Abstract
The combination of lenalidomide and low-dose dexamethasone is an effective treatment for multiple myeloma (MM). Addition of alkylating agents to lenalidomide or thalidomide results in increased response rates and deeper responses. We designed this trial to study the combination of cyclophosphamide, lenalidomide, and dexamethasone (CRd) as initial therapy for MM. Fifty-three patients with previously untreated symptomatic MM was enrolled. Patients received 4-week treatment cycles consisting of lenalidomide (25 mg daily for 3 weeks), dexamethasone (40 mg weekly), and cyclophosphamide (300 mg/m2 weekly for 3 weeks). A partial response or better was seen in 85% of patients including 47% with a very good partial response or better. The toxicities were manageable with over 80% of planned doses delivered; six patients went off study for toxicity. The median progression free survival (PFS) for the entire group was 28 months (95% CI: 22.7–32.6) and the overall survival (OS) at 2 years was 87% (95% CI: 78–96). Importantly, 14 patients with high-risk MM had similar PFS and OS as the standard-risk patients (n = 39). CRd is an effective and well-tolerated regimen for upfront therapy of MM with high response rates and excellent 2-year OS, and is suitable for long-term therapy. Am. J. Hematol. 86:640–645, 2011.
Introduction
Approaches to treating symptomatic multiple myeloma (MM) have undergone a paradigm change during the past decade, mostly a result of the introduction of new drugs such as thalidomide and lenalidomide and the proteasome inhibitor bortezomib [1–7]. Since then, substantial progress has been made by rationally combining them with older drugs such as corticosteroids and alkylating agents [5,6,8–16]. Compared to traditional regimens like vincristine, adriamycin, and dexamethasone (VAD), the new drug combinations provides deeper responses prior to and following high dose therapy (HDT) and autologous stem cell transplantation. Thalidomide and bortezomib have also been combined with melphalan in patients ineligible for HDT, resulting in high response rates and improved overall survival (OS) [9–12]. Melphalan is typically avoided in HDT eligible patients because of the risk of stem cell damage. In contrast, cyclophosphamide is used in these patients as part of initial therapy and as priming chemotherapy for stem cell collection [17]. Combinations of cyclophosphamide with thalidomide as well as bortezomib have previously been studied as induction therapy for newly diagnosed MM [8,18,19]. Given the potent antimyeloma activity of lenalidomide, cyclophosphamide has been studied in combination with lenalidomide for relapsed disease, with excellent efficacy and safety [20]. In this context, we present the first study on the combination of cyclophosphamide, lenalidomide and low dose dexamethasone (CRd) in previously untreated MM. The purpose of this phase II trial was to determine the response rate, time to progression, and the safety of this regimen in the frontline setting.
Patients and methods
Eligibility
Patients with previously untreated MM, requiring therapy were enrolled on this trial provided they had measurable or evaluable disease defined as having one of the following: serum M-protein ≥1.0 g/dL, urinary M-protein excretion ≥200 mg in 24 hr, serum immunoglobulin free light chain (FLC) assay with involved FLC ≥10 mg/dL AND abnormal FLC ratio, or bone marrow plasmacytosis ≥30%. Patients were required to have adequate hematologic and organ function with absolute neutrophil count (ANC) ≥1,500/μL, platelet count ≥75,000/μL, hemoglobin ≥8.0 gm/dL, serum creatinine ≤2.5 mg/dL, and AST <3 times the upper limit of normal, all obtained within 21 days of enrollment. Patients had to have an ECOG performance status of 0, 1, or 2 for inclusion in the trial. Patients who had received any prior treatment for MM and those with uncontrolled infection or another active malignancy, New York Heart Association class III or IV, or DVT that has not been therapeutically anticoagulated were excluded from the trial. Prior corticosteroid use for the treatment of MM was not permitted; prior corticosteroid use for the treatment of nonmalignant disorders was permitted but concurrent use was restricted to the equivalent of prednisone 10 mg or less per day. Pregnant or nursing women, as well as women of childbearing potential who were unwilling to use a dual method of contraception, and men who were unwilling to use a condom were not eligible for the study. Patients were required to be at least 18 years of age. The trial was performed with approval of the Mayo Clinic Institutional Review Board in accordance with the principles of the Helsinki Declaration and the trial was registered at www.clinicaltrials.gov (NCT00478218).
