Abstract
As part of our project pointed at the search of new antiparasitic agents against American trypanosomiasis (Chagas disease) and toxoplasmosis a series of 2-alkylaminoethyl-1-hydroxy-1,1-bishosphonic acids has been designed, synthesized and biologically evaluated against the etiologic agents of these parasitic diseases, Trypanosoma cruzi and Toxoplasma gondii, respectively, and also towards their target enzymes, T. cruzi and T. gondii farnesyl pyrophosphate synthase (FPPS), respectively. Surprisingly, while most pharmacologically active bisphosphonates have a hydroxyl group at the C-1 position, the additional presence of an amino group at C-3 resulted in decreased activity towards either T. cruzi cells or TcFPPS. Density functional theory calculations justify this unexpected behavior. Although these compounds were devoid of activity against T. cruzi cells and TcFPPS, they were efficient growth inhibitors of tachyzoites of T. gondii. This activity was associated with a potent inhibition of the enzymatic activity of TgFPPS. Compound 28 arises as a main example of this family of compounds exhibiting an ED50 value of 4.7 μM against tachyzoites of T. gondii and an IC50 of 0.051 μM against TgFPPS.
Introduction
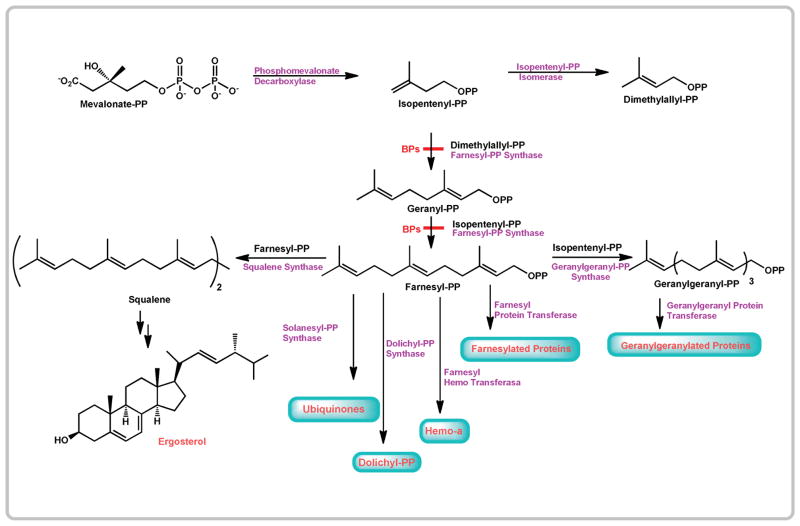
Farnesyl pyrophosphate synthase (FPPS) can be considered as a valid target not only for bone related disorders, but also for different parasitic diseases.1 FPPS catalyzes the consecutive condensation of IPP with DMAPP and with geranyl diphosphate (GPP) to produce farnesyl diphosphate (FPP). FPP is the substrate for enzymes catalyzing the first committed step for the biosynthesis of sterols, ubiquinones, dolichols, heme a, and prenylated proteins. FPP could be condensed with an additional molecule of IPP by the geranylgeranyl pyrophosphate synthase (GGPPS) to form the 20-carbon isoprenoid GGPP. In trypanosomatids, isoprenoid biosynthesis occurs via the classical mevalonate pathway (Scheme 1). The FPPS gene has been cloned from T. cruzi and T. brucei.2,3 Both of these genes are single copy. RNA interference showed that the T. brucei FPPS gene is essential.3
Scheme 1.
Isoprenoid biosynthesis in trypanosomatids.
In Apicomplexan parasites such as Toxoplasma gondii isoprenoids are biosynthesized through the DOXP/MEP pathway as illustrated in Scheme 2.4 In addition, T. gondii possesses a bifunctional FPPS/GGPPS (TgFPPS) that is able to catalyze the formation of both FPP and GGPP.5,6 The FPPS gene appears to be essential in all organisms.7,8 Comparison of the amino acid sequence of FPPSs from different organisms (bacteria to higher eukaryotes) shows the presence of seven conserved regions including two aspartate-rich domains that are very important for the catalytic action and most likely act as the binding sites for IPP and the allylic substrates. All the FPPSs that have been characterized are homodimeric enzymes, and require divalent cations such as Mg2+ or Mn2+ for activity.9
Scheme 2.
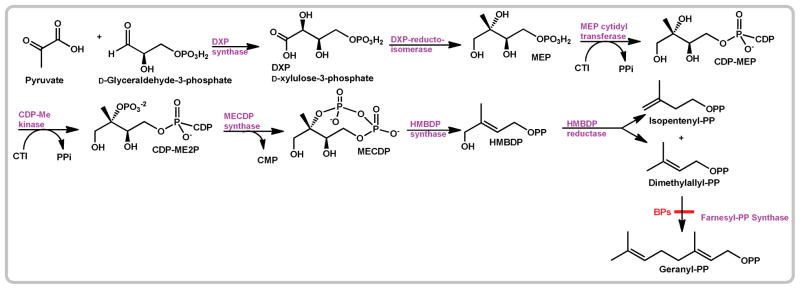
Isoprenoid biosynthesis in Apicomplexan parasites.
On the other hand, bisphosphonates (2) are pyrophosphate (1) analogues in which a methylene group replaces the oxygen atom bridge between the two phosphorus atoms of the pyrophosphate moiety. The substitution of carbon with different side chains has generated a large family of compounds. Several bisphosphonates are potent inhibitors of bone resorption and are in clinical use for the treatment of different bone disorders.10 Acidocalcisomes are equivalent in composition to the bone mineral; the accumulation of bisphosphonates in these organelles, as they do in bone mineral, facilitates their antiparasitic action.11 Aminobisphosphonates such as pamidronate (3), alendronate (4), and risedronate (5), were first found to be effective in the inhibition of T. cruzi in vitro and in vivo without toxicity to the host cells (Figure 1).12 In addition, some bisphosphonates were growth inhibitors of T. gondii, T. brucei rhodesiense, Leishmania donovani and Plasmodium falciparum.13–16 In vivo testing in mice has shown that risedronate can significantly increase the survival of mice infected by T. cruzi.17,18 All these results indicate that bisphosphonates are promising candidate drugs to treat infections by T. cruzi and other pathogenic parasites. In fact, they have already been developed to treat other diseases and consequently have low toxicity; their structures are simple and easy to synthesize; these compounds have shown effective inhibitory activity against T. cruzi in vitro12 and in vivo.17,18
Figure 1.
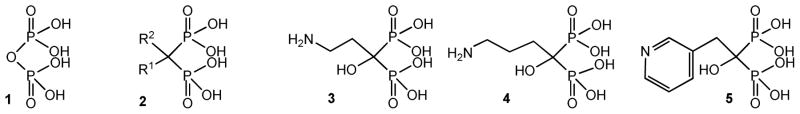
Chemical structures of representative FDA-approved bisphosphonates clinically employed for different bone disorders.
