Abstract
Objective
In a recent crossover trial, methylphenidate treatment decreased apathy in Alzheimer's disease. We further assessed this finding in the Alzheimer's Disease Methylphenidate Trial (ADMET).
Method
Six-week, randomized, double-blind, placebo-controlled multicenter trial enrolling Alzheimer's disease participants (NINCDS-ADRDA criteria) with apathy assigned to methylphenidate 20 mg daily or placebo, conducted from June 2010 to December 2011. Primary outcomes were change in Apathy Evaluation Scale (AES) score and modified Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change (ADCS-CGI-C). Secondary outcomes included change in Neuropsychiatric Inventory (NPI) apathy score, Mini-Mental State Examination (MMSE) score, and safety.
Results
60 participants were randomly assigned (29 methylphenidate, 31 placebo). At baseline, mean (SD) age = 76 (8) years, MMSE score = 20 (5), AES score = 51 (12), NPI total score = 16 (8), and 62% of the participants (n = 37) were female. After 6 weeks' treatment, mean (SD) change in AES score was −1.9 (1.5) for methylphenidate and 0.6 (1.4) for placebo (P = .23). Odds ratio for improvement in ADCS-CGI-C was 3.7 (95% CI, 1.3 to 10.8) (P = .02), with 21% of methylphenidate versus 3% of placebo rated as moderately or markedly improved. NPI apathy score improvement was 1.8 points (95% CI, 0.3 to 3.4) greater on methylphenidate than on placebo (P = .02). MMSE trended toward improvement on methylphenidate (P = .06). There were trends toward greater anxiety and weight loss > 2% in the methylphenidate-treated group.
Conclusions
Methylphenidate treatment of apathy in Alzheimer's disease was associated with significant improvement in 2 of 3 efficacy outcomes and a trend toward improved global cognition with minimal adverse events, supporting the safety and efficacy of methylphenidate treatment for apathy in Alzheimer's disease.
Alzheimer's disease is the major neurodegenerative disease of aging, affecting an estimated 5.1 million persons in the United States in 2010 and increasing to 11 to 16 million by 2050.1 Neuro-psychiatric syndromes affecting behavior are near-universal in Alzheimer's disease.2 Apathy is one of the most common syndromes, with a 5-year prevalence estimate of 71%.2 Apathy in Alzheimer's disease is described as loss of interest and motivation in daily activities in the absence of depression or other mood changes.3 Because apathy in Alzheimer's disease is associated with poorer quality of life,4,5 greater functional impairment,6 greater caregiver burden,7 increased risk of institutionalization,8 and higher costs of care,9 it is an important treatment target.
Current evidence suggests that apathy in Alzheimer's disease is associated with a decrease in dopaminergic neurotransmission. Lower dopamine transporter binding in the bilateral putamen has been associated with poor initiative.10 It has been proposed that dopaminergic circuits between the basal ganglia, anterior cingulate, and frontal cortex, which are involved in motivation and reward, are dys-functional in Alzheimer's disease patients with apathy.11 Alzheimer's disease patients with apathy have a blunted subjective response to dextroamphetamine challenge,12 a potential biomarker of the brain reward system. Therefore it has been hypothesized that treatments that enhance dopamine (such as methylphenidate) could be effective for apathy in Alzheimer's disease. Methylphenidate improved apathy more than placebo in a recent crossover trial,13 while modafinil treatment did not.14 We report the results of a larger double-blind, randomized, placebo-controlled, multicenter trial of methylphenidate for apathy in Alzheimer's disease (Alzheimer's disease methylpheni-date trial [ADMET]).
METHOD
Participants
The design and rationale of ADMET were recently published in detail.15 Participants were recruited at Johns Hopkins Bayview Medical Center (Baltimore, Maryland), Medical University of South Carolina (Charleston, South Carolina), and University of Toronto Sunnybrook Health Sciences Centre (Toronto, Ontario, Canada) between June 2010 and October 2011. Ethics review boards at all 3 institutions approved study procedures. Study participants or their legally authorized representatives gave informed consent. Inclusion criteria included (1) diagnosis of possible or probable Alzheimer's disease (National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria)16; (2) clinical stability as judged by the local investigator; (3) Mini-Mental State Examination (MMSE)17 score of at least 10; (4) clinically significant apathy for at least 4 weeks, defined as a Neuropsychiatric Inventory (NPI)18 apathy frequency of “often” or greater and an apathy severity of “moderate,” or “marked”; and (5) stable dose for the prior 3 months, if treated with a selective serotonin reuptake inhibitor (SSRI). After the 14th patient was randomized, the protocol was amended to include participants taking serotonin-noradrenergic reuptake inhibitors with at least 3 months' stable dosing, with ADMET Steering Committee approval. Exclusion criteria included (1) diagnosis of major depressive episode; (2) agitation/aggression, delusions, or hallucinations with a frequency on the NPI of “very frequently” or “frequently” and a severity of “moderate” or “marked”; or (3) treatment with psychotropic medications, with the exception of trazodone for sleep and antidepressants as stated above.