Treatment schedule
The treatment schedule consisted of 4-week cycles with lenalidomide given at 25 mg PO days 1-21, cyclophosphamide 300 mg/m2 PO given days 1, 8, and 15 and dexamethasone 40 mg PO given days 1, 8, 15, and 22 (weekly continuously). Each cycle was repeated every 4 weeks. The last 19 patients received a lower dose of cyclophosphamide (300 mg PO days 1, 8, and 15) in combination with the same doses of lenalidomide and dexamethasone, to examine if this would reduce hematological toxicities. Patients were allowed to go off treatment after four cycles of therapy to pursue stem cell transplantation if desired, but treatment beyond four cycles was permitted at physician's discretion. Stem cell mobilization protocol was not specified in the clinical trial and was typically performed using growth factor alone, with a few patients receiving cyclophosphamide pulsing followed by growth factor administration. For patients continuing on therapy, cyclophosphamide was given for a maximum of 12 cycles, but lenalidomide with or without dexamethasone could be continued until disease progression at physician discretion. Thromboprophylaxis consisted of aspirin 325 mg given daily, with low molecular weight heparin or coumadin recommended for patients with history of prior thrombotic events or in patients considered at higher risk for a thrombotic event based on presence of risk factors.
Dose adjustments were permitted based on toxicity. Lenalidomide was permanently discontinued for erythema multiforme/Stevens Johnson syndrome Grade III or higher, desquamating/blistering rash of any grade, any rash of Grade IV severity, Grade IV neuropathy or hypersensitivity, and Grade III or higher bradycardia or cardiac arrhythmia. Subjects experiencing other Grade III or greater adverse events felt related to lenalidomide or cyclophosphamide had the drug held until resolution of the adverse event and restarted at the next lower dose level. Hematologic toxicities required dose reductions of cyclophosphamide and lenalidomide, while other toxicities thought to be related to either one of the drugs only required reduction of the suspected drug. When Grade III or IV adverse events occurred prior to day 15 of a cycle and resolved to Grade II or lower severity prior to day 21 of the cycle, drugs were resumed at the next lower dose level until day 21, with the next cycle continuing at the reduced dose level. For Grade III or IV adverse events occurring on or after day 15 of a given cycle, they were held for the remainder of the cycle and reduced by one dose level beginning with the next cycle. Once the dose of any of the drugs was reduced for toxicity, no dose re-escalation was permitted. Patients unable to tolerate the lowest doses of any of the drugs needed to stop therapy with that agent permanently. Routine antibiotic, antiviral or antifungal prophylaxis was not mandated and left to the discretion of the treating physician. Bisphosphonate use for bone prophylaxis was allowed and followed standard clinical recommendations.
Response and toxicity criteria
The primary endpoint of this trial was the proportion of confirmed responses (complete response, CR; very good partial response, VGPR; or partial response, PR) noted as the objective status on two consecutive evaluations at least 2 weeks apart. Confirmed responses were evaluated using the first 4 months of treatment as well as the best response seen during the entire trial. Responses were assessed using both the EBMT criteria (per protocol, for interim efficacy analysis) [21] as well as the International Myeloma Working group (IMWG) unified response criteria [22]. All toxicities were graded and attributed according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3. Toxicity was defined as an adverse event considered being possibly, probably, or definitely related to treatment.
Statistical analysis
The primary endpoint for the study was the overall response rate at the end of four cycles. All patients meeting the eligibility criteria who signed a consent form and had begun treatment were considered evaluable for response. Secondary endpoints included the best response to therapy across all cycles of therapy, patterns and severity of toxicities across all cycles and discontinuation rate due to toxicity, stem cell collection failure rate, and engraftment kinetics among those undergoing a stem cell transplantation and the OS, progression free survival (PFS), and duration of response (DOR) from diagnosis among this group of patients receiving primary therapy with the combination. OS was defined as the time between registration date and death due to any cause, with those alive censored at the date of last follow up. PFS was defined as the time from registration to disease progression or death due to any cause, with those alive and progression free censored at the time of last follow up. DOR was defined as the time from first response until the date of progression (or date of list follow-up in patients without progression) in the subset of patients who responded to treatment. The distributions of survival time, PFS and DOR were estimated using the method of Kaplan-Meier.
