Abstract
The signs and symptoms of Graves’ ophthalmopathy (GO) result from increased volume of the orbital contents, including adipose, connective, and extraocular muscle tissues. We wanted to determine whether the expanded adipose tissue volume might be in part attributable to de novo adipogenesis. We measured levels of mRNA encoding leptin, adiponectin, peroxisome proliferator-activated receptor γ (PPARγ), preadipocyte factor-1, and TSH receptor (TSHr) genes in orbital adipose tissues from GO patients (n = 22) and normal individuals (n = 18) and in orbital preadipocyte cultures derived from GO patients (n = 6) and normal subjects (n = 3) using quantitative real-time RT PCR. We found increased leptin, adiponectin, PPARγ, and TSHr expression in GO compared with normal orbital tissue samples, with positive correlations in the GO tissues between TSHr and leptin, adiponectin and PPARγ. In vitro differentiation of GO and normal preadipocytes resulted in enhanced adiponectin, leptin, and TSHr expression, with greater expression of the latter two genes in the GO cultures. These results suggest that de novo adipogenesis within orbital tissues with parallel enhanced expression of TSHr may be important in the pathogenesis of GO, and that potential therapies for GO might include inhibition of the adipogenic pathway.
Graves’ ophthalmopathy (GO) is an autoimmune disease characterized by an increase in the volume of the orbital fatty/connective tissues and extraocular muscles within the orbit. Although the autoantigen involved in the hyperthyroidism of Graves’ disease is known to be the TSH receptor (TSHr), whether this antigen plays a primary role in the pathogenesis of GO is unclear. A prerequisite for involvement of this receptor in GO is that it be present in affected orbital tissues, and recent studies suggest that this is indeed the case. Investigators have demonstrated TSHr expression at both the mRNA and protein levels in cultured orbital fibroblasts obtained from GO patients (1–4). In addition, studies comparing relative expression in GO and normal orbital tissues and fibroblast cultures found TSHr to be more abundant in GO patients than in normal orbital cultures (5, 6). These results suggest that stimulated TSHr expression in the orbit in the setting of Graves’ disease may play a role in GO pathogenesis.
Volume measurements of the orbital adipose/connective tissue compartment and extraocular muscles using computerized tomography scans revealed enlargement of either or both in 87% of patients with clinically detectable GO (7). More recently, investigators suggested that GO can be divided into two subtypes. The first subtype is characterized by prominent extraocular muscle enlargement and diplopia. The second, without muscle enlargement, is associated with expansion of the orbital fat compartment and proptosis (8, 9). In another study, the degree of proptosis was found to correlate well with the volume of the orbital fat compartment (10). Taken together, these studies suggest that expansion of the orbital fat compartment represents a major component of the disease process. However, it is unclear whether this increased volume is secondary to enhanced adipogenesis within the orbit or to enlargement of existing orbital adipocytes.
Adipogenesis is a complex process accompanied by a dramatic and coordinated increase in the expression of several genes, including leptin, adiponectin (also known as adipocyte complement-related protein of 30 kDa), and peroxisome proliferator-activated receptor γ (PPARγ), with a concurrent decrease in the expression of preadipocyte factor-1 (pref-1). We have shown previously that orbital preadipocytes can be induced in vitro to differentiate into adipocytes bearing functional TSHr (11). We undertook the current study to ascertain whether de novo adipogenesis might be similarly enhanced within the orbit in GO.
Patients and Methods
Patients
Orbital adipose/connective tissue specimens were obtained from patients who underwent orbital decompression surgery for severe GO (n = 22) and from individuals with no history of Graves’ disease (n = 18). Four of the 22 patients with GO had optic neuropathy. The clinical characteristics of the GO patients are shown in Table 1. All GO patients were euthyroid at the time of orbital surgery. Normal orbital tissues were retrieved at very early autopsy from patients whose corneas were being harvested for transplantation. The sex ratio, body weight, and body mass index were not significantly different between the GO patients and normal individuals. However, the latter were significantly older than the patients with GO (Table 2). These studies were reviewed and approved by the Mayo Clinic institutional review board.
TABLE 1.
Clinical characteristics of patients with GO
| Time from diagnosis of hyperthyroidism (months) | 55.7 ± 14.2a |
| Duration of signs/symptoms of ophthalmopathy (months) | 44.9 ± 12.6a |
| No. with optic neuropathy | 4/22 |
| Antithyroid drug treatment | 4/22 current users |
| Treatment with corticosteroids | 4/22 currently using corticosteroids 9/22 used corticosteroids in the past |
| History of radioiodine treatment | 18/22 |
| History of orbital irradiation | 4/22 |
| Smoking | 9/22 current smokers |
Mean ± SEM.
