Abstract
Graves’ ophthalmopathy, also called Graves’ orbitopathy, is a potentially sight-threatening ocular disease that has puzzled physicians and scientists for nearly two centuries.1–3 Generally occurring in patients with hyperthyroidism or a history of hyperthyroidism due to Graves’ disease, Graves’ ophthalmopathy is also known as thyroid-associated ophthalmopathy or thyroid eye disease, because it sometimes occurs in patients with euthyroid or hypothyroid chronic autoimmune thyroiditis. The condition has an annual adjusted incidence rate of 16 women and 3 men per 100,000 population.4
This review explores the perplexing relationship between Graves’ ophthalmopathy, hyperthyroidism, and thyroid dermopathy, the associated skin condition. I examine clinical features, histologic findings, and laboratory studies, with an emphasis on mechanisms that could be targeted in the development of new treatments for this debilitating disease.
CLINICAL AND LABORATORY FEATURES
The close clinical and temporal relationships between hyperthyroidism, Graves’ ophthalmopathy, and thyroid dermopathy suggest that these conditions evolve from a single underlying systemic process with variable expression in the thyroid, eyes, and skin. Bilateral ocular symptoms and hyperthyroidism most often occur simultaneously or within 18 months of each other, although occasionally Graves’ ophthalmopathy precedes or follows the onset of hyperthyroidism by many years.5 Almost half of patients with Graves’ hyperthyroidism report symptoms of Graves’ ophthalmopathy, including a dry and gritty ocular sensation, photophobia, excessive tearing, double vision, and a pressure sensation behind the eyes. The most common clinical features of Graves’ ophthalmopathy are upper eyelid retraction, edema, and erythema of the periorbital tissues and conjunctivae, and proptosis (Fig. 1). Approximately 3 to 5% of patients with Graves’ ophthalmopathy have severe disease with intense pain, inflammation, and sight-threatening corneal ulceration or compressive optic neuropathy.6 Subclinical eye involvement is common: in nearly 70% of adult patients with Graves’ hyperthyroidism, magnetic resonance imaging or computed tomographic scanning reveals extraocular-muscle enlargement.7 Although clinically unilateral Graves’ ophthalmopathy occurs occasionally, orbital imaging generally confirms the presence of asymmetric bilateral disease.8 Thyroid dermopathy (also called pretibial myxedema), a nodular or diffuse thickening of the pretibial skin, sometimes progresses to debilitating disease. Although diagnosed on physical examination in only 13% of patients with severe Graves’ ophthalmopathy, subclinical involvement of the skin of the legs and other regions of the body occurs more commonly.9 Approximately 20% of patients with thyroid dermopathy have thyroid acropachy, which manifests as clubbing of the fingers and toes.
Figure 1. Patients with Graves’ Ophthalmopathy.
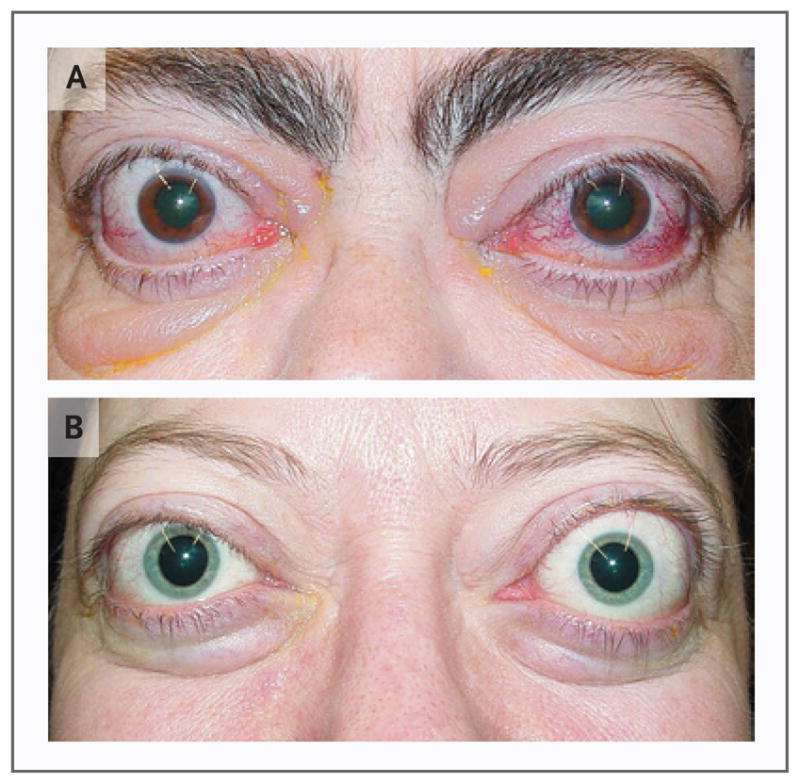
Panel A shows a 59-year-old woman with excess proptosis, moderate eyelid edema, and erythema with moderate eyelid retraction affecting all four eyelids. Conjunctival chemosis (edema) and erythema with bilateral edema of the caruncles, with prolapse of the right caruncle, are evident. Panel B shows a 40-yearold woman with excess proptosis, minimal bilateral injection, and chemosis with slight erythema of the eyelids. She also had evidence, on slit-lamp examination, of moderate superior limbic keratoconjunctivitis.
Graves’ hyperthyroidism is caused by autoantibodies that bind to the thyrotropin receptor on thyroid follicular endothelial cells and thereby stimulate excess production of thyroid hormone. 10 The presence of anti–thyrotropin-receptor antibodies in virtually all patients with Graves’ ophthalmopathy suggests that immunoreactivity against the thyrotropin receptor underlies both Graves’ ophthalmopathy and hyperthyroidism.11 The 5% of patients with Graves’ ophthalmopathy who are euthyroid or hypothyroid generally have low titers of anti–thyrotropin-receptor antibodies, which are difficult to detect in some assays. 12 Levels of anti–thyrotropin-receptor antibodies correlate positively with clinical features of Graves’ ophthalmopathy13 and influence the prognosis14; these antibody levels are especially elevated in patients with thyroid dermopathy.15
Cigarette smoking is the strongest modifiable risk factor for Graves’ ophthalmopathy (odds ratio among smokers vs. nonsmokers, 7.7), and the risk is proportional to the number of cigarettes smoked daily.16 In smokers with Graves’ ophthalmopathy, as compared with nonsmokers, severe disease is more likely to develop and is more likely to respond less well to immunosuppressive therapies.17 Smoking is associated with many autoimmune diseases, perhaps owing to nonspecific suppression of T-cell activation, reduction of natural killer T cells, and impairment of humoral and cell-mediated immunity.18 The strong association between Graves’ ophthalmopathy and smoking suggests the involvement of additional factors, including direct effects of cigarette toxins19 and trauma from heat transmitted from the ethmoid sinuses through the lamina papyracea (the thin medial orbital wall).
ANATOMICAL AND HISTOLOGIC FINDINGS
Many clinical signs and symptoms of Graves’ ophthalmopathy arise from soft-tissue enlargement in the orbit, leading to increased pressure within the bony cavity.20,21 Most patients have enlargement of both extraocular muscle and adipose tissue, with a predominance of one or the other in some (Fig. 2).22 Patients under 40 years of age tend to have fat expansion, whereas patients over 60 years of age have more extraocular-muscle swelling.23 In some patients, proptosis develops as the globe protrudes, decompressing the orbit. Patients with crowding of enlarged muscles at the orbital apex and minimal proptosis are at particular risk for compressive optic neuropathy.
Figure 2. Computed Tomographic Scans of Patients with Graves’ Ophthalmopathy and of a Normal Subject.