Results
The trial enrolled 53 patients between July 2006 and May 2008. The baseline characteristics are as shown in Table I. The primary measurable disease parameter was a serum M-protein ≥1 gm/dL, 24 hr urine M-Protein ≥200 mg or an FLC difference of ≥10 mg/dL in 74, 11, and 15% patients, respectively. The median duration on study for the entire group was 6.4 months (range: 1.6–49+) and the median number of cycles administered was five. Six patients did not complete four cycles of therapy; three of these patients went off for toxicity, treating physician switched one responding patient to alternate therapy, and two patients had progressed. Overall, 1 patient died on study and 10 patients remain on study after a median of 33 (range: 27–48) cycles of therapy. Forty-one patients were alive at the time of the analysis with a median follow up of 37 months (range: 25–49).
Table I. Patient Characteristics.
| Total (N = 53) | |
|---|---|
| Age, median (range) | 64 (37–82) |
| Age> 65 years | 23 (43%) |
| Gender, male | 27 (51%) |
| ECOG Performance Score | |
| 0 | 21 (40%) |
| 1 | 24 (45%) |
| 2 | 8 (15%) |
| ISS Stage | |
| Stage I | 18 (34%) |
| Stage II | 21 (40%) |
| Stage III | 14 (26%) |
| Isotype | |
| IgG | 36 (68%) |
| IgA | 10 (19%) |
| Light-chain | 9 (17%) |
| Parameters of Hematologic Response | |
| Serum M-Spike ≥ 1.0 g/dL | 39 (74%) |
| Serum Free Light Chain ≥ 10 mg/dL | 35 (66%) |
| Urine M-Spike ≥ 200 mg/24 hr | 15 (28%) |
| Bone Marrow Plasma Cells > 30% | 31 (58%) |
| High-risk Multiple Myeloma | 14 (26%) |
| t(4;14) | 4 (8%) |
| t(14;16) | 2 (4%) |
| Del 17p- | 4 (8%) |
| Del 13 (metaphase cytogenetics) | 3 (6%) |
| PCLI > 3% | 7 (13%) |
| Serum Creatinine, mg/dL; median (range) | 1.0 (0.6–2.1) |
| Creatinine clearance < 50 mL/mina | 8 (15%) |
| Lytic bone disease | 40 (75%) |
Calculated creatinine clearance.
N (%) unless otherwise indicated.
Response to therapy
Twenty (66%; 95%CI: 47–83) of the first 30 patients met the protocol-defined response criteria (EBMT) for confirmed response, which passed the threshold for success. The subsequent analysis uses all 53 evaluable patients using the IMWG unified response criteria. The response to therapy was assessed following administration of four cycles of therapy or prior to proceeding to HDT among those going to transplant before completing four cycles. Forty-seven patients received at least four cycles of therapy, with six discontinuing prior to four cycles for toxicity, progression or alternative treatment. The overall response to therapy at four cycles was 79% (42/53), including a VGPR or better rate of 30% (16/53) and CR rate of 2% (1/53). The depth of responses is as shown in the waterfall plot (Fig. 1). The best response achieved on trial was assessed using all patients enrolled on the trial with 45 patients achieving a PR or better with an overall response rate of 85%. A VGPR or better was seen in 25 patients (47%). Eighteen patients (34%) continued therapy beyond cycle 12. The estimated time (median, months) to first response (PR or better) for the 45 patients who responded was 1.4 months (range: 1– 8) and to a VGPR or better (N = 25) was 3.8 months (1– 19). Median DOR was 30.9 months (95%CI: 23–NA). The responses are further detailed in Table II and were not affected by reduction in the cyclophosphamide dose.
Figure 1.