Measures
Apathy Evaluation Scale
The Apathy Evaluation Scale (AES),3,19 an 18-item scale designed to measure apathy as a neuropsychiatric symptom, is the best-validated scale for measuring apathy in Alzheimer's disease. The AES informant version (administered to study partners) has good internal consistency (Cronbach α = 0.94) and test-retest reliability (Pearson r = 0.94).19
Alzheimer's Disease Cooperative Study Clinical Global Impression of Change
ADMET used a structured clinical global impression of change (CGI-C) developed by the Alzheimer's Disease Cooperative Study (ADCS)20 with the addition of a rating for the targeted behavior change similar to the approach used in several recent trials of psychotropic medications for neuropsychiatric symptoms in Alzheimer's disease.21,22
Neuropsychiatric Inventory apathy score
The NPI18 measures neuropsychiatric symptoms in dementia, and is administered as a structured interview with a knowledgeable informant who can report the patient's neuropsychiatric symptoms. The NPI surveys 12 domains of neuropsychiatric symptoms, including apathy. The apathy score was defined as the product of frequency and severity of apathy symptoms, with range 0–12; higher scores indicate more frequent and/or severe symptoms.
Mini-Mental State Examination
The Mini-Mental State Examination (MMSE)7 is a brief examination of general cognitive status widely used in assessment of cognitive impairment in the elderly; it assesses several cognitive domains on a 30-point scale.
Interventions
The randomization scheme, stratified by clinical center with permuted length blocks, assigned participants to methylphenidate or placebo in a 1:1 ratio. The coordinating center generated the treatment assignment schedule using a documented, auditable SAS program (SAS Institute Inc, Cary, North Carolina). Clinic staff obtained treatment assignments centrally using the ADMET Web site. Study drug was supplied as identical-appearing capsules containing either 5 mg methylphenidate or lactose (placebo).
Participants began treatment by taking 1 study drug capsule twice a day (10 mg/d in the methylphenidate group); if well tolerated for 3 days, dose was increased to 2 capsules twice a day (20 mg/d). All study partners and participants received the ADMET standardized psychosocial intervention similar to interventions used in comparable Alzheimer's disease trials.21,22
Adverse Events and Safety Monitoring
Data on adverse events were collected by symptom checklist for known or expected side effects of methylphenidate and by open-ended questions for unexpected side effects, results of electrolyte panels, and electrocardiogram results. Given the possible association of anorexia with methylphenidate treatment, a weight loss threshold of 7% or greater was predefined as an adverse event. Serious adverse events were defined as adverse events leading to hospitalization, emergency department visit, or death. A Data Safety and Monitoring Committee, including 3 voting members with expertise in biostatistics, psychiatry, and neurology, reviewed accumulating, unmasked data on the safety and efficacy of methylphenidate compared with placebo at 3 time points: when approximately 30, 45, and 55 participants were enrolled. No formal stopping rules were established and no correction of reported P values for these interim tests was performed.
Data Analyses
The primary assessment of efficacy was based on an intention-to-treat comparison of the difference in the change in AES scores from baseline to week 6 and the comparison at week 6 of the ratings for the CGI-C. For AES, a saturated means model (including indicators for each visit and each visit-by-treatment interaction) adjusting for stratification by clinic was created using a linear mixed effects model with random intercept for each participant to account for multiple measurements over time. All available visit data for the 60 participants were incorporated into the model, although the primary comparison was change from baseline to week 6. Because exploratory analyses suggested that there might be differences in the tails of the AES distributions, the numbers of participants with a ≥ 4-point and a ≥ 8-point improvement in AES were also compared using Fisher exact test in a post hoc analysis. We performed several prespecified sensitivity analyses for the AES outcome: (1) using multiple imputation23 to impute missing outcomes, (2) controlling for history of mood disorder in the regression analysis, and (3) performing the analysis for the subset of participants that reported taking the study drug “most of the time” or “always” at all study visits. We also tested for treatment by clinic (prespecified), treatment by sex (post hoc), and treatment by baseline AES interactions (post hoc) at week 6.
Proportional odds logistic regression was used to compare the ADCS-CGI-C ratings of change (ranging from “marked worsening” to “marked improvement” on a 7-point scale) at week 6 between the treatment groups. Change in the NPI apathy score and change in MMSE score from baseline to week 6 were assessed using a mixed effects model as described above.