Of particular interest are linear bisphosphonates, specifically, 2-alkyl(amino)ethyl derivatives, which can be considered as promising antiparasitic agents.19,20 These bisphosphonate derivatives exhibit potent cellular activity against intracellular T. cruzi, which is one of the clinically relevant forms of this parasite, having IC50 values at the low nanomolar level against the target enzyme (TcFPPS).19,20 In addition, at the present time, linear 1-hydroxy-, 1-alkyl-, and 1-amino-1,1-bisphosphonates such as 6–9 can be considered as useful structures to establish rigorous SAR studies as antiparasitic agent targeting TcFPPS.5,21–24 In fact, these compounds show a broad range of antiparasitic activity against trypanosomatids and Apicompexan. For example, 6 is a potent growth inhibitor of T. cruzi (amastigotes)21 and also against T. gondii (tachyzoites),5,24 whereas 7 is effective against P. falciparum.5 Besides, α-fluoro-1,1-bisphosphonates of formula 10 and 11 are devoid of cellular activity against T. cruzi or TcFPPS, but they are extremely potent inhibitors of the enzymatic activity of T. gondii FPPS exhibiting IC50 values of 35 nM and 60 nM, respectively.25 The high selectivity observed by these fluorine-containing bisphosphonates against TgFPPS versus TcFPPS can be rationalized by the evidence that the amino acid sequences of these enzymes have less than 50% identity.16 (Figure 2). As mentioned before, 12–14 are promising anti-T. cruzi agents. For example, 12 exhibit an ED50 value of 0.84 μM against T. cruzi (amastigotes),19 which is fifteen times more potent than the well-known antiparasitic agent WC-9 under the same assays conditions.26 In addition, 13 is an extremely potent inhibitor of the enzymatic activity of TcFPPS (IC50 = 0.058 μM) and of TgFPPS (IC50 = 0.095 μM),20 whereas the long chain length derivative 14 is an effective growth inhibitors of intracellular T. cruzi proliferation (ED50 = 0.67 μM) compared to benznidazole (ED50 = 2.77 μM).21 Sulfur-containing bisphosphonates also presents good prospective as putative lead drugs. For example, 15 and 16 are potent anti-Toxoplasma agents and, to a lesser extent, efficient anti-T. cruzi agents.27 For example, 15 has a potent cellular activity against tachyzoites of T. gondii (ED50 = 1.8 μM), which was associated with a potent inhibition of the enzymatic activity of TgFPPS (IC50 = 0.021 μM).27 Compound 15 is also effective towards TcFPPS (IC50 = 0.097 μM).27 Moreover, 16 exhibits an extremely potent inhibitory action against TgFPPS with an IC50 value as low as 0.009 μM.27 (Figure 2). It is worth mentioning that similar activity have also been reported for some lipophilic bisphosphonates, including 2-alkylaminoethyl compounds lacking the hydroxyl group at C-1, against another Apicomplexan parasite, P. falciparum.28,29
Figure 2.
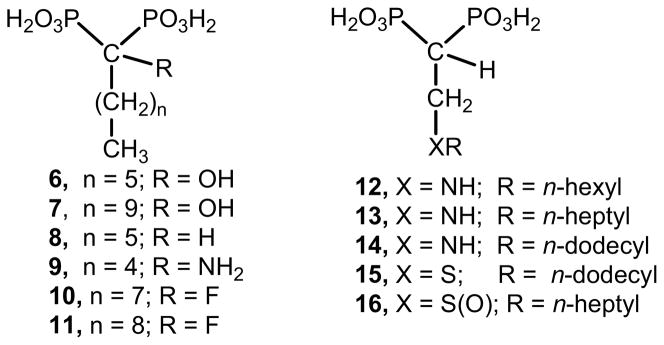
Chemical structures of representative members of bisphosphonic acids derived from fatty acids.
Rationale
The present study was motivated to get further insight into the molecular recognition processes of 2-alkyl(amino)ethyl-1,1-bisphosphonates taking compounds 12–14 as reference structures. We have recently demonstrated that TcFPPS inhibitors 12 and 13 bind to the allylic site of the enzyme with the phosphates group of the bisphosphonate unit coordinating three Mg2+ atoms,30 which bridge the compounds to the enzyme in a similar way to that observed for the physiological substrates.31,32 Binding of either 12 or 13 is enthalpically unfavorable. The favorable entropy, which dominates the favorable free-energy, results from a delicate balance between two opposing effects: the unfavorable loss of conformational entropy, due to freezing of single bond rotations of the inhibitor (and binding site side chains), and the favorable increase of entropy associated with burial of the hydrophobic alkyl chains.30 The nitrogen atom at the C-3 position is very important to maintain a potent inhibition of the enzymatic activity of TcFPPS, but does not coordinate any Mg2+ atom at the active site30 as we had been initially considered.19,20 It has a crucial role to drive the spatial alignment of the alkyl chains for better fitting.
We have envisioned that the introduction of a hydroxyl group at the C-1 position in compounds 12 and 13 is a relevant structural variation for a number of reasons: (a) it is known that the presence of an electron withdrawing group at C-1 would enhance the ability to coordinate Ca2+ or Mg2+ in a tridentate manner;32–38 (b) the presence of an electron withdrawing group at C-1 of 1,1-bisphosphonic acids would increase acidity in at least one order of magnitude compared to those where these groups are absent mimicking the pKa value of pyrophosphoric acid;39–43 (c) most of the bisphosphonates clinically in use for the treatment of bone disorders have a hydroxyl group bonded at C-1;44–46 (d) compound 6 impairs its efficiency as inhibitor of the enzymatic activity agaisnt TcFPPS when the hydroxyl group is absent as occurs with compound 8.21,22
Bearing in mind the above statements, the role of the hydroxyl group at C-1 on biological activity in a variety of bisphosphonates is still uncertain. Experimental evidence indicates that that the hydroxyl group at C-1 does not interact with Mg2+ at the active site of FPPS suggesting that the function of this group is circumvented to influence the ability of the adjacent bisphosphonic unit to coordinate Mg2+ as well as to increase the pKa of the gem-phopshonate functionality.47–49
Results and Discussion
As previously discussed, we selected 2-alkylaminoethyl-1-hydroxy-1,1-bisphosphonic acid derivatives 27–31 as the title compounds for the present study. These compounds were straightforwardly prepared starting from benzyl bromoacetate and the corresponding linear amine. Nucleophilic displacement reaction between each amine and benzyl bromoacetate in acetonitrile as a solvent, according to slightly modified published procedures,50,51 afforded the respective benzyl n-alkylaminoacetates 17–21 in yields ranging 29–84%. Benzyl groups were cleaved by catalytic hydrogenation employing palladium on charcoal as catalyst to give the respective free acids 22–26 in 46–98% yields. These 2-(n-alkylamino)acetic acids were the substrates to prepare the title compounds 27–31. Then, in independent experiments, on treatment with phosphorous acid and phosphorous trichloride employing benzenesulfonic acid as a solvent at 65 °C followed by hydrolysis, 22–26 were converted into 27–31 according to the widely employed method for the preparation of 1-hydroxy-1,1-bisphosphonic acids from carboxylic acids (Scheme 3).52
Scheme 3.
