Abstract
Importance
People with substance dependence have health consequences, high healthcare utilization and frequent comorbidity but often receive poor quality care overall and for dependence. Chronic care management has been proposed as an approach to improve care and outcomes.
Objective
To determine whether chronic care management (CCM) for alcohol and other drug (AOD) dependence improves substance use outcomes compared to usual primary care.
Design, Setting, and Participants
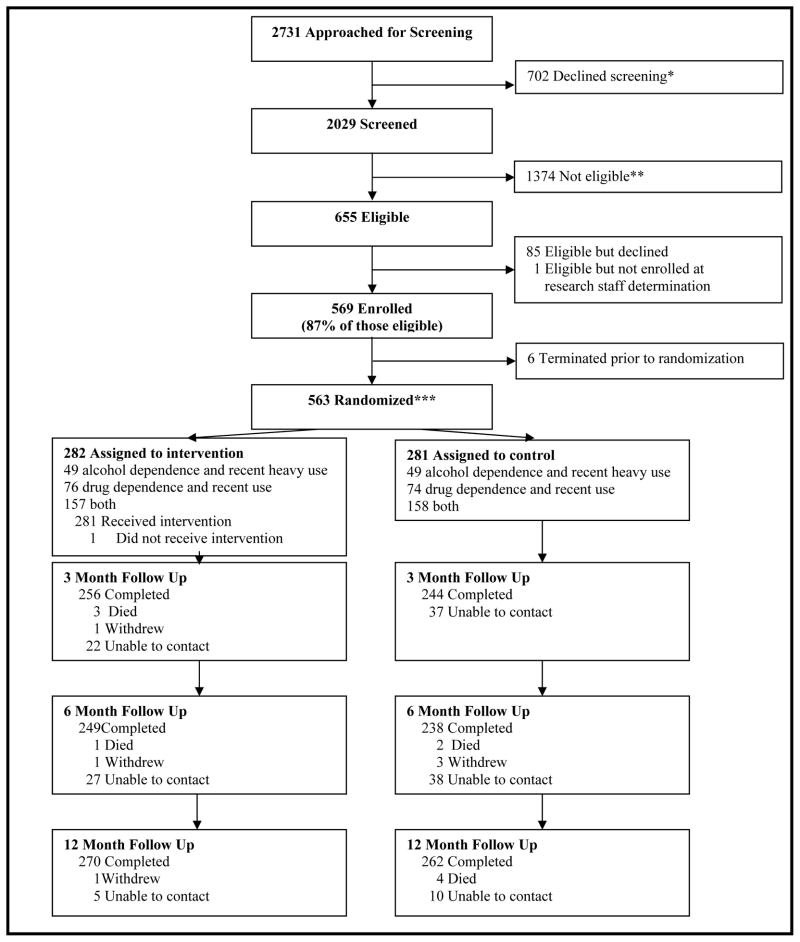
The AHEAD study was a randomized trial in people with AOD dependence, not necessarily seeking treatment, at a Boston hospital-based primary care practice. Of the 655 eligible participants, 563 (86%) were randomized. Study participants were recruited from September 2006 to September 2008 from a free-standing residential detoxification unit (74%) and referrals from an urban teaching hospital and advertisements (26%). Participants were randomized to CCM (n=282) or no CCM (n=281).
Intervention
CCM included longitudinal care coordinated with a primary care clinician, motivational enhancement therapy, relapse prevention counseling, and on-site medical, addiction and psychiatric treatment, social work assistance and referrals (including mutual help). The no CCM group received a primary care appointment, and a list of treatment resources including a phone number to arrange counseling.
Main Outcome and Measure
The primary outcome was self-reported abstinence from opioids, stimulants or heavy drinking. Biomarkers were secondary outcomes. We employed longitudinal analyses for data from 3, 6 and 12 months (last interview January 21, 2010).
Results
Of 563 participants, 95% completed 12-month follow-up. Baseline characteristics of the study participants were similar across randomization groups, but differed significantly for race and depressive symptoms. There was no significant difference in abstinence from opioids, stimulants or heavy drinking between the CCM (44%) and control (42%) groups (adjusted odds ratio 0.84; 95% confidence interval (CI) 0.65–1.10; p=0.21). No significant differences were found for secondary outcomes: addiction severity, health-related quality of life or drug problems. No subgroup effects were found except among those with alcohol dependence in whom CCM was associated with fewer alcohol problems (mean 10 vs. 13, incidence rate ratio 0.85, 95% CI 0.72–1.00, p=0.048).
Conclusions and Relevance
Among persons with AOD dependence, CCM compared with a primary care appointment but no CCM did not increase self-reported abstinence over 12 months. Whether more intensive or longer duration CCM is effective would require further investigation.
INTRODUCTION
Alcohol and other drug dependence can be chronic diseases but they are usually treated episodically.1 Few seek treatment,2 and most who do don’t complete it.3 Barriers to care range from impaired motivation to seek help, to healthcare organizational impediments, including poor coordination of care for common co-occurring conditions.4,5
Treatments for substance dependence, particularly longitudinal ones, have efficacy.6 Although primary care settings are designed to address most health care needs with longitudinal, comprehensive and coordinated care, and are therefore logical settings in which to manage chronic illness like addiction, they have not adequately addressed substance dependence.5 The main approach to care – referral to addiction treatment programs – has been unsuccessful largely because patients do not go to them.4
Chronic care management (CCM) has efficacy for chronic medical and mental health conditions.7–12 Current health care reform approaches to improving care quality and lowering costs for patients with chronic illness have turned to CCM as a solution.13,14 The focus for implementation has been the primary care patient-centered medical home.15 CCM is multidisciplinary patient-centered pro-active care, a way to organize services that provides coordination and expertise, and has been effective for depression, medical illnesses, and tobacco dependence (a substance use disorder).9–12 Trials of integrated medical and addiction care have shown some success and suggest CCM has potential for addiction,16–19 particularly since care elements long known to be effective for addiction overlap with CCM approaches. We have made the case for why it should be implemented in primary care and be effective,7 but no large randomized trials have been published testing the effectiveness of CCM in primary care for substance dependence.18
METHODS
Study design
The Addiction Health Evaluation And Disease management (AHEAD) study was a randomized controlled trial comparing the effect of CCM versus usual primary care for patients with alcohol or drug dependence. The study was originally designed as two—a study of CCM for alcohol dependence, and a study of CCM for drug dependence. For efficiency in implementation and to maximize power, the studies were implemented as one, enrolling participants with alcohol or drug dependence.
Participants
Study participants were recruited from September 2006 to September 2008 from a free-standing residential detoxification unit (74%) and referrals from an urban teaching hospital and advertisements (26%).
Inclusion criteria were: (1) age 18 years or older; (2) alcohol dependence (determined by the Composite International Diagnostic Interview-Short Form [CIDI-SF])20 and heavy drinking in the past 30 days (for men, ≥5 drinks (13.7 grams of ethanol each) on one occasion at least twice or ≥22 drinks per week in an average week; ≥4 and ≥15, respectively, for women) or CIDI-SF diagnosis of drug dependence and past 30-day use of psychostimulants (cocaine, methamphetamine or prescription amphetamine misuse), heroin or prescription opioid misuse (with misuse defined as use without a prescription, in larger amounts than prescribed, or for a longer period of time than prescribed); and (3) willingness to continue or establish primary care at an urban hospital-based practice. Exclusion criteria were: (1) inability to be interviewed due to acute illness; (2) breath alcohol ≥100 mg/dL (Alco-sensor IV Breathalyzer (Intoximeter, Inc. St. Louis, MO); (3) inability to provide contact information for 2 persons; (4) lack of fluency in English or Spanish; (5) cognitive impairment (score of less than 21 of 30 on the Mini-Mental State Examination (MMSE);21 and (6) pregnancy.