The proportion of adverse events was compared between treatment groups using logistic regression or Fisher exact test, controlling for baseline report of the same symptom if necessary due to baseline imbalance. No patients experienced the prespecified level of 7% weight lost during the 6 weeks, so a post hoc definition of weight loss of 2% was also compared by treatment group. Additionally, the change in MMSE scores was evaluated as part of the safety assessment. Time to early (before week 6) treatment termination was compared using a log rank test. Adherence was also assessed by pill counts from returned medication bottles. The proportion of returned bottles and the proportion of pills presumed to have been taken (by pill counts in returned bottles) were compared using t tests.
Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) and R version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria). All P values are 2-sided, and P < .05 was used as the threshold for statistical significance. No adjustments were made for multiple comparisons.
Power Calculations
Using information from our preliminary study,13 we calculated that with 60 participants the power to detect a difference on the AES of 3.3 in the change from baseline to week 6 was > 80%, assuming a 2-sided type I error of 5%.
For CGI-C, we calculated power by using standard 2-sample power estimations for comparing 2 proportions and assumed that 20%–30% of participants assigned to placebo would show moderate or marked improvement as has been reported in previous trials of interventions for neuropsychiatric symptoms in Alzheimer's disease.24 With 60 participants, the study would have > 80% power to detect a difference of 35% between the proportion of participants who improve (or worsen) in the methylphenidate group compared with the placebo group.
RESULTS
Clinical Characteristics
Baseline characteristics of the participants are listed in Table 1. The participants were on average in their late 70s; 62% were female (n = 37); the majority were predominantly white, non-Hispanic; and all had been diagnosed with dementia for a mean of 3 years. Forty-seven (78%) lived in their own home, 6 (10%) lived in a caregiver or family member's home, 5 (8.3%) lived in assisted living, and 2 (3.3%) lived in other settings. Forty-seven (78%) were married, 9 (15%) were widowed, 3 (5%) were divorced, and 1 (2%) had never married. The severity of dementia averaged from mild to moderate (Table 1). We observed high scores for baseline AES (mean [SD] = 51 [12]) and NPI apathy (mean [SD] = 7 [2]). There were no statistically significant differences in baseline characteristics between treatment groups, except a slightly higher proportion of patients in the placebo group reported a history of mood disorder. While half of the participants had hyper-tension, the prevalence of other cardiovascular conditions was relatively low. The distribution of cardiovascular medication use was typical of older cohorts. While only 13% of the participants (n = 8) had a past diagnosis of major depression, over one third (n = 22) were taking SSRIs at baseline. Forty-three (72%) were taking acetylcholinesterase inhibitors and 37 (62%) were taking memantine.
Table 1.
Baseline Characteristics of 60 Patients With Alzheimer's Disease Randomly Assigned to Methylphenidate or Placebo
| Characteristic | Total (n = 60) | Methylphenidate (n = 29) | Placebo (n = 31) |
|---|---|---|---|
| Age, mean (SD), y | 76 (8) | 78 (8) | 75 (9) |
| Women, n (%) | 37 (62) | 17 (59) | 20 (65) |
| Racial/ethnic group, n (%) | |||
| White, non-Hispanic | 55 (92) | 28 (97) | 27 (87) |
| African-American, non-Hispanic | 5 (8) | 1 (3) | 4 (13) |
| Hispanic/Latino | 0 (0) | 0 (0) | 0 (0) |
| Other, non-Hispanic | 0 (0) | 0 (0) | 0 (0) |
| Highest education, n (%) | |||
| No high school diploma | 7 (12) | 3 (10) | 4 (13) |
| High school diploma | 17 (28) | 5 (17) | 12 (39) |
| Some college/associate's degree | 13 (22) | 6 (21) | 7 (23) |
| Bachelor's degree | 13 (22) | 8 (28) | 5 (16) |
| Professional/graduate degree | 9 (15) | 6 (21) | 3 (10) |
| Missing | 1 (2) | 1 (3) | 0 (0) |
| Blood pressure, mean (SD), mm Hg | |||
| Systolic | 133 (16) | 135 (14) | 130 (18) |
| Diastolic | 75 (11) | 75 (10) | 75 (12) |
| Abnormal ECG results, n (%) | 36 (60) | 18 (62) | 18 (58) |
| Duration of dementia, mean (SD), y | 3 (3) | 3 (3) | 3 (3) |
| Concomitant medications, n (%) | |||
| Selective serotonin reuptake inhibitors | 22 (37) | 11 (38) | 11 (35) |
| Cholinesterase inhibitors | 43 (72) | 21 (72) | 22 (71) |
| Memantine | 37 (62) | 21 (72) | 16 (52) |
| History of mood disorder before Alzheimer's disease, n (%) | 8 (13) | 2 (7) | 6 (19) |
| Apathy Evaluation Scale score, mean (SD) | 51 (12) | 50 (13) | 51 (11) |
| Neuropsychiatric Inventory, mean (SD) | |||
| Total score | 16 (8) | 15 (6) | 17 (9) |
| Apathy subscore | 7 (2) | 7 (2) | 8 (2) |
| Depression subscore | 2 (2) | 1 (2) | 2 (3) |
| MMSE score, mean (SD) | 20 (5) | 19 (5) | 20 (5) |
Abbreviation: MMSE = Mini-Mental State Examination.
