Abstract
Rifampicin and caffeine are widely used drugs with reported protective effect against Alzheimer’s disease (AD). However, the mechanism underlying this effect is incompletely understood. In this study, we have hypothesized that enhanced amyloid-β (Aβ) clearance from the brain across the blood-brain barrier (BBB) of wild-type mice treated with rifampicin or caffeine is caused by both drugs potential to upregulate low-density lipoprotein receptor related protein-1 (LRP1) and/or P-glycoprotein (P-gp) at the BBB. Expression studies of LRP1 and P-gp in brain endothelial cells and isolated mice brain microvessels following treatment with rifampicin or caffeine demonstrated both drugs as P-gp inducers, and only rifampicin as an LRP1 inducer. Also, brain efflux index (BEI%) studies conducted on C57BL/6 mice treated with either drug to study alterations in Aβ clearance demonstrated the BEI% of Aβ in rifampicin (82.4 ± 4.3%) and caffeine (80.4 ± 4.8%) treated mice were significantly higher than those of control mice (62.4 ±6.1%, p <0.01). LRP1 and P-gp inhibition studies confirmed the importance of both proteins to the clearance of Aβ, and that enhanced clearance following drugs treatment was caused by LRP1 and/or P-gp upregulation at the mouse BBB. Furthermore, our results provided evidence for the presence of a yet to be identified transporter/receptor that plays significant role in Aβ clearance and is upregulated by caffeine and rifampicin. In conclusion, our results demonstrated the upregulation of LRP1 and P-gp at the BBB by rifampicin and caffeine enhanced brain Aβ clearance, and this effect could explain, at least in part, the protective effect of rifampicin and caffeine against AD.
Keywords: Alzheimer’s disease, amyloid-β, caffeine, LRP1, P-gp, rifampicin
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease that affects about 30 million people worldwide and has an increasing socioeconomic impact [1, 2]. The pathogenesis of AD is complex and involves neuropathological lesions that remain incompletely understood. However, the accumulation of extracellular amyloid-β (Aβ) and the formation of senile plaques of this short peptide is a major hallmark found in the brains of AD patients [3, 4]. Aβ peptides are 39–43 amino acid peptides derived from proteolytic hydrolysis of amyloid-β protein precursor (AβPP) in neurons and other cells [5]. These peptides (mainly Aβ40 and Aβ42) accumulate in the cortical and hippocampal regions of the brain, leading to cognitive decline and memory loss [3, 6, 7]. The level of Aβ in the brain is controlled by its production and clearance; chronic imbalance between these two processes may resultin accumulation of Aβ in the brain [8]. Increased Aβ production represent only a small percentage of early-onset cases of AD (familial AD) caused by inherited mutations in the AβPP gene or in presenilin 1 or 2 genes. The majority of AD patients, including late-onset cases (sporadic AD) and some familial AD cases, do not show increased Aβ production [9]. Instead, increasing evidence demonstrates that increased Aβ level is a result of its faulty clearance across the blood-brain barrier (BBB) [10]. Given that Aβ is a peptide, it is expected that its clearance across the BBB is mediated by a receptor(s) or transporter(s). Aβ efflux from the brain across the BBB is facilitated mainly by low density lipoprotein receptor-related protein-1 (LRP1), P-glycoprotein (P-gp) [11–13], and the recently reported multidrug resistance-associated protein 1 (MRP1) [14]. On the other hand, receptor for advanced glycation end products (RAGE) and low density lipoprotein receptor-related protein-2 (LRP2) are the major proteins that regulate Aβ influx to the brain [15, 16].
Increasing number of studies suggested alteration of expression and functional activity of LRP1 and P-gp contribute to the accumulation of Aβ in the brain, and lead to increased risk for developing AD [17–19]. Moreover, recent evidence indicated a progressive decline in the levels of LRP1 and P-gp at the BBB during normal aging, and this decline was positively correlated with accumulation of Aβ in AD [20].
LRP1 is a multifunctional receptor that is located at the abluminal side of the brain endothelial cells, which are the main cellular constituent of BBB [21]. The role of LRP1 in AD was first suggested by genetic evidence linked LRP1 gene to late-onset AD [22]. Subsequently, Zlokovic and coworkers proved the role of LRP1 in the clearance of Aβ across the BBB by several in vivo studies [11, 23]. P-gp, on the other hand, is a transporter protein functions as an ATP-driven efflux pump. In the brain, P-gp is located mainly at the luminal side of brain endothelial cells and is responsible for extrusion of drugs and toxins from the brain [24]. A previous in vivo study suggested the role of P-gp in the clearance of Aβ from the brain of P-gp null mice [13]. Subsequent evidence using isolated brain capillaries indicated that the last step in the clearance of Aβ across BBB is governed by P-gp [25].
Since the discovery of the role of Aβ in the pathogenesis of AD, numerous attempts have been made to decrease Aβ accumulation, and enhanced Aβ clearance across BBB was one potential therapeutic approach that has been discussed [2]. Parallel to these attempts, many clinically used drugs and herbal medicines were reported to have a protective effect against AD [26, 27]. However, the underlying mechanism that is responsible for this protective effect is still unclear for most of these drugs. For instance, rifampicin, a widely used antibiotic, attenuated the rate of cognitive decline in mild to moderate AD patients after 3-month course of 300 mg/day dose, and this neuroprotective activity was unrelated to the antibiotic activity of rifampicin and still not clear [26]. Another important agent that is proved to have beneficial effect against AD is caffeine. Caffeine is a central nervous system stimulant that is highly concentrated in coffee and is probably the most widely used drug that has protective effect against AD [28–30]. Previous case-control study demonstrated that patients with AD had consumed less caffeine compared to individuals who have normal cognitive function during the 20 years that proceeded diagnosis of AD [31].
In addition to their established protective effect, both drugs have been reported as potent inducers of P-gp [17,32]; however, studies investigating rifampicin and caffeine as LRP1 inducers are still lacking. Collectively, in the current study we have hypothesized that enhanced Aβ clearance from the brain across the BBB of wild-type mice treated with rifampicin or caffeine is caused by both drugs potential to upregulate LRP1 and/or P-gp at the BBB, thus exhibiting their protective effect against AD. Based on the encouraging results of this study, and on the fact that many drugs induce LRP and/or P-gp activities, it is possible to explain the protective effect of many clinically used drugs against AD on the basis of enhanced Aβ clearance machinery at the BBB. In addition, the results of this study support the premise that enhanced Aβ clearance across BBB as a promising therapeutic strategy to decrease Aβ load in the brain of AD patients [2, 25].
MATERIALS AND METHODS
Reagents and antibodies
Rifampicin, caffeine, and 1,1,1,3,3,3-Hexafluoro-2-propanol (HFP) were purchased from Sigma-Aldrich (St. Louis, MO). Synthetic monoiodinated and non-oxidized 125I-Aβ40 (human, 2200 Ci/mmol) was purchased from PerkinElmer (Boston, MA). 14C-Inulin (2.2mCi/g) was purchased from American Radiolabeled Chemicals (St. Louis, MO). The human receptor associated protein (RAP) was purchased from Oxford Biomedical Research (Oxford, MI). Valspodar was obtained from XenoTech (Lenexa, KS). The reagents and supplements required for western blotting were purchased from Bio-Rad (Hercules, CA). For western blot, the mouse monoclonal antibody C-219 against P-gp was obtained from Covance Research Products (Dedham, MA); mouse monoclonal antibody against light chain LRP1 was obtained from Calbiochem (Gibbstown, NJ); mouse monoclonal antibody against MRP1 was obtained from Abcam (Cambridge, MA); mouse monoclonal antibody against RAGE, goat polyclonal antibodies against actin (C-11) and HRP-labeled secondary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). For immunohistochemistry, the rabbit polyclonal antibody against P-gp was purchased from Rockland Immunochemicals Inc. (Gilbertsville, PA). Alexa Flour 594 was purchased from Invitrogen (Carlsbad, CA), and goat anti-mouse IgG-FITC labeled secondary antibody was obtained from Santa Cruz Biotechnology. Ketamine and xylazine for mice anesthetization were purchased from Henry Schein Inc. (Melville, NY). All other reagents and supplies were purchased from VWR (West Chester, PA).
In vitro induction of LRP1 and P-gp expression by rifampicin and caffeine in mouse brain endothelial (bEnd3) cells
The mouse brain endothelial cells (bEnd3; ATCC, Manassas, VA), passage 28–32, were cultured in 10-mm cell culture dishes (Corning, NY, USA) at a seeding density of 10×105 cells per dish. Cultures were maintained in a humidified atmosphere (5% CO2/95% air) at 37°C with medium comprising DMEM supplemented with 10% fetal bovine serum (FBS), and the antibiotics penicillin G (100 units/ml) and streptomycin (100 µg/ml), 1% w/v nonessential amino acids, glutamine 2 mM. Media was changed every other day. Cells were allowed to grow up to 50% confluence and then treated with rifampicin, caffeine, or control (DMSO, 0.1%). Stock solutions of rifampicin and caffeine were diluted to a final concentration of 50 µM in growth medium before use [17, 32]. Vehicle and drug containing media were added to the respective treatment cells and were then incubated for 72 h at 37°C/5% CO2. Vehicle and drug-containing media were changed every day for the duration of the experiment. At the end of treatment period, cells were harvested and protein and RNA were extracted for western blot and real time PCR (RT-PCR) analyses of LRP1 and P-gp.
Western blot analysis of LRP1 and P-gp proteins in bEnd3 cells
At the end of treatment period with rifampicin, caffeine, or control (DMSO), total protein was extracted from bEnd3 cells with lysis buffer (RIPA buffer; 25 mM Tris.HCl, 150mM NaCl, 1% NP-40, 1% sodium deoxylate, 0.1% SDS, pH 7.6) containing protease inhibitors.