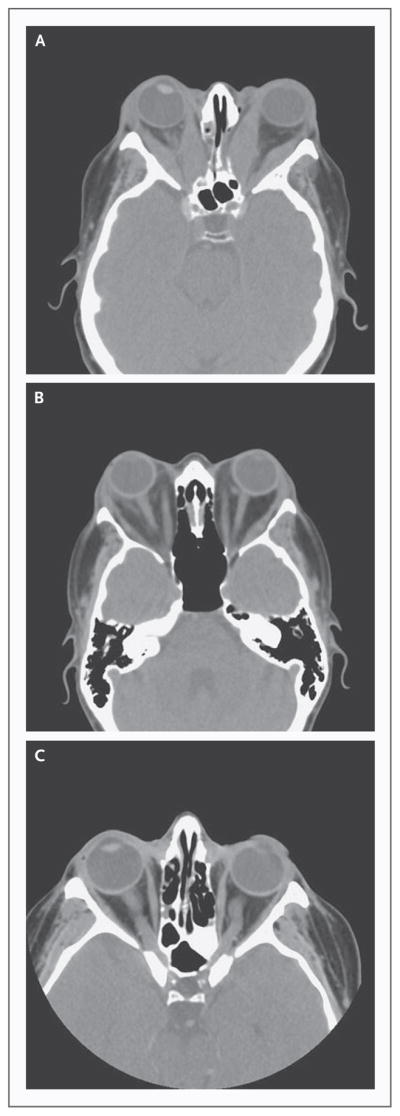
Axial images of patients with Graves’ ophthalmopathy reveal generalized enlargement of the extraocular muscles with marked bilateral proptosis (Panel A) and marked bilateral proptosis and asymmetric involvement of the extraocular muscles with expansion of the orbital fat bilaterally (Panel B). Normal orbits are shown (Panel C) for comparison.
Diplopia develops from inflammation and swelling of the extraocular muscles and is generally restrictive rather than paralytic. The inferior rectus is the most commonly involved muscle. Upper-eyelid retraction is caused by increased sympathetic stimulation of Müller’s muscle, overaction of the levator muscle as it contracts against a tight inferior rectus, or scarring between the levator and surrounding tissues. Symptomatic corneal dryness is due to eyelid retraction, decreased blinking, increased tear evaporation, and incomplete eyelid closure during sleep.
Anatomical and mechanical features of the orbit and lower extremities may be important in expression of the disease. Periorbital edema is primarily congestive and most likely reflects decreased venous drainage due to vascular compression within the orbital space. Similarly, dependent edema after prolonged standing, when venous and lymphatic flow is compromised, can worsen thyroid dermopathy.24 Moreover, individual anatomical variability, such as the volume or shape of the orbits25 or variations in venous or lymphatic drainage, may increase the risk of extrathyroidal manifestations in patients with Graves’ disease. In Graves’ ophthalmopathy, trauma itself may be a stimulus; it has long been observed that thyroid dermopathy may develop at sites of trauma to the skin of the shoulders, arms, or other regions.9
Histologic studies of Graves’ ophthalmopathy have focused on extraocular muscles, owing to their obvious enlargement in patients with the disease. However, electron microscopy reveals intact extraocular muscle fibers in such patients. 26 The extraocular muscles are widely separated by an amorphous accumulation of granular material consisting primarily of collagen fibrils and glycosaminoglycans, among which hyaluronan predominates (Fig. 3).27 The polyanionic charge and extremely high osmotic pressure of this matrix substance make it extremely hydrophilic and capable of binding many times its weight in water. Consequently, the muscle bodies become edematous and may enlarge to many times their normal size. In inactive disease, atrophy and fibrosis of muscle bundles are evident, with extension of fibrous strands into adjacent adipose tissues. Histologic features of thyroid dermopathy are similar to those seen in the orbit, with hyaluronan accumulation in the reticular dermis, although with less abundant lymphocytic infiltration and no evidence of fat expansion.9
Figure 3. Histologic Appearance of Extraocular Muscle in Graves’ Ophthalmopathy (Hematoxylin and Eosin).
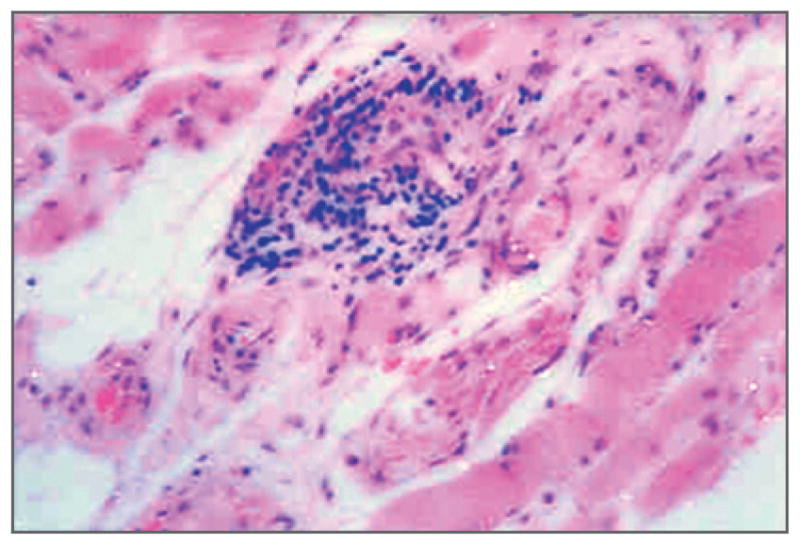
The focal and perivascular interstitial inflammatory mononuclear cell infiltrate is in close association with intact striated extraocular muscle fibers widely separated by amorphous granular material.
Focal and diffuse mononuclear-cell infiltration occurs within the extraocular and levator muscles, lacrimal glands, and adipose tissues in active Graves’ ophthalmopathy.28 These cells are primarily CD4+ T cells, but there are also minor populations of CD8+ cells, B cells, plasma cells, and macrophages.29,30 In early stages of the disease, type 1 helper T cells predominate and produce the cytokines interleukin-2, interferon-γ, and tumor necrosis factor (TNF), indicating ongoing cell-mediated immunity within the orbit. In disease of longer duration, type 2 helper T cells that produce interleukin-4, interleukin-5, and interleukin-10 are dominant and propagate autoantibody production.31 Macrophages, fibroblasts, and adipocytes produce other inflammatory mediators, including interleukins 1, 6, and 16 and transforming growth factor β (TGF-β) within the orbit.32,33
CELLULAR ORIGINS
Current evidence points to orbital fibroblasts as the target cells in Graves’ ophthalmopathy and suggests that their normal functions are dysregulated through autoimmune mechanisms.34,35 The concept that fibroblast proteins are autoantigens in Graves’ ophthalmopathy derives in part from the finding that orbital T cells obtained from patients with Graves’ ophthalmopathy proliferate when exposed in vitro to autologous orbital fibroblast proteins.36 These orbital fibroblasts secrete large quantities of hyaluronan in response to various cytokines,34 and a subgroup of orbital fibroblasts can differentiate into mature adipocytes37 that have increased expression of thyrotropin receptor.38,39 These cellular changes lead to the characteristically enlarged eye muscles and expansion of orbital fat of patients with Graves’ ophthalmopathy.
