and protein re-expression. Functionality of the reactivated ERα was shown by expression of ERα response genes like PgR as well as enhanced activity of a reporter gene linked to ERE when cells were grown in estrogen-containing conditions.139 ChIP assays of the ERα promoter in MDA-MB-231 cells that re-express ERα after 5-azaDc treatment showed depletion of DNMT1 protein and release of DNMT1, DNMT3b, MBD1, MBD2 and MeCP2 from the ERα promoter.
However, other factors might also be in play because of a decrease in DNMT levels due to either adduct formation resulting in activation of apoptotic pathways or failure to repair the adducts. A role for inhibition of DNMT1 specifically in the reactivation of silenced ERα in ERα negative breast cancers was evaluated through an antisense oligonucleotide (ASO98) against DNMT1. ASO98 treatment (but not treatment with a scrambled oligonucleotide) led to specific suppression of DNMT1 mRNA and protein expression in MDA-MB-231 and Hs578t cells, both of which have epigenetically silenced ERα. Such treatment resulted in expression of an ERα whose function could be inhibited with fulvestrant. However, there was no change in the methylation status of ERα by MSP analysis. This suggests then that re-expression of ERα after DNMT inhibition is not solely through ERα promoter demethylation but may also result from disruption of repressive transcription complexes on the ERα promoter mediated through DNMT1.142
New DNMT inhibitors are under development. One such agent is zebularine. Unlike 5-azaDc and azaC, zebularine is highly stable and shows little toxicity in preclinical testing models although it is associated with hepatic and renal toxicity in primates.143 Treatment of ERα negative MDA-MB-231 cells with zebularine resulted in ERα reactivation in association with conversion to an active chromatin at the ERα promoter as evidenced by ChIP. A limitation of zebularine is that relatively high doses of zebularine are required for ERα re-expression. However, little cytotoxicity is seen with these doses, making zebularine a potential candidate for combination therapy with other DNMT inhibitors or other types of agents.144 Alternatively novel zebularine-related inhibitors may emerge via structure-activity studies.
The possibility that natural products, especially dietary constituents, might serve as epigenetic modifiers is also under study. For example, catechol-containing dietary polyphenols and DNMTs use the same source of S-adenosyl methionine (SAM) for their reactions and treatment with polyphenols can deplete SAM and increase the levels of S-adenosyl-L-homocysteine (SAH), which is a potent inhibitor of DNA methylation.145–149 Two common coffee polyphenols, caffeic acid and chlorogenic acid, are natural DNA methylation inhibitors, and treatment of MDA-MB-231 and MCF-7 cells with these compounds partially inhibited methylation of the promoter region of retinoic acid receptor- β (RAR-β) by non-competitive inhibition of DNMTs through increased formation of SAH.150 However, the effect of these drugs and other potential demethylating dietary components on ERα reactivation is unexplored.
HDAC Inhibitors
Treatment with any of several hydroxamic acid HDAC inhibitors including trichostatin A (TSA), LBH589 and vorinostat can lead to re-expression of ERα in ERα-negative cells like MDA-MB-231 cells. TSA treatment led to a time and dose-dependent re-expression of ERα mRNA with no alteration of ERα promoter methylation. Rather TSA-activated gene transcription was associated with increased sensitivity of the ERα promoter to DNase 1 treatment (indicative of active chromatin) suggesting that inactive chromatin is a major factor for epigenetic silencing.151 ChIP assay showed that TSA treatment led to accumulation of acetylated H3 and H4 and release of HDAC1 from the ERα promoter.120
Detailed studies with LBH589 show reactivation of ERα mRNA and protein expression occurs as early as 24 hours after treatment and re-expression was stable for at least 96 hours after withdrawal of treatment. ChIP assay of the ERα promoter showed that LBH589 released DNMT1, HDAC1 and the H3-K9 methyltransferase SUV39H1 from the ERα promoter. These changes were associated with an active chromatin conformation manifested as accumulation of acetylated histone H3 and H4, decrease in methylated H3-K9, and impaired binding of heterochromatin protein 1 (HP1α) at the promoter.124 Thus, studies with several HDAC inhibitors have shown that re-expression of ERα through HDAC inhibition does not alter the methylation status of the ER promoter. It appears that HDAC inhibitor-induced re-expression of the ER is likely mediated through the reorganization of heterochromatin associated proteins without a change in the promoter hypermethylation.124,151 Such ERα is functional as LBH589 treatment of MDA-MB-231 cells enhanced tamoxifen sensitivity.124 However, HDAC inhibitors are likely to have a multitude of other effects in ERα-negative breast cancer cells as well including promotion of DNMT1 degradation by interrupting DNMT1 association with HSP90 as discussed earlier.82 In addition, the possibility that HDAC inhibitors might sensitize ERα-negative cell lines to tamoxifen by activation of ER β has also been proposed.152 It is noteworthy that HDAC inhibitor-induced ERα expression has not been uniformly observed by all investigators; this may reflect differences in tissue culture cells that have evolved over time or variations in culture conditions.127,153
Of note, the treatment of ERα-positive human breast cancer cell lines with HDAC inhibitors like MS-275, vorinostat, LBH529 or TSA decreases ERα expression and may also sensitize these cells to tamoxifen. Indeed Bicaku et al. have reported that HDAC inhibitor treatment of human breast cancer cell lines of diverse phenotypes with regard to ERα and HER-2 expression can sensitize these cells to tamoxifen, presumably by multiple mechanisms.127 One potential mechanism appears to be that treatment with several different HDAC inhibitors can induce hsp90 hyperacetylation, thereby decreasing its binding to ERα, leading to increased polyubiquitylation and depletion of ERα levels.123,124,127,153,–155 The role of individual HDAC isoenzymes on ERα and PgR expression was evaluated using pharmacological inhibitors or siRNA. This study suggests that depletion of HDAC1 and HDAC6 was associated with downregulation of ERα but not PgR in ER-positive cells while depletion of HDAC2 downregulated ERα and PgR and potentiated tamoxifen’s effects.127
Combination Epigenetic Modifier Approaches
