Abstract
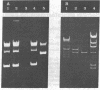
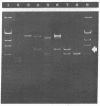
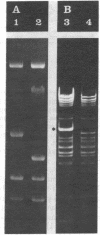
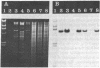
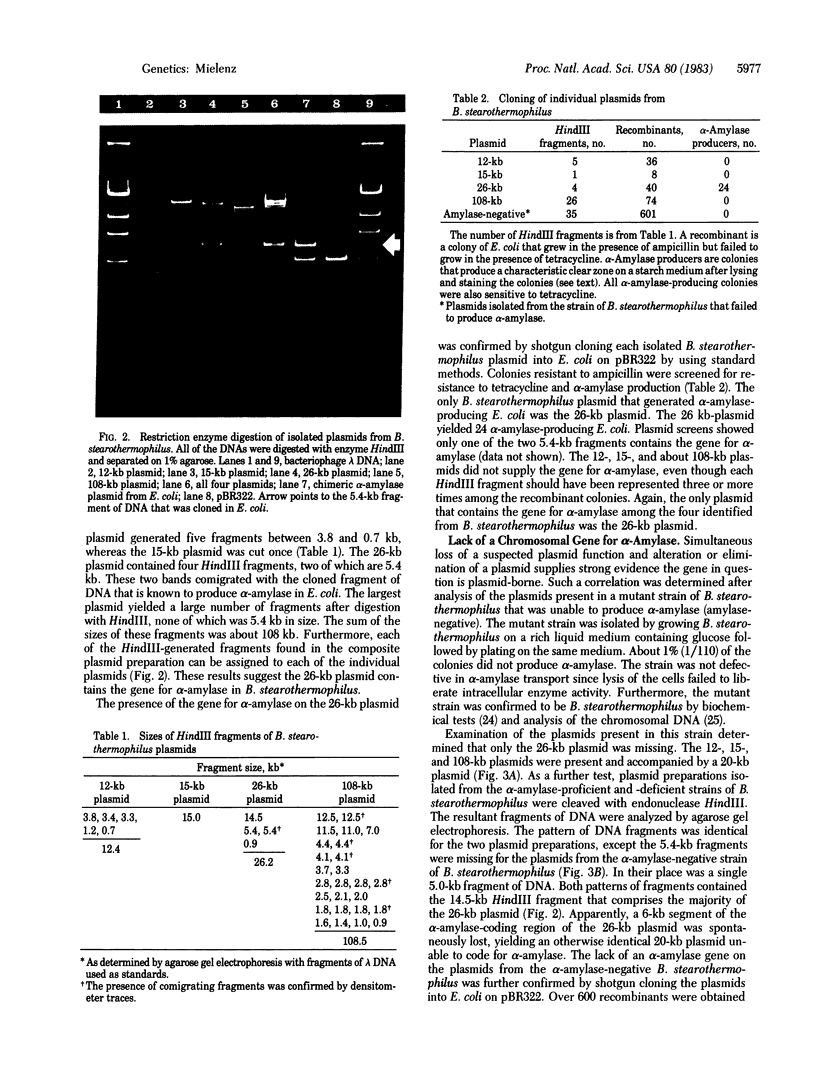
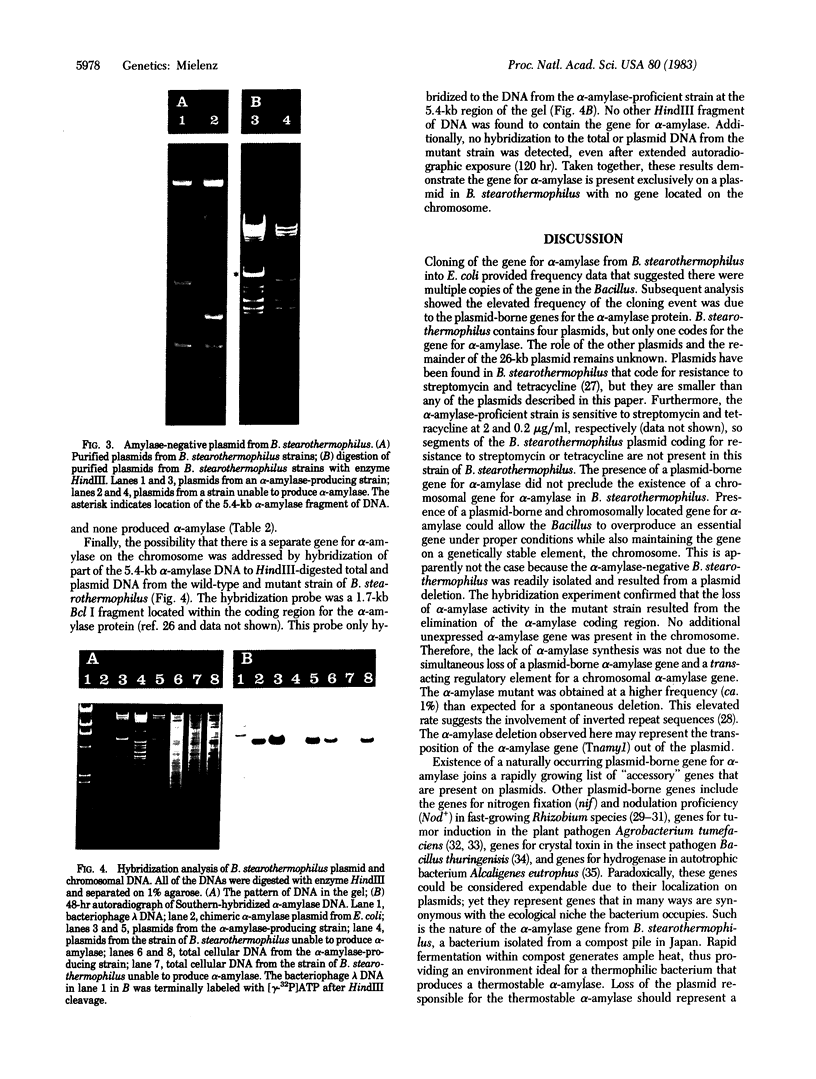
The gene for thermostable alpha-amylase from the thermophilic bacterium Bacillus stearothermophilus has been cloned and expressed in Escherichia coli. Each alpha-amylase-producing colony contained at least a 9.7-kilobase-pair (kb) chimeric plasmid composed of the vector pBR322 and a common 5.4-kb HindIII fragment of DNA. B. stearothermophilus contains four plasmids with sizes from 12 kb to over 108 kb. Restriction endonuclease analysis of these naturally occurring plasmids showed they also contain a 5.4-kb HindIII fragment of DNA. Cloning experiments with the four plasmids yielded alpha-amylase-producing E. coli that contained the same 9.7-kb chimeric plasmid. Restriction endonuclease analysis and further recombinant DNA experiments identified a 26-kb plasmid that contains the gene for alpha-amylase. A spontaneous mutant of B. stearothermophilus unable to produce alpha-amylase was missing the 26-kb plasmid but contained a 20-kb plasmid. A 6-kb deletion within the region of the 5.4-kb HindIII fragment yielded the 20-kb plasmid unable to code for alpha-amylase. A nick-translated probe for the alpha-amylase coding region did not hybridize to either plasmid or total cellular DNA from this mutant strain of B. stearothermophilus. These results demonstrate the gene for alpha-amylase is located exclusively on a 26-kb plasmid in B. stearothermophilus with no genetic counterpart present on the chromosome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham A. H., Bruton C. J., Atkinson T. Isolation and partial characterization of four plasmids from antibiotic-resistant thermophilic bacilli. J Gen Microbiol. 1979 Oct;114(2):401–408. doi: 10.1099/00221287-114-2-401. [DOI] [PubMed] [Google Scholar]
- Blin N., von Gabain A., Bujard H. Isolation of large molecular weight DNA from agarose gels for further digestion by restriction enzymes. FEBS Lett. 1975 Apr 15;53(1):84–86. doi: 10.1016/0014-5793(75)80688-0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Friedrich C. G., Friedrich B. Regulation of hydrogenase formation is temperature sensitive and plasmid coded in Alcaligenes eutrophus. J Bacteriol. 1983 Jan;153(1):176–181. doi: 10.1128/jb.153.1.176-181.