Orbital adipose tissue is a unique fat depot that supports and cushions the globe, extraocular muscles, and other orbital structures. Most orbital disorders are inflammatory,40 suggesting that orbital fat may be especially prone to robust inflammatory reactions. Indeed, as compared with fibroblasts from other sites, orbital fibroblasts show exaggerated inflammatory responses to various stimuli.41 Although orbital fibroblasts produce high levels of the proinflammatory cytokine interleukin-1, their expression of the neutralizing interleukin-1–receptor antagonist is relatively low.42 Likewise, orbital fibroblasts treated with interferon-γ or leukoregulin synthesize particularly high levels of prostaglandin E2, a mediator with important roles in inflammation.43
The orbit contains subpopulations of fibroblasts exhibiting phenotypic and functional heterogeneity. The cell-surface marker Thy-1 (CD90) is overexpressed in orbital tissues in Graves’ ophthalmopathy44 and defines a population of fibroblasts capable of cytokine-induced production of prostaglandin E2, interleukin-8, and hyaluronan. 45 When exposed to TGF-β, these fibroblasts differentiate into myofibroblasts with prominent cytoplasmic actin filaments that can participate in inflammation, repair, and fibrosis. Although fibroblasts within the extraocular muscles are almost exclusively Thy-1+, approximately half the fibroblasts within the adipose tissue lack this marker and are preadipocytes capable of differentiation into mature fat cells. Within the orbit, relative proportions of Thy-1+ and Thy-1− fibroblasts and their degree of exposure to TGF-β may affect disease expression, including whether muscle or fat expansion predominates and the extent of fibrosis that develops.46
MOLECULAR MECHANISMS
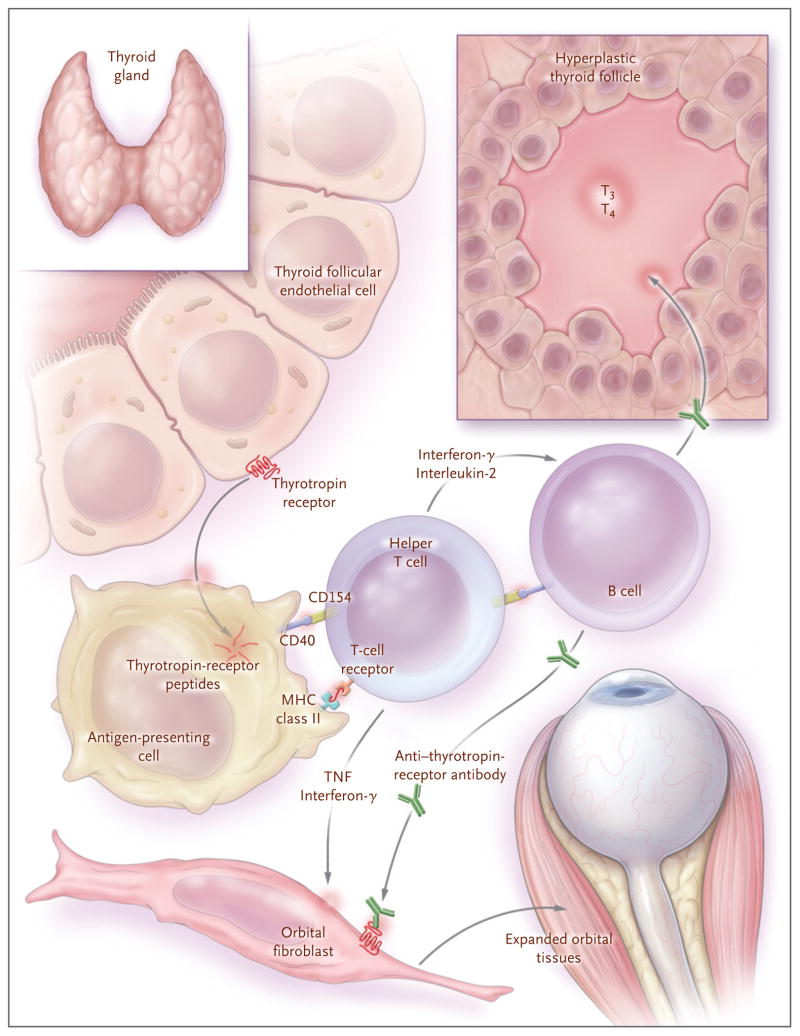
Investigators have long postulated that the thyrotropin receptor is a target of autoimmunity within the orbit and that its recognition by a circulating thyrotropin-like factor explains the link between hyperthyroidism and Graves’ ophthalmopathy. 47–49 The cloning of the thyrotropin receptor50,51 enabled direct assessment of the expression of this receptor in extrathyroidal tissues, and several groups reported a low abundance of thyrotropin receptor in cultured orbital fibroblasts52– 54 and in normal orbital-adipose tissues.55 Subsequent studies showed elevated thyrotropin-receptor expression in orbital tissues in patients with Graves’ ophthalmopathy,39,56 with the highest levels in those with clinically active disease.57 These findings, along with the close association between Graves’ ophthalmopathy and Graves’ hyperthyroidism and the consistently elevated levels of anti–thyrotropin-receptor antibodies in Graves’ ophthalmopathy, support the concept that the thyrotropin receptor is the primary autoantigen in Graves’ ophthalmopathy (Fig. 4). A low abundance of thyrotropin receptor can also be detected in several extrathyroidal tissues, including skin, adrenal gland, kidney, and thymus.58–60 In the pretibial skin of patients with thyroid dermopathy, the level of the thyrotropin receptor is elevated.61
Figure 4. Model of the Initiation of Thyrotropin-Receptor Autoimmunity in Graves’ Ophthalmopathy and Its Consequences.
A failure of T cells to tolerate the thyrotropin receptor, for unknown reasons, allows for the development of autoimmunity directed against this receptor. The thyrotropin receptor is internalized and degraded by antigen-presenting cells that present thyrotropin-receptor peptides, in association with major histocompatibility complex (MHC) class II antigens, to helper T cells. These cells become activated, interact with autoreactive B cells through CD154–CD40 bridges, and secrete interleukin-2 and interferon-γ. These cytokines induce the differentiation of B cells into plasma cells that secrete anti–thyrotropin-receptor antibodies. These antibodies stimulate the thyrotropin receptor on thyroid follicular epithelial cells, leading to hyperplasia and increased production of the thyroid hormones triiodothyronine (T3) and thyroxine (T4). Anti-thyrotropin-receptor antibodies also recognize the thyrotropin receptor on orbital fibroblasts and, in conjunction with the secreted type 1 helper T cytokines interferon-γ and tumor necrosis factor (TNF), initiate the tissue changes characteristic of Graves’ ophthalmopathy.
In animal models of Graves’ disease, various methods have been used to induce the production of anti–thyrotropin-receptor antibodies, and the results have provided valuable insights into the pathogenesis of hyperthyroidism.62 However, reproducible ocular changes resembling those in Graves’ ophthalmopathy have not been reported, 63 perhaps owing partly to differences in orbital anatomy between humans and rodents. Similarly, much has been learned about the genetic basis of thyroid autoimmunity, but it is unclear whether there is a genetic susceptibility to Graves’ ophthalmopathy apart from that identified for Graves’ disease.64
Adipogenesis requires growth arrest and the induction of transcriptional regulators, including peroxisome-proliferator–activated receptor γ (PPAR-γ).65 Evidence that adipogenesis is active in the orbit in Graves’ ophthalmopathy includes the results of microarray studies showing upregulation of immediate early adipogenic genes66 and other adipocyte-related genes, including those encoding PPAR-γ, interleukin-6, adiponectin, and leptin.67 PPAR-γ agonists stimulate adipogenesis and expression of the thyrotropin receptor in cultured orbital preadipocytes.46,68 Several reports have described progressive proptosis in patients with a history of Graves’ ophthalmopathy, as well as in those without such a history, after the treatment of type 2 diabetes mellitus with PPAR-γ agonists of the thiazolidinedione type.69,70 Therefore, particular sensitivity to PPAR-γ ligation may be a characteristic of orbital preadipocytes.
Factors that might stimulate adipogenesis within the orbit in patients with Graves’ ophthalmopathy include naturally occurring PPAR-γ ligands, which are proadipogenic prostaglandins produced by activated T cells through the activity of cyclooxygenase-2.71 This enzyme is upregulated in the orbit of patients with Graves’ ophthalmopathy,72 and T cells from such patients produce prostaglandins that induce adipogenesis in orbital fibroblasts.73 Thyrotropin can stimulate adipogenesis in mouse embryonic stem cells, even in the absence of other adipogenic factors, suggesting that activation of the thyrotropin receptor initiates new fat-cell development.74 In orbital fibroblasts that were transfected with an activating mutant thyrotropin-receptor construct, 75,76 both early adipocyte differentiation and hyaluronan production were stimulated, suggesting that thyrotropin-receptor ligation by anti– thyrotropin-receptor antibodies within the orbit directly contributes to the soft-tissue changes characteristic of Graves’ ophthalmopathy.
