Abstract
Objective
To assess metabolic and endocrine defects in girls genetically predisposed to polycystic ovary syndrome (PCOS).
Design
Controlled cross-sectional study.
Setting
University hospital.
Patients
Nine girls aged 8–14 years having a first-degree relative diagnosed with PCOS (PCOSr) and 10 age-matched girls unrelated to PCOS.
Intervention
None.
Main outcome measure
Insulin sensitivity determined by frequently sampled intravenous glucose tolerance testing (ISFSivGTT) and insulin-induced non-esterified fatty acid suppression (NEFAsupp), estimated by the log-linear slope of NEFA levels during the first 20 min of FSivGTT.
Results
In comparison to controls, PCOSr had higher body mass index Z-score (BMI-z), waist circumference and waist/height ratio. Levels of the androgen 17α-hydroxyprogesterone (17OHPg) were significantly increased in PCOSr, independently of adiposity, and inversely correlated with ISFSivGTT. ISFSivGTT was decreased and NEFAsupp was less steep in PCOSr as compared to controls, independently of BMI-z and 17OHPg. NEFAsupp was more pronounced with increasing ISFSivGTT, independently of adiposity.
Conclusions
Girls at high risk of developing PCOS display increased adiposity and 17OHPg levels, but are mainly characterized by global insulin resistance and resistance to insulin-induced suppression of lipolysis that were independent of adiposity and 17OHPg levels. Therefore, genetic predisposition to PCOS may be related to early insulin resistance and adipocyte dysfunction.
Keywords: Polycystic ovary syndrome, Non-esterified fatty acids, Insulin resistance, Adipose Tissue Metabolism and Adolescents/Children
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a frequent, multifaceted endocrine disorder affecting 6– 10% of women of reproductive age (1). According to the 2009 Androgen Excess-PCOS Task Force report, PCOS is defined by hyperandrogenism (clinical and/or biochemical) and ovarian dysfunction (oligo-anovulation or polycystic ovaries) (2). Although the full clinical spectrum of PCOS does not appear until puberty, frequently following significant pubertal weight gain (3), hyperandrogenism often emerges earlier, during childhood or in the peripubertal years (4–7).
Substantial evidence suggests a genetic predisposition for developing PCOS. First, the incidence of PCOS rises from 6–10% in the general population to 20–40% in families of women with PCOS (8;9). Secondly, daughters and sisters of women with PCOS present higher levels of adrenal and ovarian androgens during puberty or adulthood (1;4–7;10); while daughters, sisters, brothers, mothers and fathers display insulin resistance, hyperinsulinemia and/or lower glucose tolerance (6;7;10–12).
The higher prevalence of metabolic disturbances in first-degree relatives of PCOS women, including brothers (11) and fathers (10;12) with normal androgen levels, and the development of early metabolic anomalies in daughters of PCOS women before completion of puberty (6) suggest that PCOS heritability and early development may be more related to metabolic factors than intrinsic defects of the gonadal axis. Among metabolic factors, lipotoxicity resulting from overflow of nonesterified free fatty acids (NEFA) towards non-adipose tissue is an interesting candidate for PCOS pathogenesis. Lipotoxicity, due to NEFA spillover from defective adipose tissue and/or impaired intracellular metabolism in non-adipose tissues, is recognized as an important factor in the development of insulin resistance and type 2 diabetes (13). Moreover, experimentally increased levels of NEFA have been found to promote androgen production in vivo in healthy women (14) and in vitro in primary culture of bovine adrenal cells (15).
In light of these findings, we hypothesized that metabolic anomalies develop early in girls at risk for PCOS, before the development of ovarian hyperandrogenism and dysfunction, and mainly affect insulin action and adipose tissue function. As mentioned, previous reports characterized the endocrine and metabolic milieu in young girls genetically at risk for developing PCOS (5–7;10), but none comprehensively assessed insulin sensitivity and secretion, nor evaluated NEFA metabolism. Therefore, in order to appropriately test our hypothesis, we performed a cross-sectional study contrasting young girls having a first degree PCOS relative with aged-matched controls using robust techniques to measure insulin sensitivity and insulin-mediated suppression of plasma NEFA, a surrogate of adipose tissue insulin sensitivity (16).