Initiation and maintenance of epigenetic silencing is a consequence of complex interactions between DNA methylation and histone modifications to determine chromatin structure and transcriptional status.66 This implies that interventions that target both DNA methylation and histone modifications might be more effective than either strategy alone in altering transcription. It is reported that simultaneous demethylation and histone deacetylation inhibition with 5-azaDc and TSA synergistically re-expresses genes commonly hypermethylated and silenced in colorectal cancer cells.156 Similarly, treatment of ERα negative MDA-MB-231 human breast cancer cells with both DNMT and HDAC inhibitors resulted in synergistic functional ERα reactivation compared with either agent alone. Treatment with 5-azaDc alone induced the ERα transcript by 30–40-fold, while TSA increased ERα mRNA expression by ten-fold. But treatment with the two drugs in sequence led to a 300–400-fold increase in ERα mRNA expression in these cell lines. This was associated with a marked decrease in DNMT1 protein expression and activity, partial ERα CpG island demethylation and increased acetylation of H3 and H4.102,140 Combination studies in MDA-MB-231 and Hs578t cells of 5-azaDc with another HDAC inhibitor, Scriptaid, for 18 hours also showed an enhanced effect on cell growth inhibition, increased acetylation of H3 and H4, and synergistic activation of ERα mRNA and protein when compared to either individual drug given alone.141 ChIP assays have permitted a detailed dissection of changes in the protein complexes in the ER promoter with monotherapy or combined DNMT and HDAC inhibitor therapy. For example, in MDA-MB-231 cells dissociation of MeCP2, MBD1, MBD2, DNMT1 and DNMT3b was more evident after combination therapy with 5-azaDc and TSA as compared to individual drug treatments. The association of acetylated histones H3 and H4 was no greater with combination therapy than with TSA alone but combination therapy led to greater alterations in the association of methylated histones than either drug alone.120 Whether these types of combinations lead to enhanced sensitivity to targeted agents like tamoxifen over treatment with a single DNMT or HDAC inhibitor has not been carefully explored.
Future Studies
A number of opportunities for new research in this area exist. First, the availability of two DNMT inhibitors and several drugs that are felt to have HDAC inhibitory effects (including vorinostat) has facilitated the first breast cancer clinical trials with these agents. As with all biological agents, significant questions remain about the appropriate clinical scenarios for testing of a new agent and the definition for success. A traditional Phase II study of vorinostat in women with refractory breast cancer has shown disease stability in a fraction of patients but no evidence of response by classic criteria.157 A Phase II study of vorinostat and tamoxifen in women with metastatic breast cancer has established the safety of the combination and now evidence for efficacy is being sought.158 Unfortunately this single arm design does not permit an evaluation of the role of the combination over single agent therapy. Finally a “window” study of vorinostat for three days before definitive breast surgery for women with newly diagnosed untreated breast cancer has completed accrual. This brief duration of therapy will not allow any evaluation of tumor response but tissues and blood recovered before and after therapy should provide an excellent platform for confirmation of biomarker modulation observed in the preclinical studies and discovery of new targets.
While these proof of principle clinical trials with established drugs are in progress, much work is also focused on developing new agents and identifying new targets in the epigenetic pathways. As mentioned earlier one such agent is zebularine. Although its clinical development is in doubt, other epigenetically targeted agents are under evaluation. For example, polycomb group proteins like histone methyltransferase EZH2 are often overexpressed in cancers. EZH2 is reported to be associated with H3 trimethylated lysine 27 (H3K27me3) at the promoters of several silenced genes in colon, prostate and breast cancer.159–161 Whether such polycomb proteins play a role in ERα silencing is not yet understood. Lysine demethylase inhibition has also emerged as a strategy for modulating epigenetic gene silencing and certain polyamine analogues appear to function as lysine demethylase inhibitors. Their use has been associated with the re-expression of epigenetically silenced genes in colon cancer models; whether these same agents that are in some cases known to inhibit human breast cancer cell growth might also re-express epigenetically silenced ERα is not known. In addition, the silencing of other components of corepressor complexes like the MBDs, which read and interpret DNA methylation without modifying DNA, resulted in reactivation of specific genes associated with MBDs.130 Development of drugs that target MBDs and other components of corepressor complexes localized on the ERα promoter also potentially represent a novel strategy for ERα re-expression with minimal toxicity.
Finally, although most work in the field of epigenetic modulation focuses on the treatment of established cancer with synthetic drugs, it is also imperative that research into non-drug interventions, especially dietary components, continue. These approaches are especially attractive as they would theoretically be devoid of toxicity and widely applicable through the diet. Evidence that caffeic acid and EGCG might function as DNMT inhibitors is emerging.162 In addition, it is reported that dietary components like sulphoraphane, the active chemopreventive compound from cruciferous vegetables, acts as an HDAC inhibitor in vitro in human breast cancer cells.163 That these dietary components might act in concert is suggested by a report that treatment with EGCG and sulphoraphane can synergistically enhance transcriptional activation of AP-1 in HC-29 cells.164 In addition, a well established negative relationship exists between the micronutrient selenium and breast cancer.165 Indeed, breast carcinoma cell lines are more sensitive to selenium induced apoptosis than non-tumorigenic mammary epithelial cells.166 It has recently been reported that sodium selenite, a selenium derivative, is both a DNMT and HDAC inhibitor in LNCaP prostate cancer cells. Exposure results in demethylation and reactivation of genes commonly silenced in cancers like glutathione-S-transferase, adenomatous polyposis coli and cellular stress response 1.167 In sum these studies hold promise for the development of dietary components as epigenetically targeted agents although their value in breast cancer generally and estrogen receptor expression specifically is untested. It seems likely, however, that the value of such approaches will be appreciated largely in the prevention rather than in the treatment setting.
Finally, several reports have speculated that a methyl-deficient diet may be a risk factor for breast cancer since CpG methylation is an early event in cancer incidence and a methyl-deficient diet can result in CpG methylation.149 Because the epidemiological evidence specifically points to a relationship between a methyl-group deficient diet and incidence of ERα negative breast cancer, it was postulated that such dietary deficiency would be associated with epigenetic silencing in the ERα gene. A small epidemiological study in African-American women was not able to establish that link however.148
Acknowledgements
This work was supported by NIH CA88843, Department of Defense Predoctoral Award BC050707 and the Breast Cancer Research Foundation.