The type I insulin-like growth factor receptor (IGF-IR) may be another important autoantigen in Graves’ ophthalmopathy. Orbital fibroblasts in patients with the disease express higher levels of IGF-IR than normal fibroblasts.77 In addition, the IgG fraction of pooled serum samples obtained from patients with Graves’ disease contains autoantibodies that stimulate orbital fibroblasts from patients with Graves’ ophthalmopathy to synthesize interleukin-16 and the chemokine RANTES (regulated upon activation normal T-cell expressed and secreted), factors that enhance trafficking of CD4+ T cells.78 This effect was inhibited by a specific monoclonal antibody that blocks IGF-IR and by transfecting fibroblasts with a dominant-negative mutant IGF-IR, suggesting that signaling through IGF-IR mediates the process. Moreover, IgG from patients with Graves’ disease stimulates hyaluronan synthesis by orbital fibroblasts from patients with Graves’ ophthalmopathy but not by normal orbital-cell cultures.79 Tsui and colleagues80 have proposed that there are physical and functional relationships between IGF-IR and the thyrotropin receptor, as suggested by colocalization studies and the finding that a monoclonal antibody that specifically blocks IGF-IR inhibits thyrotropin-induced kinase signaling. Although a role for IGF-IR and antibodies targeting this receptor in Graves’ ophthalmopathy is potentially important, it has yet to be confirmed by other investigators.
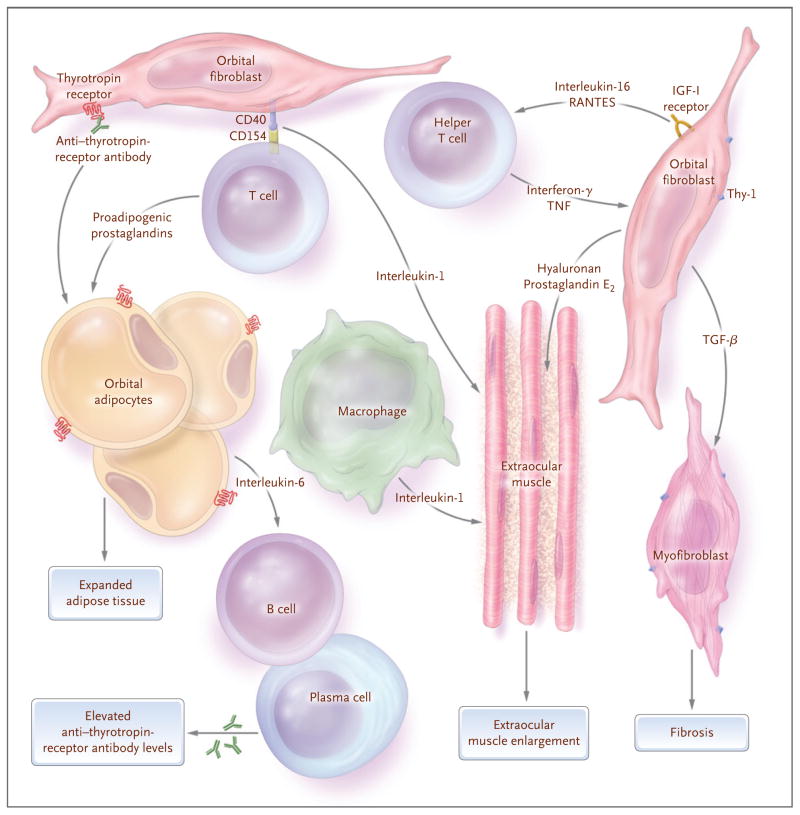
Orbital fibroblasts participate in the autoimmune process within the orbit in Graves’ ophthalmopathy (Fig. 5). These cells express CD40, a costimulatory protein present on the surface of many types of cells, including macrophages, lymphocytes, and thyrocytes.81 CD4+ T cells expressing the CD40 ligand (also called CD154) directly activate orbital fibroblasts through the formation of CD40–CD154 bridges; as a result, such fibroblasts proliferate82 and produce high levels of interleukin-1 and interleukin-6.83 In addition, leukoregulin and interferon-γ (products of activated T cells) and interleukin-1 (secreted by resident fibroblasts and macrophages) increase the synthesis of hyaluronan and prostaglandin E2 by orbital fibroblasts.84–86 Interleukin-1 and interferon-γ, as well as IgG obtained from patients with Graves’ disease, can also stimulate orbital fibroblasts to express adhesion molecules that promote direct interaction between target cells and inflammatory cells.
Figure 5. Model of Interactions between Orbital Fibroblasts and the Autoimmune Process Leading to the Tissue Changes Characteristic of Graves’ Ophthalmopathy.
When activated by anti–thyrotropin-receptor antibodies, a subgroup of orbital fibroblasts (termed preadipocytes) begins to differentiate into adipocytes with increased thyrotropin-receptor expression, while others bearing the antigen Thy-1 are stimulated by cytokines, including interferon-γ and tumor necrosis factor (TNF), to increase their production of hyaluronan. Similarly, stimulation of the insulin-like growth factor receptor (IGF-I receptor) expressed on orbital fibroblasts results in the secretion of the chemokines interleukin-16 and RANTES (regulated upon activation normal T-cell expressed and secreted), which enhance recruitment of activated T cells and other mononuclear immune cells into the orbit. The expression of CD154 in T cells allows for their direct interaction with orbital fibroblasts through the formation of CD40–CD154 bridges, resulting in fibroblast production of interleukin-1. Activated type 1 helper T cells in patients with early Graves’ ophthalmopathy produce interferon-γ and TNF, and resident macrophages secrete interleukin-1. These cytokines stimulate orbital fibroblasts to produce high levels of prostaglandin E2 and hydrophilic hyaluronan that accumulates between the intact extraocular muscle fibers and within the orbital adipose tissues to enlarge the volume of these tissues. Activated T cells in patients with Graves’ ophthalmopathy also produce proadipogenic prostaglandins that stimulate preadipocytes to differentiate into mature fat cells, further expanding the tissue volume. Adipocytes and fibroblasts produce interleukin-6, which augments B-cell maturation and increases the production of anti–thyrotropin-receptor antibodies by plasma cells within the orbit. Orbital fibroblasts also produce transforming growth factor β (TGF-β), which stimulates both production of hyaluronan and differentiation of the Thy-1+ subgroup into myofibroblasts that participate in the development of fibrosis, especially in late stages of the disease.
The disease-producing effects of some soluble mediators within the orbit in Graves’ ophthalmopathy are opposed by others that mitigate these effects. Interleukin-6, a product of activated T cells, macrophages, fibroblasts, and adipocytes, is proinflammatory and enhances B-cell differentiation and antibody production but also reduces the strength of many inflammatory activities of interleukin-1.87 TGF-β, TNF, and interferon-γ inhibit adipogenesis and thyrotropin-receptor expression in cultured orbital fibroblasts88 and thus may counteract PPAR-γ ligation within the orbit. Although some endogenous PPAR-γ ligands produced by activated T cells trigger adipocyte differentiation,73 others oppose inflammation by inhibiting production of inflammatory cytokines by macrophages, monocytes, and adipocytes.89 Finally, TGF-β produced by fibroblasts most likely acts in an autocrine fashion to stimulate hyaluronan synthesis84 and the differentiation of the Thy-1+ subgroup of fibroblasts into myofibroblasts46 while it also inhibits adipogenesis in these cells.
THERAPEUTIC IMPLICATIONS
Our understanding of Graves’ ophthalmopathy points to several potential therapeutic targets and suggests difficulties in designing effective immunotherapy for this disease.90 The immune mediators that cause pathogenic orbital changes have additional roles within the adaptive immune network. Therefore, it is difficult to predict whether the biologic agents that are effective in, say, rheumatoid arthritis91 will be useful in treating Graves’ ophthalmopathy. Answers will come from randomized clinical trials designed to account for the unique features of Graves’ ophthalmopathy, such as the self-limited duration of active disease and the central role of tissue expansion rather than destruction.