For western blot analysis, 25 µg of cellular protein was resolved on 7.5% SDS-polyacrylamide gels and transferred onto nitrocellulose membrane. Blotting membranes were blocked with 2% BSA and incubated overnight with either monoclonal antibodies for LRP1 (light chain), P-gp (C-219), or β-actin (C-11) at dilutions 1:200, 1:1500, and 1:3000, respectively. For proteins detection, the membranes were washed and incubated with HRP-labeled secondary IgG antibody for LRP1, P-gp (anti-mouse) and β-actin (anti-goat) at 1: 5000 dilution. The bands were visualized using Pierce chemiluminescence detection kit (Thermo Scientific; Rockford, IL). Quantitative analysis of the immunoreactive bands was performed using GeneS-nap luminescent image analyzer (Scientific Resources Southwest Inc., Stafford, TX) and band intensity was measured by densitometric analysis. Three independent western blotting experiments were carried out for each treatment group.
RT-PCR analysis of LRP1 and P-gp mRNA in bEnd3 cells
For RT-PCR analysis, total RNA was extracted from bEnd3 cells using RNeasy Mini Kit (Qiagen Sciences Inc.) according to the manufacturer’s instruction and was processed directly to produce cDNA using TaqMan reverse transcription reagents kit (Applied Biosystems, Branchburg, NJ). The cDNA was then used for RT-PCR to quantify LRP1 and P-gp gene expression using IQ SYBR Green SuperMix with MyiQRT-PCR Detection System from Bio-Rad. The PCR primers were: LRP1 forward primer 5′-GAGTGTTTCCGTGTATGGCAC-3′ and reverse primer 5′-GATGCCTTGGATGATGGTC-3′; MDR1 forward primer 5′-TCCTTCCCGCAGTGGCTCT TGA-3′ and reverse primer 5′-CACCTCCGGG TTTCCTTCGCA-3′. GAPDH was used as an internal control for normalization using the forward primer 5′-ACAGCCGCATCTTCTTGTGCAGTG-3′ and reverse primer 5′-GGCCTTGACTGTGCCGTTGAA TTT-3′. All the primers were screened for specificity by using the PubMed BLAST database and custom-synthesized by Invitrogen. Real time PCR reaction was performed using the cDNA with an initial denaturation step at 94°C for 10min, followed by 35 cycles of denaturation at 95°C for 1 min, annealing at 64°C for LRP1 and 72°C for MDR1 and GAPDH for 1 min, and extension at 72°C for 30 s. Specific amplification was confirmed by melting curve analysis. The Ct for each sample was normalized against that of GAPDH. Folds change was determined relative to the control using the comparative threshold cycle method (2−ΔΔCt). All the results were expressed as means and standard deviation and compared to control.
Animals
C57BL/6 wild-type male mice were purchased from Harlan Laboratories (Houston, TX, USA). The mice were 6–7 weeks old with an average body weight of 20 g. Mice were kept under standard environmental conditions (22°C, 35% relative humidity, 12 h dark/light cycle) with free access to tap water and standard rodent food. After shipping, mice were allowed to adapt to the new environment for one week before initiating the experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Louisiana at Monroe and all surgical and treatment procedures were consistent with the IACUC policies and procedures.
In vivo induction of LRP1 and P-gp expression by rifampicin and caffeine
Animals were divided into three treatment groups: control, rifampicin, and caffeine groups. Each group contained at least 4 animals. Drug administration was started at 7–8 weeks of age and continued for 3 weeks in all experiments except for caffeine group (treated only for 2 weeks due to weight loss). Mice of control group received intraperitoneal normal saline once daily. Mice of rifampicin group received intraperitoneal rifampicin at dose of 20 mg/kg once daily. Mice of caffeine group received intraperitoneal caffeine at dose of 40 mg/kg once daily. Treatments were given intraperitoneally to avoid variability associated with oral administration when given with food. In this case the administered dose will be influenced by the amount of food ingested by each mouse, as well as drugs stability, especially for rifampicin.
Brain efflux index study
At the end of treatment period, mice were prepared for brain efflux index study (BEI) to assess the clearance of Aβ 3 h after the last dose of drug. In separate experiments, BEI was performed after 24 h washout period of the drugs. This design of the experiments was used to differentiate between protein induction and possible inhibition effects of rifampicin and caffeine on Aβ clearance across the BBB of the mice.
The in vivo Aβ clearance experiments (BEI%) were performed using the intracerebral microinjection technique reported previously [33–35]. In brief, mice were anesthetized with intraperitoneal xylazine and ketamine (20 and 125 mg/kg, respectively) and placed in a stereotaxic apparatus (Stoelting Co., Wood Dale, IL) to determine the coordinates of the mice brain that coincide within the right caudate nucleus. A stainless steel guide cannula was implanted stereo-taxically at 0.9 mm anterior, 1.9 mm lateral to bregma, and 2.9 mm below the surface of the brain as previously reported [11]. The guide cannula and screw were fixed to the skull with binary dental cement, and a stylet was introduced into the guide cannula. Once the cement was firm, the animal was removed from the stereotaxic device, and the wound was closed anterior and posterior to the guide assembly using 1.75 mm Michel suture clips. Animals were then allowed for 12 h recovery from acute brain injury due to insertion of guide cannula in order to restore BBB integrity [34]. Animals were re-anesthetized with intraperitoneal xylazine/ketamine and an injector cannula connecting via Teflon tubing to a 1.0 µl gas-tight Hamilton microsyringe was inserted into the guide cannula. The applied tracer fluid (0.5 ul) containing 125I-Aβ40 (30 nM) and 14C-inulin (0.02 µCi) prepared in extracellular fluid buffer (ECF; 122 mM NaCl, 25 mM NaHCO3, 3 mM KCl, 1.4 mM CaCl2, 1.2 mM MgSO4, 0.4 mM K2HPO4,10 mM D-glucose, and 10 mM HEPES, pH 7.4) was administered into the caudate nucleus over a period of 3 min. To prevent aggregation, 125I-Aβ40 was initially solubilized in HFP, dried, and resolubilized in ECF buffer prior to the experiment. The intactness and quality of 125I-Aβ40 was initially confirmed by trichloacetic acid (TCA) precipitation assay [11]. After the microinjection, the microsyringe was left in place for 3 min to minimize any backflow. At the designated time, 30 min post 125I-Aβ40 injection [11, 13], plasma and brain tissues were rapidly collected for Aβ and drug levels measurements. To characterize the role of LRP1 and P-gp function, 0.5 µl of ECF containing RAP (an LRP1 inhibitor [11, 36]) or valspodar (a well-established P-gp inhibitor [37]) were intracerebrally pre-administered 5min prior to 125I-Aβ40 injection at concentrations of 1 µM (19.5ng/0.5µl injection volume) and 40 µM (24 ng/0.5 µl injection volume), respectively.
Calculation of 125I-Aβ40 clearance
125I-Aβ40 is characterized by its rapid clearance across the BBB [38], thus brain was collected 30 min post-injection to determine Aβ BEI% [11, 13]. Brain tissues were excised and homogenized by Dounce tissue grinder with 7 strokes in two volumes of DPBS buffer (2.7 mM KCl, 1.46 mM KH2PO4, 136.9 mM NaCl, 8.1 mM Na2HPO4, 0.9 mM CaCl2, and 0.5 mM MgCl2 supplemented with 5mM D-glucose and 1 mM sodium pyruvate, pH 7.4) containing mammalian protease inhibitor cocktail. About half of the brain homogenate was used for 125I-Aβ40 and 14C-inulin quantification and the second half for microvessels isolation and protein expression study. TCA precipitation method was used to calculate the amount of intact125I-Aβ40 remained in the brain [11]. To measure intact 125I-Aβ40, one volume of TCA (20%) was added to the sample, and then samples were vortexed, incubated in ice for 30 min, and centrifuged at 14,000 rpm (4°C) for 30 min. Following centrifu-gation, gamma radioactivity of precipitated 125I-Aβ40 (intact peptide) and TCA supernatant (degraded peptide) were measured using Wallac 1470 Wizard Gamma Counter (PerkinElmer Inc., Waltham, MA). The supernatant and precipitate were then mixed with 5 ml scintillation cocktail and the beta radioactivity of 14C-inulin was measured using Wallac 1414 Win Spec-tral Liquid Scintillation Counter (PerkinElmer Inc.). The BEI% was defined by equation 1 and the percentage of substrate remaining in the brain (100 – BEI%) was determined using equation 2 [36].
| (1) |
| (2) |
The percent degradation of 125I-Aβ40 for each sample was calculated by dividing the supernatant cpm by the total cpm (sum of the cpm in the precipitate and the supernatant cpm) and the resulting percent were subtracted from the percent of free 125I which determined from pre-injected sample by spiking mice brain homogenate with 0.5 µl injectate solution and then processed as sample homogenate.
Isolation of brain microvessels
Brain microvessels were isolated as described previously [39]. Briefly, after decapitation of mouse, brain was immediately put in ice-cold normal saline, homogenized and divided into halves as described above. One volume Ficoll (30%) was added to the half of brain homogenate to a final concentration of 15% and the mixture was mixed, and then centrifuged (5000 rpm for 10 min, 4°C). The resulting pellets were suspended in ice-cold DPBS containing 1% bovine serum albumin (BSA) and passed over glass bead column. Microvessels adhering to the glass beads were collected by gentle agitation in 1% BSA in DPBS. Isolated microvessels were used for western blotting studies of LRP1, P-gp, and RAGE, and immunohistochemistry studies of LRP1 and P-gp.