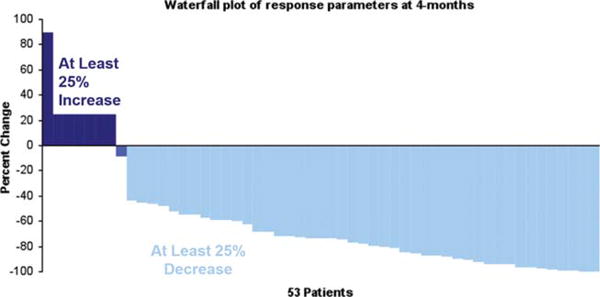
Figure presents the actual decrease in the M-protein measurements as a proportion of the baseline values using a waterfall plot of response depth at the end of four cycles of therapy. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II. Response to Therapy.
| Full dose cyclophosphamide N (%) | Reduced dose cyclophosphamide N (%) | All Patients N (%) | |
|---|---|---|---|
| Total number of patients | 34 | 19 | 53 |
| Overall response rates at 4 cycles (>=PR) | 26 (77) | 16 (84) | 42 (79) |
| ≥ VGPR | 11 (32) | 5 (26) | 16 (30) |
| CR | 0 | 1 | 1 |
| VGPR | 11 | 4 | 15 |
| PR | 15 | 11 | 26 |
| Best Response across all cycles (≥PR) | 29 (85%) | 16 (84%) | 45 (85%) |
| ≥ VGPR | 15 (44) | 10 (53) | 25 (47) |
| CR/sCR | 3 | 4 | 7 |
| VGPR | 12 | 6 | 18 |
| PR | 14 | 6 | 20 |
Response rate was calculated using the International Myeloma Working Group unified response criteria for all evaluable patients.
Toxicity of the combination
The most common toxicities seen across the trial were hematologic, with nearly 60% of patients having at least one episode of Grade 3 or 4 neutropenia. The most common non-hematological toxicity seen across the study was fatigue. The overall frequency and highest grade of various toxicities, considered at least possibly related to therapy, among the 53 patients enrolled on the study is as shown in Fig. 2A. The frequency and the grade of toxicities across all the 699 cycles of therapy administered are shown in Fig. 2B. Six patients discontinued study therapy due to toxicity after a median of four cycles of therapy. There were 63 (9%) instances of treatment delays, involving 30 patients, across 699 cycles administered. The most common reason for delay in re-treatment was neutropenia (68%). The overall tolerability of the regimen was excellent with over 80% of the targeted dose of lenalidomide and cyclophosphamide delivered as planned. The proportion of patients with dose reductions and reasons for reductions are summarized in Table III. The reduction in the cyclophosphamide doses did not have any clear impact on the toxicity. The median total white count, neutrophil count and platelet count across the initial 12 cycles are presented in Fig. 2C and does not suggest any cumulative toxicity.
Figure 2.
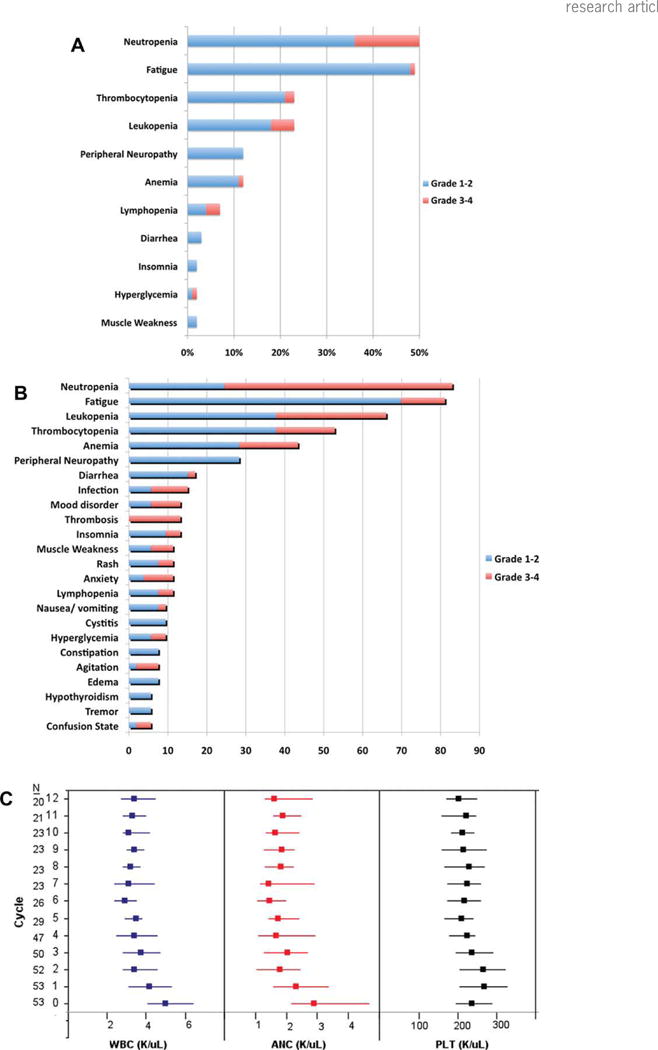
A: Figure provides information on the frequency and grade of various toxicities seen across the trial, grouped by patients (n = 53). The maximum grade of an individual toxicity seen in any given patient is presented. If a patient had multiple occurrences of a particular toxicity, only the highest grade is presented. B: Figure provides estimates of frequency and grade of various toxicities seen across all cycles (n = 699) grouped by toxicities. If