References
- 1.ACS. Cancer Facts & Figures. Atlanta: American Cancer Society; 2008. 2008. [Google Scholar]
- 2.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 3.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.McGuire WL. Hormone receptors: their role in predicting prognosis and response to endocrine therapy. Semin Oncol. 1978;5:428–433. [PubMed] [Google Scholar]
- 5.McGuire WL, Horwitz KB, Zava DT, Garola RE, Chamness GC. Hormones in breast cancer: update 1978. Metabolism. 1978;27:487–501. doi: 10.1016/0026-0495(78)90103-8. [DOI] [PubMed] [Google Scholar]
- 6.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- 8.Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- 9.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 10.Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- 11.Song RX, Fan P, Yue W, Chen Y, Santen RJ. Role of receptor complexes in the extranuclear actions of estrogen receptor alpha in breast cancer. Endocr Relat Cancer. 2006;13:3–13. doi: 10.1677/erc.1.01322. [DOI] [PubMed] [Google Scholar]
- 12.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Nakao M. Epigenetics: interaction of DNA methylation and chromatin. Gene. 2001;278:25–31. doi: 10.1016/s0378-1119(01)00721-1. [DOI] [PubMed] [Google Scholar]
- 14.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 15.Pedram A, Razandi M, Wallace DC, Levin ER. Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell. 2006;17:2125–2137. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CL. Cross-talk between peptide growth factor and estrogen receptor signaling pathways. Biol Reprod. 1998;58:627–632. doi: 10.1095/biolreprod58.3.627. [DOI] [PubMed] [Google Scholar]
- 17.Yager JD, Chen JQ. Mitochondrial estrogen receptors—new insights into specific functions. Trends Endocrinol Metab. 2007;18:89–91. doi: 10.1016/j.tem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Johnston SR, Dowsett M, Smith IE. Towards a molecular basis for tamoxifen resistance in breast cancer. Ann Oncol. 1992;3:503–511. doi: 10.1093/oxfordjournals.annonc.a058251. [DOI] [PubMed] [Google Scholar]
- 19.Lapidus RG, Nass SJ, Davidson NE. The loss of estrogen and progesterone receptor gene expression in human breast cancer. J Mammary Gland Biol Neoplasia. 1998;3:85–94. doi: 10.1023/a:1018778403001. [DOI] [PubMed] [Google Scholar]
- 20.Roodi N, Bailey LR, Kao WY, Verrier CS, Yee CJ, Dupont WD, et al. Estrogen receptor gene analysis in estrogen receptor-positive and receptor-negative primary breast cancer. J Natl Cancer Inst. 1995;87:446–451. doi: 10.1093/jnci/87.6.446. [DOI] [PubMed] [Google Scholar]
- 21.Lapidus RG, Nass SJ, Butash KA, Parl FF, Weitzman SA, Graff JG, et al. Mapping of ER gene CpG island methylation-specific polymerase chain reaction. Cancer Res. 1998;58:2515–2519. [PubMed] [Google Scholar]
- 22.Tang Z, Treilleux I, Brown M. A transcriptional enhancer required for the differential expression of the human estrogen receptor in breast cancers. Mol Cell Biol. 1997;17:1274–1280. doi: 10.1128/mcb.17.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Lynch MD, Cariati M, Purushotham AD. Breast cancer, stem cells and prospects for therapy. Breast Cancer Res. 2006;8:211. doi: 10.1186/bcr1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006;12:296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- 27.Weissman I. Stem cell research: paths to cancer therapies and regenerative medicine. Jama. 2005;294:1359–1366. doi: 10.1001/jama.294.11.1359. [DOI] [PubMed] [Google Scholar]
- 28.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 29.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesen-chymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boerner JL, Gibson MA, Fox EM, Posner ED, Parsons SJ, Silva CM, et al. Estrogen negatively regulates epidermal growth factor (EGF)-mediated signal transducer and activator of transcription 5 signaling in human EGF family receptor-overexpressing breast cancer cells. Mol Endocrinol. 2005;19:2660–2670. doi: 10.1210/me.2004-0439. [DOI] [PubMed] [Google Scholar]
- 32.Mosesson Y, Yarden Y. Oncogenic growth factor receptors: implications for signal transduc-tion therapy. Semin Cancer Biol. 2004;14:262–270. doi: 10.1016/j.semcancer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Revillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34:791–808. doi: 10.1016/s0959-8049(97)10157-5. [DOI] [PubMed] [Google Scholar]
- 34.Revillion F, Lhotellier V, Hornez L, Bonneterre J, Peyrat JP. ErbB/HER ligands in human breast cancer, and relationships with their receptors, the bio-pathological features and prognosis. Ann Oncol. 2008;19:73–80. doi: 10.1093/annonc/mdm431. [DOI] [PubMed] [Google Scholar]
- 35.Oh AS, Lorant LA, Holloway JN, Miller DL, Kern FG, El-Ashry D. Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. Mol Endocrinol. 2001;15:1344–1359. doi: 10.1210/mend.15.8.0678. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson S, Halcrow P, Sainsbury JR, Angus B, Chambers P, Farndon JR, et al. Epidermal growth factor receptor (EGFr) status associated with failure of primary endocrine therapy in elderly postmenopausal patients with breast cancer. Br J Cancer. 1988;58:810–814. doi: 10.1038/bjc.1988.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson S, Richard J, Sainsbury C, Halcrow P, Kelly P, Angus B, et al. Epidermal growth factor receptor (EGFr); results of a 6 year follow-up study in operable breast cancer with emphasis on the node negative subgroup. Br J Cancer. 1991;63:146–150. doi: 10.1038/bjc.1991.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholson S, Sainsbury JR, Halcrow P, Chambers P, Farndon JR, Harris AL. Expression of epidermal growth factor receptors associated with lack of response to endocrine therapy in recurrent breast cancer. Lancet. 1989;1:182–185. doi: 10.1016/s0140-6736(89)91202-6. [DOI] [PubMed] [Google Scholar]