TABLE 2.
Demographic characteristics of GO patients and normal individuals
| Parameter | GO | Normal | P value |
|---|---|---|---|
| Males/females | 9/13 | 8/10 | NS |
| Age (yr) | 56.7 ± 3.05a | 70.7 ± 3.61a | <0.05 |
| Weight (kg) | 82 ± 3.49a | 75.8 ± 4.55a | NS |
| BMI (kg/m2) | 29.3 ± 1.12a | 26.9 ± 2.5a | NS |
BMI, Body mass index.
Mean ± SEM.
Orbital tissues
Orbital adipose/connective tissue was obtained during the course of transantral orbital decompression surgery for GO, placed in a sterile container on saline-soaked gauze, and transported at room temperature to our laboratory, where it was frozen at −70 C. Normal orbital tissue was frozen immediately upon harvesting.
Cell cultures
GO (n = 6) and normal orbital adipose tissue (n = 3) samples were minced and placed directly in plastic culture dishes, allowing preadipocyte fibroblasts to proliferate as described previously (12). Cells were propagated in medium 199 containing 20% fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT), penicillin (100 U/ml), and gentamicin (20 μg/ml) in a humidified 5% CO2 incubator at 37 C and were maintained in 80-mm2 flasks with medium 199 containing 10% FBS and antibiotics.
To initiate adipocyte differentiation, orbital cells were grown to confluence in six-well plates in medium 199 with 10% FBS. Differentiation was carried out as reported previously (11); cultures were changed to serum-free DMEM/Ham’s F-12 (1:1; Sigma-Aldrich Corp., St. Louis, MO) supplemented with biotin (33 μM), pantothenic acid (17 μM), trans-ferrin (10 μg/ml), T3 (0.2 nM), insulin (1 μM), carbaprostacyclin (0.2 μM; Calbiochem, La Jolla CA), and, for the first 4 d only, dexamethasone (1 μM) and isobutylmethylxanthine (0.1 mM). The differentiation protocol was continued for 10 d, during which time the medium was replaced every 3–4 d. Undifferentiated cultures were derived from fibroblasts obtained from the same patient’s orbital tissues and were maintained for the same period of time in medium lacking several of the components necessary for complete adipocyte differentiation (i.e. carbaprostacyclin, dexamethasone, and isobutylmethylxanthine).
Real-time RT-PCR
Total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. cDNA was synthesized using 200 ng total RNA incubated with random hexamers, followed by a 100-μl RT reaction with 6.25 U Multiscribe Reverse Transcriptase (PE Applied Biosystems, Foster City, CA). Conditions used were 25 C for 10 min, 37 C for 60 min, and 95 C for 5 min.
Oligonucleotide primers and TaqMan probes were designed using the computer program Primer Express (PE Applied Biosystems). To avoid amplification of genomic DNA, one of the two primers or the probe for TSHr, leptin, PPARγ, and Pref-1 was placed at the junction between two exons. For adiponectin, primers and probes could not be designed across an exon intron junction, and hence, no RT controls were used to detect amplification of genomic DNA. Expression of 18S ribosomal RNA (rRNA) was used to correct for differences in the amount of total RNA added to a reaction and to compensate for different levels of inhibition during RT of RNA and PCR. Primers and probes for TSHr and 18S rRNA were designed and supplied by PE Applied Biosystems (Assays-on-Demand and predeveloped TaqMan assay reagents, respectively). The PCR primers and probes for leptin, adiponectin, PPARγ, and pref-1 are shown in Table 3.
TABLE 3.
Primers and probes for adipogenesis-related genes
| Leptin | |
| Forward primer | TCACCAGGATCAATGACATTTCAC |
| Reverse primer | CCCAGGAATGAAGTCCAAACC |
| Probe | CACGCAGTCAGTCTCCTCCAAACAGAAAG |
| Adiponectin | |
| Forward primer | CCACTATGATGGCTCCACTGGTA |
| Reverse primer | AGACTGTGATGTGGTAGGCAAAGTAG |
| Probe | ATTCCACTGCAACATTCCTGGGCTGT |
| PPARγ | |
| Forward primer | TGACAGCGACTTGGCAATATTT |
| Reverse primer | TCTTCAATGGGCTTCACATTCA |
| Probe | CAAACCTGGGCGGTCTCCACTGAG |
| pref-1 | |
| Forward primer | TCCTGCGCGTCCTCTTG |
| Reverse primer | GAAGCATTCAGCCCCATAGG |
| Probe | CTGCTGGCTTTCGGCCACAGC |
Quantitative PCR reactions were performed in a 96-well optical reaction plate. Amplification reactions contained cDNA equivalent of 2 ng total RNA for orbital tissues and 200 ng total RNA for orbital cultures, 900 nM of the forward and reverse primers, and 250 nM of the probes in a volume of 25 μl using the Universal TaqMan 2× PCR MasterMix (PE Applied Biosystems). The thermal cycling conditions used were 2 min at 50 C for optimal AmpErase UNG activity, 10 min at 95 C to activate AmpliTaq Gold DNA polymerase, followed by 40 cycles at 95 C for 15 sec and 60 C for 1 min. The target genes and 18S rRNA were amplified in separate wells. All reactions were performed in duplicate in the ABI PRISM 7700 sequence detector (PE Applied Biosystems), and the data were pooled. Thyroid tissue was used as the positive control for TSHr, and normal adipose tissue was used as the positive control for leptin, adiponectin, and PPARγ. A human liposarcoma cell line was used as the positive control for pref-1. Reaction mixture, without the cDNA, was used as the negative control in each run.