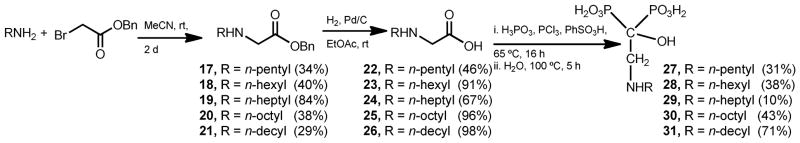
Synthetic approach for the preparation of modified alkylaminoethyl bisphosphonates.
Biological evaluation of 2-alkylaminoethyl-1-hydroxy-1,1-bisphosphonates has lead to surprising results. Contrary to it was expected, all of these compounds were almost devoid of biological activity as inhibitors of T. cruzi proliferation and also as inhibitor of the enzymatic activity of TcFPPS confirming our previous finding in compound 29.27 However, some of these compounds showed an extremely potent inhibition of the enzymatic activity of TgFPPS. For example 28 and 30 are potent inhibitors of TgFPPS exhibiting IC50 values of 0.051 μM and 0.039 μM, respectively. This enzymatic activity was associated with an efficient cellular activity showing ED50 values of 4.7 μM and 2.0 μM, respectively. Risedronate was used as positive control (ED50 = 2.4 μM). This selectively observed towards the target enzymes (TcFPPS versus TgFPPS) has previously been observed and it can be justified by the fact that sequences of these enzymes have less than 50% identity.16 Moreover, these results are more comprehensible taking into account that TgFPPS is a bifunctional enzyme, hence as TgFPPS also catalyzes formation of both FPP (C-15) and GGPP (C-20),5,6 it is reasonable to assume that its enzymatic activity could be inhibited by compounds of long chain length, which are structurally rather similar to GGPP in contrast to TcFPPS, the enzyme that catalyses formation of FPP as final product exclusively. The biological evaluation is presented in Table 1.
Table 1.
Biological activity of 2-alkylaminoethyl-1-hydroxy-1,1-bisphosphonic acids against TcFPPS, TgFPPS, T. cruzi (amastigotes), and tachyzoites of T. gondii.
| Compound | TcFPPS IC50 (μM) | ED50 T. cruzi amastigotes (μM) | TgFPPS IC50 (μM) | ED50 T. gondii tachyzoites (μM) |
|---|---|---|---|---|
| 27 | > 10 | > 20 | 0.067 ± 0.064 | > 10 |
| 28 | > 10 | > 20 | 0.051 ± 0.006 | 4.68 ± 1.19 |
| 29 | > 10 | > 20 | > 1.0 | 0.00% at 10 μM |
| 30 | > 10 | 46% at 10 μM cytotoxic at 10 μM | 0.039 ± 0.033 | 2.00 ± 0.95 |
| 31 | > 10 | 42% at 10 μM | 0.125 ± 0.023 | 14.9% at 10 μM |
| Benznidazole | 1.44 ± 0.97 | |||
| Risedronate | 0.027 ± 0.0153 | 55.0 ± 5.053 | 0.074 ± 0.0175 | 2.4 ± 0.724 |
The lack of biological activity of this family of compounds against T. cruzi is quite unexpected and cannot be attributable simply to the presence of a hydroxyl group at C-1. In fact, either compound 6 or risedronate (5), both bearing a hydroxyl group at C-1, are effective inhibitors of TcFPPS.21,22,53 Why our title compounds are devoid of antiparasitic activity against T. cruzi? In risedronate the nitrogen atom is bonded one position further than is bonded in our compounds. Evidently, the position of this nitrogen atom has a strong influence in the observed biological activity. Removal of the hydroxyl group keeping the nitrogen atom at C-3 results in extremely potent 2-alkylaminoethyl bisphosphonates, such as 12–14. Therefore, these two groups did not produce the expected synergistic effect. On the contrary, the combination of these two groups reduced their activity. We have attempted to rationalize this behavior using density functional theory (DFT) calculations. Analogs of 12–14 (12a) and 27–31 (27a) carrying, for the sake of simplicity, N-methylamino groups instead of longer alkylamino moieties were submitted to energy minimizations at the B3LYP/6-311+G(d,p) level, simulating the presence of water as solvent with the polarizable continuum method (PCM). In order to reproduce better the possible conformation of these compounds in the biological environment, the molecules 12a and 27a were considered to have the acidic hydrogen atoms expected to appear at physiological pH (≈ 6.5), i.e. two hydrogen atoms for 12a, and only one for the more acidic 27a.39,40 One magnesium atom was added to complete each molecule (Figure 3).
Figure 3.
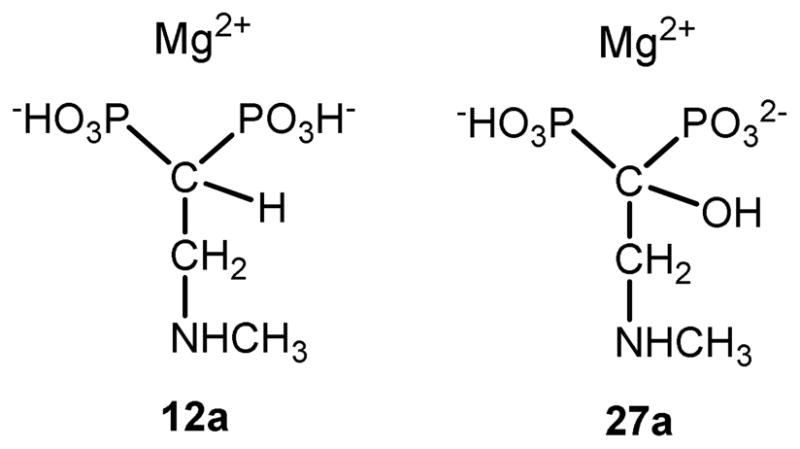
Simplified models of 12–14 (12a) and 27–31 (27a) to carry out molecular modeling studies.
Results show that the minimization always leads to the formation of a six-membered ring containing C-1, both P atoms, two O atoms and the Mg atom, being the distance between each O and the Mg of 1.91–1.96 Å. However, the “exocyclic” atoms generate different hydrogen bond patterns (even in the simulated water environment) with distinct geometries and energies. It is known that the strength of the hydrogen bonds depends, under the geometric criteria, on acceptor-hydrogen distances as short as possible (for very strong bonds, the distance can be even shorter than 2 Å), and donor-hydrogen-acceptor angles as close to 180° as possible.54,55 Table 1 shows the results for the main conformers of each analog. For 12a, the most stable geometry shows a strong “1,3-diaxial” hydrogen bond between an axial O-H and an axial O bonded to different P atoms (dH-O = 1.82 Å), and a very strong hydrogen bond between the other acidic hydrogen (equatorial) and the nearby nitrogen atom (dH-N = 1.68 Å), which fixes the conformation of the side chain (Figure 4). Another conformer, with similar energy, has the same hydrogen bond features but a different conformation of the carbon chain. On the other hand, compound 27a does not have two acidic hydrogens. Thus, the only acidic hydrogen should be involved in either the 1,3-diaxial H-bond interaction or the interaction with the N. The most stable geometry (Figure 4, Table 1) shows the strong diaxial interaction (dH-O = 1.62 Å, θ = 161°), and two weaker interactions (Table 1), as deduced from directional factors (θ = 124–134°). Other conformations, with higher energies show other bonding patterns: conformer 4 shows a similar pattern, whereas conformers 2 and 3 show a strong hydrogen bond between the acidic hydrogen and the nitrogen atom, as occurred with 12a (Table 1). However, these conformers have energies surpassing in about 2 kcal/mol that of the most stable conformer. The strength of the bond in conformer 3 of 27a is equivalent to that observed for the main conformer of 12a, but the hydrogen bond in conformer 2 is slightly weaker, as deduced from the distance and angles (Table 1).