Agents that neutralize cytokine-induced inflammation or production of hyaluronan by orbital fibroblasts, such as anti-TNF agents92 and agents targeting the interleukin-1 receptor93 or the interleukin-6 receptor94 (Table 1), are attractive potential treatments for Graves’ ophthalmopathy. Targeting TNF in patients with Graves’ ophthalmopathy might affect the production by preadipocytes of chemoattractant protein 1, a macrophage-attracting protein.113 The findings of a case report95 and a small, open study96 should encourage the performance of randomized trials of anti-TNF therapy in patients with Graves’ ophthalmopathy. Antioxidants such as selenium may be useful, since they have a beneficial effect on autoimmunity in Graves’ disease.101 Neutralizing the profibrotic effects of TGF-β may also be of benefit, especially in patients with inactive Graves’ ophthalmopathy, in whom countering the antiinflammatory and antiadipogenic effects of this cytokine may be less detrimental than in those with the active disease.100
Table 1.
Potential Therapeutic Targets in Graves’ Ophthalmopathy.*
| Target | Current Agent | Description | Potential Benefit | Reference |
|---|---|---|---|---|
| TNF | Infliximab, adalimumab | TNF-specific monoclonal antibodies | Reduction in inflammation, leukocyte recruitment, and hyaluronan production | Feldmann,92 Durrani et al.95 |
| TNF receptor | Etanercept | TNF receptor–IgG Fc fusion molecule | Reduction in inflammation, leukocyte recruitment, and hyaluronan production | Feldmann,92 Paridaens et al.96 |
| Interleukin-1 receptor | Anakinra | Interleukin-1–receptor antagonist | Reduction in inflammation, leukocyte recruitment, and hyaluronan production | Mertens and Singh,97 Tan et al.98 |
| Interleukin-6 receptor | Tocilizumab | Interleukin-6 receptor–specific mono-clonal antibody | Reduction in inflammation, leukocyte recruitment, and hyaluronan production | Smolen et al.99 |
| TGF-β | Lerdelimumab, GC1008 | TGF-β–specific monoclonal antibodies | Reduction in fibrosis | Pohlers et al.100 |
| Oxygen free radicals | Selenium | Essential trace element | Antiinflammatory activity | Wertenbruch et al.101 |
| CD20 | Rituximab, ocrelizumab, ofatumumab | Partially or fully humanized CD20-specific monoclonal antibodies | Decreased antigen presentation and T-cell activation; possible modulation of anti–thyrotropin-receptor antibody production | Tsokos,102 El Fassi et al.,103 Kwan-Morley and Albert,104 Salvi et al.105 |
| CD3 | ChAglyCD3 | Fc-mutated CD3-specific monoclonal antibody | Induction of tolerance | Keymeulen et al.106 |
| CD28 | Abatacept | CTLA-4–immunoglobulin recombinant protein | Modulation of costimulatory pathways | Kremer et al.107 |
| CD154 | IDEC-131 | Humanized CD154-specific monoclonal antibody | Modulation of costimulatory pathways | Kalunian et al.108 |
| PPAR-γ | Selective PPAR modulators | Novel selective PPAR-γ antagonists | Reduction in inflammation and orbital adipogenesis | Knouff and Auwerx,109 Straus and Glass110 |
| Somatostatin receptor | SOM230 | Synthetic high-affinity somatostatin analogue | Inhibition of orbital preadipocyte proliferation | Cozma et al.111 |
| Thyrotropin receptor | NIDDK/CEB-52 | Low-molecular-weight thyrotropin-receptor antagonist | Inhibition of orbital adipogenesis and hyaluronan production | Neumann et al.112 |
CTLA denotes cytotoxic T-lymphocyte–associated antigen 4, PPAR peroxisome-proliferator–activated receptor, TGF transforming growth factor, and TNF tumor necrosis factor.
Targeting autoreactive B cells with rituximab, an anti-CD20 monoclonal antibody, is another possible approach.114 In a study of Graves’ hyperthyroidism, rituximab decreased the total anti– thyrotropin-receptor antibody levels only minimally but markedly reduced (66%) the subgroup of stimulatory anti–thyrotropin-receptor antibodies. 115 Several case reports have suggested that rituximab might be effective in active Graves’ ophthalmopathy,103,116 and randomized, controlled trials of this agent in Graves’ ophthalmopathy are being led by Bahn117 and Salvi (Salvi M: personal communication). The important role of cellular immunity in Graves’ ophthalmopathy suggests that targeting CD3 on T cells106 may hold promise. Similarly, inhibitors of the cellular interactions necessary for T-cell signaling, which potentially decrease production of both autoantibodies and inflammatory cytokines, may be of benefit.107 A recent study of a mouse model of Graves’ disease showed that pretreatment with the thyrotropin receptor-A–subunit protein, before immunization with an adenovirus bearing the same subunit, attenuated subsequent hyperthyroidism by directing the autoantibody response away from pathogenic epitopes.118 Although administration of the subunit did not reverse established hyperthyroidism, tolerance to particular thyrotropin-receptor epitopes was achieved; therefore, this approach may be useful for disease prevention in predisposed persons.
CONCLUSIONS
Although recent studies suggest that there are novel mechanisms involved in the development of Graves’ ophthalmopathy, much remains to be learned. The thyrotropin receptor on orbital fibroblasts is likely to be an important autoimmune target in the disease, and early evidence suggests that immunoreactivity directed against IGF-IR may also be involved. Many studies support a role for cellular immunity, and recent findings suggest that autoantibodies may also directly affect the orbital disease process. The recent demonstration that some anti–thyrotropin-receptor antibodies signal within the thyrocyte through a unique signaling cascade not used by thyrotropin suggests that various antibodies may activate somewhat different signaling networks.119 It is therefore possible that various clinical phenotypes, such as euthyroid Graves’ ophthalmopathy or the predominance of muscle or fat enlargement, may be affected by the molecular signature of the particular anti–thyrotropin-receptor antibodies or other autoantibodies produced. Delineation of the functional heterogeneity of anti–thyrotropin-receptor antibodies and the signaling pathways involved will improve our understanding of orbital disease mechanisms and may suggest new approaches to therapy, including perhaps the use of low-molecular-weight thyrotropin-receptor antagonists. 112
Unraveling the Graves’ ophthalmopathy conundrum would enable the design of effective means of prevention or treatment. The quality of life is markedly decreased in patients with Graves’ ophthalmopathy,120 and now we have the means to precisely measure the effect of new therapies on quality of life and specific clinical end points. The recent, excellent consensus statement on therapy for Graves’ ophthalmopathy developed by the European Group on Graves’ Orbitopathy121 emphasizes the importance of performing randomized, controlled, multicenter trials of current treatments and new therapies on the horizon. These might be tailored to specific disease phenotypes or stages and use combination therapies to improve outcomes. Although major challenges remain, patients with Graves’ ophthalmopathy should benefit from these efforts.
Acknowledgments
Supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK77814).