The standard curve method was used to quantify the expression of the various genes and 18S rRNA in each sample. The normalized results were expressed as the ratio of RNA (picograms) of the target gene to RNA (picograms) of 18S rRNA. For each experimental sample, a gene was considered not to be expressed if amplification was not detected by cycle 40. The relative expression levels of these genes in orbital adipose tissue specimens from patients with GO were compared with those in specimens from normal controls. The number of orbital tissue specimens examined differed slightly between the genes studied (TSHr: n =18 for GO, n =15 for normal subjects; leptin, adiponectin, and PPARγ: n =22 for GO, n =18 for normal subjects; pref-1: n = 13 for GO, n = 8 for normal subjects;).
Statistical analysis
The Mann-Whitney rank-sum test was used to assess statistically significant differences between the groups. Additional analyses were performed using Spearman rank order correlation to study correlations between expression levels of TSHr and the various adipogenesis-related genes.
Results
Tissue specimens
TSHr mRNA was amplified in each of the 18 GO orbital tissue specimens and in 14 of 15 normal orbital tissues. The expression of TSHr, normalized to 18S rRNA, was significantly higher in orbital adipose tissues from GO patients than in normal adipose tissues (P < 0.05; Fig. 1A). TSHr expression did not correlate with the duration of GO, type of prior GO treatment, smoking history, time since diagnosis of hyperthyroidism, or age of the individual. We found no difference in TSHr expression between males and females (P = 0.543). TSHr mRNA levels were significantly lower (100- to 1000-fold) in GO orbit than in normal thyroid tissue.
Fig. 1.
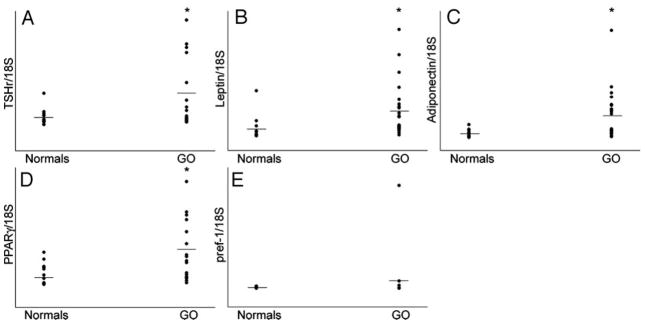
Relative expression of TSHr and adipogenesis-related genes (normalized to 18S rRNA) in orbital adipose tissues from patients with GO and normal individuals. A, TSHr (GO, n = 18; normal, n = 15); B, leptin (GO, n = 22; normal, n = 18); C, adiponectin (GO, n = 22; normal, n = 18); D, PPARγ (GO, n = 22; normal, n = 18); E, pref-1 (GO, n = 13; normal, n = 8). Horizontal lines represent mean values for each group. *,P < 0.05 compared with normal values.
Leptin, adiponectin, and PPARγ, were amplified in each of the 22 GO orbital tissues and in the 18 normal orbital tissues. In contrast, pref-1 was amplified in only 11 of 13 GO tissues and 5 of 8 normal adipose tissues. As was found for TSHr, the expression of leptin, adiponectin, and PPARγ, normalized to 18S rRNA, was increased in orbital adipose tissues from GO patients compared with normal adipose tissue samples (P < 0.001 for leptin and adiponectin; P < 0.003 for PPARγ; Fig. 1, B–D). The expression levels of the genes did not correlate with the duration of GO, type of prior GO treatment, smoking history, time since diagnosis of hyperthyroidism, or age of the individual. Because adiponectin serum levels are known to vary inversely with weight and to be greater in females than in males, it is important to note that the mean weights of the normal subjects and GO patients were not significantly different (P = 0.283), and that there was a nearly equal proportion of females in both groups (55% in normal subjects and 60% in GO). Results for pref-1 showed wide variability of expression both within and between the 13 GO and 8 normal adipose tissues examined (Fig. 1E); thus, we found no significant differences between these groups.