Figure 4.
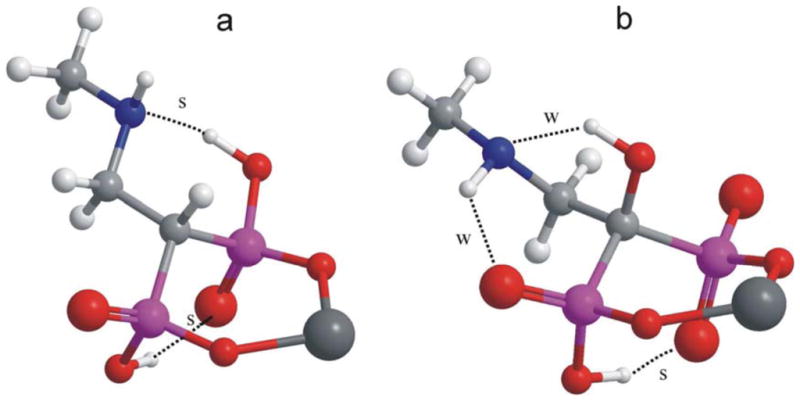
Most stable geometries of simple models 12a (a) and 27a (b). Hydrogen bonds were labelled as strong (s) when dAcceptor-H < 2 Å and θdonor-H-Acceptor > 145°, or as weak (w) if they do not meet both of these criteria.
These results might be a clue that in compounds like 12–14, having two acidic hydrogen atoms, the flexibility of the carbon chain is strongly reduced, to the point of giving an almost “fixed” conformation carrying the nitrogen atom in a favorable arrangement for biological action. On the other hand, compounds like 27–31, which carry both a hydroxyl group at C-1 and a N atom on C-2 display a higher flexibility of the carbon chain, thus generating a manifold of conformations, for which only some (less stable) display the arrangement needed for an optimal molecular recognition.
NMR analyses support the above statement. Compound 32 is an interesting example of an isosteric analog of compounds 12–14.56 It has been demonstrated that 32 forms a rather stable six-membered ring via a hydrogen bond based on the chemical shift of the phosphorus atom at the C-3 position as illustrated in Figure 5.56 In addition, proton NMR data of 28 and the n-propyl derivative of 12–14 (compound 33), performed in anhydrous deuterated DMSO, indicated the occurrence of this conformational restriction on these compounds. Two signals of protons bonded to heteroatoms were observed in the 1H NMR spectrum of 33 at 7.70 ppm and 8.95 ppm, respectively. The first signal moved upfield (5.40 ppm) when one equivalent of Mg2+ was added. A similar behavior was observed from the proton NMR spectrum of 28. Once again, two signals that appeared at 6.69 ppm and 9.11 ppm. The first one moved upfield (4.41 ppm) when one equivalent of Mg2+ was added.
Figure 5.
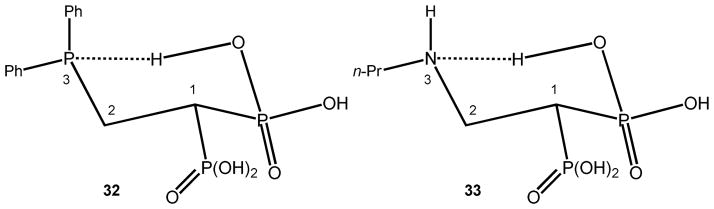
Chemical structures of compound 32, exhibiting a typical six-membered hydrogen bond (see ref. 56), and its isosteric analogue 33.
In summary, it can be concluded that the activity against T. cruzi of compounds of type 12–14 can be attributed due to these compounds adapt a restricted conformation having no tension of torsion that would be benefit for molecular recognition, whereas the 1-hydroxy derivatives 27–31 present a manifold of conformations that would not lead to a successful interaction with the target enzyme. Nevertheless, these bisphophonate derivatives exhibited a selective and potent inhibitory action towards TgFPPS. Efforts in optimizing lead structures 12–14 are currently being pursued in our laboratory.
Experimental Section
General
The glassware used in air- and/or moisture-sensitive reactions was flame-dried and reactions were carried out under an argon atmosphere. Unless otherwise noted, chemicals were commercially available and used without further purification. Solvents were distilled before use. Acetonitrile was distilled from phosphorus pentoxide.
Nuclear magnetic resonance spectra were recorded using a AM-500 MHz spectrometer. Chemical shifts are reported in parts per million (δ) relative to tetramethylsilane. Coupling constants are reported in Hertz. 13C NMR spectra were fully decoupled. 31P NMR spectra are referenced with respect to the peak of 85% H3PO4 as external reference. Splitting patterns are designated as s, singlet; d, doublet; t, triplet; q, quartet.
High-resolution mass spectra were obtained using a hybrid quadrupole time of flight mass spectrometer with MS/MS capability.
Melting points are uncorrected. Analytical TLC was performed on commercial 0.2 mm aluminum-coated silica gel plates (F254) and visualized by 254 nm UV or immersion in an aqueous solution of (NH4)6Mo7O24•4H2O (0.04 M), Ce(SO4)2 (0.003 M) in concentrated H2SO4 (10%). Elemental analyses were conducted by UMYMFOR (CONICET-FCEyN). The results were within ±0.4% of the theoretical values.
Synthesis of 2-(n-Alkylamino)acetic acids
General Procedure
To a solution of the corresponding n-alkylamine (1.00 g, 10 mmol) in anhydrous acetonitrile (15 mL) cooled at 0 °C was added dropwise benzyl bromoacetate (2.29 g, 10 mmol). Then, triethylamine (2.7 mL, 19.4 mmol) was added and the reaction mixture was stirred overnight. The solvent was evaporated and the residue was purified by column chromatography (sílica gel) eluting with a mixture of hexane–EtOAc (19:1) to afford the corresponding benzyl esters 17–21 as colorless oils. Then, a solution of respective benzyl ester (8.0 mmol) in ethyl acetate (50 mL) in the presence of palladium on charcoal (50 mg) was treated with hydrogen at 3 atm in a Parr apparatus. The reaction mixture was shaken for 6 h and the mixture was filtered through a fritted glass funnel. The solvent was evaporated to yield the corresponding free 2-(n-alkylamino) acetic acids that were used in the next step without further purification.