Study Characteristics
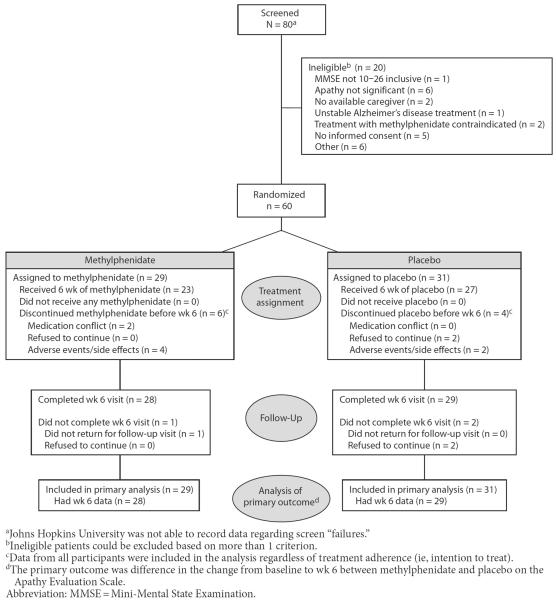
Participant flow is illustrated in Figure 1. Enrollment was from June 2010 to October 2011 and the last follow-up visit was completed in December 2011. Sixty patients were randomly assigned (29 to methylphenidate and 31 to placebo), and all were included in the analyses. Fifty-seven participants (95%) completed the week 6 visit, and 50 participants (83%) completed 6 weeks of treatment on study drug (79% methylphenidate, n = 23; 87% placebo, n = 27). The time to early treatment termination did not differ significantly by group (log rank χ21 = 0.5, P = .48). More than 95% of study bottles were returned with adherence by pill count of 88.3% for methylphenidate and 86.8% for placebo (P = .79, t test for difference). By participant/caregiver self-report, 72.4% of the methylphenidate subjects (n = 21) and 83.9% of the placebo subjects (n = 26) reported adherence “most of the time” or “always” at all visits (χ21 = 1.2, P = .28). Twenty-four methylphenidate participants (83%) and 25 placebo participants (81%) were taking the target dose of 4 capsules (20 mg in methylphenidate group) at the week 6 visit (Fisher exact P = 1.0). Only 2 participants (both in the placebo group) were taking 3 capsules. Protocol deviations included enrollment of 3 participants who took excluded classes of medications and 4 with diagnoses of glaucoma. Two participants were discontinued from study medication due to clinicians' perception of conflict with antidepressant treatment and cardiovascular medications respectively.
Figure 1.
Participant Flow, CONSORT Diagram
Apathy Outcomes
Table 2 presents the change in apathy outcomes between baseline and week 6 from the mixed models. The difference in the change in AES was an estimated −2.5 (95% CI, −6.5 to 1.6) points at 6 weeks (negative differences favor methylphenidate), which was not statistically significant (P = .23). The figure and the results from the mixed model indicate that both groups had their lowest mean AES scores at week 2, although the greatest difference in the change in AES between the groups was −4.0 (95% CI, −8.0 to 0.1; P = .06) at week 4. There was no significant treatment by clinic, treatment by sex, or treatment by baseline AES interactions. The results were similar for all sensitivity analyses (data not shown). The proportion of participants with AES improvement ≥ 4 points and ≥ 8 points was numerically greater in the methylphenidate group but the differences were not statistically significant.
Table 2.