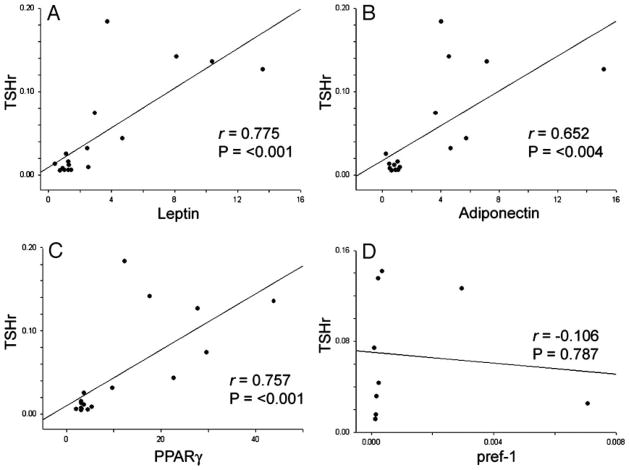
We analyzed our data to determine whether correlations exist between the expression of TSHr and that of any of the adipogenesis-related genes. Within the GO tissues, we found significant positive correlations between expression levels of TSHr and leptin (P < 0.001; r = 0.775; Fig. 2A), TSHr and adiponectin (P < 0.004; r = 0.652; Fig. 2B), and TSHr and PPARγ (P < 0.001; r = 0.757; Fig. 2C). In contrast, no correlations were found between expression levels of pref-1 and any of the other genes studied in GO tissues. Additionally, no correlations existed in normal orbital tissues between the expression of TSHr and any of the other genes studied.
Fig. 2.
Correlations between TSHr mRNA and levels of mRNA encoding adipogenesis-related genes (normalized to 18S rRNA) in orbital adipose tissues from patients with GO. A, Leptin (n = 17); B, adiponectin (n = 17); C, PPAR-γ (n = 17); D, pref-1 (n = 10). Correlation coefficients and P values are included in each graph.
Cell cultures
TSHr mRNA expression increased upon differentiation in each of the six GO cultures (mean, 11.9-fold; range, 4.2- to 44.4-fold; P = 0.002) and in two of three normal orbital cultures (mean, 1.6-fold; range, 0.9- to 2-fold; Fig. 3). The degree of increase in expression of TSHr was significantly greater in GO cultures compared with normal cultures (P = 0.024). Similarly, leptin mRNA expression increased upon differentiation in all GO (mean, 16.1-fold; range, 3.7- to 40.3-fold; P = 0.002) and normal cultures (mean, 3.2-fold; range, 2.1- to 5.3-fold), and the degree of increase in expression of this gene was also significantly higher in GO cultures than in normal cultures (P = 0.048). Adiponectin expression also increased upon differentiation in both GO (mean, 12.9-fold; range, 1.4- to 60-fold; P = 0.002) and normal (mean, 9.1-fold; range, 2.3- to 22.6-fold) preadipocyte cultures. However, the degree of increase in expression of this gene was not significantly different between GO and normal cultures.
Fig. 3.
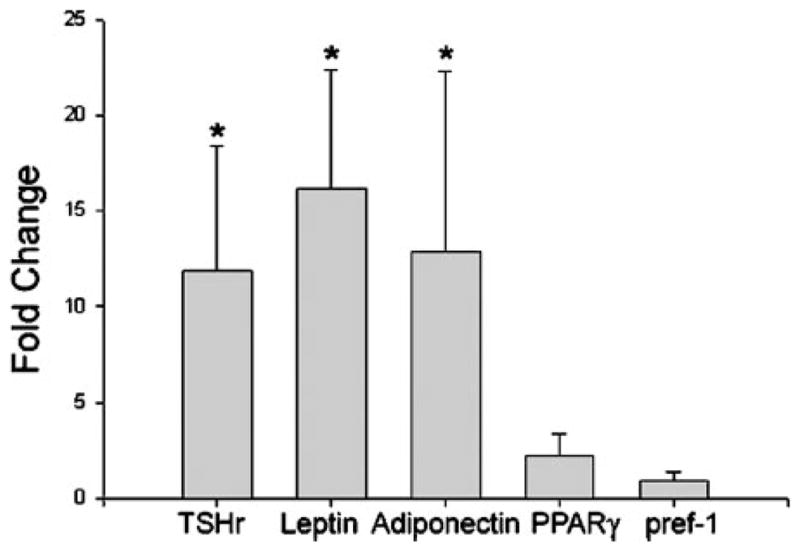
Fold change (mean ± SEM) in the expression levels of TSHr and adipogenesis-related genes in differentiated orbital preadipocyte cultures from patients with GO (n = 6) and normal individuals (n = 3). Results are expressed relative to parallel undifferentiated orbital cultures from the same individuals. *, P < 0.05.