Western blot analysis of LRP1, P-gp, RAGE, and MRP1 in isolated brain microvessels
Protein expression levels in isolated brain microvessels were analyzed by western blotting as described below. Total protein was extracted from isolated brain microvessels by homogenization with lysis buffer (RIPA buffer) containing protease inhibitors. Homogenized samples were centrifuged at 13,000 rpm for 10 min and supernatant was used for further western blot analysis. Expression of LRP1, P-gp, and RAGE were determined in isolated brain microvessels, while MRP1 levels in brain homogenate were analyzed instead as its expression in isolated microvessels was not detectable. Western blot was done as described above. The primary antibodies for RAGE and MRP1 were used at dilution 1:500, and secondary antibodies for RAGE and MRP1 were anti-mouse at 1:5000 dilution.
Immunofluorescence staining of LRP1 and P-gp in isolated brain microvessels
Isolated brain microvessels were transferred to glass slide and washed 3 times with ice-cold DPBS. Microvessels were then fixed for 15 min with 4% paraformaldehyde/0.2% glutaraldehyde at room temperature. Microvessels were washed 3 times with ice-cold DPBS and permeabilized with 0.1% (v/v) Triton X-100 in DPBS for 30min. Next, microvessels were blocked with 1% BSA in DPBS for 1 h and then incubated overnight at 4°C with monoclonal antibody against LRP1 or polyclonal antibody against P-gp, at 1:800 and 1:200 dilutions, respectively. After washing with DPBS, microvessels were incubated for 1 h at room temperature with anti-mouse IgG-FITC for LRP1 detection (1:200) or anti-rabbit Alexa flour 594 conjugated secondary IgG for P-gp (1:200 dilution). Nuclei were stained with DAPI (0.5 µg/ml). Negative controls for each treatment that were processed without primary antibody showed negligible background fluorescence (data not shown). Images for LRP1and P-gp were captured using Zeiss LSM 5 Pascal con-focal microscope equipped with 488 nm line Argon laser and 543 nm line of HeNe Laser and 63X oil immersion objective lens with numerical aperture = 1.4 (Carl Zeiss MicroImaging, LLC, Thornwood, NY). For each treatment, confocal images of 5 randomly selected microvessels for LRP1 quantification and 5 microvessels for P-gp quantification were acquired. LRP1 and P-gp immunofluorescence was quantified using ImageJ version 1.44 software (Research Services Branch, NIMH/NIH, Bethesda, MD). Fluorescence intensity for each microvessel was the mean of five randomly selected measurements per microvessel.
Measurement of drugs concentration in plasma and brain homogenate samples
Rifampicin
Rifampicin levels in plasma and brain homogenate were quantified using LC/MS/MS system. Mice plasma and brain homogenate were mixed with methanol to precipitate proteins in a ratio of 1:2 (v/v). The samples were vortexed for 30 s and cen-trifuged at 13,000 rpm for 10 min at 4°C. The clear supernatant was directly injected into the LC/MS/MS system. Chromatographic separation of rifampicin was performed on Kinetex XB-C18 reversed phase column (100×4.6 mm i.d., 2.6 µm; Phenomenex, Torrance, CA) with an ODS guard column (4×3 mm; Phenomenex) using Agilent 1100 series LC system (Agilent Technologies, Santa Clara, CA) and 3200 QTRAP LC/MS/MS system (Applied Biosys-tems/MDS Sciex, Foster City, CA) at flow rate of 0.5ml/min. The injection volume was 10 µl. The mobile phase used was methanol/ammonium acetate 10 mM, pH 6.8 (70: 30, v/v). The analyte was detected using electrospray ionization interface operated in positive mode. Instrument control and data acquisition were carried out by the Analyst 1.4.1 software (Applied Biosystems). The analyte was detected and quantified by MS/MS in multiple-reaction monitoring (MRM) method. The peak signals of transition from the parent ion to its major fragment m/z 823.3→791 was measured.
Caffeine
Caffeine levels in plasma and brain homogenate were quantified using HPLC. In brief, mice plasma or brain homogenate were mixed with methanol to precipitate proteins in a ratio of 1:2 (v/v). The samples were vortexed for 30 s and centrifuged at 13,000 rpm for 10 min at room temperature. The clear supernatants were directly injected into the HPLC system. Quantification of caffeine was conducted using isocratic Shimadzu LC-20AB liquid chromatograph equipped with the Shimadzu SIL-20A HT autosampler and LC-20AB pump connected to a Dgu-20A3 degasser (Columbia, MD). Data acquisition was achieved by LC Solution software version 1.22 SP1 (Shimadzu). The column used was a reversed-phase, ZorbaxODS XDB-C18 column (150 × 4.6 mm i.d., 5µm; Agilent Technologies) with an ODS guard column (4×3 mm; Phenomenex) at flow rate of 1 ml/min. The injection volume was 30 µl. The mobile phase composed of water/acetonitrile (90:10, v/v). Detection was carried out by Shimadzu UV SPD-20A detector set at 275 nm.
The analytical methods for rifampicin and caffeine were found to be accurate and precise and the recoveries of drugs from plasma or brain homogenate were more than 80%. The results were used to calculate the brain uptake index (Kp) which is defined as the ratio of brain to plasma drug concentration.
Statistical analysis
Unless otherwise indicated, the data were expressed as mean ± SEM. Wherever possible, the experimental results were statistically analyzed for significant difference using two-tailed Student’s t-test to evaluate differences between controls and treated groups. The data were statistically analyzed for normality and variance homogeneity using F-test. The results showed that data are normally distributed around the mean and the variances are homogenous (p > 0.05). A p -value less than 0.05 was considered to be statistically significant.
RESULTS
LRP1 and P-gp upregulation in bEnd3 cells after rifampicin and caffeine treatment
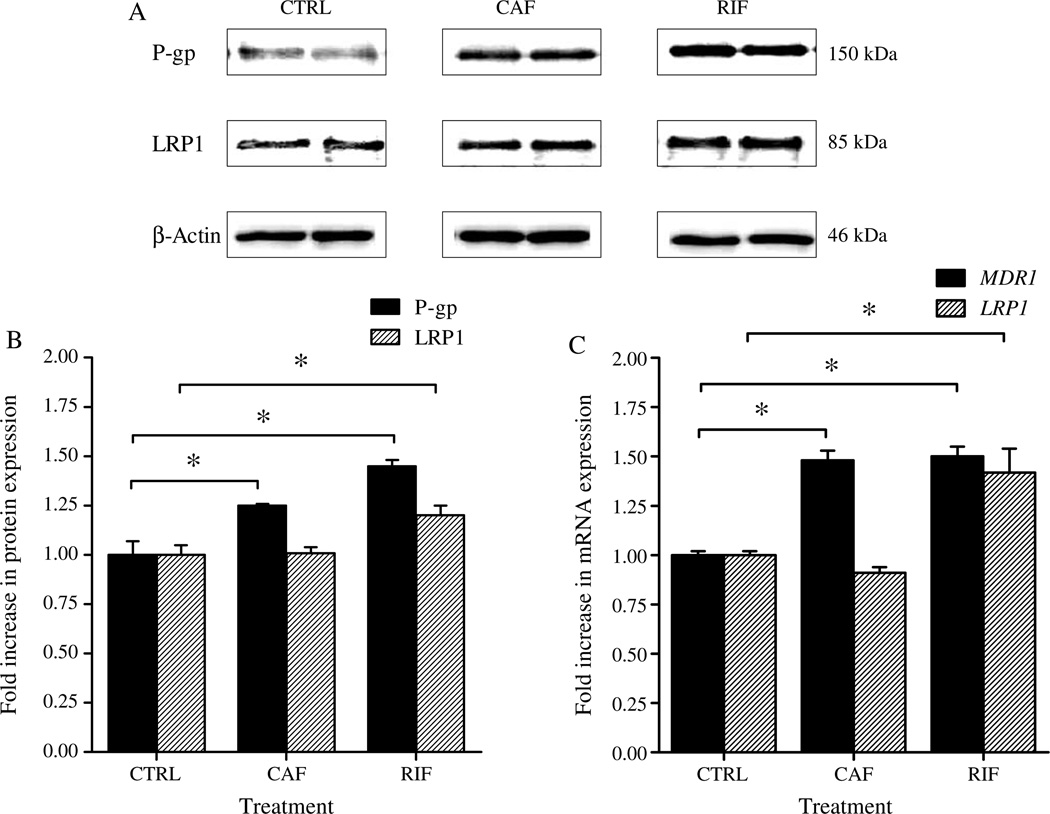
In vitro expression experiments using bEnd3 cells, as a representative model of mouse BBB, were first conducted to examine whether treatment with rifampicin or caffeine will upregulate LRP1 and P-gp in mouse brain endothelial cells. In these experiments, we measured the levels of LRP1 and P-gp in bEnd3 cells by western blot after 3 days treatment with rifampicin or caffeine. The results of these experiments demonstrated both rifampicin and caffeine upregu-lated P-gp protein and gene expression; however, only rifampicin was able to upregulate LRP1 protein and mRNA levels (Fig. 1).
Fig. 1.
A) Representative western blot lanes for P-gp, LRP1, and protein loading control (β-actin) from bEnd3 cells treated with rifampicin (RIF), caffeine (CAF), and vehicle (CTRL). B) Quantitative fold increase in P-gp and LRP1 expressions in control and drug treated bEnd3 cells. C) Real time PCR analysis for the mRNA expression of MDR1 and LRP1 in bEnd3 cells treated with 50 µM of rifampicin and caffeine. Significantly higher expression level of P-gp and MDR1 was detected in caffeine and rifampicin treated cells compared to control cells. Significant upregulation of LRP1 and its encoding gene was only detected in rifampicin treated cells. The data are expressed as mean ± SEM of n = 3 independent experiments (*p < 0.05).