- 39.Nicholson S, Wright C, Sainsbury JR, Halcrow P, Kelly P, Angus B, et al. Epidermal growth factor receptor (EGFr) as a marker for poor prognosis in node-negative breast cancer patients: neu and tamoxifen failure. J Steroid Biochem Mol Biol. 1990;37:811–814. doi: 10.1016/0960-0760(90)90424-j. [DOI] [PubMed] [Google Scholar]
- 40.Sainsbury JR, Farndon JR, Harris AL, Sherbet GV. Epidermal growth factor receptors on human breast cancers. Br J Surg. 1985;72:186–188. doi: 10.1002/bjs.1800720309. [DOI] [PubMed] [Google Scholar]
- 41.Sainsbury JR, Farndon JR, Needham GK, Malcolm AJ, Harris AL. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987;1:1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- 42.Toi M, Osaki A, Yamada H, Toge T. Epidermal growth factor receptor expression as a prognostic indicator in breast cancer. Eur J Cancer. 1991;27:977–980. doi: 10.1016/0277-5379(91)90262-c. [DOI] [PubMed] [Google Scholar]
- 43.Gusterson BA. Identification and interpretation of epidermal growth factor and c-erbB-2 overexpression. Eur J Cancer. 1992;28:263–267. doi: 10.1016/0959-8049(92)90429-6. [DOI] [PubMed] [Google Scholar]
- 44.Sivaraman VS, Wang H, Nuovo GJ, Malbon CC. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salh B, Marotta A, Matthewson C, Ahluwalia M, Flint J, Owen D, et al. Investigation of the Mek-MAP kinase-Rsk pathway in human breast cancer. Anticancer Res. 1999;19:731–740. [PubMed] [Google Scholar]
- 46.Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 47.Davidson NE, Gelmann EP, Lippman ME, Dickson RB. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol Endocrinol. 1987;1:216–223. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- 48.Sheikh MS, Shao ZM, Chen JC, Li XS, Hussain A, Fontana JA. Expression of estrogen receptors in estrogen receptor-negative human breast carcinoma cells: modulation of epidermal growth factor-receptor (EGF-R) and transforming growth factor alpha (TGFalpha) gene expression. J Cell Biochem. 1994;54:289–298. doi: 10.1002/jcb.240540305. [DOI] [PubMed] [Google Scholar]
- 49.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17:309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- 50.Eppenberger-Castori S, Kueng W, Benz C, Caduff R, Varga Z, Bannwart F, et al. Prognostic and predictive significance of ErbB-2 breast tumor levels measured by enzyme immunoassay. J Clin Oncol. 2001;19:645–656. doi: 10.1200/JCO.2001.19.3.645. [DOI] [PubMed] [Google Scholar]
- 51.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 52.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 53.Perren TJ. c-erbB-2 oncogene as a prognostic marker in breast cancer. Br J Cancer. 1991;63:328–332. doi: 10.1038/bjc.1991.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayliss J, Hilger A, Vishnu P, Diehl K, El-Ashry D. Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clin Cancer Res. 2007;13:7029–7036. doi: 10.1158/1078-0432.CCR-07-0587. [DOI] [PubMed] [Google Scholar]
- 55.Baeuerle PA, Baltimore D. NFkappaB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh S, May MJ, Kopp EB. NFkappaB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 57.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC. The NFkappaB pathway and endocrine-resistant breast cancer. Endocr Relat Cancer. 2005;12:37–46. doi: 10.1677/erc.1.00977. [DOI] [PubMed] [Google Scholar]
- 59.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NFkappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tyree CM, Zou A, Allegretto EA. 17beta-Estradiol inhibits cytokine induction of the human E-selectin promoter. J Steroid Biochem Mol Biol. 2002;80:291–297. doi: 10.1016/s0960-0760(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 62.Valentine JE, Kalkhoven E, White R, Hoare S, Parker MG. Mutations in the estrogen receptor ligand binding domain discriminate between hormone-dependent transactivation and transrepression. J Biol Chem. 2000;275:25322–25329. doi: 10.1074/jbc.M002497200. [DOI] [PubMed] [Google Scholar]
- 63.Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, Iglehart JD. NFkappaB activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA. 2004;101:10137–10142. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biswas DK, Iglehart JD. Linkage between EGFR family receptors and nuclear factor kappaB (NFkappaB) signaling in breast cancer. J Cell Physiol. 2006;209:645–652. doi: 10.1002/jcp.20785. [DOI] [PubMed] [Google Scholar]
- 65.Pianetti S, Arsura M, Romieu-Mourez R, Coffey RJ, Sonenshein GE. Her-2/neu overex-pression induces NFkappaB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IkappaB-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–1299. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- 66.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones PA. DNA methylation and cancer. Oncogene. 2002;21:5358–5360. doi: 10.1038/sj.onc.1205597. [DOI] [PubMed] [Google Scholar]
- 68.Bestor T. Structure of mammalian DNA methyltransferase as deduced from the inferred amino acid sequence and direct studies of the protein. Biochem Soc Trans. 1988;16:944–947. doi: 10.1042/bst0160944. [DOI] [PubMed] [Google Scholar]
- 69.Chen T, Li E. Establishment and maintenance of DNA methylation patterns in mammals. Curr Top Microbiol Immunol. 2006;301:179–201. doi: 10.1007/3-540-31390-7_6. [DOI] [PubMed] [Google Scholar]