Participants provided written informed consent and received compensation. The Institutional Review Board of Boston University Medical Campus and Boston Medical Center approved the study including follow-up of incarcerated participants, and we obtained a Certificate of Confidentiality from the National Institutes of Health. Subjects were compensated upon completion of study procedures (not for any clinical visits) ($35 at baseline, $50 at 3-month, $50 at 6-month, and $75 at 12-month research contacts), and $2 each time they updated their contact information. Subjects were offered a meal and reimbursement for transportation at each study visit.
Assessment at baseline
The baseline interview assessed: demographics (including race/ethnicity by self-report), 30-day Timeline Follow-back for alcohol use,22 Addiction Severity Index (ASI, range 0–1, where 1 is greatest severity),23 Short Inventory of Problems (SIP-2L, range 0–45, where higher score indicates greater problems),24 Short Inventory of Problems - Drugs (SIP-D, range 0–45),25 readiness to change visual analogue scales (1–10, where 10 is greater readiness),26 Short Form Health Survey (SF-12, see below for ranges),27 Patient Health Questionnaire-9 (PHQ-9) (depressive symptoms, range 1–28; ≥10 consistent with depression diagnosis),28 sex and drug risk behaviors (HIV Risk Assessment Battery (RAB; range 1–33; higher scores represent more risk behaviors),29 healthcare utilization,30 and medical comorbidity (any vs. none).31 To encourage truth telling and discourage enrollment of those ineligible, participants enrolled outside of the detoxification unit had breath alcohol testing and, if they reported drug dependence and recent use, saliva drug testing (see below).
Randomization
After the baseline assessment and via a central secure website (providing allocation concealment), participants were randomly assigned in a 1:1 ratio to either intervention (CCM) or control (usual primary care) using random permuted blocks of size 6 and 8 stratified by dependence and recent use status (i.e. alcohol dependence, drug dependence, or both).
Intervention (CCM)
CCM for substance dependence was delivered by the AHEAD study clinic located in a primary care clinic. CCM included longitudinal care for substance dependence and related medical and psychiatric comorbidities, and coordination of specialty medical, psychiatric and addiction care with primary medical care as needed, facilitated by a shared electronic health record that had specifically created forms. Clinicians maintained a registry and pro-actively re-engaged patients who missed follow-up for any reason.
The AHEAD Clinic staff was a multidisciplinary team separate from any primary care staff: a nurse care manager (NCM), a social worker, and internists (who did not deliver primary care for these participants) and a psychiatrist with addictions expertise. All clinic staff were on site 2 half-days a week for new and follow-up visits. The NCM and social worker were on site the remaining weekdays; physicians were available for consultation.
Intervention participants were asked to attend two AHEAD clinic visits (90 minutes each), separated by 3–4 days, receiving substance use, psychiatric, medical and social assessments by all 4 clinicians. The main focus of these visits was to engage with participants so they would return for ongoing care. Treatments for addiction and for medical and psychiatric conditions were begun depending on the participant’s diagnoses and readiness/priorities. Clinicians were provided with the CIDI-SF and PHQ-9 results but no other research assessment results. Participants were escorted to their first visit as soon as possible after randomization. Participants were offered 4 sessions of motivational enhancement therapy with a social worker (who used MMSE, SIP and liver enzyme results for patient feedback),32 relapse prevention counseling at every contact by whichever clinician they saw, usually the NCM or social worker (which includes assessment of substance use),33 a primary care appointment, and referral to specialty addiction treatment and mutual help groups, all tailored to clinical needs and patient preferences. Addiction (naltrexone, acamprosate, disulfiram, buprenorphine, referral for methadone) and psycho-pharmacotherapy were offered as appropriate. Continuing care was delivered over the follow-up period, including clinic visits, NCM contacts by phone, facilitated referrals to addiction specialty care, drop-in care, and 24-hour pager access. Because participants had varied diagnoses, severity, priorities and readiness for treatments, care was individualized and there was no set number of visits (which could be counterproductive if required against a participant’s desires). In general, however, it was common for participants to return in a week after the first 2 visits to check on progress, complete paperwork needed for social services, to transition to additional addiction treatment, to begin addiction or psychiatric pharmacotherapy, to receive addiction or mental health counseling. If patients did not appear for visits in a month, the NCM contacted them to re-engage.
Control (usual primary care)
Controls were given a timely appointment with a named primary care physician (PCP) and a list of addiction treatment resources. They had no access to the AHEAD Clinic. They were also given a phone number to access 4 motivational enhancement therapy sessions. The rationale for this access was to have all services available to both groups so the trial would test CCM, not specific clinical interventions, and motivational enhancement therapy was not routinely available outside the study; 9 (3%) control participants had a session.
Participant assessment at follow-up
Assessments were at 3, 6 and 12 months after enrollment, usually in person. The last participant follow-up assessment was on January 21, 2010. At 6 months, carbohydrate-deficient transferrin (%CDT) and gamma-glutamyltransferase (GGT) tests were done, and saliva and hair samples were tested for drugs (saliva for opioids, cocaine, methamphetamines, benzodiazepines and THC by ELISA [Friends Medical Laboratory, Inc.], 1–3 day window;34 hair for opioid and cocaine by ELISA and GC/MS [Psychemedics Corp.], 90-day window).
In the first year of the study, %CDT and GGT were obtained only for those with baseline heavy alcohol use and dependence, and hair and saliva were tested for those with drug use and dependence; thereafter all were tested because it became financially feasible to do so and having data on all subsequent participants was thought to be better than not having it.
Outcomes
The primary outcome was self-reported 30-day abstinence from stimulants, opioids and heavy alcohol use (≥4 13.7g ethanol drinks for women, ≥5 for men in a day) at 3, 6 and 12 months. Stimulant (cocaine, amphetamine) and opioid (heroin, other opioid misuse) use were assessed by the ASI.23 Alcohol use was assessed using the 30-day Timeline Follow-back calendar method.22 Additional outcomes of particular interest were 30-day abstinence from stimulants, opioids, and any alcohol use; alcohol and drug problems (SIP-2L and SIP-D); any hospitalization; and any emergency department visits. Other outcomes were: %CDT ≥1.7, GGT ≥66 IU/L; detection of opioids or cocaine by hair testing and cocaine, opioids or methamphetamine by saliva testing; alcohol and drug addiction severity (ASI); number of heavy drinking days; health-related quality of life mental and physical component summary scales (SF-12 MCS and PCS, range 0–100; 100 represents best health); and addiction treatment utilization (including mutual help group meeting attendance [e.g. Alcoholics Anonymous, Narcotics Anonymous], inpatient or outpatient addiction treatment, and medication for addiction (e.g. buprenorphine, methadone, naltrexone, acamprosate, disulfiram).
Statistical analysis
While longitudinal regression models were used in the analyses, for the purposes of power calculations a simpler setting utilizing a single time point was considered (we anticipated this was conservative as the power for the longitudinal analysis would be higher). It was assumed that 30% of controls would be abstinent at follow-up. This estimate was based on both the literature6,16,17,35 and a previous randomized clinical trial conducted by the authors testing the effectiveness of a multidisciplinary clinic at a detoxification unit.36 It was hypothesized that the proportion in the intervention group with abstinence would be 50% (i.e. an absolute difference of 20% between groups). Allowing for 25% attrition from 320 participants in each of the alcohol and drug dependent subgroups, the study provided 86% power to detect a 20% difference in the proportions with abstinence from drug and heavy alcohol use between randomized arms for each subgroup (2-sided α=0.05), The study was therefore expected to have greater power to detect the same effect size in the full sample. Recruitment did not continue to the originally planned 640 since there were participants who had both alcohol and drug dependence. The combined study exceeded the originally planned sample sizes (and follow-up rates) for each of the separate subsamples (413 with alcohol dependence; 465 with drug dependence).