1-[(n-Alkylamino)ethyl]-1-hydroxy-1,1-bisphosphonic acids
General Procedure
To a flame dried 100 mL three neck flask having an addition funnel and a reflux condenser through which water at 0 °C was circulated the corresponding 2-(n-alkylamino) acetic acid (2.9 mmol), H3PO3 acid (273 mg, 2.9 mmol), and anhydrous benzenesulfonic acid (1.0 g, 6.3 mmol) under argon atmosphere. The reaction mixture was heated to 65 °C, then PCl3 (500 μL, 5.8 mmol) was added dropwise with vigorous stirring. The reaction was stirred at 65 °C for 16 h, and then allowed to cool to room temperature. Then, cold water (60 mL) was added and the reaction was stirred at 100 °C for an additional 5 h. The reaction was cooled to room temperature and the pH was adjust to 4.3 with a 50% aqueous NaOH solution. Acetone (20 mL) was added, and the resulting mixture was cooled to 0 °C for 24 h. The product was filtrated and crystallized from water–ethanol.
2-(n-Pentylamino)acetic acid (22)
White solid; 46% yield; mp 185–190 °C (desc.); 1H NMR (500.13 MHz, CD3OD) δ 0.94 (t, J = 6.8 Hz, 3H), 1.37 (m, 4H), 1.68 (p, J = 7.5 Hz, 2H), 2.97 (dist t, J = 7.9 Hz, 2H), 3.47 (s, 2H); 13C NMR (125.77 MHz, CD3OD) δ 14.1, 23.2, 26.9, 29.7, 48.7, 50.6, 170.9. HRMS (ESI) calcd for C7H16O2NNa [M+Na]+ 168.1000; found: 168.1010.
1-[(n-Pentylamino)ethyl]-1-hydroxy-1,1-bisphosphonic Acid (27)
White solid; 31% yield; mp 186–190 °C; 1H NMR (500.13 MHz, D2O) δ 0.79 (t, J = 7.1 Hz, 3H), 1.26 (m, 4H), 1.62 (p, J = 7.4 Hz, 2H), 3.02 (t, J = 7.5 Hz, 2H), 3.40 (t, J = 11.7 Hz, 2H); 13C NMR (125.77 MHz, D2O) δ 13.0, 21.4, 25.0, 27.7, 48.2, 49.9, 70.3 (t, J = 137.5 Hz); 31P NMR (202.46 MHz, D2O) δ 14.82. HRMS (ESI) calcd for C7H19O7NP2Na [M+Na]+ 314.0534; found: 314.0527.
2-(Hexylamino)acetic acid (23)
White solid; 91% yield; mp 196–199 °C (desc.); 1H NMR (500.13 MHz, CD3OD) δ 0.92 (t, J = 6.9 Hz, 3H), 1.35 (m, 4H), 1.39 (p, J = 7.8 Hz, 2H), 1.67 (p, J = 7.6 Hz, 2H), 2.97 (dist t, J = 7.9 Hz, 2H), 3.46 (s, 2H); 13C NMR (125 MHz, CD3OD) δ 14.3, 23.5, 27.2, 27.3, 32.4, 48.7, 50.6, 170.9. HRMS (ESI) calcd for C8H18O2N [M+H]+ 160.1338; found: 160.1343.
1-[(n-Hexylamino)ethyl]-1-hydroxy-1,1-bisphosphonic Acid (28)
White solid; 38% yield; mp 189–190 °C (desc.); 1H NMR (500.13 MHz, D2O) δ 0.77 (t, J = 7.2 Hz, 3H), 1.25 (m, 4H), 1.29 (p, J = 6.6 Hz, 2H), 1.61 (p, J = 7.5 Hz, 2H), 3.02 (t, J = 7.5 Hz, 2H), 3.39 (t, J = 11.6 Hz, 2H); 13C NMR (125.77 MHz, D2O) δ 13.2, 21.6, 25.2,25.3, 30.4, 48.2, 50.0, 70.4 (t, J = 138.4 Hz); 31P NMR (202.46 MHz, D2O) δ 14.69. HRMS (ESI) calcd for C8H21O7NP2Na [M+Na]+ 328.0691; found: 328.0684.
2-(n-Heptylamino)acetic acid (24)
White solid; 67% yield; mp = 187–191 °C; 1H NMR (500.13 MHz, CD3OD) δ 0.91 (t, J = 7.0 Hz, 3H), 1.32 (m, 4H), 1.37 (m, 4H), 1.67 (p, J = 7.5 Hz, 2H), 2.97 (m, 2H); 3.46 (s, 2H); 13C NMR (125.77 MHz, D2O) δ 13.6, 22.2, 28.5, 28.7, 29.4, 31.3, 47.4, 49.2, 169.5. HRMS (ESI) calcd for C9H20O2N [M+H]+ 174.1494; found: 174.1510. Anal. Calcd. for (C9H19O2N): C, 62.39; H, 11.05; N, 8.08. Found C, 62.05; H, 10.62; N, 7.74.
1-[(n-Heptylamino)ethyl]-1-hydroxy-1,1-bisphosphonic Acid (29)
White solid; 10% yield; mp 155–159 °C; 1H NMR (500.13 MHz, CDCl3) δ 0.90 (t, J = 7.0 Hz, 3H), 1.18 (m, 6H), 1.24 (m, 2H), 1.60 (p, J = 7.4 Hz, 2H), 3.01 (t, J = 7.6 Hz, 2H), 3.39 (t, J = 11.7 Hz, 2H); 13C NMR (125.77 MHz, D2O) δ 13.3, 21.8, 25.3,25.5, 27.8, 30.7, 48.3, 49.9, 70.3 (t, J = 137.7 Hz); 31P NMR (D2O) δ 15.31. HRMS (ESI) calcd for C9H24O7NP20 [M+H]+ 320.1030; found: 320.1037. Anal. Calcd. for (C9H23O7NP2.1.50H2O): C, 31.22; H, 7.57; N, 4.05. Found C, 31.53; H, 7.75; N, 4.36.
2-(Octylamino)acetic acid (25)
White solid; 96% yield; 1H NMR (500.13 MHz, CD3OD) δ 0.90 (t, J = 6.8 Hz, 3H), 1.33 (m, 10H), 1.68 (p, J = 7.5 Hz, 2H), 2.97 (dist t, J = 7.9 Hz, 2H), 3.46 (s, 2H); 13C NMR (125.77 MHz, CD3OD) δ 14.4, 23.7, 27.2, 27.6, 30.17, 30.19, 32.9, 48.7, 50.6, 170.9. HRMS (ESI) calcd for C10H22O2N [M+H]+ 188.1651; found: 188.1669.