- 70.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 71.Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 72.Piva R, Castagnoli A, Maestri I, Rimondi AP, Giovannini G, del Senno L. Hypomethylation of the human estrogen receptor gene in primary human breast cancers. Int J Biol Markers. 1988;3:65. doi: 10.1177/172460088800300114. [DOI] [PubMed] [Google Scholar]
- 73.Piva R, Kumar LV, Hanau S, Maestri I, Rimondi AP, Pansini SF, et al. The methylation pattern in the 5’ end of the human estrogen receptor gene is tissue specific and related to the degree of gene expression. Biochem Int. 1989;19:267–275. [PubMed] [Google Scholar]
- 74.Piva R, Rimondi AP, Hanau S, Maestri I, Alvisi A, Kumar VL, et al. Different methylation of oestrogen receptor DNA in human breast carcinomas with and without oestrogen receptor. Br J Cancer. 1990;61:270–275. doi: 10.1038/bjc.1990.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinzone JJ, Stevenson H, Strobl JS, Berg PE. Molecular and cellular determinants of estrogen receptor alpha expression. Mol Cell Biol. 2004;24:4605–4612. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- 77.Yao J, Huang Q, Zhang X, Fu W. Promoter CpG methylation of estrogen receptors in leukemia. Biosci Rep. 2008 doi: 10.1042/BSR20080140. [DOI] [PubMed] [Google Scholar]
- 78.Agoston AT, Argani P, Yegnasubramanian S, De Marzo AM, Ansari-Lari MA, Hicks JL, et al. Increased protein stability causes DNA methyltransferase 1 dysregulation in breast cancer. J Biol Chem. 2005;280:18302–18310. doi: 10.1074/jbc.M501675200. [DOI] [PubMed] [Google Scholar]
- 79.Ferguson AT, Vertino PM, Spitzner JR, Baylin SB, Muller MT, Davidson NE. Role of estrogen receptor gene demethylation, DNA methyltransferase DNA adduct formation in 5-aza-2’deoxycytidine-induced cytotoxicity in human breast cancer cells. J Biol Chem. 1997;272:32260–32266. doi: 10.1074/jbc.272.51.32260. [DOI] [PubMed] [Google Scholar]
- 80.Szyf M. DNA methylation patterns: an additional level of information? Biochem Cell Biol. 1991;69:764–767. doi: 10.1139/o91-117. [DOI] [PubMed] [Google Scholar]
- 81.Nass SJ, Ferguson AT, El-Ashry D, Nelson WG, Davidson NE. Expression of DNA methyl-transferase (DMT) and the cell cycle in human breast cancer cells. Oncogene. 1999;18:7453–7461. doi: 10.1038/sj.onc.1203138. [DOI] [PubMed] [Google Scholar]
- 82.Zhou Q, Agoston AT, Atadja P, Nelson WG, Davidson NE. Inhibition of histone deacety-lases promotes ubiquitin-dependent proteasomal degradation of DNA methyltransferase 1 in human breast cancer cells. Mol Cancer Res. 2008;6:873–883. doi: 10.1158/1541-7786.MCR-07-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Girault I, Lerebours F, Amarir S, Tozlu S, Tubiana-Hulin M, Lidereau R, et al. Expression analysis of estrogen receptor alpha coregulators in breast carcinoma: evidence that NCOR1 expression is predictive of the response to tamoxifen. Clin Cancer Res. 2003;9:1259–1266. [PubMed] [Google Scholar]
- 84.Gosden JR, Middleton PG, Rout D. Localization of the human oestrogen receptor gene to chromosome 6q24—q27 by in situ hybridization. Cytogenet Cell Genet. 1986;43:218–220. doi: 10.1159/000132325. [DOI] [PubMed] [Google Scholar]
- 85.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kos M, Reid G, Denger S, Gannon F. Minireview: genomic organization of the human ERalpha gene promoter region. Mol Endocrinol. 2001;15:2057–2063. doi: 10.1210/mend.15.12.0731. [DOI] [PubMed] [Google Scholar]
- 87.Grandien KF, Berkenstam A, Nilsson S, Gustafsson JA. Localization of DNase I hypersensitive sites in the human oestrogen receptor gene correlates with the transcriptional activity of two differentially used promoters. J Mol Endocrinol. 1993;10:269–277. doi: 10.1677/jme.0.0100269. [DOI] [PubMed] [Google Scholar]
- 88.Grandien K, Backdahl M, Ljunggren O, Gustafsson JA, Berkenstam A. Estrogen target tissue determines alternative promoter utilization of the human estrogen receptor gene in osteoblasts and tumor cell lines. Endocrinology. 1995;136:2223–2229. doi: 10.1210/endo.136.5.7720671. [DOI] [PubMed] [Google Scholar]
- 89.Hayashi S, Imai K, Suga K, Kurihara T, Higashi Y, Nakachi K. Two promoters in expression of estrogen receptor messenger RNA in human breast cancer. Carcinogenesis. 1997;18:459–464. doi: 10.1093/carcin/18.3.459. [DOI] [PubMed] [Google Scholar]
- 90.Donaghue C, Westley BR, May FE. Selective promoter usage of the human estrogen receptor-alpha gene and its regulation by estrogen. Mol Endocrinol. 1999;13:1934–1950. doi: 10.1210/mend.13.11.0366. [DOI] [PubMed] [Google Scholar]
- 91.Keaveney M, Klug J, Gannon F. Sequence analysis of the 5’ flanking region of the human estrogen receptor gene. DNA Seq. 1992;2:347–358. doi: 10.3109/10425179209020816. [DOI] [PubMed] [Google Scholar]
- 92.Piva R, Gambari R, Zorzato F, Kumar L, del Senno L. Analysis of upstream sequences of the human estrogen receptor gene. Biochem Biophys Res Commun. 1992;183:996–1002. doi: 10.1016/s0006-291x(05)80289-x. [DOI] [PubMed] [Google Scholar]
- 93.Tang B, Jiang J. Study of the CpG methylation status of ER alpha gene in estrogen receptor alpha-negative breast cancer cell lines and the role of hydralazine demethylation. Zhonghua Bing Li Xue Za Zhi. 2005;34:283–287. [PubMed] [Google Scholar]
- 94.Issa JP, Baylin SB, Belinsky SA. Methylation of the estrogen receptor CpG island in lung tumors is related to the specific type of carcinogen exposure. Cancer Res. 1996;56:3655–3658. [PubMed] [Google Scholar]