Densitometric analysis of western blot bands showed significant increasein P-gp level of bEnd3 cells after treatment with rifampicin (1.45-fold increase, p < 0.05, Fig. 1B) and caffeine (1.25-fold increase, p < 0.05, Fig. 1B). On the other hand, densito-metric analysis showed that LRP1 level increased significantly after treatment with rifampicin (1.2-fold increase, p < 0.05, Fig. 1B) but not caffeine. To confirm these results, we conducted quantitative RT-PCR experiment to measure the increase in mRNA levels of LRP1 and MDR1 after rifampicin or caffeine treatment. Consistent with the results of western blot, RT-PCR showed 1.5-fold increase in the mRNA of MDR1 after treatment with rifampicin and caffeine (Fig. 1C), while mRNA level of LRP1 was increased by 1.4-fold after rifampicin treatment however caffeine did not show any effect on the level of LRP1 mRNA (Fig. 1C).
LRP1 and P-gp induction at blood-brain barrier of C57BL/6 mice
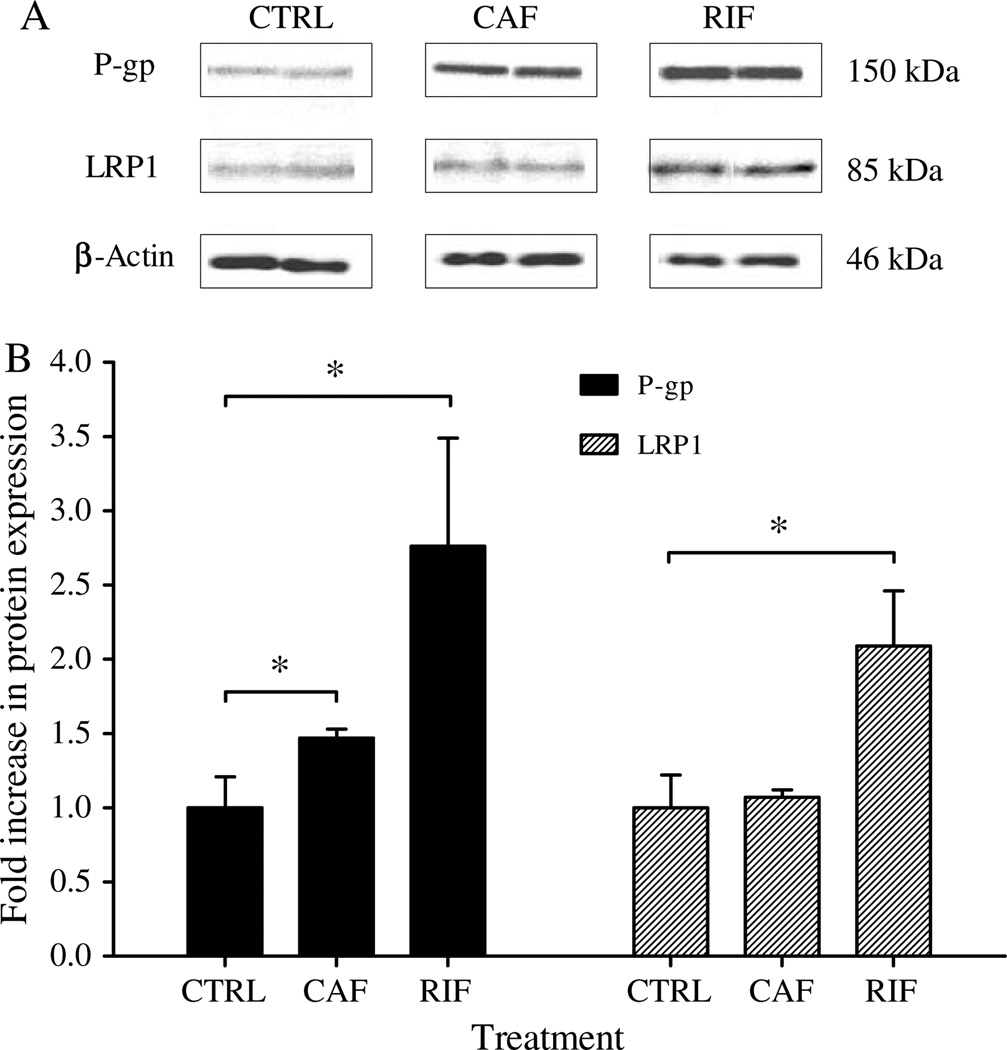
The ability of rifampicin and caffeine to induce LRP1 and P-gp expression in wild-type C57BL/6 mice brain microvessels was assessed by western blotting and fluorescent micrographs. In line with the in vitro results, western blot analysis (Fig. 2) revealed increase in the expression of LRP1 and P-gp in the brain microvessels of wild-type C57BL/6 mice by rifampicin or caffeine treatment. Densitometric analysis of the bands showed that rifampicin and caffeine increased P-gp expression by 2.7- and 1.5-fold compared to control, respectively. However, only rifampicin increased LRP1 expression by 2.1-fold compared to control; while caffeine did not cause any significant change in LRP1 expression (Fig. 2).
Fig. 2.
Western blot analysis of P-gp and LRP1 in brain microvessels of C57BL/6 wild-type mice. Significantly higher expression level of P-gp was detected in rifampicin (RIF) and caffeine (CAF) treated mice compared to control group (CTRL). Significant upregulation of LRP1 was only detected in rifampicin treated mice. A) Representative western blot lanes for P-gp, LRP1 and protein loading control (β-actin). B) Quantitative fold increase in P-gp and LRP1 expressions. The data are expressed as mean ± SEM of n = 3 independent experiments (*p < 0.05).
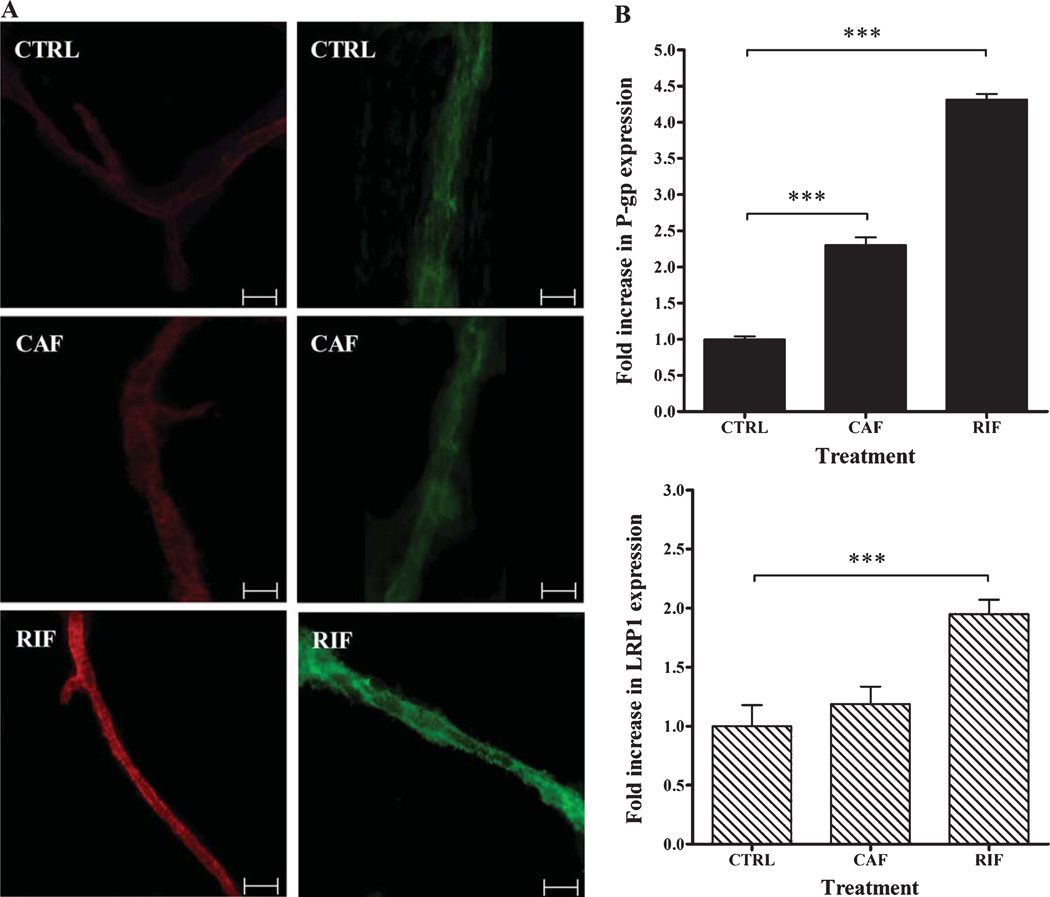
Figure 3 illustrates representative fluorescent micrograph images of LRP1and P-gp expressions in control, rifampicin, and caffeine treated mice brain microvessels. Fluorescence of secondary antibodies against LRP1 and P-gp was located mainly on the membrane of brain microvessels. Consistent with western blots results, quantitative analysis of LRP1and P-gp showed significant increase in LRP1and P-gp expression in the microvessels of rifampicin-treated mice compared to control. Rifampicin caused 1.9 and 4.5-fold increase in LRP1and P-gp, respectively (p <0.05; Fig. 3B), while caffeine showed a 2.3-fold increase in P-gp expression, and only a modest, but insignificant, increase in LRP1 (1.2-fold, p > 0.05).
Fig. 3.