Measures of Apathy at 6 Weeks in 60 Patients With Alzheimer's Disease Randomly Assigned to Methylphenidate or Placebo
| Measure | Methylphenidate (n = 29) | Placebo (n = 31) | P Value |
|---|---|---|---|
| No. of patients with wk 6 data, n | 28 | 29 | |
| Apathy Evaluation Scale (AES) | |||
| Estimated score at 6 wk, mean (SE)a | 48.9 (2.1) | 52.0 (2.1) | |
| Estimated change from baseline to 6 wk, mean (SE)a | −1.9 (1.5) | 0.6 (1.4) | |
| Estimated treatment effect (methylphenidate – placebo), mean (95% CI)a | −2.5 (−6.5 to 1.6) | .23 | |
| ≥ 4-point improvement in AES from baseline to 6 wk, n (%) | 9 (32) | 8 (28) | .78 |
| ≥ 8-point improvement in AES from baseline to 6 wk, n (%) | 5 (18) | 3 (10) | .47 |
| ADCS-CGI-C change in apathy, n (%) | |||
| Marked improvement | 2 (7) | 0 (0) | |
| Moderate improvement | 4 (14) | 1 (3) | |
| Minimal improvement | 10 (36) | 7 (24) | |
| No change | 11 (39) | 20 (69) | |
| Minimal worsening | 1 (4) | 1 (3) | |
| Moderate worsening | 0 (0) | 0 (0) | |
| Marked worsening | 0 (0) | 0 (0) | |
| Estimated treatment effect (methylphenidate vs placebo), OR (95% CI)b | 3.7 (1.3 to 10.8) | .02 | |
| Neuropsychiatric Inventory apathy subscale | |||
| Estimated score at 6 wk, mean (SE)a | 2.9 (0.5) | 5.1 (0.5) | |
| Estimated change from baseline to 6 wk, mean (SE)a | −4.4 (0.6) | −2.6 (0.6) | |
| Estimated treatment effect (methylphenidate – placebo), mean (95% CI)a | −1.8(−3.4 to −0.3) | .02 | |
The score, change, and treatment effect are the model-based estimates (calculated using mixed model regression). The treatment effect is the comparison of within treatment group change from enrollment (the difference of differences) controlling for the stratification variable. A negative number favors methylphenidate. The number of people with ≥ 4- and ≥ 8-point improvement in AES score was compared using Fisher exact test.
The treatment effect is the odds ratio (calculated using proportional odds logistic regression) of being at or better than a given ADCS-CGI-C category for methylphenidate vs placebo controlling for the stratification variable. A number greater than 1 favors methylphenidate.
Abbreviations: ADCS-CGI-C = Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change, CI = confidence interval, OR = odds ratio, SE = standard error.
The CGI-C results favored methylphenidate over placebo, with 21% of methylphenidate participants having moderate or marked improvement at 6 weeks vs 3% of placebo participants. The odds ratio of being at or better than a given ADCS-CGI-C category for methylphenidate vs placebo was 3.7 (95% CI, 1.3 to 10.8), strongly favoring methylphenidate over placebo (P = .02). Similarly, the difference in the change in NPI apathy scores was −1.8 (95% CI, −3.4 to −0.3) favoring methylphenidate at week 6 (P = .02).
Adverse Events
Side effects and adverse events are presented in Table 3. Two patients experienced serious adverse events (abdominal pain and drop in hemoglobin), 1 in each treatment group, and there were no deaths. Two participants in the methylphenidate group and none in the placebo group experienced significant hallucinations or delusions defined as NPI delusions and/or hallucination frequency by severity score of ≥ 6. There were trends toward methylphenidate participants experiencing > 2% weight loss (OR = 3.7; 95% CI, 0.9 to 19.4; P = .06) and anxiety (OR = 2.7; 95% CI, 0.9 to 7.8; P = .07), and placebo participants experienced more arthralgia (OR = 0.3; 95% CI, 0.1 to 0.9; P = .03). Reports of both anxiety and arthralgia were slightly imbalanced at baseline, but controlling for this imbalance resulted in little difference in results (data not shown). No other differences were noted in reports of side effects between methylpheni-date and placebo groups. Adverse events leading to study drug discontinuation included hypertension, nervousness, nausea, and anxiety in 4 methylphenidate participants, and insomnia and drop in hemoglobin in 2 placebo participants.
Table 3.