- 95.Issa JP, Zehnbauer BA, Civin CI, Collector MI, Sharkis SJ, Davidson NE, et al. The estrogen receptor CpG island is methylated in most hematopoietic neoplasms. Cancer Res. 1996;56:973–977. [PubMed] [Google Scholar]
- 96.Sasaki M, Kaneuchi M, Fujimoto S, Tanaka Y, Dahiya R. Hypermethylation can selectively silence multiple promoters of steroid receptors in cancers. Mol Cell Endocrinol. 2003;202:201–207. doi: 10.1016/s0303-7207(03)00084-4. [DOI] [PubMed] [Google Scholar]
- 97.Sasaki M, Tanaka Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto S, et al. Methylation and inactivation of estrogen, progesterone and androgen receptors in prostate cancer. J Natl Cancer Inst. 2002;94:384–390. doi: 10.1093/jnci/94.5.384. [DOI] [PubMed] [Google Scholar]
- 98.Pilat MJ, Schwab ED, Yao KL, Pienta KJ. Examination of the DNA methylation properties in nontumorigenic and tumorigenic breast epithelial cell lines. Anticancer Res. 1998;18:2575–2582. [PubMed] [Google Scholar]
- 99.Shekhar PV, Chen ML, Werdell J, Heppner GH, Miller FR, Christman JK. Transcriptional activation of functional endogenous estrogen receptor gene expression in MCF10AT cells: a model for early breast cancer. Int J Oncol. 1998;13:907–915. doi: 10.3892/ijo.13.5.907. [DOI] [PubMed] [Google Scholar]
- 100.Sogon T, Masamura S, Hayashi S, Santen RJ, Nakachi K, Eguchi H. Demethylation of promoter C region of estrogen receptor alpha gene is correlated with its enhanced expression in estrogen-ablation resistant MCF-7 cells. J Steroid Biochem Mol Biol. 2007;105:106–114. doi: 10.1016/j.jsbmb.2006.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshida T, Eguchi H, Nakachi K, Tanimoto K, Higashi Y, Suemasu K, et al. Distinct mechanisms of loss of estrogen receptor alpha gene expression in human breast cancer: methylation of the gene and alteration of trans-acting factors. Carcinogenesis. 2000;21:2193–2201. doi: 10.1093/carcin/21.12.2193. [DOI] [PubMed] [Google Scholar]
- 102.Wei M, Xu J, Dignam J, Nanda R, Sveen L, Fackenthal J, et al. Estrogen receptor alpha, BRCA1 and FANCF promoter methylation occur in distinct subsets of sporadic breast cancers. Breast Cancer Res Treat. 2008;111:113–120. doi: 10.1007/s10549-007-9766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC, et al. Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene. 2003;22:7600–7606. doi: 10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]
- 104.Lapidus RG, Ferguson AT, Ottaviano YL, Parl FF, Smith HS, Weitzman SA, et al. Methylation of estrogen and progesterone receptor gene 5’ CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin Cancer Res. 1996;2:805–810. [PubMed] [Google Scholar]
- 105.Nass SJ, Herman JG, Gabrielson E, Iversen PW, Parl FF, Davidson NE, et al. Aberrant methylation of the estrogen receptor and E-cadherin 5’ CpG islands increases with malignant progression in human breast cancer. Cancer Res. 2000;60:4346–4348. [PubMed] [Google Scholar]
- 106.Archey WB, McEachern KA, Robson M, Offit K, Vaziri SA, Casey G, et al. Increased CpG methylation of the estrogen receptor gene in BRCA1-linked estrogen receptor-negative breast cancers. Oncogene. 2002;21:7034–7041. doi: 10.1038/sj.onc.1205844. [DOI] [PubMed] [Google Scholar]
- 107.Foulkes WD, Metcalfe K, Sun P, Hanna WM, Lynch HT, Ghadirian P, et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade and histological type. Clin Cancer Res. 2004;10:2029–2034. doi: 10.1158/1078-0432.ccr-03-1061. [DOI] [PubMed] [Google Scholar]
- 108.Widschwendter M, Siegmund KD, Muller HM, Fiegl H, Marth C, Muller-Holzner E, et al. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64:3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- 109.Skliris GP, Munot K, Bell SM, Carder PJ, Lane S, Horgan K, et al. Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol. 2003;201:213–220. doi: 10.1002/path.1436. [DOI] [PubMed] [Google Scholar]
- 110.Chen Z, Ko A, Yang J, Jordan VC. Methylation of CpG island is not a ubiquitous mechanism for the loss of oestrogen receptor in breast cancer cells. Br J Cancer. 1998;77:181–185. doi: 10.1038/bjc.1998.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bird AMolecular biology. Methylation talk between histones and DNA. Science. 2001;294:2113–2115. doi: 10.1126/science.1066726. [DOI] [PubMed] [Google Scholar]
- 112.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 113.Lachner M, Jenuwein T. The many faces of histone lysine methylation. Curr Opin Cell Biol. 2002;14:286–298. doi: 10.1016/s0955-0674(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 114.Kouzarides T. Histone methylation in transcriptional control. Curr Opin Genet Dev. 2002;12:198–209. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- 115.Richards EJ, Elgin SC. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell. 2002;108:489–500. doi: 10.1016/s0092-8674(02)00644-x. [DOI] [PubMed] [Google Scholar]
- 116.Fahrner JA, Eguchi S, Herman JG, Baylin SB. Dependence of histone modifications and gene expression on DNA hypermethylation in cancer. Cancer Res. 2002;62:7213–7218. [PubMed] [Google Scholar]
- 117.Lehnertz B, Ueda Y, Derijck AA, Braunschweig U, Perez-Burgos L, Kubicek S, et al. Suv39 h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol. 2003;13:1192–1200. doi: 10.1016/s0960-9822(03)00432-9. [DOI] [PubMed] [Google Scholar]