In contrast, there was no significant alteration in the expression of either PPARγ (GO: mean, 2.2-fold; range, 0.9- to 7.6-fold; P = 0.394; normal: mean, 1.8-fold; range, 1.2- to 2.1-fold) or pref-1 (GO: mean, 0.89-fold; range, 0.1- to 3-fold; normal: mean, 0.3-fold; range, 0.04- to 0.9-fold) after differentiation in either GO or normal cultures (Fig. 3). Further, the degree of change in TSHr after differentiation did not correlate significantly with any of the adipogenesis-related genes in either GO or normal cultures. However, in GO cultures, we noted a trend toward significance in the correlation between changes in TSHr and adiponectin (r = 0.829; P = 0.0583) and between changes in TSHr and PPARγ (r = 0.829; P = 0.0583; data not shown).
Discussion
The hypothesis that TSHr is an important autoantigen in GO rests on evidence that this receptor is present in the orbit. In addition, it raises the question of whether TSHr might be expressed to a greater degree in GO patients than in normal orbital tissues. Studies from several laboratories have identified TSHr mRNA or a variant TSHr transcript in human orbital tissues from GO patients and derivative cell cultures using RT-PCR (1–4, 13, 14). However, the results of these studies were difficult to interpret because this method is not quantitative, and RNA transcripts detected only by RT-PCR-based amplification of cDNA may not have physiological relevance. Later studies used more direct methods, including a ribonuclease protection assay (5) or Northern blotting (6), to assess relative TSHr expression. Although only a very small number of normal and GO orbital tissue samples were assessed in these studies, the results were in general agreement that TSHr mRNA, although present in both normal and GO orbital tissues, is more abundant in the tissues of affected patients. Our current study used real-time PCR, a newer quantitative and more sensitive technique, to assess TSHr expression in a large number of patient and normal orbital tissue samples (n = 18 for GO; n = 15 for normal). Results showed orbital TSHr expression to be significantly increased in patients with GO, while remaining some 100- to 1000-fold lower than that found in thyroid tissue. Additionally, we found that differentiation of orbital preadipocyte fibroblasts was associated with a greater degree of increase in expression of TSHr mRNA in GO than in normal cultures. Although factors responsible for this stimulated expression are unknown at present, these findings are consistent with the hypothesis that orbital TSHr expression is involved in GO pathogenesis. It may be that enhanced receptor expression plays a role in initiation of the orbital autoimmune process. Alternately, elevated levels of TSHr at the site of clinical disease may arise secondarily to an ongoing autoimmune process. As such, although not directly involved in the earliest stages of disease, the presence of relatively abundant TSHr in the GO orbit might be important in disease progression.
Although we and others have found TSHR expression levels in thyroid and GO orbital adipose tissues to be the highest measured (5, 6), TSHR mRNA is detectable in adrenal, kidney, thymus (15), pituitary (16), human abdominal adipose tissue (17), skin, liver, leukocytes, lymph nodes, brain, gut, and pancreas (18). The finding of low level TSHR expression in diverse tissues suggests that extrathyroidal TSHR expression may not have physiological relevance in normal individuals. However, in the setting of Graves’ disease with circulating antibodies and T cells directed against this antigen, there may be autoimmune reactivity directed against TSHr in adipose and connective tissues throughout the body (19). Patients in whom GO or pretibial dermopathy become clinically apparent might represent a subset affected by a combination of environmental and local factors (20, 21). Potentially important factors might include gravitational dependency and trauma, smoking, anatomic constraints of the bony orbit, dysfunction of efferent lymphatic channels, and elaboration within these tissues of particular cytokines. These anatomic regions appear to be particularly susceptible to various cytokine effects due to site-specific phenotypic characteristics of fibroblasts (22).