The primary outcome was analyzed using generalized estimating equation (GEE) logistic regression models adjusting for dependence and recent use status (alcohol, drug or both, the randomization stratification variable) and time. The time-averaged effect of the intervention was the main interest in this study and the results reported in the primary analyses are main effects from models that do not include interaction terms. An independence working correlation was used and empirical standard errors are reported for all GEE analyses. Confirmatory analyses were performed adjusting for race and depressive symptoms, two factors significantly different between groups at baseline. Additional binary outcomes were analyzed using the same approach. For the continuous outcomes MCS and PCS scores, we fit linear mixed effects regression models. Number of heavy drinking days was analyzed using GEE overdispersed Poisson models. For alcohol and drug problems (SIP-alcohol and SIP-drug scores) and for ASI drug and alcohol scores, the distributions were non-normal and appropriate transformations were not found. Therefore SIP-alcohol and SIP-drug scores, non-negative integers, were analyzed using GEE overdispersed Poisson models as the variance exceeded the mean. Confirmatory analyses were also performed using negative binomial regression models and linear mixed effects models, and the results were consistent across all models for both the SIP-alcohol and SIP-drug scores. For ASI drug and alcohol scores, each outcome was categorized into multiple ordered categories and analyzed using GEE proportional odds models. Biological outcomes were analyzed using logistic regression models. All analyses were conducted on an intention-to-treat basis, where study participants were analyzed according to randomized group regardless of whether they received their assigned intervention. Missing data were not imputed, only the observed data were used. However, a sensitivity analysis was conducted using multiple imputation to address missing follow-up data for the primary outcome abstinence from stimulants, opioids, and heavy alcohol use. Baseline variables used in the imputation were dependence and recent use status (alcohol, drug, or both), randomized group, age, sex, and race. A priori defined subgroup analyses for the above outcomes were conducted among those with alcohol dependence and those with drug dependence.
In post-hoc defined subgroups we analyzed intervention effects among baseline opioid and stimulant users in the drug dependent subgroup separately. In analyses of the primary outcome we also tested interactions between the intervention and time, medical comorbidity, substance-abuse related medical comorbidities, intention to change alcohol or drug use, homelessness, MCS, past 3 month addiction treatment, and recruitment site, but there were no meaningful interactions (see eTables 1–4). In an exploratory analysis, we tested the effect of the number of AHEAD Clinic visits using the longitudinal regression models described above. All analyses were conducted using 2-sided tests and a significance level of 0.05. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., NC, USA).
RESULTS
Enrollment and follow-up
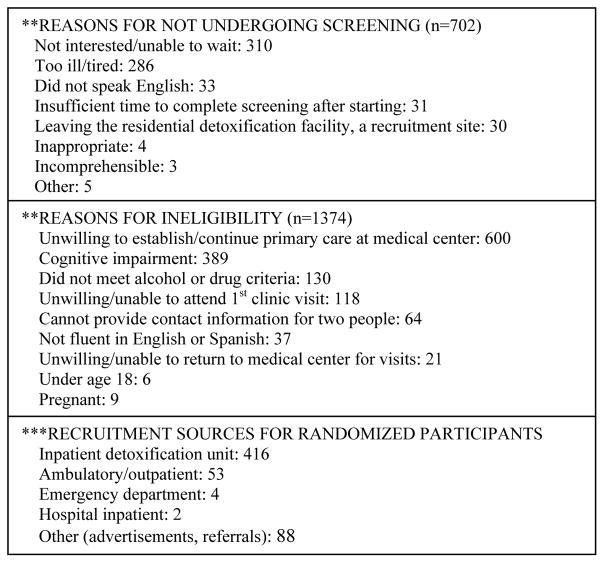
Of the 2,029 people screened, 1,374 were ineligible (Figure 1). Of the 655 eligible participants, 563 (86%) were randomized. At least one follow up interview was conducted for 98% (553/563) of participants (no significant difference between groups). Baseline characteristics of the study participants (Table 1) were similar across randomization groups, but differed significantly for race and depressive symptoms. Both groups improved over time on a number of measures.
Figure 1.
Addiction Health Evaluation And Disease management (AHEAD) study CONSORT (CONsolidated Standards of Reporting Trials) diagram
Table 1.
Characteristics of AHEAD study participants with alcohol or other drug dependence at baseline and 12 months
| BASELINE | 12-MONTH FOLLOW-UP | |||||
|---|---|---|---|---|---|---|
| Characteristic | Overall n=563 |
Intervention n=282 |
Control n=281 |
Overall n=532 |
Intervention n=270 |
Control n=262 |
| Age (mean years (SD)) | 38 (10) | 39 (10) | 38 (11) | -- | -- | -- |
| Female sex (No. (%)) | 154 (27%) | 84 (30%) | 70 (25%) | -- | -- | -- |
| Race/ethnicity (No. (%))* | ||||||
| White | 264 (47%) | 132 (47%) | 132 (47%) | -- | -- | -- |
| Black | 179 (32%) | 93 (33%) | 86 (31%) | -- | -- | -- |
| Hispanic | 75 (13%) | 28 (10%) | 47 (17%) | -- | -- | -- |
| Other | 45 (8%) | 29 (10%) | 16 (5%) | -- | -- | -- |
| Education (No. (%)) | ||||||
| < High school | 133 (24%) | 73 (26%) | 60 (21%) | -- | -- | -- |
| High school | 277 (49%) | 137 (49%) | 140 (50%) | -- | -- | -- |
| Beyond high school | 153 (27%) | 72 (25%) | 81 (29%) | -- | -- | -- |
| Homeless (1+ night in the past 3 months) (No. (%)) | 332 (59%) | 159 (56%) | 173 (62%) | 156 (29%) | 75 (28%) | 81 (31%) |
| Health insurance (No. (%)) | 446 (79%) | 221 (79%) | 225 (80%) | 472 (89%) | 237 (88%) | 235 (90%) |
| Ever incarcerated (No. (%)) | 439 (78%) | 224 (80%) | 215 (77%) | -- | -- | -- |
| Incarcerated in the past 3 months (No. (%)) | 95 (17%) | 55 (19%) | 40 (14%) | 114 (21%) | 59 (22%) | 55 (21%) |
| Depressive symptoms score (PHQ-9) ≥10* (No. (%)) | 465 (83%) | 224 (79%) | 241 (87%) | 222 (42%) | 107 (40%) | 115 (44%) |
| Mental health related quality of life score (mean (SD)) | 30 (10) | 31 (10) | 30 (10) | 39 (10) | 39 (9) | 39 (10) |
| Physical health related quality of life score (mean (SD)) | 42 (8) | 41 (9) | 42 (8) | 43 (8) | 43 (8) | 42 (8) |
| Any medical comorbidity (No. (%)) | 184 (33%) | 103 (37%) | 81 (29%) | -- | -- | -- |
| Dependence (No. (%)) | ||||||
| Alcohol | 68 (12%) | 29 (10%) | 39 (14%) | -- | -- | -- |
| Drug | 129 (23%) | 71 (25%) | 58 (21%) | -- | -- | -- |
| Both | 366 (65%) | 182 (65%) | 184 (65%) | -- | -- | -- |
| Any heroin use, past 30 days (No. (%)) | 335 (60%) | 168 (60%) | 167 (59%) | 118 (22%) | 64 (24%) | 54 (21%) |
| Any cocaine use, past 30 days (No. (%)) | 379 (67%) | 189 (67%) | 190 (68%) | 142 (27%) | 72 (27%) | 70 (27%) |