RESEARCH DESIGN AND METHODS
Study participants
Between January 2007 and August 2009, daughters or sisters (PCOSr) of women with PCOS and control subjects without family history of PCOS. PCOSr girls were recruited as part of a multicenter longitudinal study lead by Dr DH Geller, but only results from subjects assessed in Sherbrooke are reported in this article. Girls of the control group were followed in general pediatric clinics for stable conditions, mainly well-replaced hypothyroidism. It was a convenient sample of girls interested to participate. PCOSr girls between the ages of 8 and 14 years were enrolled and controls were recruited in order to match with at least one at-risk girl for age (±1.5 year, 1:1 or 2:1 match). Girls with histories of precocious pubarche or puberty, those taking medications known to affect carbohydrate metabolism or following a specific diet or exercise program affecting metabolic status, and those diagnosed with diabetes mellitus or any other uncontrolled chronic disease were excluded. Finally, all girls were born at term from singleton pregnancies. This study was approved by the human research ethical committee of the Centre hospitalier universitaire de Sherbrooke (CHUS), and written informed consent was obtained from all participants’ parental authority and assent was signed by all girls.
PCOS probands were diagnosed and followed-up at the Reproductive Endocrinology Clinic of the CHUS by Dr Baillargeon. All PCOS probands met established diagnostic criteria (2): oligomenorrhea (≤8 menstrual periods during the preceding year) or confirmed oligo-anovulation; clinical or biochemical signs of hyperandrogenism (acnea, hirsutism, serum total testosterone >78ng/dL or calculated free testosterone >1.15ng/dL) (17); and exclusion of secondary causes: non-classical congenital adrenal hyperplasia (early morning 17α-hydroxyprogesterone (17OHPg) >220ng/dL (18)), abnormal thyroid function, hyperprolactinemia, evidence of androgen-secreting tumours, Cushing’s syndrome or acromegaly, and having taken medications known to affect levels of testosterone or 17OHPg within 3 months of testing.
Clinical assessment of subjects
A complete physical examination was performed, including the following anthropometric measurements: weight, height, waist and hip circumferences, and body fat percentage assessed by foot-to-foot bioimpedance (TANITA, Arlington Heights, IL, USA). Body mass index (BMI; kg/m2) was calculated and BMI Z-score (calculated according to the USCDC growth-chart reference values of 2000 (19)) was determined. Waist circumference has been shown to relate closely to visceral adiposity and metabolic risks in adults (20), but must be corrected for the stature in developing kids. Therefore, ratios of waist to hip circumferences and waist circumference to height (all in cm) were calculated for this study.
Birth weight, height and gestational age as well as age at menarche were recorded. Experienced observers (B.M., J.S–R, J–P.B) assessed pubertal stage of development according to Tanner criteria (21). Hirsutism was evaluated using the modified Ferriman-Gallwey score (22) and presence of acne or acanthosis nigricans was assessed, but none of the participant presented these clinical manifestations.
Experimental protocol
Participation required two visits: at visit 1, complete physical examination and anthropometric measures were performed, followed by a 2-h oral glucose tolerance test (OGTT); at visit 2, a 3.5-h insulin-modified frequently sampled intravenous glucose tolerance test (FSivGTT) (23) was performed. For each visit, girls were admitted to the Clinical Research Center of CHUS at approximately 8h30, after a 12-h overnight fasting period. Subjects remained fasted throughout study period. Baseline fasting sample at the 1st visit was used to measure concentrations of insulin, glucose, androgens, 17β-estradiol, SHBG, triglycerides and high-density lipoprotein-cholesterol (HDL-C).