Adipose tissue volume increases through a combination of increased cell number (hyperplasia) and expanded cell size (hypertrophy). New adipocyte formation, or adipogenesis, plays an on-going role in adipose tissue enlargement throughout life (23). Sorisky and colleagues (24) demonstrated that cultures derived from human orbital adipose/ connective tissue contain adipocyte precursor cells (comprising 5–10% of the total), capable of differentiating into lipid-filled adipocytes when cultured under conditions known to stimulate adipogenesis in fibroblasts from other sites. Adipogenesis is a complex process associated with activation of several adipocyte-specific genes, including leptin, adiponectin, and PPARγ, and inhibition of the preadipocyte gene pref-1. PPARγ is a nuclear hormone receptor that is highly expressed in adipose tissue (25, 26). The activation of this receptor is critical for the complex process of adipogenesis, and several ligands of this receptor that have profound effects on this process as well as on insulin sensitivity have been developed (27, 28). Leptin is a protein produced and secreted exclusively by mature adipocytes (29). Similarly, adiponectin is a recently identified, adipose tissue-derived, soluble protein produced solely by mature adipocytes (30). This protein has important metabolic effects related to whole body insulin sensitivity and also possesses antiatherogenesis properties. Serum levels of adiponectin decrease with obesity and are higher in females than in males. Both adiponectin expression and secretion are stimulated by activators of PPARγ (31).
We found significantly increased expression of all three gene markers of adipocyte differentiation (leptin, adiponectin, and PPARγ) in orbital adipose tissue from patients with GO compared with normal orbital tissue. In addition, the expression of each of these genes correlated positively with TSHr gene expression. We reported previously that PPARγ is expressed in orbital adipose tissue from GO patients and that both adipogenesis and PPARγ are stimulated in cultures of GO orbital fibroblasts treated with a PPARγ agonist (32). The potential clinical significance of this observation was suggested by investigators who reported the case of a patient with GO who experienced exacerbation of his eye disease, with expansion of the orbital fat, after treatment of diabetes mellitus type 2 with the PPARγ agonist pioglitazone (33).
In the current study we found higher levels of leptin and adiponectin, genes produced exclusively by mature adipocytes, in uncultured orbital adipose tissue specimens from patients with GO compared with normal orbital tissue specimens. Although effects of prior treatment for GO cannot be entirely ruled out, these results suggest that there may be a relatively greater number of mature adipocytes in GO than in normal orbital tissues. This may result from the stimulation of adipogenesis in orbital preadipocytes by some unknown factor present in Graves’ disease. The positive correlations noted between TSHr and adipogenesis-related gene expression in uncultured GO tissues further suggest that the expanded orbital adipose tissue volume in GO stems from adipogenesis rather than lipid accumulation in existing mature adipocytes. That similar correlations were not found in normal orbital adipose tissues that had lower levels of TSHr, leptin, adiponectin, and PPARγ further supports the concept that enhanced orbital adipogenesis is a component of the disease process in GO.
We found that GO preadipocytes respond more vigorously to culture conditions inducing differentiation than do normal orbital cultures, as measured by increased leptin and TSHr expression. A similar, but not statistically significant, trend was apparent for adiponectin expression in GO compared with normal orbital cultures. These in vitro observations parallel our findings of increased adipocyte and TSHr gene expression in GO orbital adipose tissue specimens compared with normal tissue samples. These results suggest that some circulating or local factor present in the orbit in GO, the effects of which are maintained in tissue culture, may render preadipocytes especially susceptible to differentiation. The nature of these putative stimulators is unclear and is the subject of current investigation.
Orbital fibroblasts derived from the connective tissue/ adipose depot in the posterior orbit are a distinct subpopulation from those investing the extraocular muscles (34). The preadipocyte fibroblasts studied in this investigation are of the former variety, as they are derived from orbital fat removed in the course of orbital decompression surgery for GO. The fibroblasts investing the extraocular muscles do not differentiate into adipocytes in culture conditions similar to those we used in this study, but are similarly capable of glycosaminoglycan synthesis (35). This heterogeneity in orbital fibroblast phenotype may help to explain why some patients with GO experience predominant enlargement of the extraocular muscles with accumulation of glycosamino-glycans, whereas in other patients expansion of the orbital adipose tissues represents the major disease process (34).
In summary, de novo adipogenesis within the orbit, with parallel enhanced expression of TSHr in orbital adipocytes, may be of central importance in the pathogenesis of GO. This process would result in increased expression of TSHr in the orbit, perhaps allowing this receptor to act as an autoantigen there. In addition, the concomitant expansion of orbital adipose tissue volume would lead to the development of the clinical signs and symptoms of GO. Factors responsible for stimulation of orbital adipogenesis are unknown, but various cytokines or other immune factors known to be present in the GO orbit may be involved (20, 36). It is perplexing that this process appears clinically to be limited to the orbit, with similar, although not identical, histopathological changes found in the pretibial tissues of patients with pretibial dermopathy. This relative anatomic site specificity might be explained by local mechanical factors and by phenotypic characteristics of fibroblasts residing in these anatomic sites (22, 34). Finally, these findings suggest that inhibition of orbital adipogenesis by antagonism of various components of the adipogenic pathway might be of benefit in the treatment of GO.