Immunohistochemical analyses of P-gp (red, left micrographs) and LRP1 (green, right micrographs) in brain microvessels of C57BL/6 wild-type mice following rifampicin (RIF), caffeine (CAF), and vehicle (CTRL) treatments. Rifampicin and caffeine significantly upregulated P-gp expression compared to control group, while significant upregulation of LRP1 was only observed in rifampicin group. A) Representative fluorescent micrographs of P-gp (red, left micrographs) and LRP1 (green, right micrographs) for control, caffeine and rifampicin treated groups. B) Quantitative fold increase in P-gp (upper panel) and LRP1 (lower panel) expressions. The data are expressed as mean±SEM of n = 3 independent experiments (***p < 0.001). The white scale bar indicates 50 µm length.
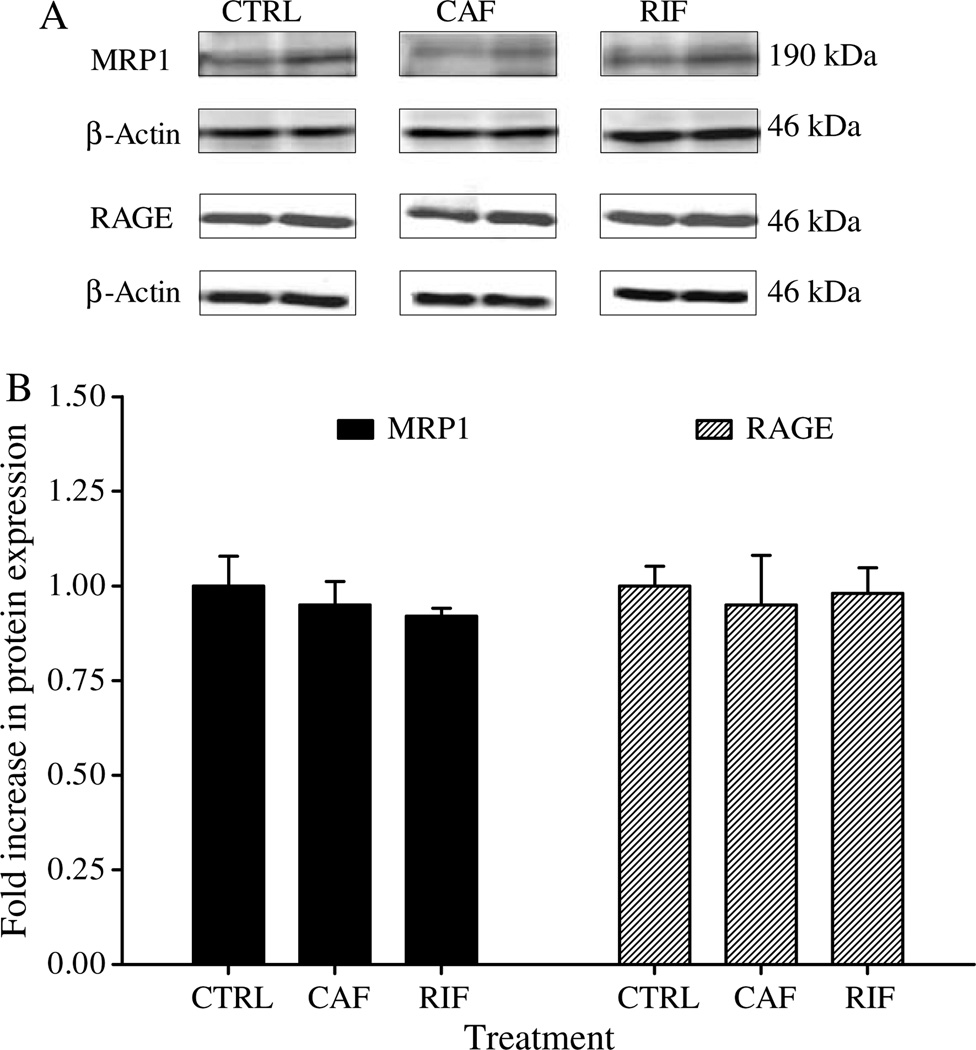
In contrast to LRP1 and P-gp, results from western blots demonstrated that rifampicin and caffeine treatments did not alter microvessels’ RAGE and brain homogenate’s MRP1 expressions (Fig. 4).
Fig. 4.
Western blot analysis of RAGE and MRP1 in brain microves-sels of C57BL/6 wild-type mice. Insignificant differences were observed in the expression level of both proteins in rifampicin (RIF), caffeine (CAF), and control (CTRL) treated mice. A) Representative western blot lanes for MRP1, RAGE, and protein loading control (β-actin). B) Quantitative fold increase in MRP1 and RAGE expressions. The data are expressed as mean ± SEM of n = 3 independent experiments.
Effect of rifampicin and caffeine treatment on Aβ40 clearance across mice BBB
The in vivo clearance of 125I-Aβ40 from mice brain was examined using the BEI% method with 14C-inulin being the reference compound. Although Aβ42 is more prone to aggregation compared to Aβ40, both Aβ peptides have been implicated in the pathogenesis of AD [3], and participate in the formation of senile plaques with neurotoxicity potentials [40,41]. Aβ40 and Aβ42 are substrates for P-gp and LRP1 [11, 13], thus both peptides clearance is expected to be altered by these transport proteins modulation. In this study, Aβ40 was used in the BEI experiments for practicality reasons as it has much faster clearance rate than Aβ42 [36, 38].
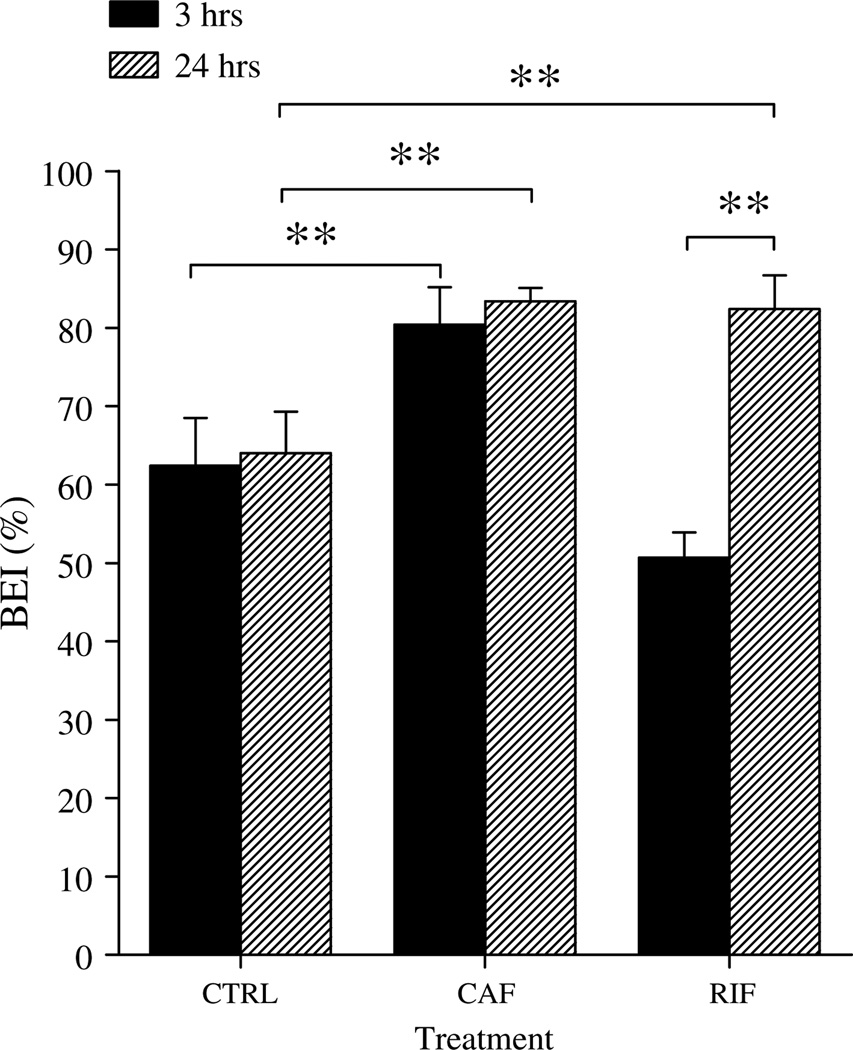
Clearance BEI% experiments were performed at 3 and 24 h after the last injection of rifampicin, caffeine, or normal saline. After 24 h of the last injection of the drugs (plasma drugs concentrations approaching zero), BEI% analysis showed about 20% increase in the clearance of Aβ40 in rifampicin and caffeine treated mice comparing to control mice (Fig. 5). In contrast, after 3h from the last drug injection where rifampicin and caffeine plasma concentrations were 7.4 ±3.9 and 20 ± 5.4 µg/ml, respectively (Table 1), only caffeine had about 20% increases in the clearance of Aβ, while rifampicin-treated mice had approximately 10% decrease in the clearance of Aβ (Fig. 5).
Fig. 5.
Brain efflux index (BEI) of 125I-Aβ40 in control (CTRL), rifampicin (RIF), and caffeine (CAF) treated groups measured at 3 and 24 h after the last injection of rifampicin, caffeine, or normal saline. Significantly higher BEI% was observed in rifampicin and caffeine treated mice compared to control group after complete washout of the drugs from mice body (24 h). In contrast, following 3 h of injection, only caffeine treated mice showed significant increase in Aβ clearance compared to control. The data are expressed as mean ± SEM of n = 4–6 (**p < 0.01).
Table 1.