I thank Drs. Michael Brennan, Marius Stan, James Garrity, and George Bartley for their review of the manuscript and very helpful suggestions; the members of my laboratory who have studied Graves’ ophthalmopathy with me over many years; and the patients with this disease who have taught me so much.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Graves R. Newly observed affection of the thyroid: clinical lectures. Lond Med Surg J. 1835;7:516–7. [Google Scholar]
- 2.Parry C. Diseases of the heart. Elements Pathol Ther. 1825;2:111–28. [Google Scholar]
- 3.von Basedow K. Exophthalmos durch hypertrophie des zellgewebes in der augenhöhle. Wochenschr Heilkunde. 1840;6:197–204. [Google Scholar]
- 4.Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477–588. [PMC free article] [PubMed] [Google Scholar]
- 5.Wiersinga WM, Smit T, van der Gaag R, Koornneef L. Temporal relationship between onset of Graves’ ophthalmopathy and onset of thyroidal Graves’ disease. J Endocrinol Invest. 1988;11:615–9. doi: 10.1007/BF03350193. [DOI] [PubMed] [Google Scholar]
- 6.Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12:855–60. doi: 10.1089/105072502761016476. [DOI] [PubMed] [Google Scholar]
- 7.Enzmann DR, Donaldson SS, Kriss JP. Appearance of Graves’ disease on orbital computed tomography. J Comput Assist Tomogr. 1979;3:815–9. [PubMed] [Google Scholar]
- 8.Wiersinga WM, Smit T, van der Gaag R, Mourits M, Koornneef L. Clinical presentation of Graves’ ophthalmopathy. Ophthalmic Res. 1989;21:73–82. doi: 10.1159/000266782. [DOI] [PubMed] [Google Scholar]
- 9.Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295–309. doi: 10.2165/00128071-200506050-00003. [DOI] [PubMed] [Google Scholar]
- 10.Rapoport B, McLachlan SM. The thyrotropin receptor in Graves’ disease. Thyroid. 2007;17:911–22. doi: 10.1089/thy.2007.0170. [DOI] [PubMed] [Google Scholar]
- 11.Khoo DH, Eng PH, Ho SC, et al. Graves’ ophthalmopathy in the absence of elevated free thyroxine and triiodothyronine levels: prevalence, natural history, and thyrotropin receptor antibody levels. Thyroid. 2000;10:1093–100. doi: 10.1089/thy.2000.10.1093. [DOI] [PubMed] [Google Scholar]
- 12.Eckstein AK, Lösch C, Glowacka D, et al. Euthyroid and primarily hypothyroid patients develop milder and significantly more asymmetrical Graves ophthalmopathy. Br J Ophthalmol. 2009;93:1052–6. doi: 10.1136/bjo.2007.137265. [DOI] [PubMed] [Google Scholar]
- 13.Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 2000;52:267–71. doi: 10.1046/j.1365-2265.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein AK, Plicht M, Lax H, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–70. doi: 10.1210/jc.2005-2813. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC, III, Hay ID, Nelson RE, Jiang NS. Clinical utility of thyrotropin-receptor antibody assays: comparison of radioreceptor and bioassay methods. Mayo Clin Proc. 1988;63:707–17. doi: 10.1016/s0025-6196(12)65533-5. [DOI] [PubMed] [Google Scholar]
- 16.Prummel MF, Wiersinga WM. Smoking and risk of Graves’ disease. JAMA. 1993;269:479–82. [PubMed] [Google Scholar]
- 17.Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21:168–99. doi: 10.1210/edrv.21.2.0393. [DOI] [PubMed] [Google Scholar]
- 18.Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15:737–45. doi: 10.1177/0961203306069344. [DOI] [PubMed] [Google Scholar]
- 19.Cawood TJ, Moriarty P, O’Farrelly C, O’Shea D. Smoking and thyroid-associated ophthalmopathy: a novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92:59–64. doi: 10.1210/jc.2006-1824. [DOI] [PubMed] [Google Scholar]
- 20.Bahn RS, Heufelder AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med. 1993;329:1468–75. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 21.Otto AJ, Koornneef L, Mourits MP, Deen-van Leeuwen L. Retrobulbar pressures measured during surgical decompression of the orbit. Br J Ophthalmol. 1996;80:1042–5. doi: 10.1136/bjo.80.12.1042. Erratum, Br J Ophthalmol 1997;81:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes G, Gorman CA, Brennan MD, Gehring DG, Ilstrup DM, Earnest F., IV Ophthalmopathy of Graves’ disease: computerized volume measurements of the orbital fat and muscle. AJNR Am J Neuroradiol. 1986;7:651–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson RL, Tweeten JP, Patrinely JR, Garland PE, Thiese SM. Dysthyroid optic neuropathy without extraocular muscle involvement. Ophthalmic Surg. 1989;20:568–74. [PubMed] [Google Scholar]
- 24.Rapoport B, Alsabeh R, Aftergood D, McLachlan SM. Elephantiasic pretibial myxedema: insight into and a hypothesis regarding the pathogenesis of the extrathyroidal manifestations of Graves’ disease. Thyroid. 2000;10:685–92. doi: 10.1089/10507250050137761. [DOI] [PubMed] [Google Scholar]
- 25.Chan LL, Tan HE, Fook-Chong S, Teo TH, Lim LH, Seah LL. Graves ophthalmopathy: the bony orbit in optic neuropathy, its apical angular capacity, and impact on prediction of risk. AJNR Am J Neuroradiol. 2009;30:597–602. doi: 10.3174/ajnr.A1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroll AJ, Kuwabara T. Dysthyroid ocular myopathy: anatomy, histology, and electron microscopy. Arch Ophthalmol. 1966;76:244–7. doi: 10.1001/archopht.1966.03850010246017. [DOI] [PubMed] [Google Scholar]
- 27.Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989;10:366–91. doi: 10.1210/edrv-10-3-366. [DOI] [PubMed] [Google Scholar]
- 28.Riley FC. Orbital pathology in Graves’ disease. Mayo Clin Proc. 1972;47:975–9. [PubMed] [Google Scholar]
- 29.Pappa A, Calder V, Ajjan R, et al. Analysis of extraocular muscle-infiltrating T cells in thyroid-associated ophthalmopathy (TAO) Clin Exp Immunol. 1997;109:362–9. doi: 10.1046/j.1365-2249.1997.4491347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckstein AK, Quadbeck B, Tews S, et al. Thyroid associated ophthalmopathy: evidence for CD4(+) γδ T cells, de novo differentiation of RFD7(+) macrophages, but not of RFD1(+) dendritic cells, and loss of γδ and αβ T cell receptor expression. Br J Ophthalmol. 2004;88:803–8. doi: 10.1136/bjo.2003.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aniszewski JP, Valyasevi RW, Bahn RS. Relationship between disease duration and predominant orbital T cell subset in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2000;85:776–80. doi: 10.1210/jcem.85.2.6333. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Bahn RS. Relative overexpression of macrophage-derived cytokines in orbital adipose tissue from patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2003;88:4246–50. doi: 10.1210/jc.2003-030380. [DOI] [PubMed] [Google Scholar]
- 33.Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2000;85:1194–9. doi: 10.1210/jcem.85.3.6433. [DOI] [PubMed] [Google Scholar]
- 34.Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003;24:802–35. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]
- 35.Bahn RS. Clinical review 157: pathophysiology of Graves’ ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab. 2003;88:1939–46. doi: 10.1210/jc.2002-030010. [DOI] [PubMed] [Google Scholar]
- 36.Otto EA, Ochs K, Hansen C, Wall JR, Kahaly GJ. Orbital tissue-derived T lymphocytes from patients with Graves’ ophthalmopathy recognize autologous orbital antigens. J Clin Endocrinol Metab. 1996;81:3045–50. doi: 10.1210/jcem.81.8.8768872. [DOI] [PubMed] [Google Scholar]
- 37.Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab. 1996;81:3428–31. doi: 10.1210/jcem.81.9.8784110. [DOI] [PubMed] [Google Scholar]
- 38.Valyasevi RW, Erickson DZ, Harteneck DA, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2557–62. doi: 10.1210/jcem.84.7.5838. [DOI] [PubMed] [Google Scholar]
- 39.Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. Adipose thyrotrophin receptor expression is elevated in Graves’ and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol. 2003;30:369–80. doi: 10.1677/jme.0.0300369. [DOI] [PubMed] [Google Scholar]