| Heavy drinking days, past 30 days (median (25th, 75th percentiles)) | 11 (1, 26) | 10 (1, 26) | 13 (1, 26) | 0 (0, 3) | 0 (0, 3) | 0 (0, 3) |
| Readiness to change alcohol use (1–10) (median (25th, 75th)) | 9 (6, 10) | 9 (6, 10) | 9 (6.5, 10) | 4 (0, 9) | 2 (0, 9) | 5 (0, 9) |
| Readiness to change drug use (1–10) (median (25th, 75th)) | 10 (8, 10) | 10 (8, 10) | 10 (8, 10) | 7 (0, 10) | 7 (0, 10) | 7 (0, 10) |
| Alcohol severity index (median (25th, 75th) | 0.5 (0.1, 0.8) | 0.5 (0.1, 0.8) | 0.5 (0.1, 0.8) | 0.2 (0, 0.3) | 0.2 (0, 0.3) | 0.2 (0, 0.4) |
| Drug severity index (median (25th, 75th) | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 0.1 (0, 0.2) | 0.1 (0, 0.2) | 0.1 (0, 0.2) |
| Alcohol related problem score, past 3 months (median (25th, 75th)) | 21 (2, 34) | 20 (1.5, 33) | 22 (3, 35) | 0 (0, 15) | 0 (0, 11.5) | 1 (0, 19) |
| Drug related problem score, past 3 months (median (25th, 75th)) | 33 (21, 40) | 32 (18, 39) | 33 (22, 40) | 5 (0, 26) | 5 (0, 24) | 5 (0, 28) |
| Ever overdosed requiring medical attention (No. (%)) | 169 (30%) | 84 (30%) | 85 (30%) | -- | -- | -- |
| Overdose requiring medical attention, past 3 months (No. (%)) | 42 (7%) | 21 (7%) | 21 (7%) | 13 (2%) | 6 (2%) | 7 (3%) |
| Sex and drug risk behavior score (median (25th, 75th)) | 7 (5, 12) | 7 (5, 11) | 7 (5, 12) | 5 (2, 6) | 5 (2, 6) | 5 (2, 7) |
| Any episode of vaginal or anal sex without a condom, past 3 months (No. (%)) | 309 (59%) | 156 (60%) | 153 (59%) | 233 (49%) | 119 (49%) | 114 (49%) |
| Any sex in exchange for drugs or money, past 3 months (No. (%)) | 191 (34%) | 102 (37%) | 89 (32%) | 92 (18%) | 46 (17%) | 46 (18%) |
| Any injection drug use, past 3 months (No. (%)) | 256 (46%) | 126 (45%) | 130 (47%) | 126 (24%) | 66 (25%) | 60 (23%) |
p < 0.05
AHEAD=Addiction Health Evaluation And Disease management
SD=standard deviation
PHQ-9=9-item Patient Health Questionnaire. A score of 10 or greater is consistent with a diagnosis of major depressive disorder.
Mental health related quality of life score is the score of the 12-item Short Form Health Survey (SF-12) Mental Component Summary (MCS) scale
Physical health related quality of life score is the score of the 12-item Short Form Health Survey (SF-12) Physical Component Summary (PCS) scale
Medical comorbidity determined by the Katz Comorbidity Index (score range 0–8 in this sample; “any”=score >0)
Substance dependence determined using the Composite International Diagnostic Interview (CIDI) Short Form
Drug use was determined using the Addiction Severity Index, alcohol use by the Timeline Follow-back method
Readiness to change assessed by 1–10 visual analogue scale; a higher score represents greater readiness to change
Alcohol and drug related problems assessed by the Short Inventory of Problems (SIP) and SIP-Drug (SIP-D); scores range from 0–45, with higher scores representing more and more frequent problems related to substance use
Sex and drug risk behavior score calculated from the HIV Risk Assessment Battery (RAB); scores range from 1 to 33, with higher scores representing more HIV sex and drug risk behaviors
Receipt of the intervention
Of the 282 subjects assigned to the intervention group, 281 (99.6%) attended at least one CDM clinic visit, 75.9% attended at least two, and 64.5% attended three or more visits (median 6, interquartile range 2–16). Most reported scores consistent with receipt of high quality CCM at 12 months (75% had scores ≥3.3 on a scale adapted to assess addiction CCM, possible range 1–5).37 Most (62%) received ≥1 motivational enhancement therapy sessions, and 27% completed 4 sessions.
Main results
For the primary outcome abstinence from stimulants, opioids, and heavy drinking, there was no significant difference between the AHEAD CCM intervention and the control group (44% vs. 42%, respectively, at 12 months, adjusted odds ratio for intervention vs. control across the 12 month follow-up: 0.84; 95% confidence interval: 0.65–1.10; p=0.21) (Table 2). There were also no significant differences in other outcomes.
Table 2.
Main results: Effects of chronic care management intervention for substance dependence on, favorable addiction status (1-category improvement in addiction severity index), substance use related problems, health related quality of life and acute healthcare utilization1,2
| BASELINE | 12-MONTH FOLLOW-UP | |||||
|---|---|---|---|---|---|---|
| Outcome | Intervention | Control | Intervention | Control | Parameter† and 95% confidence interval | p |
| All participants | n=282 | n=281 | n=270 | n=262 | n=553 | |
| Abstinence from stimulants, opioids, and heavy drinking, past 30 days (No. (%)) | *** | *** | 120 (44%) | 109 (42%) | OR 0.84 (0.65, 1.10) § | 0.21 |
| Abstinence from stimulants, opioids, and any drinking, past 30 days (No. (%)) | *** | *** | 109 (40%) | 95 (36%) | OR 0.89 (0.68, 1.17) § | 0.40 |
| Alcohol Addiction Severity Index score > 0.4* (No. (%)) | 170 (60%) | 175 (63%) | 53 (20%) | 58 (22%) | OR 1.01 (0.78, 1.31) ¥ | 0.93 |
| Drug Addiction Severity Index score >0.2** (No. (%)) | 205 (73%) | 208 (74%) | 58 (21%) | 54 (21%) | OR 0.90 (0.71, 1.14) ¥ | 0.38 |
| Mental health related quality of life (MCS score)(mean (95% CI)) | 30.8 (29.7, 31.9) | 30.0 (28.8, 31.2) | 39.4 (38.3, 40.5) | 39.1 (37.9, 40.3) | β (95% CI) −0.14 (−1.49, 1.21) ¤ | 0.84 |
| Physical health related quality of life (PCS score)(mean (95% CI)) | 41.4 (40.4, 42.4) | 42.0 (41.0, 42.9) | 43.1 (42.2, 44.0) | 42.4 (41.5, 43.4) | β (95% CI) 0.48 (−0.70, 1.66) ¤ | 0.42 |
| Any nights in hospital (medical, psychological, detox) past 3 months (No. (%)) | 76 (27%) | 84 (30%) | 46 (17%) | 39 (15%) | OR 1.07 (0.78, 1.46) § | 0.67 |
| Any days in emergency department past 3 months (No. (%)) | 146 (52%) | 158 (56%) | 81 (30%) | 80 (31%) | OR 0.96 (0.74, 1.23) § | 0.73 |
| Alcohol dependence subgroup | n=206 | n=207 | n=199 | n=195 | n=409 | |
| Abstinence from heavy drinking, past 30 days (No. (%)) | *** | *** | 109 (55%) | 97 (50%) | OR 1.05 (0.78, 1.43) § | 0.74 |
| Number of heavy drinking days in past 30 days (mean (95% CI)) | 17.4 (15.9, 18.8) | 18.6 (17.2, 20.0) | 5.1 (3.8, 6.4) | 5.7 (4.4, 7.1) | IRR 0.95 (0.73, 1.23) # | 0.69 |