Plasma and brain levels of rifampicin and caffeine after 3 h washout period, and Kp values in C57BL/6 wild-type male mice (n =12 for each treatment group)
| Group | Drug concentration (µg/ml) |
Kp | |
|---|---|---|---|
| Plasma | Brain | ||
| Rifampicin | 7.4± 3.9 | 0.15 ± 0.08 | 0.03 |
| Caffeine | 20 ± 5.4 | 11.8 ± 3.6 | 0.5 |
Thirty minutes post 125I-Aβ40 microinjection, the BEI% values in rifampicin (82.4 ±4.3%) and caffeine (83.4 ± 1.7%) treated mice after 24 h washout period were significantly higher than control mice (64.0 ±5.3%, p <0.01). The BEI% values of control and caffeine-treated mice 3 h after completion of drug dosing (80.4 ± 4.8% versus 62.4 ±6.1% respectively, p <0.01) were comparable and consistent to those obtained after 24 h washing. However, the BEI% values of rifampicin treated mice 3 h after completion of drug dosing were significantly lower than control mice (50.7 ± 3.2% versus 62.4 ±6.1% respectively, p <0.01). Collectively, these results indicated that clearance of Aβ40 from the brain-to-blood was enhanced by caffeine and rifampicin treatment. However, presence of high concentration of rifampicin (7.4 ± 3.9 µg/ml; 3 h post dose) significantly decreased Aβ clearance, which is expected as rifampicin is a potent inhibitor for P-gp [42]. In subsequent studies, BEI% values of 3 and 24 h wash-out period after the last administered dose of caffeine and rifampicin, respectively, were used.
Effect of rifampicin and caffeine treatment on Aβ40 metabolism
To characterize the effect of rifampicin and caffeine treatment on the metabolism of Aβ40 in C57BL/6 mice brain, we performed TCA degradation assay. This method demonstrated an excellent correlation between TCA and HPLC methods [11]. The percent of degraded peptide (cpm in supernatant) were almost equal in three treatment groups (40.5 ± 4.3% and 41.4 ± 7.1% in rifampicin- and caffeine-treated group respectively, versus 41.1 ±2.9% in control-treated group, p > 0.05). Thus, rifampicin and caffeine treatment did not modulate the metabolism of Aβ40 in mice brain, therefore enhanced metabolism does not provide explanation to the increased Aβ40 clearance observed following drugs treatments.
Influence of rifampicin and caffeine treatment on BBB efflux of Aβ40
According to the results of Aβ metabolism and to the fact that BEI% method evaluates clearance of compound specifically across BBB [35], it is clear that the enhancement in the brain Aβ clearance after rifampicin or caffeine treatment is related to increase in the activity of Aβ efflux system at the BBB of C57BL/6. Because RAGE and MRP1 expression were not changed by rifampicin and caffeine treatment, their association with increase Aβ40 clearance was excluded from further analysis and the focus was shifted toward the contribution of LRP1 and P-gp to Aβ40 enhanced clearance. To further study whether the enhanced brain clearance of Aβ40 was associated with increased expression of LRP1 and P-gp at the BBB, inhibitory studies of each protein were performed.
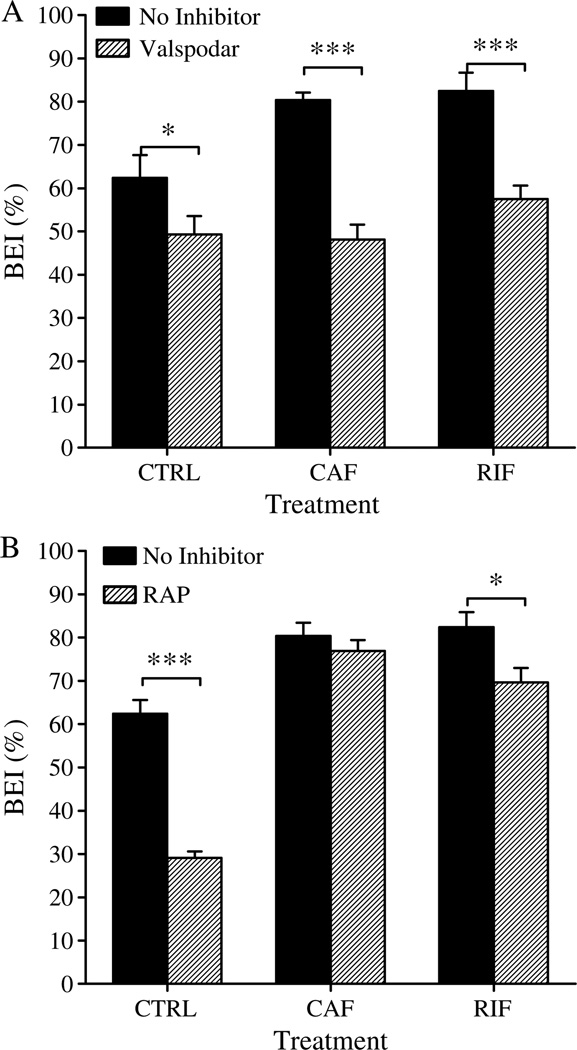
To characterize the role of LRP1 on Aβ40 clearance across the BBB of C57BL/6 mice, a specific LRP1 inhibitor (RAP) was pre-injected into the brain 5min before intracerebral microinjection of 125I-Aβ40/14C-inulin solution and the BEI% was measured as described above. RAP significantly reduced the Aβ BEI% (p <0.05, Fig. 6A) in control and rifampicin-treated mice, whereas no significant difference was observed in the BEI% of caffeine-treated mice compared to those without RAP (Fig. 6A). Pretreatment of control, rifampicin-, and caffeine-treated mice with RAP resulted in about 33% (from 62.4 ±6.1% to 29.1 ±1.5%; p <0.001), 13% (82.4±4.3% to 69.7 ±3.3%; p <0.05), and 3% (80.4 ±4.8% to 76.9 ±2.5%; p >0.05) reduction in BEI% values of 125I-Aβ40, respectively.
Fig. 6.
A) Effect of P-gp inhibition by valspodar (24 ng/0.5 µl injection), and (B) effect of LRP1 inhibition by RAP (19.5 ng/0.5 µl injection) on BEI% of 125I-Aβ40 in control (CTRL), rifampicin (RIF), and caffeine (CAF) treated groups. Valspodar caused a significant reduction in the BEI% in all treatment groups, while RAP caused a significant reduction in the BEI% of control and rifampicin treated groups but not caffeine group. The data are expressed as mean ± SEM of n = 4–6 (***p < 0.001, and *p < 0.05).
On the other hand, pre-injected valspodar, a P-gp inhibitor; into the brain of C57BL/6 mice 5 min before intracerebral microinjection of 125I-Aβ40/14C-inulin solution significantly lowered BEI% value of Aβ in all treatment groups (Fig. 6B). Thirty minutes post microinjection of 125I-Aβ40, the pretreatment of control, rifampicin-, and caffeine-treated mice with valspodar resulted in about 18% (from 62.4 ±6.1% to 49.3±4.6%; p <0.05), 25% (82.4±4.3% to 57.5± 1.1%; p <0.001), and 32% (80.4±4.8% to 48.1 ±2.3%; p <0.001), reduction in BEI% values, respectively.
DISCUSSION
Rifampicin and caffeine, structurally and pharmacologically unrelated molecules, are clinically used drugs and in post-hoc analyses seem to show protective effect against AD. In addition, several studies, including ours, have demonstrated both drugs as potent P-gp inducers [17, 32]. In this study, we further investigated the effect of either drug on LRP1 where rifampicin treatment upregulated LRP1 but not caffeine. Thus, in the current study we aimed first to investigate the association of both drugs’ neuroprotective effect to their ability to induce LRP1 and/or P-gp and Aβ clearance across the BBB. Then to evaluate the effect of both proteins, upregulation (by rifampicin) was compared to only P-gp upregulation (by caffeine) on Aβ clearance, which should highlight the benefits of P-gp and LRP1 upregulation as therapeutic approach compared to individual P-gp or LRP1 upregulation. Accordingly, rifampicin and caffeine were both studied.
While the exact underlying mechanism(s) is (are) still incompletely understood, several studies have suggested different mechanisms [26, 28–30, 43–45]. However, the ability of rifampicin and caffeine to increase brain Aβ clearance via the upregulation of LRP1 and/or P-gp at BBB is investigated for the first time by this study. We hypothesized that enhanced Aβ clearance from the brain across the BBB of wild-type mice treated with rifampicin or caffeine is caused by the potential of both drugs to upregulate LRP1 and/or P-gp at the BBB, thus affecting, at least in part, their protective effect against AD. To investigate this hypothesis, we evaluated the ability of rifampicin and caffeine to upregulate LRP1 and P-gp in the mouse brain endothelial cell line bEnd3, and in mice brain microvessels. Then, we investigated the effect of such upregulation on the brain-to-blood efflux clearance of Aβ using BEI% studies.
The results of this study demonstrated that rifampicin is a P-gp inducer in bEnd3 cells, and in microvessels isolated from brains of treated mice. Although this finding is in line with previous results obtained from in vitro [32] and in vivo [42] studies, a previous study reported that the induction of P-gp by rifampicin is associated with increased pregnane X receptor (PXR) activity in humans and rats but not mice PXR [39]. While this discrepancy could be related to differences in administered dose, treatment duration, and/or mice strain [42], the above results suggest that rifampicin may have an alternative pathway(s) for P-gp upregulation other than PXR activation in mouse brain endothelial cells, and demonstrate that, regardless of the mechanism, rifampicin upregulates P-gp expression and activity in both the human [32] and mouse origin cell lines. Mechanistic studies to investigate P-gp upregulation is beyond the scope of this study.