- 40.Rootman J. Pathophysiologic patterns of orbital disease. In: Rootman J, editor. Diseases of the orbit: a multidisciplinary approach. 2. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 43–51. [Google Scholar]
- 41.Smith TJ, Tsai CC, Shih MJ, et al. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thyroid. 2008;18:983–8. doi: 10.1089/thy.2007.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao HJ, Han R, Smith TJ. Robust induction of PGHS-2 by IL-1 in orbital fibroblasts results from low levels of IL-1 receptor antagonist expression. Am J Physiol Cell Physiol. 2003;284:C1429–C1437. doi: 10.1152/ajpcell.00354.2002. [DOI] [PubMed] [Google Scholar]
- 43.Smith TJ. The putative role of prostaglandin endoperoxide H synthase-2 in the pathogenesis of thyroid-associated orbitopathy. Exp Clin Endocrinol Diabetes. 1999;107(Suppl 5):S160–S163. doi: 10.1055/s-0029-1212175. [DOI] [PubMed] [Google Scholar]
- 44.Khoo TK, Coenen MJ, Schiefer AR, Kumar S, Bahn RS. Evidence for enhanced Thy-1 (CD90) expression in orbital fibroblasts of patients with Graves’ ophthalmopathy. Thyroid. 2008;18:1291–6. doi: 10.1089/thy.2008.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163:1291–300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87:385–92. doi: 10.1210/jcem.87.1.8164. [DOI] [PubMed] [Google Scholar]
- 47.Dobyns BM. Present concepts of the pathologic physiology of exophthalmos. J Clin Endocrinol Metab. 1950;10:1202–30. doi: 10.1210/jcem-10-10-1202. [DOI] [PubMed] [Google Scholar]
- 48.Mullin BR, Lee G, Ledley FD, Winland RJ, Kohn LD. Thyrotropin interactions with human fat cell membrane preparations and the finding of soluble thyrotropin binding component. Biochem Biophys Res Commun. 1976;69:55–62. doi: 10.1016/s0006-291x(76)80271-9. [DOI] [PubMed] [Google Scholar]
- 49.Davies TF, Teng CS, McLachlan SM, Smith BR, Hall R. Thyrotropin receptors in adipose tissue, retro-orbital tissue and lymphocytes. Mol Cell Endocrinol. 1978;9:303–10. doi: 10.1016/0303-7207(78)90072-2. [DOI] [PubMed] [Google Scholar]
- 50.Parmentier M, Libert F, Maenhaut C, et al. Molecular cloning of the thyrotropin receptor. Science. 1989;246:1620–2. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- 51.Nagayama Y, Kaufman KD, Seto P, Rapoport B. Molecular cloning, sequence and functional expression of the cDNA for the human thyrotropin receptor. Biochem Biophys Res Commun. 1989;165:1184–90. doi: 10.1016/0006-291x(89)92727-7. [DOI] [PubMed] [Google Scholar]
- 52.Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297–300. doi: 10.1089/thy.1993.3.297. [DOI] [PubMed] [Google Scholar]
- 53.Mengistu M, Lukes YG, Nagy EV, et al. TSH receptor gene expression in retroocular fibroblasts. J Endocrinol Invest. 1994;17:437–41. doi: 10.1007/BF03347732. [DOI] [PubMed] [Google Scholar]
- 54.Crisp MS, Lane C, Halliwell M, Wynford-Thomas D, Ludgate M. Thyrotropin receptor transcripts in human adipose tissue. J Clin Endocrinol Metab. 1997;82:2003–5. [PubMed] [Google Scholar]
- 55.Feliciello A, Porcellini A, Ciullo I, Bonavolonta G, Avvedimento EV, Fenzi G. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet. 1993;342:337–8. doi: 10.1016/0140-6736(93)91475-2. [DOI] [PubMed] [Google Scholar]
- 56.Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 57.Wakelkamp IM, Bakker O, Baldeschi L, Wiersinga WM, Prummel MF. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves’ ophthalmopathy patients. Clin Endocrinol (Oxf) 2003;58:280–7. doi: 10.1046/j.1365-2265.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 58.Endo T, Ohno M, Kotani S, Gunji K, Onaya T. Thyrotropin receptor in nonthyroid tissues. Biochem Biophys Res Commun. 1993;190:774–9. doi: 10.1006/bbrc.1993.1116. [DOI] [PubMed] [Google Scholar]
- 59.Dutton CM, Joba W, Spitzweg C, Heufelder AE, Bahn RS. Thyrotropin receptor expression in adrenal, kidney, and thymus. Thyroid. 1997;7:879–84. doi: 10.1089/thy.1997.7.879. [DOI] [PubMed] [Google Scholar]
- 60.Paschke R, Metcalfe A, Alcalde L, Vassart G, Weetman A, Ludgate M. Presence of nonfunctional thyrotropin receptor variant transcripts in retroocular and other tissues. J Clin Endocrinol Metab. 1994;79:1234–8. doi: 10.1210/jcem.79.5.7962314. [DOI] [PubMed] [Google Scholar]
- 61.Daumerie C, Ludgate M, Costagliola S, Many MC. Evidence for thyrotropin receptor immunoreactivity in pretibial connective tissue from patients with thyroid-associated dermopathy. Eur J Endocrinol. 2002;146:35–8. doi: 10.1530/eje.0.1460035. [DOI] [PubMed] [Google Scholar]
- 62.McLachlan SM, Nagayama Y, Rapoport B. Insight into Graves’ hyperthyroidism from animal models. Endocr Rev. 2005;26:800–32. doi: 10.1210/er.2004-0023. [DOI] [PubMed] [Google Scholar]
- 63.Baker G, Mazziotti G, von Ruhland C, Ludgate M. Reevaluating thyrotropin receptor-induced mouse models of Graves’ disease and ophthalmopathy. Endocrinology. 2005;146:835–44. doi: 10.1210/en.2004-1015. [DOI] [PubMed] [Google Scholar]
- 64.Bednarczuk T, Gopinath B, Ploski R, Wall JR. Susceptibility genes in Graves’ ophthalmopathy: searching for a needle in a haystack? Clin Endocrinol (Oxf) 2007;67:3–19. doi: 10.1111/j.1365-2265.2007.02854.x. [DOI] [PubMed] [Google Scholar]
- 65.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 66.Lantz M, Vondrichova T, Parikh H, et al. Overexpression of immediate early genes in active Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2005;90:4784–91. doi: 10.1210/jc.2004-2275. [DOI] [PubMed] [Google Scholar]
- 67.Kumar S, Leontovich A, Coenen MJ, Bahn RS. Gene expression profiling of orbital adipose tissue from patients with Graves’ ophthalmopathy: a potential role for secreted frizzled-related protein-1 in orbital adipogenesis. J Clin Endocrinol Metab. 2005;90:4730–5. doi: 10.1210/jc.2004-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-γ (PPARγ), and thyrotropin receptor by PPARγ agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2002;87:2352–8. doi: 10.1210/jcem.87.5.8472. [DOI] [PubMed] [Google Scholar]
- 69.Starkey K, Heufelder A, Baker G, et al. Peroxisome proliferator-activated receptor-gamma in thyroid eye disease: contraindication for thiazolidinedione use? J Clin Endocrinol Metab. 2003;88:55–9. doi: 10.1210/jc.2002-020987. [DOI] [PubMed] [Google Scholar]
- 70.Dorkhan M, Lantz M, Frid A, Groop L, Hallengren B. Treatment with a thiazolidinedione increases eye protrusion in a subgroup of patients with type 2 diabetes. Clin Endocrinol (Oxf) 2006;65:35–9. doi: 10.1111/j.1365-2265.2006.02542.x. [DOI] [PubMed] [Google Scholar]
- 71.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxydelta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 72.Konuk EB, Konuk O, Misirlioglu M, Menevse A, Unal M. Expression of cyclo-oxygenase-2 in orbital fibroadipose connective tissues of Graves’ ophthalmopathy patients. Eur J Endocrinol. 2006;155:681–5. doi: 10.1530/eje.1.02280. [DOI] [PubMed] [Google Scholar]
- 73.Feldon SE, O’Loughlin CW, Ray DM, Landskroner-Eiger S, Seweryniak KE, Phipps RP. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol. 2006;169:1183–93. doi: 10.2353/ajpath.2006.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu M, Lin RY. TSH stimulates adipogenesis in mouse embryonic stem cells. J Endocrinol. 2008;196:159–69. doi: 10.1677/JOE-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, Baker G, Janus D, Paddon CA, Fuhrer D, Ludgate M. Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Invest Ophthalmol Vis Sci. 2006;47:5197–203. doi: 10.1167/iovs.06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L, Bowen T, Grennan-Jones F, et al. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J Biol Chem. 2009;284:26447–55. doi: 10.1074/jbc.M109.003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith TJ. The putative role of fibroblasts in the pathogenesis of Graves’ disease: evidence for the involvement of the insulin-like growth factor-1 receptor in fibroblast activation. Autoimmunity. 2003;36:409–15. doi: 10.1080/08916930310001603000. [DOI] [PubMed] [Google Scholar]