1-[(n-Octylamino)ethyl]-1-hydroxy-1,1-bisphosphonic Acid (30)
White solid; 43% yield; 1H NMR (500.13 MHz, DMSO-d6) δ 0.85 (t, J = 6.9 Hz, 3H), 1.25 (m, 10H), 1.54 (p, J = 7.1 Hz, 2H), 2.90 (dist. t, J = 7.1 Hz, 2H), 3.22 (t, J = 10.7 Hz, 2H); 13C NMR (125.77 MHz, DMSO-d6) δ 13.9, 22.1, 25.6, 25.9, 28.46, 28.48, 31.1, 47.4, 49.8, 69.4 (t, J = 132.3 Hz); 31P NMR (DMSO-d6) δ 14.85. HRMS (ESI) calcd for C10H25O7NP2Na [M+Na]+ 356.1004; found: 356.0994.
2-(Decylamino)acetic acid (26)
White solid; mp 184–187 °C; 1H NMR (500.13 MHz, CD3OD) δ 0.89 (t, J = 6.9 Hz, 3H), 1.24 (m, 14H), 1.67 (p, J = 7.5 Hz, 2H), 2.97 (dist t, J = 8.9 Hz, 2H), 3.46 (s, 2H); 13C NMR (125.77 MHz, CD3OD) δ 14.4, 23.7, 27.2, 27.6, 30.2, 30.4, 30.5, 30.6, 33.0, 48.7, 50.6, 170.9. HRMS (ESI) calcd for C12H26O2N [M+H]+ 216.1964; found: 216.1995.
1-[(n-Decylamino)ethyl]-1-hydroxy-1,1-bisphosphonic Acid (31)
White solid; mp 188–190 °C; 1H NMR (500.13 MHz, DMSO-d6) δ 0.84 (t, J = 6.7 Hz, 3H), 1.25 (m, 14H), 1.54 (m, 2H), 2.90 (dist. t, J = 7.0 Hz, 2H), 3.22 (t, J = 11.1 Hz, 2H); 13C NMR (125.77 MHz, DMSO-d6) δ 13.9, 22.1, 25.6, 25.9, 28.5, 28.7, 28.8, 28.9, 31.3, 47.3, 50.0, 69.2 (t, J = 130.0 Hz); 31P NMR (DMSO-d6) δ 15.19. HRMS (ESI) calcd for C12H30O7NP2 [M+H]+ 362.1498; found: 362.1493.
Drug Screening
T. cruzi amastigote assays
Gamma-irradiated (2,000 Rads) Vero cells (3.4 × 104 cells/well) were seeded in 96 well plates (black, clear bottom plates from Greiner Bio-One) in 100 μL RPMI media (Sigma) with 10% FBS. Plates were incubated overnight at 35 °C and 7% CO2. After overnight incubation, Vero cells were challenged with 3.4 × 105 trypomastigotes/well (CL strain overexpressing a tdTomato red fluorescent protein) in 50 μL volume and incubated for 5 h at 35 °C and 7% CO2. After infection, cells were washed once with Hanks solution (150 μL/well) to eliminate any extracellular parasites and compounds were added in serial dilutions in RPMI media in 150 μL volumes. Each dilution was tested in quadruplicate. Each plate also contained controls with host cells and no parasites (for background check), and controls with parasites and no drugs (positive control). Drugs were tested on T. cruzi at 1.56 μM, 3.125 μM, 6.25 μM, 12.5 μM, 25 μM. For each set of experiments, benznidazole was also used as a positive control 0.39 μM, 0.78 μM, 1.56 μM, 3.125 μM, and 6.25 μM. After drug addition, plates were incubated at 35 °C and 7% CO2. At day 3 post-infection, plates were assayed for fluorescence.57 IC50 values were determined by non-linear regression analysis using SigmaPlot. There was no evident cytotoxicity on the host cells (visual assay) with any of the drugs tested at concentrations as high as 25 μM.
T. gondii tachyzoites assays
Experiments on T. gondii tachyzoites were carried out as described previously58 using T. gondii tachyzoites expressing red fluorescent protein.59 Cells were routinely maintained in hTerT cells grown in High Glucose Dulbecco’s modified Eagle’s medium (DMEM-HG) supplemented with 1% fetal bovine serum, 2 mM glutamine, 1 mM pyruvate, at 37 °C in a humid 5% CO2 atmosphere. Confluent monolayers grown in 96-well black plates with optical bottoms (black, clear bottom plates from Greiner Bio-One) were used and drugs dissolved in the same medium and serially diluted in the plates. Freshly isolated tachyzoites were filtered through a 3 μm filter and passed through a 22 gauge needle, before use. The cultures were inoculated with 104 tachyzoites/well in the same media. The plates were incubated at 37 °C and read daily in a Molecular Devices fluorescence plate reader. To preserve sterility the plates were read with covered lids, and both excitation (510 nm) and emission (540 nm) were read from the bottom.59 For the calculation of the EC50, the percent of growth inhibition was plotted as a function of drug concentration by fitting the values to the function: I = Imax C / (EC50 + C), where I is the percent inhibition, Imax = 100% inhibition, C is the concentration of the inhibitor, and EC50 is the concentration for 50% growth inhibition. There was no evident cytotoxicity on the host cells with any of the drugs tested (visual assay).
Computational methods
All the DFT calculations were performed using the Gaussian09 program,60 the B3LYP61 functional and the 6-311+G(d,p) basis set. Calculations were carried out with full geometry optimization, using standard termination conditions, and including in all cases the effect of the solvent (water) through the Tomasi’s polarized continuum model (PCM)62 as implemented in Gaussian09. Several different input geometries were used in order to find the most important conformers; the Mg atom always appeared coordinated to two oxygen atoms, and the hydrogen bonding usually drove the energy minimization processes.
Supplementary Material
Table 2.
Main conformersa of 12a and 27a, calculated by B3LYP/6-311+G(d,p) with PCM in water: energies and geometrical features of the hydrogen bonds.
| Conformer | Energy (kcal/mol) | Carbon chainb | Hydrogen bonds Atoms involved | dH-Acc (Å) | θDon-H-Acc (°) |
|---|---|---|---|---|---|
| 12a | |||||
| 1 | 0.00 | equat. | H(POax) -Oax(P′) | 1.82 | 154 |
| H(POeq)-N | 1.68 | 157 | |||
| 2 | 0.59 | axial | H(POax) -Oax(P′) | 1.89 | 154 |
| H(POeq)-N | 1.73 | 155 | |||
| 3 | 6.50 | equat. | H(POax) -Oax(P′) | 1.86 | 154 |
| H(N)-Oeq(P) | 2.25 | 139 | |||
| 4 | 8.49 | equat. | H(N)-Oeq(P) | 2.19 | 139 |
| 27a | |||||
| 1 | 0.00 | equat. | H(POax) -Oax(P′) | 1.62 | 161 |
| H(Oax)-N | 2.01 | 124 | |||
| H(N)-Oeq(P) | 2.24 | 134 | |||
| 2 | 1.77 | equat. | H(Oax)-Oeq(P) | 1.91 | 135 |
| H(POeq)-N | 1.78 | 153 | |||
| 3 | 2.17 | axial | H(Oeq) -Oax(P) | 1.95 | 133 |
| H(POeq)-N | 1.67 | 157 | |||
| 4 | 3.75 | equat. | H(POax) -Oax(P′) | 1.62 | 162 |
| H(Oax)-Oeq(P) | 2.82 | 108 | |||
| H(N)-Oeq(P) | 2.14 | 142 | |||
| 5 | 4.86 | axial | H(POax) -Oax(P′) | 1.59 | 160 |
| H(Oeq)-Oeq(P) | 2.25 | 127 | |||
For each of the nine H-bond/carbon chain arrangements, the conformer with the orientation of the N-methyl group with lower energy is shown.