| Alcohol related problem score (mean (95% CI)) | 25.1 (23.3, 26.9) | 26.4 (24.7, 28.1) | 10.4 (8.5, 12.3) | 13.1 (11.0, 15.1) | IRR 0.85 (0.72, 1.00) # | 0.048 |
| Alcohol Addiction Severity Index score > 0.4* (No. (%)) | 168 (82%) | 173 (84%) | 53 (27%) | 57 (29%) | OR 1.08 (0.80, 1.45) ¥ | 0.62 |
| Mental health related quality of life (MCS score) (mean (95% CI)) | 31.2 (29.9, 32.5) | 30.0 (28.6, 31.4) | 39.8 (38.5, 41.1) | 38.7 (37.2, 40.1) | β (95% CI) 0.47 (−1.08, 2.02) ¤ | 0.55 |
| Physical health related quality of life (PCS score) (mean (95% CI)) | 41.7 (40.6, 42.9) | 41.6 (40.4, 42.7) | 43.1 (42.1, 44.1) | 42.0 (40.8, 43.2) | β (95% CI) 1.06 (−0.29, 2.40) ¤ | 0.12 |
| Any nights in hospital (medical, psychological, detox) past 3 months (No. (%)) | 59 (29%) | 67 (32%) | 40 (20%) | 35 (18%) | OR 1.06 (0.74, 1.50) § | 0.76 |
| Any days in emergency department past 3 months (No. (%)) | 108 (52%) | 121 (58%) | 61 (31%) | 58 (30%) | OR 1.00 (0.74, 1.35) § | 0.99 |
| Drug dependence subgroup | n=233 | n=232 | n=224 | n=217 | n=458 | |
| Abstinence from stimulants and opioids, past 30 days (No. (%)) | *** | *** | 117 (52%) | 111 (51%) | OR 0.85 (0.64, 1.14) § | 0.28 |
| Drug related problem score (mean (95% CI)) | 32.1 (30.7, 33.4) | 33.3 (32.1, 34.5) | 15.6 (13.6, 17.7) | 16.0 (13.8, 18.1) | IRR 1.03 (0.92, 1.16) # | 0.62 |
| Drug Addiction Severity Index score >0.2** (No. (%)) | 202 (87%) | 204 (88%) | 57 (25%) | 54 (25%) | OR 0.87 (0.67, 1.12) ¥ | 0.27 |
| Mental health related quality of life (MCS score) (mean (95% CI)) | 30.3 (29.1, 31.5) | 29.6 (28.4, 30.8) | 39.1 (37.8, 40.4) | 39.1 (37.8, 40.4) | β (95% CI) −0.65 (−2.14, 0.84) ¤ | 0.39 |
| Physical health related quality of life (PCS score) (mean (95% CI)) | 41.3 (40.2, 42.4) | 42.3 (41.2, 43.3) | 43.3 (42.3, 44.3) | 42.7 (41.7, 43.7) | β (95% CI) 0.23 (−1.04, 1.50) ¤ | 0.72 |
| Any nights in hospital (medical, psychological, detox) past 3 months (No. (%)) | 60 (26%) | 59 (25%) | 38 (17%) | 28 (13%) | OR 1.18 (0.83, 1.66) § | 0.35 |
| Any days in emergency department past 3 months (No. (%)) | 115 (49%) | 129 (56%) | 68 (30%) | 67 (31%) | OR 0.86 (0.66, 1.14) § | 0.30 |
Analyses were adjusted for dependence type (alcohol, drug or both, the randomization stratification variable) and time
ASI=Addiction Severity Index
CI=confidence interval
MCS=Mental Component Summary scale score of the 12-item Short Form Health Survey (SF-12)
PCS=Physical Component Summary scale score of the 12-item Short Form Health Survey (SF-12)
Alcohol and drug related problems assessed by the Short Inventory of Problems (SIP) and SIP-Drug (SIP-D)
Alcohol ASI score >0.4 represents the top two of five ordered categories used for analysis. Odds ratio is for a 1-category improvement (i.e. lower ASI alcohol score) in alcohol addiction severity
Drug ASI score >0.2 represents the top two of five ordered categories used for analysis. Odds ratio is for a 1-category improvement (i.e. lower ASI drug score) in drug addiction severity
All participants reported recent substance use at baseline
OR (odds ratio) and (95% confidence interval, CI) from Generalized Estimating Equations (GEE) logistic regression model
OR (95% CI) from GEE proportional odds regression model predicting a lower addiction severity score category
β (mean difference) between randomized groups (95% CI) from linear mixed effects regression model
IRR (incidence rate ratio) (95% CI) from GEE overdispersed Poisson regression model
Corresponds to the main effect of the intervention in models that do not include interaction terms
Results of analyses adjusting for race and depressive symptoms were similar.
Exploratory analyses suggested an interaction between intervention and time (p=0.05) for the outcome abstinence from drugs and heavy drinking, but not in any hypothesized direction: the intervention group had lower odds of abstinence (drugs and heavy drinking) at 3 months (AOR 0.69, 95% CI 0.48–0.99), but no significant differences were observed at 6 or 12 months. No other outcomes had a statistically significant intervention by time interaction.
In the alcohol and drug dependent subgroups (Table 2), there were no significant differences across time except fewer alcohol problems (SIP) in the intervention group among those with alcohol dependence (mean 10.4 vs. 13.1 at 12 months, incidence rate ratio of 0.85; p=0.048).
In sensitivity analyses of the primary outcome abstinence from drugs and heavy drinking using multiple imputation to account for missing observations, no significant difference was observed for the intervention vs. control groups (OR 0.87, 95% CI 0.71–1.07; p=0.19).
Opioid and stimulant subgroups
Among those with drug dependence and recent use of opioids (n=369), intervention was associated with a lower odds of opioid abstinence across follow-up (OR 0.71 (95% CI 0.51–0.98), 52% vs. 54% at 12 months) but had no effect on days of opioid use (IRR 1.19 (95% CI 0.94–1.52), mean 16.7 vs. 14.0 days for intervention and control, at 12 months respectively, an analysis adjusted for baseline use). Among those with drug dependence and recent use of stimulants (n=364), there were no significant intervention effects on stimulant abstinence (OR 0.77 (95% CI 0.56–1.07), 51% vs. 55% at 12 months) or days of stimulant use (IRR 1.05 (95% CI 0.81–1.37), mean 11.0 vs. 12.4 days at 12 months, an analysis adjusted for baseline use).
Biological tests
All biomarker analyses (hair and saliva drug tests, %CDT and GGT at 6 months) showed similar nonsignificant results. These included subgroup analyses by substance dependence as well as separate analyses of baseline opioid and stimulant users in the drug dependent sample). In the full sample, ORs for the association between intervention and a negative test were 1.20 (95% CI 0.76–1.90; n=417, 30% intervention vs. 27% control) for hair, 1.07 (95% CI 0.70–1.62; n=491, 74% intervention vs. 73% control) for saliva, 1.27 (95% CI 0.77–2.08; n=420, 80% intervention vs. 78%) for %CDT, and 0.92 (95% CI 0.54–1.54; n=428, 83% intervention vs. 85%) for GGT.