a patient had multiple occurrences of any particular toxicity, each will be counted. C: Median (interquartile range; IQR) for total white cell count (WBC), ANC, and platelet counts (PLT) during the first 12 cycles of treatment are presented. There is no progressive decline in the counts over the 12 cycles, beyond that seen in the initial cycles, suggesting lack of any cumulative hematological toxicity with the combination of lenalidomide and cyclophosphamide.
Table III. Treatment Administration.
| Full dose cyclophosphamide (N = 34) | Reduced dose cyclophosphamide (N = 19) | All Patients | |
|---|---|---|---|
| Lenalidomide | |||
| No. of Cycles Administered | 428 | 267 | 695 |
| Median % of Targeted Dose (Range) | 80% (12–100) | 80% (19–100) | 80% (12–100) |
| No. of Patients with Reductions | 22 (65%) | 12 (63%) | 34 (64%) |
| Total Reductions | 41 | 26 | 67 |
| Reasons for Reductions | |||
| Neutropenia | 23 | 13 | 36 |
| Thrombocytopenia | 1 | 0 | 1 |
| Nonblistering rash | 3 | 6 | 9 |
| Grade 3+ Adverse Event | 8 | 0 | 8 |
| Other | 6 | 7 | 13 |
| Cyclophosphamide | |||
| No. of Cycles Administered | 219 | 171 | 390 |
| Median % of Targeted Dose (Range) | 96% (0–102) | 100% (11–100) | 96% (0–102) |
| No. of Patients with Reductions | 19 (56%) | 9 (47%) | 28 (53%) |
| Total Reductions | 28 | 12 | 40 |
| Reasons for Reductions | |||
| Neutropenia | 16 | 12 | 28 |
| Grade 3 + Adverse Event | 6 | 0 | 6 |
| Other | 6 | 0 | 6 |
| Dexamethasone | |||
| No. of Cycles Administered | 362 | 249 | 611 |
| Median % of Targeted Dose (Range) | 75% (0–100) | 50% (0–100) | 75% (0–100) |
| No. of Patients with Reductions | 12 (35%) | 11 (58%) | 23 (43%) |
| Total Reductions | 25 | 29 | 54 |
| Reasons for Reductions | |||
| Hyperglycemia | 0 | 6 | 6 |
| Muscle Weakness | 2 | 4 | 6 |
| Confusion/Mood Alteration | 14 | 1 | 15 |
| Insomnia | 4 | 3 | 7 |
| Other | 5 | 15 | 14 |
Stem cell collection
Thirty-one patients (58%) underwent a stem collection attempt, of whom seven patients failed to mobilize and had too few peripheral blood CD34 cells (< 10/μL) to initiate apheresis, and another patient collected only 1.63 million CD34 cells/kg, for an overall failure rate of 25%. Five of these eight patients were successfully collected subsequently with cyclophosphamide-based mobilization or with plerixafor; the remaining three patients did not attempt another collection. No relationship was seen between the number of cycles and likelihood of a failed attempt. Among the 23 patients who successfully collected adequate stem cells in the first attempt, the median CD34 yield was 7.2 million CD34 cells/kg (range; 2.9–13.1). Eighteen of the 31 patients have so far proceeded to a stem cell transplant, including 10 patients who went off study for an early transplant.