In the current study, the induction effect of rifampicin was associated with an intraperitoneal dose that resulted in an average plasma level of 7.4 µg/ml. Rifampicin free fraction (unbound) was determined to be 0.89 µg/ml which is comparable to that detected in humans (1.08 µg/ml) after administration of 6.4 mg/kg dose [46]. Thus, dosing mice with 20 mg/kg rifampicin daily caused a significant upregulation of P-gp and LRP1 at plasma level of free drug that is comparable to those seen in patients receiving therapeutic doses of rifampicin.
Caffeine effect on LRP1 and P-gp expressions in bEnd3 cells and in mice brain microvessels is investigated for the first time by this study. According to our western blot results, confirmed by RT-PCR and fluorescent imaging, it is clear that caffeine upregulated P-gp but not LRP1 expression in both bEnd3 cells and in the isolated microvessels. These results in bEnd3 cells are consistent with those obtained using cell line of human origin [32] suggesting that caffeine effect on P-gp and LRP1 in humans and mice is comparable. Such increase in P-gp expression by caffeine has been obtained from 40mg/kg/day, a dose which is lower than that reported by Arendash et al. that is associated with cognitive protective effect and reduction in mice brain Aβ levels [47]. Yet, the level of caffeine obtained in our study (20 µg/ml, Table 1) was associated with a significant upregulation of P-gp and resulted in a significant reduction in Aβ levels in the brains of treated mice.
The Kp value of rifampicin (0.03, Table 1) was much lower than that of caffeine (0.5, Table 1) indicating rifampicin poor brain permeability compared to caffeine, which could be related to differences in plasma protein binding of both drugs as rifampicin has higher protein binding (88%) [48] compared to caffeine (10–37%) [49, 50]. While it is difficult to correlate plasma or brain levels of both drugs to the degree of enhanced expression of either transport protein, rifampicin and caffeine may not need to cross the BBB to upregulate LRP1or P-gp [13].
The second aim of this study was to investigate the effect of rifampicin and caffeine treatment on the clearance of Aβ from mice brains. Herein, we repot that treatment of wild-type mice with rifampicin or caffeine resulted in a significant increase (~20%, p<0.01) in Aβ40 clearance from the brain, as indicated by the enhanced BEI% (Fig. 5).
Aβ is cleared from the brain by non-saturable and saturable pathways [51]. Non-saturable pathway involves passive removal of soluble Aβ through bulk outflow of cerebrospinal fluid (i.e., cerebrospinal fluid turnover); however, in this study we used the brain efflux index method to study brain Aβ clearance, and this method is specific for clearance across the BBB [35]. Saturable pathways involve low affinity metabolism and high affinity BBB efflux components. Our results indicated that rifampicin and caffeine did not affect metabolism of Aβ and thus argue against the likelihood that enhanced Aβ clearance and protective effect of rifampicin and caffeine against AD are mediated by metabolism of Aβ upon drug treatment.
On the other hand, increased Aβ clearance was associated with upregulation of LRP1 and/or P-gp expressions in the brain microvessels. Although previous studies have demonstrated the roles of LRP1and P-gp in the clearance of Aβ [11, 13], our study is the first to investigate the effect of in vivo upregulation of LRP1 and P-gp on the clearance of Aβ from the brain of wild-type mice after rifampicin and caffeine treatment. In this study, we investigated Aβ clearance at two different drugs plasma levels. While plasma level of caffeine did not affect the clearance of Aβ, it is clear from our results that at high plasma concentration of rifampicin the clearance of Aβ is reduced, while at very low rifampicin plasma level (i.e., after a 24 h washout period) the clearance of Aβ was significantly enhanced. The effect of high rifampicin plasma levels on Aβ clearance is attributable to P-gp inhibition by rifampicin, which further confirms the important contribution of P-gp to the clearance of Aβ across BBB.
LRP1 inhibition studies with RAP demonstrated a significant reduction in Aβ clearance from the brains of control mice (BEI% decreased from 62.4% to 29.1%). These results are consistent with those of a previous study that investigated the role of LRP1 on the clearance of Aβ [11]. On the other hand, the inhibition of LRP1 in rifampicin treated mice decreased the clearance of Aβ by 10% (p <0.05). Interestingly, in caffeine treated mice, inhibition of LRP1 by RAP did not cause any significant change in the clearance of Aβ, which is consistent with the protein expression studies where caffeine did not upregulate LRP1. Yet, caffeine and rifampicin treated mice cleared Aβ more rapidly than control mice even in the presence of RAP suggesting the presence of an unknown mechanism contributing to the clearance of Aβ. This mechanism could be either an unknown transporter(s)/receptor(s), most likely at the abluminal side, or enhanced proteolysis of Aβ as a result of the treatments. However, the possibility of enhanced Aβ proteolysis was ruled out because caffeine and rifampicin treatment did not affect Aβ metabolism. Our findings, rather, suggest the contribution of a yet to be identified abluminal transporter/receptor that is upregulated by rifampicin and caffeine and contributed to the enhanced Aβ clearance, and compensated for LRP1 inhibition by RAP. A recent study has demonstrated the significant role of MRP1 transporter in the clearance of Aβ [14, 52]. However, our results of protein expression demonstrated that neither rifampicin nor caffeine upregulated MRP1 expression. Consequently, such assumption was not further considered.
The specific contribution of P-gp to the enhanced clearance of Aβ was investigated by the addition of valspodar, a selective P-gp inhibitor [53]. Valspodar inhibited Aβ clearance by 23% and 32% in mice treated with rifampicin and caffeine, respectively, compared to the control where valspodar inhibited Aβ clearance by only 16% (Fig. 4). Such results further corroborate the assumption that either drug enhances Aβ clearance by enhancing P-gp expression/activity at the BBB. Decrease in Aβ clearance after inhibition by valspodar was comparable to the reduction in Aβ clearance by rifampicin (when only a 3 h washout was allowed instead of 24 h) confirming the specific role and contribution of P-gp to Aβ clearance across the BBB. Interestingly, although the upregulation of P-gp expression in caffeine-treated mice is less than rifampicin-treated mice, the reduction in Aβ clearance as a result of P-gp inhibition by valspodar was significantly higher in the caffeine group (32% versus 25% reduction in BEI, p < 0.05), which could be related to the upregulation of LRP1 by rifampicin but not caffeine. LRP1 upregulation by rifampicin enhanced Aβ transport from the abluminal side of the endothelial cells, thus compensated for the decrease in P-gp activity caused by valspodar. This finding could emphasize the advantage of both transporters simultaneous upregulation for the efficient removal of Aβ from the brain and support the hypothesis of vectorial efflux of Aβ as suggested previously [25]. In control treated mice, inhibition of LRP1 caused greater reduction in the clearance of Aβ than inhibition of P-gp (33% versus 16%). This observation supports the importance of LRP1 as a first step in the clearance of Aβ from the brain, and our results suggest that efflux system at the abluminal side (LRP1 and other unknown transport mechanism(s)) may interact with P-gp at the luminal side in a vectorial manner. This finding also confirms the important contribution of P-gp in the final step of Aβ clearance from the brain which is supported by the results from inhibition studies by valspodar wherein a reduction in Aβ clearance in all treated groups including the control group was observed. Further studies are required to confirm the possible existence of, and nature of, vectorial interaction between LRP1 and P-gp in Aβ clearance across the BBB.
In conclusion, our findings demonstrated that the beneficial effect of rifampicin and caffeine against AD could be extended to their ability to induce LRP1 and/or P-gp, which are responsible for Aβ clearance. While enhanced Aβ clearance across BBB by upregulation of LRP1 and/or P-gp is probably not the only mechanism to explain the protective effect of rifampicin and caffeine against AD, this effect is of great importance because a number of commonly used drugs and herbal medicines have been reported as upregulators of P-gp and/or LRP1 and may be beneficial for mitigating the progression of AD. Also, this study provides evidence for the pivotal role of both LRP1 and P-gp in the clearance of Aβ from the brain, and extends the hypothesis of upregulation of P-gp at the BBB as a therapeutic strategy in AD to include upregulation of both LRP1 and P-gp as a therapeutic strategy to delay or slow progression of AD. Furthermore, this study suggests the presence of (an) unknown transporter(s)/receptor(s) that act in the same direction as LRP1 in Aβ clearance, and proposes the presence of a vectorial interaction between LRP1, located at the abluminal side, and P-gp, located at the luminal side, of the BBB.
ACKNOWLEDGMENTS
This project was supported by grants from the National Center for Research Resources (5P20RR016456–11) and the National Institute of General Medical Sciences (8 P20GM103424–11) from the National Institutes of Health.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=1245).