Oral glucose tolerance test (OGTT)
OGTTs were performed using one catheter placed on the forearm for sampling purpose. Blood samples were collected at times −15,−5,0,15,30,60,90 and 120min after glucose load (40g/m2 body surface area) for the measurement of glucose, insulin, C-peptide, NEFA and ghrelin. Total and high molecular weight adiponectin and leptin concentrations were assessed at 0 and 120min. Areas under the curves (AUC; measured by the trapezoidal integration) were calculated for NEFA, insulin, C-peptide and ghrelin. Based on OGTT results, the following indices were calculated: Matsuda insulin sensitivity index (ISIMatSuda=10,000/[square root (fasting glucose (mg/dL)×fasting insulin (µU/mL))×(mean glucose (mg/dL)×mean insulin ((µU/mL))]) (23), corrected insulin response to glucose at 30 min (CIR30= [insulin 30min (µU/mL)]/[glucose 30min (mg/dL)−70]) (24) and the corresponding disposition index (DIOGTT=ISlMatsuda×CIR30). Since DI reflects insulin secretion adjusted for the level of insulin sensitivity, it is an estimation of beta-cell function (25).
Suppression of NEFA blood levels induced by the insulin surge occurring after glucose load was estimated by the slope of the log-linear decrease of NEFA levels during the entire course of the OGTT (Ln(NEFA) Slope), as adapted from our previously published method (16). Therefore, a more pronounced NEFA suppression following glucose load translates into a steeper reduction of NEFA levels during OGTT and consequently, in a more negative slope. Another method to estimate insulin-induced NEFA suppression is the time required to suppress 50% of baseline NEFA levels, i.e. T50nefa Finally, since NEFA suppression depends on circulating insulin levels, Ln(NEFA) Slope was corrected for the amount of circulating insulin measured during the same period, using the ratio of Ln(NEFA) Slope/AUCinsulin.
FSivGTT
FSivGTT study required two catheters placed in contralateral forearms. After three baseline samples over 15min, a bolus of dextrose (11.4g/m2 of body surface area) was injected at time 0 followed by 0.02U/kg human insulin (Humulin Regular, Eli Lilly) at time 20 min (23). A total of 26 blood samples were collected over 210min for glucose, insulin and NEFA assays. Insulin sensitivity (ISFSivgtt) (23), insulin secretion (acute insulin response to glucose (AIRg)) (26) and the corresponding disposition index (DIFsrvGTT=ISFsrvGTT×AIRg) (27) were calculated using the minimal model of Bergman (MINMOD computer program version Millennium 6.02, Richard N. Bergman, 2004) (28). As with the OGTT, AUC insulin, insulin-induced NEFA suppression (Ln(NEFA) Slope), NEFA suppression corrected for circulating insulin amount (Ln(NEFA) Slope/AUCinsulin) and T50 (T50nefa) were calculated during the early phase of the FSivGTT, before exogenous insulin injection.
Assays
Acquired samples were rapidly centrifuged and frozen at −80°C for later analysis. Plasma glucose concentrations were assayed by the glucose hexokinase technique (Raichem). Human insulin was dosed by solid phase sandwich immunoassay (Wallace/Perkin-Elmer). C-peptide and leptin were measured by radioimmunoassays (Millipore, Billerica, MA, USA) and ghrelin and sex hormones binding globulin (SHBG), by immunoradiometric assay (SIEMENS, Los Angeles, CA). Total and high molecular weight adiponectin were quantified by ELISA (Adiponectin multimeric, Alpco Diagnostics, Salem, NH). NEFAs were assayed by the only validated method available; a colorimetric assay (Wako Chemicals, Richmond, VA) having a minimum detectable level of 0.0014mEq/L. Total cholesterol, HDL-C, and triglyceride concentrations were assessed by a colorimetric method (Johnson & Johnson Clinical Diagnostics, Rochester, NY). The following androgens were assayed by liquidchromatography combined to tandem mass spectrometry (Quest Diagnostic-Nichols Institute, San Juan Capistrano, CA): total testosterone, dehydroepiandrosterone (DHEA), androstenedione, 17β-estradiol and 17OHPg. Inter- and intra-assay coefficients of variation are less than 10% for total testosterone and less than 8.5% for all other steroid hormones. Free testosterone was calculated by the Sodergard formula (29).
Statistical analyses
Because of the relatively small sample size, non-parametrical tests were used for main statistical analyses and results are reported as medians (25th–75th percentiles). Comparisons of continuous variables between groups were performed using Wilcoxon rank sum tests and dichotomous variables were compared using Fisher’s exact tests. Relationships between continuous variables were tested using non-parametric Spearman tests.