Long-term follow up and survival outcomes
The median OS from diagnosis for the entire group was not reached with a median follow up of 37 months for patients alive at last follow up (Fig. 3A). The estimated OS rate at 2 years for the entire group was 87% (95% CI: 78–96). All ten patients who went off study for a transplant were alive at the time of the last follow up. The median PFS for the entire group was 28 months (95% CI: 22.7–32.6).
Figure 3.
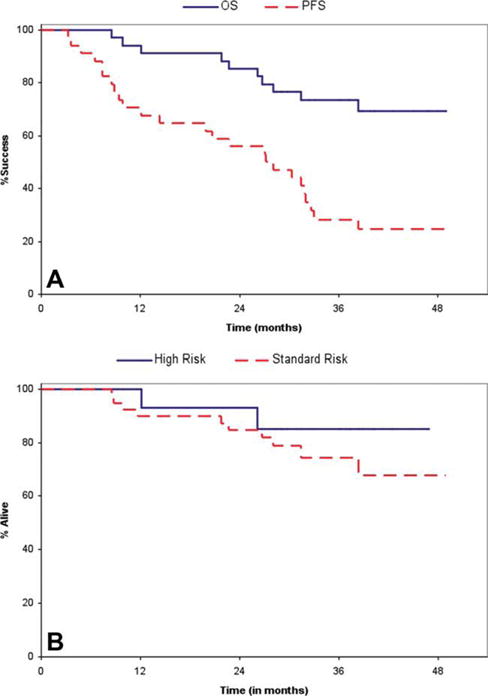
A: Figure depicts the Kaplan Meier plots for OS and PFS for all patients enrolled on the study (N = 53). B: Figure depicts the Kaplan Meier plots for OS and PFS for all patients grouped by risk status (mSMART high-risk criteria); 14 patients had high-risk myeloma and 39 patients were considered as standard risk. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fourteen patients were considered to have high-risk MM as defined by mSMART criteria. The overall response rate was 93% for the high-risk patients and 79% for the standard-risk patients. The 2-year PFS was 57% and 61% for the high and standard-risk patients, respectively (P = NS). The OS of high-risk patients were similar to that observed for the standard-risk patients (n = 39; Fig. 3B). One patient with plasma cell leukemia obtained a CR from therapy and continued on study for 36 cycles, at which time she developed progressive disease.
Discussion
Lenalidomide, either in combination with dexamethasone or as part of multidrug combinations, is increasingly becoming a part of the initial therapy of myeloma [6,23–27]. Given the excellent activity of lenalidomide seen in the phase 3 trials of lenalidomide and dexamethasone, and the promising results seen with the combination of thalidomide with alkylating agents, we designed this study to examine the efficacy of adding cyclophosphamide to lenalidomide and dexamethasone (CRd) [10,11,14]. We saw excellent activity for the combination with an overall response rate of 85% with a VGPR or better seen in over half of the patients. The overall response rate and depth of response at four cycles is higher than what was observed with the lenalidomide plus low dose dexamethasone arm (Rd), reflecting the contribution of cyclophosphamide [6]. The four cycles responses rates with the Rd in the phase three trials was 68% at four cycles with 24% having a VGPR or better. The best overall response of 85% with 47% VGPR or better was also higher than that seen with Rd (70% with 40% VGPR or better) in the phase 3 trial. Cyclophosphamide has also been combined with bortezomib for treating newly diagnosed MM, with similar response rates (ORR of 88% with ≥VGPR of 61%) [8]. However, this regimen used high dose dexamethasone and had high rates of neuropathy within the first four cycles of therapy.