- 118.Tariq M, Saze H, Probst AV, Lichota J, Habu Y, Paszkowski J. Erasure of CpG methylation in Arabidopsis alters patterns of histone H3 methylation in heterochromatin. Proc Natl Acad Sci USA. 2003;100:8823–8827. doi: 10.1073/pnas.1432939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Macaluso M, Montanari M, Noto PB, Gregorio V, Bronner C, Giordano A. Epigenetic modulation of estrogen receptor-alpha by pRb family proteins: a novel mechanism in breast cancer. Cancer Res. 2007;67:7731–7737. doi: 10.1158/0008-5472.CAN-07-1476. [DOI] [PubMed] [Google Scholar]
- 120.Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol. 2005;19:1740–1751. doi: 10.1210/me.2004-0011. [DOI] [PubMed] [Google Scholar]
- 121.Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci USA. 2008;105:14867–14872. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 123.Sharma D, Saxena NK, Davidson NE, Vertino PM. Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res. 2006;66:6370–6378. doi: 10.1158/0008-5472.CAN-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou Q, Atadja P, Davidson NE. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER) gene expression without loss of DNA hypermethylation. Cancer Biol Ther. 2007;6:64–69. doi: 10.4161/cbt.6.1.3549. [DOI] [PubMed] [Google Scholar]
- 125.Kawai H, Li H, Avraham S, Jiang S, Avraham HK. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor alpha. Int J Cancer. 2003;107:353–358. doi: 10.1002/ijc.11403. [DOI] [PubMed] [Google Scholar]
- 126.Varshochi R, Halim F, Sunters A, Alao JP, Madureira PA, Hart SM, et al. ICI182,780 induces p21Waf1 gene transcription through releasing histone deacetylase 1 and estrogen receptor alpha from Sp1 sites to induce cell cycle arrest in MCF-7 breast cancer cell line. J Biol Chem. 2005;280:3185–3196. doi: 10.1074/jbc.M408063200. [DOI] [PubMed] [Google Scholar]
- 127.Bicaku E, Marchion DC, Schmitt ML, Munster PN. Selective inhibition of histone deacetylase 2 silences progesterone receptor-mediated signaling. Cancer Res. 2008;68:1513–1519. doi: 10.1158/0008-5472.CAN-07-2822. [DOI] [PubMed] [Google Scholar]
- 128.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 129.Yang XJ, Gregoire S. Class II histone deacetylases: from sequence to function, regulation and clinical implication. Mol Cell Biol. 2005;25:2873–2884. doi: 10.1128/MCB.25.8.2873-2884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lopez-Serra L, Ballestar E, Ropero S, Setien F, Billard LM, Fraga MF, et al. Unmasking of epigenetically silenced candidate tumor suppressor genes by removal of methyl-CpG-binding domain proteins. Oncogene. 2008;27:3556–3566. doi: 10.1038/sj.onc.1211022. [DOI] [PubMed] [Google Scholar]
- 131.Sansom OJ, Maddison K, Clarke AR. Mechanisms of disease: methyl-binding domain proteins as potential therapeutic targets in cancer. Nat Clin Pract Oncol. 2007;4:305–315. doi: 10.1038/ncponc0812. [DOI] [PubMed] [Google Scholar]
- 132.Kumar R, Wang RA, Mazumdar A, Talukder AH, Mandal M, Yang Z, et al. A naturally occurring MTA1 variant sequesters oestrogen receptor-alpha in the cytoplasm. Nature. 2002;418:654–657. doi: 10.1038/nature00889. [DOI] [PubMed] [Google Scholar]
- 133.Macaluso M, Cinti C, Russo G, Russo A, Giordano A. pRb2/p130-E2F4/5-HDAC1-SUV39H1-p300 and pRb2/p130-E2F4/5-HDAC1-SUV39H1-DNMT1 multimolecular complexes mediate the transcription of estrogen receptor-alpha in breast cancer. Oncogene. 2003;22:3511–3517. doi: 10.1038/sj.onc.1206578. [DOI] [PubMed] [Google Scholar]
- 134.Singh S, Shi Q, Bailey ST, Palczewski MJ, Pardee AB, Iglehart JD, et al. Nuclear factor-kappaB activation: a molecular therapeutic target for estrogen receptor-negative and epidermal growth factor receptor family receptor-positive human breast cancer. Mol Cancer Ther. 2007;6:1973–1982. doi: 10.1158/1535-7163.MCT-07-0063. [DOI] [PubMed] [Google Scholar]
- 135.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NFkappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappaB activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cardoso F, Ross JS, Picart MJ, Sotiriou C, Durbecq V. Targeting the ubiquitin-proteasome pathway in breast cancer. Clin Breast Cancer. 2004;5:148–157. doi: 10.3816/cbc.2004.n.020. [DOI] [PubMed] [Google Scholar]
- 138.Hehner SP, Hofmann TG, Droge W, Schmitz ML. The antiinflammatory sesquiterpene lactone parthenolide inhibits NFkappaB by targeting the IkappaB kinase complex. J Immunol. 1999;163:5617–5623. [PubMed] [Google Scholar]
- 139.Ferguson AT, Lapidus RG, Baylin SB, Davidson NE. Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res. 1995;55:2279–2283. [PubMed] [Google Scholar]
- 140.Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001;61:7025–7029. [PubMed] [Google Scholar]
- 141.Keen JC, Yan L, Mack KM, Pettit C, Smith D, Sharma D, et al. A novel histone deacetylase inhibitor, scriptaid, enhances expression of functional estrogen receptor alpha (ER) in ER negative human breast cancer cells in combination with 5-aza 2’-deoxycytidine. Breast Cancer Res Treat. 2003;81:177–186. doi: 10.1023/A:1026146524737. [DOI] [PubMed] [Google Scholar]
- 142.Yan L, Nass SJ, Smith D, Nelson WG, Herman JG, Davidson NE. Specific inhibition of DNMT1 by antisense oligonucleotides induces reexpression of estrogen receptor-alpha (ER) in ER-negative human breast cancer cell lines. Cancer Biol Ther. 2003;2:552–556. doi: 10.4161/cbt.2.5.469. [DOI] [PubMed] [Google Scholar]
- 143.Johnson WD, Harder JB, Naylor J, McCormick DL, Detrisac CJ, Glaze ER, et al. A pharmacokinetic/pharmacodynamic approach to evaluating the safety of zebularine in non-human primates. Washington DC: American Association of Cancer Research; 2006. [Google Scholar]