Addiction treatment utilization
The intervention was significantly associated with greater receipt of addiction treatment and addiction medication but not mutual help group attendance (Table 3).
Table 3.
Effects of chronic care management intervention for substance dependence on mutual help meeting attendance and addiction treatment utilization
| BASELINE | 12-MONTH FOLLOW-UP | |||||
|---|---|---|---|---|---|---|
| Outcome | Intervention | Control | Intervention | Control | Odds ratio† (95% confidence interval) | p |
| All participants | n=282 | n=281 | n=270 | n=262 | n=553 | |
| Any mutual help meeting attendance (No. (%)) | 136 (48%) | 133 (48%) | 147 (54%) | 147 (56%) | 0.91 (0.69, 1.19) § | 0.49 |
| Any addiction treatment (No. (%)) | 95 (34%) | 111 (40%) | 132 (49%) | 116 (44%) | 1.41 (1.09, 1.83) § | 0.01 |
| Any inpatient addiction treatment (No. (%)) | 60 (21%) | 70 (25%) | 49 (18%) | 48 (18%) | 0.97 (0.73, 1.30) § | 0.86 |
| Any addiction medications (No. (%)) | 13 (5%) | 20 (7%) | 58 (21%) | 39 (15%) | 1.88 (1.28, 2.75) § | 0.001 |
| Alcohol dependence subgroup | n=206 | n=207 | n=199 | n=195 | n=409 | |
| Any mutual help meeting attendance (No. (%)) | 103 (50%) | 94 (46%) | 106 (53%) | 103 (53%) | 1.04 (0.76, 1.44) § | 0.79 |
| Any addiction treatment (No. (%)) | 68 (33%) | 82 (40%) | 86 (43%) | 81 (42%) | 1.36 (1.01, 1.84) § | 0.04 |
| Any inpatient addiction treatment (No. (%)) | 42 (20%) | 53 (26%) | 33 (17%) | 37 (19%) | 1.00 (0.71, 1.41) § | 1.00 |
| Any addiction medications (No. (%)) | 8 (4%) | 16 (8%) | 32 (16%) | 19 (10%) | 2.12 (1.29, 3.48) § | 0.002 |
| Drug dependence subgroup | n=233 | n=232 | n=224 | n=217 | n=458 | |
| Any mutual help meeting attendance (No. (%)) | 114 (49%) | 113 (49%) | 126 (56%) | 126 (58%) | 0.91 (0.68, 1.22) § | 0.53 |
| Any addiction treatment (No. (%)) | 81 (35%) | 96 (41%) | 119 (53%) | 99 (46%) | 1.47 (1.10, 1.96) § | 0.008 |
| Any inpatient addiction treatment (No. (%)) | 54 (23%) | 60 (26%) | 43 (19%) | 43 (20%) | 0.96 (0.70, 1.31) § | 0.79 |
| Any addiction medications (No. (%)) | 11 (5%) | 15 (6%) | 54 (24%) | 36 (17%) | 1.97 (1.30, 3.00) § | 0.001 |
Outcomes over the past 3 months were assessed at 3, 6 and 12 month follow-up
Corresponds to the main effect of the intervention in models that do not include interaction terms
from GEE Logistic Model
Exposure to intervention
AHEAD clinic visit exposure was significantly associated with the secondary abstinence outcome (less with 1–2 vs. 0 visits, more with ≥3 vs. 1–2 visits) but not other outcomes (Table 4).
Table 4.
Odds ratios for abstinence and favorable addiction status (1-category improvement in addiction severity index) in relation to number of visits to AHEAD chronic care management for substance dependence clinic (among the full sample, n=553)
| Outcome | No. (%) at 12 months | Odds ratio† (95% confidence interval) | p |
|---|---|---|---|
|
| |||
| Abstinence from stimulants, opioids, and heavy drinking (No. (%)) | |||
| 0 visits (reference group) | 109 (42%) | 1.00 | 0.07 |
| 1–2 visits | 39 (41%) | 0.64 (0.43, 0.96) § | |
| ≥3 visits | 81 (46%) | 0.99 (0.73, 1.34) § | |
|
| |||
| Abstinence from stimulants, opioids, and any drinking (No. (%)) | |||
| 0 visits (reference group) | 95 (36%) | 1.00 | 0.03 |
| 1–2 visits | 33 (35%) | 0.62 (0.40, 0.95) §§ | |
| ≥3 visits | 76 (43%) | 1.08 (0.79, 1.46) §§ | |
|
| |||
| Alcohol Addiction Severity Index score > 0.4* (No. (%)) | |||
| 0 visits (reference group) | 58 (22%) | 1.00 | 0.23 |
| 1–2 visits | 23 (24%) | 0.76 (0.52, 1.09) ¥ | |
| ≥3 visits | 30 (17%) | 1.07 (0.81, 1.42) ¥ | |
|
| |||
| Drug Addiction Severity Index score >0.2** (No. (%)) | |||
| 0 visits (reference group) | 54 (21%) | 1.00 | 0.33 |
| 1–2 visits | 22 (23%) | 0.76 (0.53, 1.10) ¥ | |
| ≥3 visits | 36 (20%) | 0.90 (0.70, 1.17) ¥ | |
AHEAD=Addiction Health Evaluation And Disease management
Outcomes over the past 30 days were assessed at 3, 6 and 12 month follow-up
The number of participants with 0 visits at 12 months was 262, 1–2 visits 94, 3+visits 176
ASI=Addiction Severity Index
Corresponds to the main effect of the intervention in models that do not include interaction terms
OR (odds ratio) (95% confidence interval, CI) from Generalized Estimating Equation (GEE) logistic regression model adjusting for time point, age, sex, race, homelessness, alcohol and drug related problems, physical health related quality of life and the 9-item Patient Health Questionnaire (depressive symptoms). For ≥ 3 visits versus 1–2 visits, the OR was 1.54 (95% CI 1.00, 2.38).
OR (odds ratio) (95% confidence interval, CI) from Generalized Estimating Equation (GEE) logistic regression model adjusting for time point, age, sex, race, homelessness, alcohol and drug related problems, physical health related quality of life and the 9-item Patient Health Questionnaire (depressive symptoms). For ≥ 3 visits versus 1–2 visits, the OR was 1.74 (95% CI 1.12, 2.71).
OR (95% CI) from GEE proportional odds regression model adjusting for the same covariates predicting a lower addiction severity score category
Alcohol ASI score >0.4 represents the top two of five ordered categories used for analysis. Odds ratio is for a 1-category improvement (i.e. lower ASI alcohol score) in alcohol addiction severity
Drug ASI score >0.2 represents the top two of five ordered categories used for analysis. Odds ratio is for a 1-category improvement (i.e. lower ASI drug score) in drug addiction severity
DISCUSSION
This study did not find an effect of chronic care management (CCM) for substance dependence on substance use, related consequences (with the exception of a small effect on alcohol problems among those with dependence), health-related quality of life, or acute healthcare utilization.