The OS seen in this study is comparable to that seen in the recent studies of new drug combinations in untreated MM with over 85% 2-year survival rates. While a direct comparison is not feasible, the PFS seen in the current trial of 28 months is higher than that reported with Rd in the E4A03 trial (24 months), with a similar patient population enrolled [6]. The OS among those proceeding to a transplant was excellent, with no deaths observed among those patients compared to those who did not get a transplant, likely reflecting the better outcome in general for transplant eligible patients. The comparable OS and PFS in the current trial among the high-risk patients and standard-risk patients compares favorably to Rd, where high-risk status was associated with inferior outcomes in the newly diagnosed MM [28]. However, in patients with relapsed MM undergoing treatment with lenalidomide and dexamethasone, patients with high-risk features other than p53 abnormalities had outcomes similar to standard-risk patients [29]. It is conceivable that the combination with alkylating agent may have contributed to the ability to improve the outcome of high-risk patients.
The toxicities could be managed with dose reductions and no cumulative toxicities were seen from a hematological perspective, and overall more than 80% of the intended dose could be delivered across all cycles. However, the incidence of neutropenia appears to be considerably higher than was seen with lenalidomide and dexamethasone. The higher rate of toxicity can be explained by the addition of cyclophosphamide, even though this effect does not seem to depend entirely on the dose of cyclophosphamide, given that no difference was seen following reduction of cyclophosphamide dose. Another factor that has likely contributed to the higher degree of neutropenia seen here is the lack of use of growth factors. We did not allow growth factor use because of the concerns of continued alkylator therapy on the face of growth factor stimulation. Fatigue was a common side effect and is likely related to lenalidomide as has been seen in other studies as well.
The combination did adversely impact the success of growth factor based stem cell mobilization and harvest. The failure rate seen here is slightly higher compared to what others and we have previously seen in the context of lenalidomide and dexamethasone therapy and likely reflects the additional myelotoxic effect of cyclophosphamide [30–32]. However, it is important to note that all patients who underwent a salvage mobilization with cyclophosphamide or plerixafor did manage to collect successfully and enabled them to undergo transplant when desired.
In summary, the addition of cyclophosphamide to Rd leads to higher response rates and deeper responses as well as PFS compared to lenalidomide and low-dose dexamethasone in previous studies. While the addition led to increased rates of hematological toxicity, the side effects were manageable with dose adjustments and overall drug discontinuation rate was low. Results also suggest that the addition of cyclophosphamide may improve the outcome of patients with high-risk myeloma. The impact on stem cell collection can be overcome by cyclophosphamide or plerixafor based approaches and should be used in these patients. These results should form the basis for prospective evaluation of CRd combination comparing it with other front line regimens in phase 3 trials.
Acknowledgments
We would like to acknowledge the assistance of Tammy Mc Carty, Lori Rhodes, and Carol Schimek with protocol development and assistance with study conduct.
Contract grant sponsor: Hematologic Malignancies Program; Contract grant number: CA90628 is from NIH; Contract grant sponsor: ASCO Career Development Program; Contract grant sponsor: Celgene Corporation.
Footnotes
Presented in part at the Annual Meeting of the American Society of Hematology, 2009.
Author Contributions: S.K.K. and S.V.R. designed the clinical trial, enrolled patients, and wrote the manuscript; M.Q.L., S.R.H., K.S., F.K.B., P.R.G., J.A.L., M.A.G., S.V.R., S.R.Z., P.L.B., C.B.R., T.E.W., R.F., S.J.R., J.R.M., D.D., and A.D. enrolled patients and critically reviewed the manuscript; J.A. and K.L. performed the data analysis and reviewed the manuscript.
Conflict of interest: M.Q.L., S.K.K., J.R.M., and A.D. sponsored research funded by Celgene; S.R.H., F.K.B., P.R.G., J.A.L., S.V.R., S.R.Z., P.L.B., C.B.R., T.E.W., R.F., S.J.R., and D.D. have nothing to disclose relevant to current manuscript. K.S., R.F., and M.A.G. have received consulting fees from Celgene. R.F. has received a patent for the prognostication of M.M. based on genetic categorization of the disease.