Acknowledgments
This work was supported in part by NIH Grant EYO8819 (to R.S.B.) from the National Eye Institute.
Abbreviations
- FBS
Fetal bovine serum
- GO
Graves’ ophthalmopathy
- PPARγ
peroxisome proliferator-activated receptor γ
- pref-1
preadipocyte factor-1
- rRNA
ribosomal RNA
- TSHr
TSH receptor
References
- 1.Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297–300. doi: 10.1089/thy.1993.3.297. [DOI] [PubMed] [Google Scholar]
- 2.Hiromatsu Y, Sato M, Inoue Y, Koga M, Miyake I, Kameo J, Tokisawa S, Yang D, Nonaka K. Localization and clinical significance of thyrotropin receptor mRNA expression in orbital fat and eye muscle tissues from patients with thyroid-associated ophthalmopathy. Thyroid. 1996;6:553–562. doi: 10.1089/thy.1996.6.553. [DOI] [PubMed] [Google Scholar]
- 3.Feliciello A, Porcellini A, Ciullo I, Bonavolonta G, Avvedimento EV, Fenzi G. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet. 1993;342:337–338. doi: 10.1016/0140-6736(93)91475-2. [DOI] [PubMed] [Google Scholar]
- 4.Mengistu M, Lukes YG, Nagy EV, Burch HB, Carr FE, Lahiri S, Burman KD. TSH receptor gene expression in retroocular fibroblasts. J Endocrinol Invest. 1994;17:437–441. doi: 10.1007/BF03347732. [DOI] [PubMed] [Google Scholar]
- 5.Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 6.Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. Adipose thyrotropin receptor expression is elevated in Graves’ and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol. 2003;30:369–380. doi: 10.1677/jme.0.0300369. [DOI] [PubMed] [Google Scholar]
- 7.Forbes G, Gorman CA, Brennan MD, Gehring DG, Ilstrup DM, Earnest Ft. Ophthalmopathy of Graves’ disease: computerized volume measurements of the orbital fat and muscle. AJNR Am J Neuroradiol. 1986;7:651–656. [PMC free article] [PubMed] [Google Scholar]
- 8.Hiromatsu Y, Yang D, Bednarczuk T, Miyake I, Nonaka K, Inoue Y. Cytokine profiles in eye muscle tissue and orbital fat tissue from patients with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2000;85:1194–1199. doi: 10.1210/jcem.85.3.6433. [DOI] [PubMed] [Google Scholar]
- 9.Gunji K, De Bellis A, Li AW, Yamada M, Kubota S, Ackrell B, Wengrowicz S, Bellastella A, Bizzarro A, Sinisi A, Wall JR. Cloning and characterization of the novel thyroid and eye muscle shared protein G2s: autoantibodies against G2s are closely associated with ophthalmopathy in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1641–1647. doi: 10.1210/jcem.85.4.6553. [DOI] [PubMed] [Google Scholar]
- 10.Peyster RG, Ginsberg F, Silber JH, Adler LP. Exophthalmos caused by excessive fat: CT volumetric analysis and differential diagnosis. AJR Am J Roentgenol. 1986;146:459–464. doi: 10.2214/ajr.146.3.459. [DOI] [PubMed] [Google Scholar]
- 11.Valyasevi RW, Erickson DZ, Harteneck DA, Dutton CM, Heufelder AE, Jyonouchi SC, Bahn RS. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2557–2562. doi: 10.1210/jcem.84.7.5838. [DOI] [PubMed] [Google Scholar]
- 12.Bahn RS, Gorman CA, Johnson CM, Smith TJ. Presence of antibodies in the sera of patients with Graves’ disease recognizing a 23 kilodalton fibroblast protein. J Clin Endocrinol Metab. 1989;69:622–628. doi: 10.1210/jcem-69-3-622. [DOI] [PubMed] [Google Scholar]
- 13.Paschke R, Tonacchera M, Van Sande J, Parma J, Vassart G. Identification and functional characterization of two new somatic mutations causing constitutive activation of the thyrotropin receptor in hyperfunctioning autonomous adenomas of the thyroid. J Clin Endocrinol Metab. 1994;79:1785–1789. doi: 10.1210/jcem.79.6.7989485. [DOI] [PubMed] [Google Scholar]
- 14.Chang TC, Wu SL, Hsiao YL, Kuo ST, Chien LF, Kuo YF, Change CC, Chang TJ. TSH and TSH receptor antibody-binding sites in fibroblasts of pretibial myxedema are related to the extracellular domain of entire TSH receptor. Clin Immunol Immunopathol. 1994;71:113–120. doi: 10.1006/clin.1994.1059. [DOI] [PubMed] [Google Scholar]