The designation of substituents as axial or equatorial was made considering the presence of a six-membered cycle formed by the atoms C-1–P-1–O–Mg–O–P-2.
Acknowledgments
This work was supported by grants from the National Research Council of Argentina (PIP 1888 to J.B.R. and 559/10 to C.A.S.), ANPCyT (PICT 2008 #1690 to J.B.R.), and the Universidad de Buenos Aires (200201001003801 to J.B.R. and W-759 to C.A.S.), the Bunge & Born Foundation to S.H.S, and the U.S. National Institutes of Health to R.D. (AI-082542) and S.N.J.M. (AI-102254.
Footnotes
Supporting Information. Copies of the 1H NMR, 13C NMR and 31P NMR spectra as well as Cartesian coordinates of compounds 12a and 27a are included as supporting information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun S, McKenna CE. Expert Opin Ther Patents. 2011;21:1433–1451. doi: 10.1517/13543776.2011.593511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montalvetti A, Bailey BN, Martin MB, Severin GW, Oldfield E, Docampo R. J Biol Chem. 2001;276:33930–33937. doi: 10.1074/jbc.M103950200. [DOI] [PubMed] [Google Scholar]
- 3.Montalvetti A, Fernandez A, Sanders JM, Ghosh S, Van Brussel E, Oldfield E, Docampo R. J Biol Chem. 2003;278:17075–17083. doi: 10.1074/jbc.M210467200. [DOI] [PubMed] [Google Scholar]
- 4.Moreno SNJ, Li Z-H. Expert Opin Ther Targets. 2008;12:253–263. doi: 10.1517/14728222.12.3.253. [DOI] [PubMed] [Google Scholar]
- 5.Ling Y, Li Z-H, Miranda K, Oldfield E, Moreno SN. J Biol Chem. 2007;282:30804–30816. doi: 10.1074/jbc.M703178200. [DOI] [PubMed] [Google Scholar]
- 6.Li ZH, Cintrón R, Koon NA, Moreno SNJ. Biochemistry. 2012;51:7533–7540. doi: 10.1021/bi3005335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchard L, Karst F. Gene. 1993;125:185–189. doi: 10.1016/0378-1119(93)90326-x. [DOI] [PubMed] [Google Scholar]
- 8.Song L, Poulter CD. Proc Natl Acad Sci USA. 1994;91:3044–3048. doi: 10.1073/pnas.91.8.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogura K, Koyama T. Chem Rev. 1998;98:1263–1276. doi: 10.1021/cr9600464. [DOI] [PubMed] [Google Scholar]
- 10.Rodan GA. Annu Rev Pharmacol Toxicol. 1998;38:375–88. doi: 10.1146/annurev.pharmtox.38.1.375. [DOI] [PubMed] [Google Scholar]
- 11.Docampo R, Moreno SNJ. Cell Calcium. 2011;50:113–119. doi: 10.1016/j.ceca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urbina JA, Moreno B, Vierkotter S, Oldfield E, Payares G, Sanoja C, Bailey BN, Yan W, Scott DA, Moreno SNJ, Docampo R. J Biol Chem. 1999;274:33609–33615. doi: 10.1074/jbc.274.47.33609. [DOI] [PubMed] [Google Scholar]
- 13.Martin MB, Grimley JS, Lewis JC, Heath HT, III, Bailey BN, Kendrick H, Yardley V, Caldera A, Lira R, Urbina JA, Moreno SN, Docampo R, Croft SL, Oldfield E. J Med Chem. 2001;44:909–916. doi: 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- 14.Yardley V, Khan AA, Martin MB, Slifer TR, Araujo FG, Moreno SNJ, Docampo R, Croft SL, Oldfield E. Antimicrob Agents Chemother. 2002;46:929–931. doi: 10.1128/AAC.46.3.929-931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin MB, Sanders JM, Kendrick H, de Luca-Fradley K, Lewis JC, Grimley JS, Van Brussel EM, Olsen JR, Meints GA, Burzynska A, Kafarski P, Croft SL, Oldfield E. J Med Chem. 2002;45:2904–2914. doi: 10.1021/jm0102809. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez JB, Szajnman SH. Expert Opinion Ther Patents. 2012;22:311–334. doi: 10.1517/13543776.2012.668886. [DOI] [PubMed] [Google Scholar]
- 17.Garzoni LR, Waghabi MC, Baptista MM, De Castro SL, de Meirelles MN, Britto CC, Docampo R, Oldfield E, Urbina JA. Int J Antimicrob Agents. 2004;23:286–290. doi: 10.1016/j.ijantimicag.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Bouzahzah B, Jelicks LA, Morris SA, Weiss LM, Tanowitz HB. Parasitol Res. 2005;96:184–187. doi: 10.1007/s00436-005-1331-9. [DOI] [PubMed] [Google Scholar]
- 19.Szajnman SH, García Liñares GE, Li Z–H, Galizzi M, Jiang C, Bontempi E, Ferella M, Moreno SNJ, Docampo R, Rodriguez JB. Bioorg Med Chem. 2008;16:3283–3290. doi: 10.1016/j.bmc.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosso VS, Szajnman SH, Malayil L, Galizzi M, Moreno SNJ, Docampo R, Rodriguez JB. Bioorg Med Chem. 2011;19:2211–2217. doi: 10.1016/j.bmc.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szajnman SH, Bailey BN, Docampo R, Rodriguez JB. Bioorg Med Chem Lett. 2001;11:789–792. doi: 10.1016/s0960-894x(01)00057-9. [DOI] [PubMed] [Google Scholar]
- 22.Szajnman SH, Montalvetti A, Wang Y, Docampo R, Rodriguez JB. Bioorg Med Chem Lett. 2003;13:3231–3235. doi: 10.1016/s0960-894x(03)00663-2. [DOI] [PubMed] [Google Scholar]
- 23.Szajnman SH, Ravaschino EL, Docampo R, Rodriguez JB. Bioorg Med Chem Lett. 2005;15:4685–4690. doi: 10.1016/j.bmcl.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 24.Ling Y, Sahota G, Odeh S, Chan JMW, Araujo FG, Moreno SNJ, Oldfield E. J Med Chem. 2005;48:3130–3140. doi: 10.1021/jm040132t. [DOI] [PubMed] [Google Scholar]
- 25.Szajnman SH, Rosso VS, Malayil L, Smith A, Moreno SN, Docampo R, Rodriguez JB. Org Biomol Chem. 2012;10:1424–1433. doi: 10.1039/c1ob06602a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cinque GM, Szajnman SH, Zhong L, Docampo R, Schvartzapel AJ, Rodriguez JB, Gros EG. J Med Chem. 1998;41:1540–1554. doi: 10.1021/jm970860z. [DOI] [PubMed] [Google Scholar]