References
- 1.Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: Updated mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84:1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S. Multiple myeloma-current issues and controversies. Cancer Treat Rev. 2010;36(Suppl 2):S3–11. doi: 10.1016/S0305-7372(10)70006-2. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV. Treatment of myeloma: Cure vs control. Mayo Clin Proc. 2008;83:1142–1145. doi: 10.4065/83.10.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: High response rates in a phase II clinical trial. Leukemia. 2009;23:1337–1341. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 10.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): A randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 11.Palumbo A, Bertola A, Musto P, et al. Oral melphalan, prednisone, and thalidomide for newly diagnosed patients with myeloma. Cancer. 2005;104:1428–1433. doi: 10.1002/cncr.21342. [DOI] [PubMed] [Google Scholar]
- 12.Palumbo A, Dimopoulos MA, Delforge M, et al. A phase III study to determine the efficacy and safety of lenalidomide in combination with melphalan and prednisone (MPR) in elderly patients with newly diagnosed multiple myeloma. ASH Annu Meeting Abstr. 2009;114:613. [Google Scholar]
- 13.Rajkumar SV, Hayman S, Gertz MA, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002;20:4319–4323. doi: 10.1200/JCO.2002.02.116. [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 16.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 17.Giralt S, Stadtmauer EA, Harousseau JL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100) Leukemia. 2009;23:1904–1912. doi: 10.1038/leu.2009.127. [DOI] [PubMed] [Google Scholar]
- 18.Morgan GJ, Davies FE, Owen RG, et al. Thalidomide combinations improve response rates; Results from the MRC IX Study. Blood (ASH Annu Meeting Abstr) 2007;110:3593. [Google Scholar]
- 19.Morgan GJ, Davies FE, Gregory WM, et al. The addition of thalidomide to the induction treatment of newly presenting myeloma patients increases the CR rate which is likely to translate into improved PFS and OS. ASH Annu Meeting Abstr. 2009;114:352. [Google Scholar]
- 20.Schey SA, Morgan GJ, Ramasamy K, et al. The addition of cyclophosphamide to lenalidomide and dexamethasone in multiply relapsed/refractory myeloma patients; a phase I/II study. Br J Haematol. 2010;150:326–333. doi: 10.1111/j.1365-2141.2010.08250.x. [DOI] [PubMed] [Google Scholar]
- 21.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 22.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 23.Zonder JA, Crowley J, Hussein MA, et al. Superiority of lenalidomide (Len) plus high-dose dexamethasone (HD) compared to HD alone as treatment of newly-diagnosed multiple myeloma (NDMM): Results of the randomized, double-blinded, placebo-controlled SWOG trial S0232. Blood (ASH Annu Meeting Abstr) 2007;110:77. [Google Scholar]
- 24.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar SK, Flinn I, Noga SJ, et al. Bortezomib, dexamethasone, cyclophosphamide and lenalidomide combination for newly diagnosed multiple myeloma: Phase 1 results from the multicenter EVOLUTION study. Leukemia. 2010;24:1350–1356. doi: 10.1038/leu.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111:1101–1109. doi: 10.1182/blood-2007-05-090258. [DOI] [PubMed] [Google Scholar]
- 27.Jakubowiak AJ, Reece DE, Hofmeister CC, et al. Lenalidomide, bortezomib, pegylated liposomal doxorubicin, and dexamethasone in newly diagnosed multiple myeloma: Updated results of phase I/II MMRC trial. ASH Annu Meeting Abstr. 2009;114:132. [Google Scholar]
- 28.Kapoor P, Kumar S, Fonseca R, et al. Impact of risk stratification on outcome among patients with multiple myeloma receiving initial therapy with lenalidomide and dexamethasone. Blood. 2009;114:518–521. doi: 10.1182/blood-2009-01-202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reece D, Song KW, Fu T, et al. Influence of cytogenetics in patients with relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone: adverse effect of deletion 17p13. Blood. 2009;114:522–525. doi: 10.1182/blood-2008-12-193458. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Giralt S, Stadtmauer EA, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114:1729–1735. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]
- 32.Mazumder A, Kaufman J, Niesvizky R, et al. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia. 2008;22:1280–1281. doi: 10.1038/sj.leu.2405035. author reply1281–1282. [DOI] [PubMed] [Google Scholar]