- 15.Dutton CM, Joba W, Spitzweg C, Heufelder AE, Bahn RS. Thyrotropin receptor expression in adrenal, kidney, and thymus. Thyroid. 1997;7:879–884. doi: 10.1089/thy.1997.7.879. [DOI] [PubMed] [Google Scholar]
- 16.Prummel MF, Brokken LJ, Meduri G, Misrahi M, Bakker O, Wiersinga WM. Expression of the thyroid-stimulating hormone receptor in the folliculo-stellate cells of the human anterior pituitary. J Clin Endocrinol Metab. 2000;85:4347–4353. doi: 10.1210/jcem.85.11.6991. [DOI] [PubMed] [Google Scholar]
- 17.Bell A, Gagnon A, Grunder L, Parikh SJ, Smith TJ, Sorisky A. Functional TSH receptor in human abdominal preadipocytes and orbital fibroblasts. Am J Physiol. 2000;279:C335–C340. doi: 10.1152/ajpcell.2000.279.2.C335. [DOI] [PubMed] [Google Scholar]
- 18.Agretti P, Chiovato L, De Marco G, Marcocci C, Mazzi B, Sellari-Franceschini S, Vitti P, Pinchera A, Tonacchera M. Real-time PCR provides evidence for thyrotropin receptor mRNA expression in orbital as well as in extraorbital tissues. Eur J Endocrinol. 2002;147:733–739. doi: 10.1530/eje.0.1470733. [DOI] [PubMed] [Google Scholar]
- 19.Bahn RS, Heufelder AE. Role of connective tissue autoimmunity in Graves’ ophthalmopathy. Autoimmunity. 1992;13:75–79. doi: 10.3109/08916939209014638. [DOI] [PubMed] [Google Scholar]
- 20.Bahn RS. Clinical review 157: pathophysiology of Graves’ ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab. 2003;88:1939–1946. doi: 10.1210/jc.2002-030010. [DOI] [PubMed] [Google Scholar]
- 21.Rapoport B, Alsabeh R, Aftergood D, McLachlan SM. Elephantiasic pretibial myxedema: insight into and a hypothesis regarding the pathogenesis of the extrathyroidal manifestations of Graves’ disease. Thyroid. 2000;10:685–692. doi: 10.1089/10507250050137761. [DOI] [PubMed] [Google Scholar]
- 22.Smith TJ. Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002;12:197–203. doi: 10.1089/105072502753600133. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch J, Fried SK, Edens NK, Leibel RL. The fat cell. Med Clin North Am. 1989;73:83–96. doi: 10.1016/s0025-7125(16)30693-9. [DOI] [PubMed] [Google Scholar]
- 24.Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab. 1996;81:3428–3431. doi: 10.1210/jcem.81.9.8784110. [DOI] [PubMed] [Google Scholar]
- 25.Lowell BB. PPARγ: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239–242. doi: 10.1016/s0092-8674(00)81654-2. [DOI] [PubMed] [Google Scholar]
- 26.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 27.Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996;1302:93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 28.Wright HM, Clish CB, Mikami T, Hauser S, Yanagi K, Hiramatsu R, Serhan CN, Spiegelman BM. A synthetic antagonist for the peroxisome proliferator-activated receptor γ inhibits adipocyte differentiation. J Biol Chem. 2000;275:1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 30.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 31.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 32.Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-γ (PPARγ), and thyrotropin receptor by PPARγ agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2002;87:2352–2358. doi: 10.1210/jcem.87.5.8472. [DOI] [PubMed] [Google Scholar]
- 33.Starkey K, Heufelder A, Baker G, Joba W, Evans M, Davies S, Ludgate M. Peroxisome proliferator-activated receptor-γ in thyroid eye disease: con-traindication for thiazolidinedione use? J Clin Endocrinol Metab. 2003;88:55–59. doi: 10.1210/jc.2002-020987. [DOI] [PubMed] [Google Scholar]
- 34.Smith TJ, Koumas L, Gagnon AM, Bell A, Sempowski GD, Phipps RP, Sorisky A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87:385–392. doi: 10.1210/jcem.87.1.8164. [DOI] [PubMed] [Google Scholar]
- 35.Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Th-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163:1291–1300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacDougald OA, Mandrup S. Adipogenesis: forces that tip the scales. Trends Endocrinol Metab. 2002;13:5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]