- 144.Billam M, Sobolewski M, Davidson NE. AACR Annual meeting. San diego, CA: AACR; 2008. Effects of a Novel DNMT inhibitor zebularine on human breast cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu BT, Evaristus EN, Antoniak SK, Sarabia SF, Ricci MJ, Liehr JG. Metabolic deglucuronidation and demethylation of estrogen conjugates as a source of parent estrogens and catecholestrogen metabolites in Syrian hamster kidney, a target organ of estrogeninduced tumorigenesis. Toxicol Appl Pharmacol. 1996;136:186–193. doi: 10.1006/taap.1996.0023. [DOI] [PubMed] [Google Scholar]
- 146.Zhu BT, Ezell EL, Liehr JG. Catechol-O-methyltransferase-catalyzed rapid O-methylation of mutagenic flavonoids Metabolic inactivation as a possible reason for their lack of carcinogenicity in vivo. J Biol Chem. 1994;269:292–299. [PubMed] [Google Scholar]
- 147.Zhu BT, Patel UK, Cai MX, Lee AJ, Conney AH. Rapid conversion of tea catechins to monomethylated products by rat liver cytosolic catechol-O-methyltransferase. Xenobiotica. 2001;31:879–890. doi: 10.1080/00498250110079798. [DOI] [PubMed] [Google Scholar]
- 148.Zhu K, Davidson NE, Hunter S, Yang X, Payne-Wilks K, Roland CL, et al. Methyl-group dietary intake and risk of breast cancer among African-American women: a case-control study by methylation status of the estrogen receptor alpha genes. Cancer Causes Control. 2003;14:827–836. doi: 10.1023/b:caco.0000003823.97506.be. [DOI] [PubMed] [Google Scholar]
- 149.Zhu K, Williams SM. Methyl-deficient diets, methylated ER genes and breast cancer: an hypothesized association. Cancer Causes Control. 1998;9:615–620. doi: 10.1023/a:1008819210777. [DOI] [PubMed] [Google Scholar]
- 150.Lee WJ, Zhu BT. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis. 2006;27:269–277. doi: 10.1093/carcin/bgi206. [DOI] [PubMed] [Google Scholar]
- 151.Yang X, Ferguson AT, Nass SJ, Phillips DL, Butash KA, Wang SM, et al. Transcriptional activation of estrogen receptor alpha in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 2000;60:6890–6894. [PubMed] [Google Scholar]
- 152.Jang ER, Lim SJ, Lee ES, Jeong G, Kim TY, Bang YJ, et al. The histone deacetylase inhibitor trichostatin A sensitizes estrogen receptor alpha-negative breast cancer cells to tamoxifen. Oncogene. 2004;23:1724–1736. doi: 10.1038/sj.onc.1207315. [DOI] [PubMed] [Google Scholar]
- 153.Fiskus W, Ren Y, Mohapatra A, Bali P, Mandawat A, Rao R, et al. Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-alpha levels and transcriptional activity: a result of hyperacetylation and inhibition of chaperone function of heat shock protein 90. Clin Cancer Res. 2007;13:4882–4890. doi: 10.1158/1078-0432.CCR-06-3093. [DOI] [PubMed] [Google Scholar]
- 154.Hodges-Gallagher L, Valentine CD, Bader SE, Kushner PJ. Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat. 2007;105:297–309. doi: 10.1007/s10549-006-9459-6. [DOI] [PubMed] [Google Scholar]
- 155.Duong V, Licznar A, Margueron R, Boulle N, Busson M, Lacroix M, et al. ERalpha and ERbeta expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene. 2006;25:1799–1806. doi: 10.1038/sj.onc.1209102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethyla-tion and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 157.Luu TH, Morgan RJ, Leong L, Lim D, McNamara M, Portnow J, et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin Cancer Res. 2008;14:7138–7142. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Munster PN, Lacevic M, Schmitt M, Bicaku E, Marchion D, Stephens A, et al. Phase II trial of vorinostat, a histone deacetylase inhibitor to restore the hormone sensitivity to the antiestrogen tamoxifen in patients with advanced breast cancer having failed prior aromatase inhibitor therapy. Journal of Clinical Oncology (Meeting Abstracts) 2008;26:3501. [Google Scholar]
- 159.Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, et al. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet. 2008;40:741–750. doi: 10.1038/ng.159. [DOI] [PubMed] [Google Scholar]
- 160.McGarvey KM, Fahrner JA, Greene E, Martens J, Jenuwein T, Baylin SB. Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res. 2006;66:3541–3549. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- 161.McGarvey KM, Greene E, Fahrner JA, Jenuwein T, Baylin SB. DNA methylation and complete transcriptional silencing of cancer genes persist after depletion of EZH2. Cancer Res. 2007;67:5097–5102. doi: 10.1158/0008-5472.CAN-06-2029. [DOI] [PubMed] [Google Scholar]
- 162.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137:223–228. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 163.Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6:1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- 164.Nair S, Hebbar V, Shen G, Gopalakrishnan A, Khor TO, Yu S, et al. Synergistic effects of a combination of dietary factors sulforaphane and (-) epigallocatechin-3-gallate in HT-29 AP-1 human colon carcinoma cells. Pharm Res. 2008;25:387–399. doi: 10.1007/s11095-007-9364-7. [DOI] [PubMed] [Google Scholar]
- 165.el-Bayoumy K, Chae YH, Upadhyaya P, Ip C. Chemoprevention of mammary cancer by diallyl selenide, a novel organoselenium compound. Anticancer Res. 1996;16:2911–2915. [PubMed] [Google Scholar]
- 166.Jariwalla RJ, Gangapurkar B, Nakamura D. Differential sensitivity of various human tumour-derived cell types to apoptosis by organic derivatives of selenium. Br J Nutr. 2008:1–8. doi: 10.1017/S0007114508998305. [DOI] [PubMed] [Google Scholar]
- 167.Xiang N, Zhao R, Song G, Zhong W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis. 2008;29:2175–2181. doi: 10.1093/carcin/bgn179. [DOI] [PMC free article] [PubMed] [Google Scholar]