CCM has demonstrated efficacy for many medical and mental health conditions. CCM should work for substance dependence because it can help overcome system and individual barriers to care (e.g. uncoordinated services in separate locations and systems; impaired motivation to seek help; mental and physical comorbidites). Components of CCM have been effective for addictions (e.g. case management, co-location, and integration of care),7 but CCM for addiction in primary care has not been tested in a randomized trial.18 Willenbring et al. demonstrated efficacy (abstinence, mortality) of co-location of care for medically ill veterans with alcoholism in a special alcohol clinic.16 Weisner et al. demonstrated efficacy (abstinence) of delivering primary medical care at an addictions treatment program for a subgroup of patients with substance abuse-related medical conditions.17 In a secondary analysis at 5 years, integrated care was associated with abstinence or use without problems in the whole sample.38
CCM has been described as including six elements all of which are represented in the AHEAD clinic and are elements in which staff were trained: use of community resources, making the chronic illness and its management the priority, self-management support, delivery system design, decision support, and use of clinical information systems.7–10,39 The social worker addressed or connected patients to community services to assist with legal, social and financial needs. She and the NCM connected patients to addiction treatment and mutual help groups in the community with the ability for “warm handoffs” by knowing individuals who work in or go to those resources. Substance dependence was the focus of the clinic, as documented by specific care plans. Self-management was encouraged by provision of routine assessment and feedback. With psychosocial support from clinic staff, patients were encouraged to participate in their care. Motivational interviewing was used routinely emphasizing the patient’s role. CCM provided on-site services with connections to off-site services, use of patient reminders and planned visits, and multidisciplinary collaboration of team members. Decision support was available through easily accessible expert clinician consultation. Information systems were used to communicate with primary care physicians (outside the AHEAD clinic), for a standard visit template, for a registry function to track patients to encourage follow-up and to track treatments, and to monitor outcomes (e.g. substance use). The elements of CCM could be implemented differently or to a greater extent but our and our clinicians’ experience suggest that we implemented all of the components. Participant reports were consistent with delivery of high quality CCM.37 Nonetheless, future studies could test other ways of implementing CCM for addiction that might have efficacy. For example, self-management and outcome monitoring could be bolstered by routine biomarker testing, or visit schedules could be more prescriptive, or specific care pathways more detailed. Future studies should also consider the possibility that CCM is simply insufficient, and that more intensive recovery support in the community needs to be added.
Our study, however, suggests that CCM for substance dependence in primary care is not effective, at least not as implemented in this study and population. Several explanations should be considered for these unexpected findings. First, substance dependence treatment has limited efficacy; it may be difficult to detect effects of better delivery of existing treatments. Pharmacotherapy efficacy is varied—it is highly effective for opioid dependence,40 but for alcohol dependence it yields absolute risk differences for heavy drinking and abstinence of 8–11%,35 and it has no efficacy for stimulants. Psychosocial treatments have efficacy though these too are varied, and most studies lack no-treatment control groups.6 Combination psychotherapy yielded a 6% absolute risk improvement in percent days abstinent compared with medical counseling.41 Weiss et al. found no detectable benefit of drug counseling over standard medical management of buprenorphine-naloxone.42 CCM in our study did increase receipt of addiction treatment (by 7–10%) but likely this was insufficient. We believe that the small increase in use of addiction treatments, that are modestly efficacious for only some subsets of people with addictions, and limited delivery of evidence-based practices for addiction in the community were likely the main reasons for our findings.
Second, although adherence to treatment is a problem for all with chronic illnesses, it is particularly important for those with addictions. Most people with addictions do not seek help in the first place.2 Even when they do, their substance use directly affects their motivation and ability to adhere to care. Third, many people with addictions have co-occurring mental health conditions and substantial social problems. Although CCM is designed to address complex problems, it may simply not be enough to overcome the impaired motivation and myriad severe consequences experienced by patients with addictions.
Methodological considerations might also explain the findings. Most study participants were dependent on both alcohol and other drugs, recruited from a detoxification unit, had substantial mental health symptoms, had recently been homeless and were not necessarily seeking addiction treatment (despite relatively high reported readiness to change their use). The findings may not apply to addiction treatment seeking or less severely affected populations or to populations recruited elsewhere. Although plausible, our analyses found no impact on intervention efficacy of any of these factors. Furthermore, studies of CCM for other conditions have selected severely affected patients with comorbidity and social needs, because they are the ones who need the services and could benefit, and these studies have found efficacy.11 Among people with addictions seeking treatment, favorable outcomes are already good without CCM (e.g. 74% no heavy drinking or problems with alcoholism pharmacotherapy).41 The need for what CCM offers is greatest for those with severe complex problems who are not the easiest to engage in care.
As with prior trials,16,17 we assessed main outcomes by self-report. Biological tests are inadequate for detecting substance use particularly when it is not recent. Substance problems and health-related quality of life are best assessed by self-report. We used validated tools, assured participants of confidentiality, and corroborated main results with biological tests (informing participants of testing) and a range of other outcomes, all of which were consistent.
Low intervention potency seems an unlikely explanation for the results. We implemented all elements of previously successful CCM, trained experienced staff for the study, and provided systems support and ample availability for patients. Uninsurance was not a barrier. Intervention participants had on average 6 CCM visits and reported high quality CCM, and the intervention increased exposure to addiction treatment and pharmacotherapy.
Assessment effects, the list of resources, primary care appointment or the 3% of controls who received 1 or more motivational enhancement counseling sessions could have biased the study to the null. However, those minimal control group exposures and relatively less intense assessments of 6 hours over a year (compared to longer ones in positive alcohol treatment trials) are unlikely to have had major impact on a severely affected group.41 Of note, the whole group improved over time, change most likely due to many participants having been enrolled at a detoxification unit, when they were at a more severe point in their addiction and sought some help (a logical time to offer CCM). Assessment effects in treatment trials are inconsistent and poorly understood43 and often absent in studies of people not seeking treatment.44 Contamination is also an unlikely explanation of our findings since controls had no access to addictions CCM in the study or elsewhere.
Chronic care management (CCM) for substance dependence had a small effect on problems among those with alcohol dependence but was ineffective for improving substance use, related clinical outcomes or healthcare utilization. Providing more intensive or longer duration CCM might be effective, or it might be effective for less severe primary care patients or small subgroups of patients with low severity and few comorbidities or social problems who are eager to enter addictions care. It is also possible that the effects of CCM for addictions will not be seen until the health system in which it is implemented is more supportive of integrated care.
Current health care reforms in the US include a focus on CCM as a solution in patient-centered medical homes to reduce chronic disease burden, and to reduce costs (both of which are among the highest for those with addictions), in part because numerous studies have found such benefits for medical and mental health conditions.14 The model is being widely disseminated in primary care settings by private and government health plans, healthcare delivery organizations and health policy leaders anticipating accountable care organizations and new support for CCM elements. Leading national centers on both CCM13 and integrated care (www.samhsa.integration.gov) are expanding the model to address substance disorders. In the absence of randomized trials for substance dependent patients, benefits of CCM are being anticipated and implementation is proceeding. Our findings at least raise the possibility that not all chronic diseases are the same, and that CCM may not have the same impact across conditions for which complexity varies, a possibility that should be part of the conversation when models of care are implemented widely. Even though CCM is effective for a number of chronic conditions, it may be premature to assume that CCM will be the solution to improve the quality of care for and reduce costs of patients with addiction. Further research is warranted to determine whether more intensive or longer duration CCM, or CCM designed differently might do so. But in this trial of persons with AOD dependence, CCM compared to a primary care appointment but no CCM, did not decrease use or overall addiction consequences.
Supplementary Material
Acknowledgments
The AHEAD study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse; R01s AA010870 and DA010019. The NIH did not contribute to the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Dr. Cheng reports having served on Data Monitoring Committees for Johnson & Johnson/Janssen. All authors have contributed significantly to claim authorship, and have seen and approved of the manuscript and none of the authors have relevant conflicts of interest to declare. Dr. Saitz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The study protocol can be obtained from Dr. Saitz.