Patients With Alzheimer's Disease Experiencing Adverse Events During Randomized Treatment With Methylphenidate or Placeboa
| Effect | Methylphenidate (n = 29) | Placebo (n = 31) | ORb | 95% CI | P Value |
|---|---|---|---|---|---|
| No. of patients with adverse event data, nc | 29 | 29 | |||
| Event | |||||
| Death, n | 0 | 0 | |||
| Serious adverse events, n | 1 | 1 | |||
| Abdominal pain | 1 | 0 | |||
| Drop in hemoglobin | 0 | 1 | |||
| Abnormal ECG results at wk 6, n (%)d | 20 (77) | 15 (58) | 2.4 | 0.6 to 9.9 | .24 |
| Clinically significant abnormal ECG results at wk 6, n | 1e | 0 | |||
| Weight loss at week 6 > 7%, n | 0 | 0 | |||
| Weight loss at week 6 > 2%, n (%) | 10 (40) | 4 (15) | 3.7 | 0.9 to 19.4 | .06 |
| Any hallucinations and/or delusions, n (%) | 5 (18) | 4 (14) | 1.4 | 0.3 to 5.7 | .67 |
| Significant hallucinations and/or delusions, n (%) | 2 (7) | 0 (0) | … | … | .24 |
| Side effects collected via prompted questions, n (%) | |||||
| Abdominal pain | 6 (21) | 4 (14) | 1.6 | 0.4 to 6.5 | .49 |
| Aggressive behavior or hostility | 9 (31) | 5 (17) | 2.2 | 0.6 to 7.5 | .22 |
| Agitation | 17 (59) | 13 (45) | 1.7 | 0.6 to 4.9 | .29 |
| Angina | 2 (7) | 1 (3) | … | … | 1.00 |
| Anorexia | 6 (21) | 5 (17) | 1.3 | 0.3 to 4.7 | .74 |
| Anxiety, nervousness, or tension | 17 (59) | 10 (34) | 2.7 | 0.9 to 7.8 | .07 |
| Arthralgia | 6 (21) | 14 (48) | 0.3 | 0.1 to 0.9 | .03 |
| Blood pressure changes | 4 (14) | 3 (10) | 1.4 | 0.3 to 6.8 | .69 |
| Blurry vision or eyesight changes | 0 (0) | 3 (10) | … | … | .24 |
| Decreased appetite | 8 (28) | 7 (24) | 1.2 | 0.4 to 3.9 | .76 |
| Depressed mood | 12 (41) | 12 (41) | 1.0 | 0.4 to 2.8 | 1.00 |
| Distractibility | 11 (38) | 8 (28) | 1.6 | 0.5 to 4.9 | .40 |
| Dizziness | 11 (38) | 7 (24) | 1.9 | 0.6 to 6.0 | .26 |
| Drowsiness | 10 (34) | 13 (45) | 0.6 | 0.2 to 1.9 | .42 |
| Dry mouth | 7 (24) | 6 (21) | 1.2 | 0.4 to 4.2 | .75 |
| Dyskinesia | 1 (3) | 4 (14) | … | … | .35 |
| Hair loss | 2 (7) | 1 (3) | … | … | 1.00 |
| Headache | 5 (17) | 4 (14) | 1.3 | 0.3 to 5.4 | .72 |
| Hyperactivity | 5 (17) | 1 (3) | … | … | .19 |
| Impaired learning | 4 (14) | 7 (24) | 0.5 | 0.1 to 2.0 | .32 |
| Nausea | 3 (10) | 7 (24) | 0.4 | 0.1 to 1.6 | .18 |
| Skin rash, redness, or inflammation | 6 (21) | 6 (21) | 1.0 | 0.3 to 3.6 | 1.00 |
| Tics (motor or verbal) | 0 (0) | 4 (14) | … | … | .11 |
| Side effects collected via open-ended questions, n (%) | |||||
| Related to increased bleeding | 1 (3) | 3 (10) | … | … | .61 |
| Related to increased motor activity | 6 (21) | 2 (7) | … | … | .25 |
| Other, not related to bleeding or motor activity | 14 (48) | 11 (38) | 1.5 | 0.5 to 4.9 | .60 |
The following events were reported by 2 or fewer patients in both treatment groups and were not included in this table: abnormal liver function, anemia, fever, impulsivity, palpitations, pulse changes, urticaria, tachycardia, and vasculitis.
Odds ratios (ORs) and P values were calculated using logistic regression or Fisher exact (for small cell counts). A patient was counted as having the event if he/she reported the symptom during any follow-up visit.
Two randomized patients (placebo) had no data on adverse events during follow-up. One additional patient (methylphenidate) was missing data on delusions and hallucinations at week 6. Four patients in the methylphenidate group and 4 patients in the placebo group were missing baseline or week 6 weight. Three patients taking methylphenidate and 5 patients taking placebo were missing week 6 ECG.
Among those participants with normal ECG at baseline, 4 participants taking methylphenidate and 4 participants taking placebo had abnormal ECGs at week 6.
Left atrial enlargement, prolonged QT interval.
Abbreviation: ECG = electrocardiogram.
MMSE
The MMSE showed a trend toward favorable outcomes on methylpheni-date treatment: mean (SE) score increased from 19.0 (1.1) at baseline to 20.2 (1.1) at 6 weeks in the methylphenidate group, while the mean (SE) score decreased from 20.4 (1.1) at baseline to 20.1 (1.1) at 6 weeks in the placebo group, for an estimated difference of 1.5 (95% CI, −0.1 to 3.1; P = .06).