REFERENCES
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. State-specific projections through 2025 of Alzheimer disease prevalence. Neurology. 2004;62:1645. doi: 10.1212/01.wnl.0000123018.01306.10. [DOI] [PubMed] [Google Scholar]
- 2.Citron M. Alzheimer’s disease: Strategies for disease modification. Nat Rev Drug Discov. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 5.Pietrzik C, Behl C. Concepts for the treatment of Alzheimer’s disease: Molecular mechanisms and clinical application. Int J Exp Pathol. 2005;86:173–185. doi: 10.1111/j.0959-9673.2005.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehman EJ, Kulnane LS, Lamb BT. Alterations in beta-amyloid production and deposition in brain regions of two transgenic models. Neurobiol Aging. 2003;24:645–653. doi: 10.1016/s0197-4580(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 7.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommer B. Alzheimer’s disease and the amyloid cas-cadehypothesis: Ten years on. CurrOpinPharmaco. 2002;12:87–92. doi: 10.1016/s1471-4892(01)00126-6. [DOI] [PubMed] [Google Scholar]
- 9.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 10.Zlokovic BV, Frangione B. Transport-clearance hypothesis for Alzheimer’s disease and potential therapeutic implications. In: Saido TC, editor. Aβ Metabolism in Alzheimer’s Disease. Georgetown, TX: Landes Bioscience; 2003. pp. 114–122. [Google Scholar]
- 11.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-beta(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhnke D, Jedlitschky G, Grube M, Krohn M, Jucker M, Mosyagin I, Cascorbi I, Walker LC, Kroemer HK, Warzok RW, Vogelgesang S. MDR1-P-Glycoprotein (ABCB1) Mediates Transport of Alzheimer’s amyloid-beta peptides- implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007;17:347–353. doi: 10.1111/j.1750-3639.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsada-nian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Stef-fen J, Schumacher T, Bruning T, Plath AS, Alfen F, Schmidt A, Winter F, Rateitschak K, Wree A, Gsponer J, Walker LC, Pahnke J. Cerebral amyloid-beta proteostasis is regulated by the membrane transport protein ABCC1 in mice. J Clin Invest. 2011;121:3924–3931. doi: 10.1172/JCI57867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 16.Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, Frangione B, Ghiso J. Glycoprotein 330/megalin: Probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci U S A. 1996;93:4229–4234. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abuznait AH, Cain C, Ingram D, Burk D, Kaddoumi A. Up-regulation of P-glycoprotein reduces intracellular accumulation of beta amyloid: Investigation of P-glycoprotein as a novel therapeutic target for Alzheimer’s disease. J Pharm Pharmacol. 2011;63:1111–1118. doi: 10.1111/j.2042-7158.2011.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolazzo JA, Mehta DC. Transport of drugs across the blood-brain barrier in Alzheimer’s disease. Therapeutic Delivery. 2010;1:595–611. doi: 10.4155/tde.10.41. [DOI] [PubMed] [Google Scholar]
- 19.Rapposelli S, Digiacomo M, Balsamo A. P-gp transporter and its role in neurodegenerative diseases. Curr Top Med Chem. 2009;9:209–217. doi: 10.2174/156802609787521544. [DOI] [PubMed] [Google Scholar]
- 20.Silverberg GD, Messier AA, Miller MC, Machan JT, Maj-mudar SS, Stopa EG, Donahue JE, Johanson CE. Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. J Neuropathol Exp Neurol. 2010;69:1034–1043. doi: 10.1097/NEN.0b013e3181f46e25. [DOI] [PubMed] [Google Scholar]
- 21.Herz J, Strickland DK. LRP: A multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology. 1997;49:56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- 23.Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lent-ing PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Sharom FJ. The P-glycoprotein multidrug transporter: Interactions with membrane lipids, and their modulation of activity. Biochem Soc Trans. 1997;25:1088–1096. doi: 10.1042/bst0251088. [DOI] [PubMed] [Google Scholar]
- 25.Hartz AM, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer’s disease. Mol Pharmacol. 2010;77:715–723. doi: 10.1124/mol.109.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loeb MB, Molloy DW, Smieja M, Standish T, Goldsmith CH, Mahony J, Smith S, Borrie M, Decoteau E, Davidson W, McDougall A, Gnarpe J, O’DONNell M, Chernesky M. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer’s disease. J Am Geriatr Soc. 2004;52:381–387. doi: 10.1111/j.1532-5415.2004.52109.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Ghribi O, Geiger JD. Caffeine protects against disruptions of the blood-brain barrier in animal models of Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis. 2010;20(Suppl 1):S127–S141. doi: 10.3233/JAD-2010-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Gelder BM, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. Eur J Clin Nutr. 2007;61:226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: A prospective population study (the Three City Study) Neurology. 2007;69:536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- 30.Cao C, Cirrito JR, Lin X, Wang L, Verges DK, Dickson A, Mamcarz M, Zhang C, Mori T, Arendash GW, Holtzman DM, Potter H. Caffeine suppresses amyloid-beta levels in plasma and brain of Alzheimer’s disease transgenic mice. J Alzheimers Dis. 2009;17:681–697. doi: 10.3233/JAD-2009-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maia L, de Mendonca A. Does caffeine intake protect from Alzheimer’s disease? Eur J Neurol. 2002;9:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 32.Abuznait AH, Patrick SG, Kaddoumi A. Exposure of LS-180 cells to drugs of diverse physicochemical and therapeutic properties up-regulates P-glycoprotein expression and activity. J Pharm Pharm Sci. 2011;14:236–248. doi: 10.18433/j36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinohara M, Sato N, Kurinami H, Takeuchi D, Takeda S, Shi-mamura M, Yamashita T, Uchiyama Y, Rakugi H, Morishita R. Reduction of brain beta-amyloid (Abeta) by flu-vastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Abeta clearance. J Biol Chem. 2010;285:22091–22102. doi: 10.1074/jbc.M110.102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMat-tos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakee A, Terasaki T, Sugiyama Y. Brain efflux index as a novel methodofanalyzing efflux transportatthe blood-brain barrier. J Pharmacol Exp Ther. 1996;277:1550–1559. [PubMed] [Google Scholar]
- 36.Ito S, Ohtsuki S, Terasaki T. Functional characterization of the brain-to-blood efflux clearance of human amyloid-beta peptide (1–40) across the rat blood-brain barrier. Neurosci Res. 2006;56:246–252. doi: 10.1016/j.neures.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Twentyman PR, Bleehen NM. Resistance modification by PSC-833, a novel non-immunosuppressive cyclosporin [corrected] Eur J Cancer. 1991;27:1639–1642. doi: 10.1016/0277-5379(91)90435-g. [DOI] [PubMed] [Google Scholar]
- 38.Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of amyloid beta-peptide from brain: Transport or metabolism? Nat Med. 2000;6:718–719. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]
- 39.Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–1219. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 40.Chiang PK, Lam MA, Luo Y. The many faces of amyloid beta in Alzheimer’s disease. Curr Mol Med. 2008;8:580–584. doi: 10.2174/156652408785747951. [DOI] [PubMed] [Google Scholar]
- 41.Seeman P, Seeman N. Alzheimer’s disease: Beta-amyloid plaque formation in human brain. Synapse. 2011;65:1289–1297. doi: 10.1002/syn.20957. [DOI] [PubMed] [Google Scholar]
- 42.Zong J, Pollack GM. Modulation of P-glycoprotein transport activity in the mouse blood-brain barrier by rifampin. J Pharmacol Exp Ther. 2003;306:556–562. doi: 10.1124/jpet.103.049452. [DOI] [PubMed] [Google Scholar]
- 43.Bi W, Zhu L, Wang C, Liang Y, Liu J, Shi Q, Tao E. Rifampicin inhibits microglial inflammation and improves neuron survival against inflammation. Brain Res. 2011;1395:12–20. doi: 10.1016/j.brainres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Wei C, Xu C, Bennett MC, Zhang G, Li F, Tao E. Rifampicin protects PC12 cells against MPP+induced apoptosis and inhibits the expression of an alpha-Synuclein multimer. Brain Res. 2007;1139:220–225. doi: 10.1016/j.brainres.2006.12.074. [DOI] [PubMed] [Google Scholar]
- 45.Wostyn P, Van Dam D, Audenaert K, De Deyn PP. Increased cerebrospinal fluid production as a possible mechanism underlying caffeine’s protective effect against Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:617420. doi: 10.4061/2011/617420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal S, Singh I, Kaur KJ, Bhade SR, Kaul CL, Pan-chagnula R. Bioequivalence assessment of rifampicin, isoniazid and pyrazinamide in a fixed dose combination of rifampicin, isoniazid, pyrazinamide and ethambutol vs. separate formulations. Int J Clin Pharmacol Ther. 2002;40:474–481. doi: 10.5414/cpp40474. [DOI] [PubMed] [Google Scholar]
- 47.Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 48.Nannelli A, Rossignolo F, Tolando R, Rossato P, Pellegatti M, Longo V, Gervasi PG. Expression and distribution of CYP3A genes, CYP2B22, and MDR1, MRP1, MRP2, LRP efflux transporters in brain of control and rifampicin-treated pigs. Mol Cell Biochem. 2010;337:133–143. doi: 10.1007/s11010-009-0292-1. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka H, Mizojiri K. Drug-protein binding and blood-brain barrier permeability. J Pharmacol Exp Ther. 1999;288:912–918. [PubMed] [Google Scholar]
- 50.Blanchard J. Protein binding of caffeine in young and elderly males. J Pharm Sci. 1982;71:1415–1418. doi: 10.1002/jps.2600711229. [DOI] [PubMed] [Google Scholar]
- 51.Banks WA, Robinson SM, Verma S, Morley JE. Efflux of human and mouse amyloid beta proteins 1–40 and 1–42 from brain: Impairment in a mouse model of Alzheimer’s disease. Neuroscience. 2003;121:487–492. doi: 10.1016/s0306-4522(03)00474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cisternino S, Rousselle C, Lorico A, Rappa G, Scherrmann JM. Apparent lack of Mrp1-mediated efflux at the luminal side ofmouse blood-brain barrier endothelial cells. Pharm Res. 2003;20:904–909. doi: 10.1023/a:1023895404929. [DOI] [PubMed] [Google Scholar]
- 53.Tidefelt U, Liliemark J, Gruber A, Liliemark E, Sundman-Engberg B, Juliusson G, Stenke L, Elmhorn-Rosenborg A, Mollgard L, Lehman S, Xu D, Covelli A, Gustavsson B, Paul C. P-Glycoprotein inhibitor valspodar (PSC 833) increases the intracellular concentrations of daunorubicin in vivo in patients with P-glycoprotein-positive acute myeloid leukemia. J Clin Oncol. 2000;18:1837–1844. doi: 10.1200/JCO.2000.18.9.1837. [DOI] [PubMed] [Google Scholar]