Footnotes
Trial Registration: The AHEAD study was registered at ClinicalTrials.gov. Registration number: NCT00278447. URL: http://clinicaltrials.gov/ct2/show/NCT00278447.
References
- 1.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 2.Green-Hennessy S. Factors associated with receipt of behavioral health services among persons with substance dependence. Psychiatr Serv. 2002;53(12):1592–1598. doi: 10.1176/appi.ps.53.12.1592. [DOI] [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. NSDUH series H-32, DHHS publication no. SMA 07-4293. Office of Applied Studies; 2007. Results from the 2006 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- 4.Samet JH, Friedmann P, Saitz R. Benefits of linking primary medical care and substance abuse services: patient, provider, and societal perspectives. Arch Intern Med. 2001;161(1):85–91. doi: 10.1001/archinte.161.1.85. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine (US) Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders. Improving the Quality of Health Care for Mental and Substance-use Conditions: Quality Chasm Series. Washington, D.C: The National Academies Press; 2006. Available from: http://www.ncbi.nlm.nih.gov/books/NBK19830/ [PubMed] [Google Scholar]
- 6.Raistrick D, Heather N, Godfrey C. [Accessed February 5, 2013.];Review of the effectiveness of treatment for alcohol problems. http://www.nta.nhs.uk/uploads/nta_review_of_the_effectiveness_of_treatment_for_alcohol_problems_fullreport_2006_alcohol2.pdf. Updated 2006.
- 7.Saitz R, Larson MJ, Labelle C, Richardson J, Samet JH. The case for chronic disease management for addiction. J Addict Med. 2008;2(2):55–65. doi: 10.1097/ADM.0b013e318166af74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q. 1996;4(2):12–25. [PubMed] [Google Scholar]
- 9.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 10.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 11.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph AM, Fu SS, Lindgren B, et al. Chronic disease management for tobacco dependence: a randomized, controlled trial. Arch Intern Med. 2011;171(21):1894–1900. doi: 10.1001/archinternmed.2011.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katon W. Health reform, research pave way for collaborative care for mental illness. Interview by Bridge M. Kuehn. JAMA. 2013;309(23):2425–2426. doi: 10.1001/jama.2013.6494. [DOI] [PubMed] [Google Scholar]
- 14.Laiteerapong N, Huang ES. Health care reform and chronic diseases: anticipating the health consequences. JAMA. 2010;304(8):899–900. doi: 10.1001/jama.2010.1209. [DOI] [PubMed] [Google Scholar]
- 15.Bindman AB, Blum JD, Kronick R. Medicare payment for chronic care delivered in a patient-centered medical home. JAMA. 2013 doi: 10.1001/jama.2013.276525. In press. [DOI] [PubMed] [Google Scholar]
- 16.Willenbring ML, Olson DH. A randomized trial of integrated outpatient treatment for medically ill alcoholic men. Arch Intern Med. 1999;159(16):1946–1952. doi: 10.1001/archinte.159.16.1946. [DOI] [PubMed] [Google Scholar]
- 17.Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary medical care with addiction treatment: a randomized controlled trial. JAMA. 2001;286(14):1715–1723. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler M, Kane RL, McAlpine D, et al. Integration of Mental Health/Substance Abuse and Primary Care No. 173 (prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-02-0009.). AHRQ Publication No. 09-E003. Rockville, MD: Agency for Healthcare Research and Quality; Oct, 2008. [Google Scholar]
- 19.Oslin DW, Grantham S, Coakley E, et al. PRISM-E: Comparison of integrated care and enhanced specialty referral in managing at-risk alcohol use. Psychiatr Serv. 2006;57(7):954–958. doi: 10.1176/appi.ps.57.7.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen HU. The World Health Organization Composite International Diagnostic Interview Short-Form (CIDI-SF) Int J Method Psych. 1998;7(4):171–185. [Google Scholar]
- 21.Smith KL, Horton NJ, Saitz R, Samet JH. The use of the mini-mental state examination in recruitment for substance abuse research studies. Drug Alcohol Depend. 2006;82(3):231–237. doi: 10.1016/j.drugalcdep.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Sobell LC, Sobell MB. Timeline Follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ, US: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 23.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 24.Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrInC): an instrument for assessing adverse consequences of alcohol abuse. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. Project MATCH Monograph Series, Vol. 4. DHHS Publication No. 95-3911. [Google Scholar]
- 25.Alterman AI, Cacciola JS, Ivey MA, Habing B, Lynch KG. Reliability and validity of the alcohol short index of problems and a newly constructed drug short index of problems. J Stud Alcohol Drugs. 2009;70(2):304–307. doi: 10.15288/jsad.2009.70.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rollnick S, Heather N, Gold R, Hall W. Development of a short ‘readiness to change’ questionnaire for use in brief, opportunistic interventions among excessive drinkers. Br J Addict. 1992;87(5):743–754. doi: 10.1111/j.1360-0443.1992.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navaline HA, Snider EC, Petro CJ, et al. Preparations for AIDS vaccine trials. An automated version of the Risk Assessment Battery (RAB): enhancing the assessment of risk behaviors. AIDS Res Hum Retroviruses. 1994;10 (Suppl 2):S281–3. [PubMed] [Google Scholar]
- 30.Miller WR. Form 90: A structured assessment interview for drinking and related behaviors: Test manual. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 1996. [Google Scholar]
- 31.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational enhancement therapy manual. 1992. DHHS Publication No. (ADM) 92–1894. [Google Scholar]
- 33.Friedmann PD, Rose J, Hayaki J, et al. Training primary care clinicians in maintenance care for moderated alcohol use. J Gen Intern Med. 2006;21(12):1269–1275. doi: 10.1111/j.1525-1497.2006.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cone EJ, Presley L, Lehrer M, et al. Oral fluid testing for drugs of abuse: positive prevalence rates by intercept immunoassay screening and GC-MS-MS confirmation and suggested cutoff concentrations. J Anal Toxicol. 2002;26(8):541–546. doi: 10.1093/jat/26.8.541. [DOI] [PubMed] [Google Scholar]
- 35.Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction. 2004;99(7):811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 36.Samet JH, Larson MJ, Horton NJ, Doyle K, Winter M, Saitz R. Linking alcohol- and drug-dependent adults to primary medical care: a randomized controlled trial of a multi-disciplinary health intervention in a detoxification unit. Addiction. 2003;98(4):509–516. doi: 10.1046/j.1360-0443.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim TW, Saitz R, Cheng DM, Winter MR, Witas J, Samet JH. Effect of quality chronic disease management for alcohol and drug dependence on addiction outcomes. J Subst Abuse Treat. 2012;43(4):389–396. doi: 10.1016/j.jsat.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mertens JR, Flisher AJ, Satre DD, Weisner CM. The role of medical conditions and primary care services in 5-year substance use outcomes among chemical dependency treatment patients. Drug Alcohol Depend. 2008;98(1–2):45–53. doi: 10.1016/j.drugalcdep.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Ann Intern Med. 1997;127(12):1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 40.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;(2):CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 42.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clifford PR, Davis CM. Alcohol treatment research assessment exposure: A critical review of the literature. Psychol Addict Behav. 2012;26(4):773–781. doi: 10.1037/a0029747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daeppen JB, Gaume J, Bady P, et al. Brief alcohol intervention and alcohol assessment do not influence alcohol use in injured patients treated in the emergency department: a randomized controlled clinical trial. Addiction. 2007;102(8):1224–1233. doi: 10.1111/j.1360-0443.2007.01869.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.