DISCUSSION
This study further supports the hypothesis that methylphenidate, a drug that enhances dopaminergic neurotransmission, is effective in the treatment of apathy in Alzheimer's disease. Methylphenidate treatment was associated with a significant reduction in apathy symptoms in Alzheimer's disease participants treated for 6 weeks in this multicenter randomized, placebo-controlled, double-blind trial. Two outcomes, CGI-C and NPI apathy score, were clearly improved with methylphenidate treatment, with statistically significant changes over 6 weeks' treatment. The effects appear large enough to be of significance to clinical practice as well: 21% of methylphenidate-treated participants were judged to have moderate or marked improvement on the CGI-C versus 3% of placebo-treated, while the difference in NPI apathy scores was 1.8 from the baseline mean of 7 points. Results of AES also favored methylphenidate over placebo but were not statistically significant. We observed a trend toward MMSE change favoring methylphenidate treatment, suggesting the possibility that methylphenidate treatment was associated with improvement in global cognition. Adverse events and side effects were modest. These results indicate that methylphenidate treatment may have clinical utility in treating apathy in Alzheimer's disease and further suggest the possibility of improving cognition as well.
We observed a smaller change in AES (mean of 2.5) than observed in our previous trial13 (mean of 3.3); because we based our power estimate on these prior observations, ADMET was underpowered to test the hypothesis of improvement in AES. Our previous trial13 used a single site crossover design, resulting in an expected lower variance in change in outcome scores (unadjusted AES change scores-estimate [SD] in the multisite ADMET trial: methylphenidate = −2.0 [8.6], placebo = 0.4 [6.9] versus the prior study's unadjusted AES change scores–estimate [SD]: methylphenidate = −2.3 [5.1], placebo = 0.5 [3.9]). Despite these differences in study design, the results of the 2 trials are quite similar. Additionally, we observed a discordance between the modest methylphenidate-placebo differences on AES and the more robust differences on CGI-C. Placebo effect seems unlikely since 93% of placebo participants were rated as unchanged or minimally improved on the CGI-C, suggesting that the measures have differential sensitivity to apathy outcomes.
ADMET is one of the first studies that identified and recruited Alzheimer's disease participants with apathy in the absence of a major depressive episode. These results therefore support the concept that apathy can be classified as a distinct behavioral syndrome in Alzheimer's disease. It was interesting to observe that while only 17% of subjects reported a previous history of major depressive disorder, over one third of them were taking SSRIs at baseline. This suggests that antidepressants were possibly prescribed for apathy, rather than major depression, despite lack of evidence for their efficacy25 and evidence that they may even exacerbate apathy in this population.26,27
Strengths of the study include (1) randomized, double-blind, placebo-controlled design; (2) high levels of adherence; (3) low proportion of missing data; and (4) a multicenter trial, with consistent results across sites supporting the generalizability of the results. Limitations of the study include (1) small sample size; (2) limited set of outcome measures; (3) short duration of follow up; and (4) assessment of only 1 target dose (20 mg daily based on safety and efficacy noted in the pilot, crossover trial). Given the report of blunted response to methylphenidate challenge in Alzheimer's disease,12 it is possible that higher doses might have been associated with improved outcomes.
The results of ADMET suggest that methylphenidate may be a safe and effective treatment for apathy in Alzheimer's disease and is possibly associated with cognitive improvements as well. Given the consistent results with relatively small sample sizes of both our current and the previous trial, a more definitive trial is warranted.
Clinical Points
-
■
Apathy in Alzheimer's disease is common and adds to disability and caregiver burden.
-
■
Methylphenidate treatment of apathy in Alzheimer's disease may improve symptoms with a benign safety profile.
Acknowledgments
Funding/support: National Institute on Aging (R01 AG033032-01 and 1 K08 AG029157-01A1).
Trial Registration: ClinicalTrials.gov identifier: NCT01117181
Potential conflicts of interest: Dr Rosenberg has received grant/research support from Merck, Eli Lilly, Janssen, Pfizer, NIA, and AFAR and has participated in speakers/advisory boards for Janssen and Pfizer. Drs Mintzer, Lanctôt, Drye, Herrmann, Scherer, and Bachman report no additional financial or other relationships relevant to the subject of this article.
Role of sponsor: The sponsor (National Institute on Aging) had no role in data collection, analysis, or drafting of the manuscript.
Footnotes
Author contributions: Drs Rosenberg and Lanctôt are joint primary authors. All authors contributed equally to study design, data collection, data interpretation, and drafting the manuscript. Dr Drye performed the data analyses. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Previous presentation: Previously presented at the Alzheimer's Association International Conference; July 14–19, 2012; Vancouver, Canada.