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- Henner D. J., Hoch J. A. The Bacillus subtilis chromosome. Microbiol Rev. 1980 Mar;44(1):57–82. doi: 10.1128/mr.44.1.57-82.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Meyer R., Figurski D., Helinski D. R. Physical and genetic studies with restriction endonucleases on the broad host-range plasmid RK2. Mol Gen Genet. 1977 Apr 29;152(3):129–135. doi: 10.1007/BF00268809. [DOI] [PubMed] [Google Scholar]
- Mickel S., Arena V., Jr, Bauer W. Physical properties and gel electrophoresis behavior of R12-derived plasmid DNAs. Nucleic Acids Res. 1977;4(5):1465–1482. doi: 10.1093/nar/4.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielenz J. R., Jackson L. E., O'Gara F., Shanmugam K. T. Fingerprinting bacterial chromosomal DNA with restriction endonuclease EcoRI: comparison of Rhizobium spp. and identification of mutants. Can J Microbiol. 1979 Jul;25(7):803–807. doi: 10.1139/m79-118. [DOI] [PubMed] [Google Scholar]
- Ogasahara K., Imanishi A., Isemura T. Studies on thermophilic alpha-amylase from Bacillus stearothermophilus. I. Some general and physico-chemical properties of thermophilic alpha-amylase. J Biochem. 1970 Jan;67(1):65–75. doi: 10.1093/oxfordjournals.jbchem.a129235. [DOI] [PubMed] [Google Scholar]
- Pfueller S. L., Elliott W. H. The extracellular alpha-amylase of bacillus stearothermophilus. J Biol Chem. 1969 Jan 10;244(1):48–54. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- STARK E., TETRAULT P. A. Isolation of bacterial, cell-free, starch saccharifying enzymes from the medium at 70 C. J Bacteriol. 1951 Aug;62(2):247–249. doi: 10.1128/jb.62.2.247-249.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf H. E., Whiteley H. R. Cloning and expression of the Bacillus thuringiensis crystal protein gene in Escherichia coli. Proc Natl Acad Sci U S A. 1981 May;78(5):2893–2897. doi: 10.1073/pnas.78.5.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Nagata Y., Maruo B. Isolation of mutants defective in alpha-amylase from Bacillus subtilis: genetic analyses. J Bacteriol. 1974 Aug;119(2):416–424. doi: 10.1128/jb.119.2.416-424.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Yamane K., Yamaguchi K., Nagata Y., Maruo B. Transformation of Bacillus subtilis in alpha-amylase productivity by deoxyribonucleic acid from B. subtilis var. amylosacchariticus. J Bacteriol. 1974 Dec;120(3):1144–1150. doi: 10.1128/jb.120.3.1144-1150.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]