- 27.Recher M, Barboza AP, Li ZH, Galizzi M, Ferrer-Casal M, Szajnman SH, Docampo R, Moreno SNJ, Rodriguez JB. Eur J Med Chem. 2013;60:431–440. doi: 10.1016/j.ejmech.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh AP, Zhang Y, No J-H, Docampo R, Nussenzweig V, Oldfield E. Antimicrob Agents Chemother. 2010;54:2987–2993. doi: 10.1128/AAC.00198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.No JH, de Macedo Dossin F, Zhang Y, Liu Y–L, Wei Zhu W, Feng X, Yoo JA, Lee E, Wang K, Hui R, Freitas-Junior LH, Oldfield E. Proc Natl Acad Sci USA. 2012;109:4058–4063. doi: 10.1073/pnas.1118215109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aripirala S, Szajnman SH, Jakoncic J, Rodriguez JB, Docampo R, Gabelli SB, Amzel LM. J Med Chem. 2012;55:6445–6454. doi: 10.1021/jm300425y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laskovics FM, Poulter CD. Biochemistry. 1981;20:1893–1901. doi: 10.1021/bi00510a027. [DOI] [PubMed] [Google Scholar]
- 32.Poulter CD, Argyle JC, Mash EA. J Biol Chem. 1978;253:7227–7233. [PubMed] [Google Scholar]
- 33.Van Beek E, Löwik C, Que I, Papapoulos SJ. Bone Miner Res. 1996;11:1492–1497. doi: 10.1002/jbmr.5650111016. [DOI] [PubMed] [Google Scholar]
- 34.Ebetino FH, Francis MD, Rogers MJ, Russell RGG. Rev Contemp Pharmacother. 1998;9:233–243. [Google Scholar]
- 35.Jung A, Bisaz S, Fleisch H. Calcif Tissue Res. 1973;11:269–280. doi: 10.1007/BF02547227. [DOI] [PubMed] [Google Scholar]
- 36.Matczak-Jon E, Kurzak B, Kamecka A, Kafarski P. Polyhedron. 2002;21:321–332. [Google Scholar]
- 37.Gumienna-Kontecka E, Jezierska J, Lecouvey M, Leroux Y, Kozlowski H. J Inorg Biochem. 2002;89:13–17. doi: 10.1016/s0162-0134(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 38.Gumienna-Kontecka E, Silvagni R, Lipinski R, LecouveyMMarincola FC, Crisponi G, Nurchi VM, Leroux Y, Kozlowski H. Inorg Chimica Acta. 2002;339:111–118. [Google Scholar]
- 39.Marma MS, Xia Z, Stewart C, Coxon F, Dunford JE, Baron R, Kashemirov BA, Ebetino FH, Triffit JT, Russell RGG, McKenna CE. J Med Chem. 2007;50:5967–5975. doi: 10.1021/jm0702884. [DOI] [PubMed] [Google Scholar]
- 40.McKenna CE, Shen P, Org DJ. Chem. 1981;46:4573–4576. [Google Scholar]
- 41.Berkowitz DB, Bose M. J Fluorine Chem. 2001;112:13–33. [Google Scholar]
- 42.Blackburn GM, England DA, Kolkmann F. Chem Commun. 1981:930–932. [Google Scholar]
- 43.Romanenko VD, Kukhar VP. Chem Rev. 2006;106:3868–3935. doi: 10.1021/cr051000q. [DOI] [PubMed] [Google Scholar]
- 44.Roelofs AJ, Thompson K, Ebetino FH, Rogers MJ, Coxon FP. Curr Pharm Des. 2010;16:2950–2960. doi: 10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- 45.Reszka AA, Rodan GA. Mini-Rev Med Chem. 2004;4:711–717. [PubMed] [Google Scholar]
- 46.Russell RGG, Rogers MJ. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 47.Huang C-H, Gabelli SB, Oldfield E, Amzel LM. Proteins. 2010;78:888–899. doi: 10.1002/prot.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao R, Chen CK-M, Guo R-T, Wang AH-J, Oldfield E. Proteins. 2008;73:431–439. doi: 10.1002/prot.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gabelli SB, McLellan JS, Montalvetti A, Oldfield E, Docampo R, Amzel LM. Proteins. 2006;62:80–88. doi: 10.1002/prot.20754. [DOI] [PubMed] [Google Scholar]
- 50.Zuliani V, Carmi C, Rivara M, Fantini M, Lodola A, Vacondio F, Bordi F, Plazzi PV, Cavazzoni A, Galetti M, Alfieri RR, Petronini PG, Mor M. Eur J Med Chem. 2009;44:3471–3479. doi: 10.1016/j.ejmech.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 51.Kruijtzer JAW, Liskamp RMJ. Tetrahedron Lett. 1995:6969–6972. [Google Scholar]
- 52.Kieczykowski GR, Jobson RB, Melillo DG, Reinhold DF, Grenda VJ, Shinkai I. J Org Chem. 1995;60:8310–8312. [Google Scholar]
- 53.Demoro B, Caruso F, Rossi M, Benítez D, Gonzalez M, Cerecetto H, Parajón-Costa B, Castiglioni J, Galizzi M, Docampo R, Otero L, Gambino D. J Inorg Biochem. 2010;104:1252–1258. doi: 10.1016/j.jinorgbio.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan Z, Chen J, Lu G, Geng YZ, Zheng H, Ji Q. J Chem Phys. 2012;136:164313. doi: 10.1063/1.4705371. [DOI] [PubMed] [Google Scholar]
- 55.Swiatla-Wojcik D. Chem Phys. 2007;342:260. [Google Scholar]
- 56.Delain-Bioton L, Turner A, Lejeune N, Villemin D, Hix GB, Jaffrès P-A. Tetrahedron. 2005;61:6602–6609. [Google Scholar]
- 57.Canavaci AM, Bustamante JM, Padilla AM, Pereza Brandan CM, Simpson LJ, Xu D, Boehlke CL, Tarleton RL. PLOS Negl Trop Dis. 2010;4:e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gubbels MJ, Li C, Striepen B. Antimicrob Agents Chemother. 2003;43:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agrawal S, van Dooren GG, Beatty WL, Striepen B. J Biol Chem. 2009;284:33683–33691. doi: 10.1074/jbc.M109.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision C.01. Gaussian, Inc; Wallingford CT: 2009. [Google Scholar]
- 61.(a) Lee C, Yang W, Parr RG. Phys Rev B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]; (b) Becke AD. J Chem Phys. 1993;98:5648–5652. [Google Scholar]
- 62.Tomasi J, Mennucci B, Cammi R. Chem Rev. 2005;105:2999–3093. doi: 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.