Drug names: memantine (Namenda), methylphenidate (Focalin, Daytrana, and others), modafinil (Provigil and others), trazodone (Oleptro and others).
REFERENCES
- 1.Alzheimer's Association 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg M, Shao H, Zandi P, et al. Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- 4.Hurt C, Bhattacharyya S, Burns A, et al. Patient and caregiver perspectives of quality of life in dementia: an investigation of the relationship to behavioural and psychological symptoms in dementia. Dement Geriatr Cogn Disord. 2008;26(2):138–146. doi: 10.1159/000149584. [DOI] [PubMed] [Google Scholar]
- 5.León-Salas B, Logsdon RG, Olazarán J, et al. The Msu-Adru. Psychometric properties of the Spanish QoL-AD with institutionalized dementia patients and their family caregivers in Spain. Aging Ment Health. 2011;15(6):775–783. doi: 10.1080/13607863.2011.562183. [DOI] [PubMed] [Google Scholar]
- 6.Boyle PA, Malloy PF, Salloway S, et al. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):214–221. [PubMed] [Google Scholar]
- 7.Vilalta-Franch J, Calvó-Perxas L, Garre-Olmo J, et al. Apathy syndrome in Alzheimer's disease epidemiology: prevalence, incidence, persistence, and risk and mortality factors. J Alzheimers Dis. doi: 10.3233/JAD-2012-120913. [published online ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Banerjee S, Murray J, Foley B, et al. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry. 2003;74(9):1315–1316. doi: 10.1136/jnnp.74.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrmann N, Lanctôt KL, Sambrook R, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry. 2006;21(10):972–976. doi: 10.1002/gps.1594. [DOI] [PubMed] [Google Scholar]
- 10.David R, Koulibaly M, Benoit M, et al. Striatal dopamine transporter levels correlate with apathy in neurodegenerative diseases: A SPECT study with partial volume effect correction. Clin Neurol Neurosurg. 2008;110(1):19–24. doi: 10.1016/j.clineuro.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell RA, Herrmann N, Lanctôt KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer's disease. CNS Neurosci Ther. 2011;17(5):411–427. doi: 10.1111/j.1755-5949.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanctôt KL, Herrmann N, Black SE, et al. Apathy associated with Alzheimer disease: use of dextroamphetamine challenge. Am J Geriatr Psychiatry. 2008;16(7):551–557. doi: 10.1097/JGP.0b013e318170a6d1. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann N, Rothenburg LS, Black SE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296–301. doi: 10.1097/JCP.0b013e318172b479. [DOI] [PubMed] [Google Scholar]
- 14.Frakey LL, Salloway S, Buelow M, et al. A randomized, double-blind, placebo-controlled trial of modafinil for the treatment of apathy in individuals with mild-to-moderate Alzheimer's disease. J Clin Psychiatry. 2012;73(6):796–801. doi: 10.4088/JCP.10m06708. [DOI] [PubMed] [Google Scholar]
- 15.Drye LT, Scherer RW, Lanctôt KL, et al. the ADMET Research Group Designing a trial to evaluate potential treatments for apathy in dementia: The Apathy in Dementia Methylphenidate Trial (ADMET) Am J Geriatr Psychiatry. doi: 10.1016/j.jagp.2012.12.018. [published online ahead of print April 4, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 19.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38(2):143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 20.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change: the Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 21.Martin BK, Frangakis CE, Rosenberg PB, et al. Design of Depression in Alzheimer's Disease Study-2. Am J Geriatr Psychiatry. 2006;14(11):920–930. doi: 10.1097/01.JGP.0000240977.71305.ee. [DOI] [PubMed] [Google Scholar]
- 22.Drye LT, Ismail Z, Porsteinsson AP, et al. CitAD Research Group. Citalopram for agitation in Alzheimer's disease: design and methods. Alzheimers Dement. 2012;8(2):121–130. doi: 10.1016/j.jalz.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg PB, Drye LT, Martin BK, et al. DIADS-2 Research Group. Sertraline for the treatment of depression in Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):136–145. doi: 10.1097/JGP.0b013e3181c796eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003;60(7):737–746. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- 26.Wongpakaran N, van Reekum R, Wongpakaran T, et al. Selective serotonin reuptake inhibitor use associates with apathy among depressed elderly: a case-control study. Ann Gen Psychiatry. 2007;6(1):7. doi: 10.1186/1744-859X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract. 2004;10(3):196–199. doi: 10.1097/00131746-200405000-00010. [DOI] [PubMed] [Google Scholar]