- 78.Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170:6348–54. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]
- 79.Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89:5076–80. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 80.Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181:4397–405. doi: 10.4049/jimmunol.181.6.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 82.Feldon SE, Park DJ, O’Loughlin CW, et al. Autologous T-lymphocytes stimulate proliferation of orbital fibroblasts derived from patients with Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci. 2005;46:3913–21. doi: 10.1167/iovs.05-0605. [DOI] [PubMed] [Google Scholar]
- 83.Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce pro-inflammatory cytokine production. Am J Physiol. 1998;274:C707–C714. doi: 10.1152/ajpcell.1998.274.3.C707. [DOI] [PubMed] [Google Scholar]
- 84.Korducki JM, Loftus SJ, Bahn RS. Stimulation of glycosaminoglycan production in cultured human retroocular fibroblasts. Invest Ophthalmol Vis Sci. 1992;33:2037–42. [PubMed] [Google Scholar]
- 85.Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol. 1995;268:C382–C388. doi: 10.1152/ajpcell.1995.268.2.C382. [DOI] [PubMed] [Google Scholar]
- 86.Wang HS, Cao HJ, Winn VD, et al. Leukoregulin induction of prostaglandin-endoperoxide H synthase-2 in human orbital fibroblasts: an in vitro model for connective tissue inflammation. J Biol Chem. 1996;271:22718–28. [PubMed] [Google Scholar]
- 87.Nordan RP, Potter M. A macrophage-derived factor required by plasmacytomas for survival and proliferation in vitro. Science. 1986;233:566–9. doi: 10.1126/science.3726549. [DOI] [PubMed] [Google Scholar]
- 88.Valyasevi RW, Jyonouchi SC, Dutton CM, Munsakul N, Bahn RS. Effect of tumor necrosis factor-alpha, interferon-gamma, and transforming growth factor-beta on adipogenesis and expression of thyrotropin receptor in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2001;86:903–8. doi: 10.1210/jcem.86.2.7188. [DOI] [PubMed] [Google Scholar]
- 89.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–59. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 90.Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435:612–9. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- 91.Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167–79. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- 92.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–71. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 93.Dinarello CA. The role of the interleukin-1–receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732–4. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 94.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–26. doi: 10.1038/ncprheum0338. Erratum, Nat Clin Pract Rheumatol 2006;2:691. [DOI] [PubMed] [Google Scholar]
- 95.Durrani OM, Reuser TQ, Murray PI. Infliximab: a novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit. 2005;24:117–9. doi: 10.1080/01676830590912562. [DOI] [PubMed] [Google Scholar]
- 96.Paridaens D, van den Bosch WA, van der Loos TL, Krenning EP, van Hagen PM. The effect of etanercept on Graves’ ophthalmopathy: a pilot study. Eye (Lond) 2005;19:1286–9. doi: 10.1038/sj.eye.6701768. [DOI] [PubMed] [Google Scholar]
- 97.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36:1118–25. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 98.Tan GH, Dutton CM, Bahn RS. Interleukin-1 (IL-1) receptor antagonist and soluble IL-1 receptor inhibit IL-1-induced glycosaminoglycan production in cultured human orbital fibroblasts from patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1996;81:449–52. doi: 10.1210/jcem.81.2.8636247. [DOI] [PubMed] [Google Scholar]
- 99.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 100.Pohlers D, Brenmoehl J, Löffler I, et al. TGF-beta and fibrosis in different organs — molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–56. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Wertenbruch T, Willenberg HS, Sagert C, et al. Serum selenium levels in patients with remission and relapse of Graves’ disease. Med Chem. 2007;3:281–4. doi: 10.2174/157340607780620662. [DOI] [PubMed] [Google Scholar]
- 102.Tsokos GC. B cells, be gone — B-cell depletion in the treatment of rheumatoid arthritis. N Engl J Med. 2004;350:2546–8. doi: 10.1056/NEJMp048114. [DOI] [PubMed] [Google Scholar]
- 103.El Fassi D, Nielsen CH, Hasselbalch HC, Hegedüs L. Treatment-resistant severe, active Graves’ ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid. 2006;16:709–10. doi: 10.1089/thy.2006.16.709. [DOI] [PubMed] [Google Scholar]
- 104.Kwan-Morley J, Albert D. B-cell inhibitors as therapy for rheumatoid arthritis: an update. Curr Rheumatol Rep. 2007;9:401–6. doi: 10.1007/s11926-007-0064-4. [DOI] [PubMed] [Google Scholar]
- 105.Salvi M, Vannucchi G, Campi I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33–40. doi: 10.1530/eje.1.02325. [DOI] [PubMed] [Google Scholar]
- 106.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 107.Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–15. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 108.Kalunian KC, Davis JC, Jr, Merrill JT, Totoritis MC, Wofsy D IDEC-131 Lupus Study Group. Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:3251–8. doi: 10.1002/art.10681. [DOI] [PubMed] [Google Scholar]
- 109.Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- 110.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–8. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 111.Cozma I, Zhang L, Uddin J, Lane C, Rees A, Ludgate M. Modulation of expression of somatostatin receptor subtypes in Graves’ ophthalmopathy orbits: relevance to novel analogs. Am J Physiol Endocrinol Metab. 2007;293:E1630–E1635. doi: 10.1152/ajpendo.00177.2007. [DOI] [PubMed] [Google Scholar]
- 112.Neumann S, Kleinau G, Costanzi S, et al. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149:5945–50. doi: 10.1210/en.2008-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 115.El Fassi D, Banga JP, Gilbert JA, Padoa C, Hegedüs L, Nielsen CH. Treatment of Graves’ disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clin Immunol. 2009;130:252–8. doi: 10.1016/j.clim.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 116.Salvi M, Vannucchi G, Campi I, et al. Rituximab treatment in a patient with severe thyroid-associated ophthalmopathy: effects on orbital lymphocytic infiltrates. Clin Immunol. 2009;131:360–5. doi: 10.1016/j.clim.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 117.ClinicalTrials.gov. [Accessed January 21, 2010];Trial of rituximab for Graves’ ophthalmopathy. at http://clinicaltrials.gov/ct2/show/NCT00595335.
- 118.Misharin AV, Nagayama Y, Aliesky HA, Mizutori Y, Rapoport B, McLachlan SM. Attenuation of induced hyperthyroidism in mice by pretreatment with thyrotropin receptor protein: deviation of thyroid-stimulating to nonfunctional antibodies. Endocrinology. 2009;150:3944–52. doi: 10.1210/en.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morshed SA, Latif R, Davies TF. Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology. 2009;150:519–29. doi: 10.1210/en.2008-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Terwee C, Wakelkamp I, Tan S, Dekker F, Prummel MF, Wiersinga W. Long-term effects of Graves’ ophthalmopathy on health-related quality of life. Eur J Endocrinol. 2002;146:751–7. doi: 10.1530/eje.0.1460751. [DOI] [PubMed] [Google Scholar]
- 121.Bartalena L, Baldeschi L, Dickinson AJ, et al. Consensus statement of the European Group on Graves’ Orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid. 2008;18:333–46. doi: 10.1089/thy.2007.0315. [DOI] [PubMed] [Google Scholar]