In order to account for the clustering effect of family members, significant group differences were tested using mixed-model ANOVA, which considered that sisters were paired (see Tables). Significant findings were also adjusted for potential confounders using multivariate ANOVA. Because of the limited power of the study, we adjusted for only one confounder at the time, except when adjusting for both BMI-z and 17OHPg. For these parametrical tests, variables not normally distributed were log-transformed to normalize their distribution, when possible. Statistical significance was set at P≤0.05. Statistical analyses were performed using JMP© 7.0 software (SAS Institute, Cary, NC).
RESULTS
Study participants (Table 1)
Table 1.
Baseline anthropometric characteristics and sex steroid concentrations
| Controls (n = 10) | PCOSr (n = 9) | Pc | |
|---|---|---|---|
| Birth weight (g) | 3,204 (2,975–3,270) | 3,210 (3,040–3,305) | 0.62 |
| Gestational age (weeks) | 38.0(37.5–39.0) | 40.0 (39.3–40.9) | 0.003 |
| Age (yr) | 12.3(11.2–14.6) | 12.1(9.7–13.7) | 0.84 |
| Tanner stagea >2 (%) | 30.0% | 55.6% | 0.37d |
| Post-menarche (%) | 20.0% | 44.4% | 0.35d |
| Weight (kg) | 38.0(32.8–51.2) | 49.4 (33.5–62.6) | 0.19 |
| Height (m) | 1.47(1.36–1.55) | 1.39(1.35–1.59) | 0.93 |
| BMI (kg/m2) | 18.1 (16.6–18.8) | 22.2(18.0–28.7) | 0.050 |
| BMI Z-score | −0.38 (−1.08–0.66) | 0.90 (0.02–1.98) | 0.018k |
| Fat percentage (%) | 19.5 (8.0–30.0) | 25.0 (22.0–37.0) | 0.09 |
| Waist circumference (cm) | 60.5 (52.3–66.0) | 66.0 (61.3–84.8) | 0.040 |
| Hip circumference (cm) | 77.0(70.8–89.1) | 85.5 (77.0–93.8) | 0.09 |
| Waist-to-hip ratio | 0.77 (0.71–0.82) | 0.83 (0.77–0.86) | 0.11 |
| Waist/height ratio | 0.40 (0.39–0.44) | 0.47 (0.42–0.57) | 0.018j |
| 17α-hydroxyprogesterone (ng/dL) | 13 (10–23) | 28 (19–13) | 0.016e,f,k |
| DHEA (ng/dL) | 139 (101–282) | 226(176–510) | 0.086 |
| Androstenedione (ng/dL) | 36 (17–74) | 67 (23–120) | 0.29 |
| Total testosterone (ng/dL) | 9.0 (2.9–17.3) | 13.0(2.9–20.2) | 0.74 |
| Free testosterone (ng/dL)b | 0.083(0.037–0.184) | 0.180(0.055–0.320) | 0.38 |
| SHBG (ng/dL) | 2.25 (1.64–3.65) | 1.50(1.01–2.07) | 0.047j |
| 17β-estradiol (pg/mL) | 19 (2–73) | 15 (2–28) | 0.84 |
Values are expressed as median (25th –75th percentiles). To convert values for 17α-hydroxyprogesterone to nmol/L, multiply by 0.0303; for DHEA to nmol/L, multiply by 0.0347; for androstenedione to nmol/L, multiply by 0.0349; for total testosterone to nmol/L, multiply by 0.0347; and for free testosterone to pmol/L, multiply by 34.7; for SHBG to nmol/L, multiply by 34.7 and for 17β-estradiol to pmol/L, multiply by 3.67.
BMI = Body mass index; DHEA = Dehydroepiandrosterone; SHBG = Sex hormone binding globulin; and waist/height ratio = Ratio of waist circumference (cm) to height (cm).
Determined according to the highest score of its three components: pubic hair, axillary hair and breast development.
Calculated using Sodergard equation from total testosterone and SHBG values (29).
Comparisons between controls and PCOSr using Wilcoxon tests.
Comparison between groups using Fisher’s Exact test.
P≤0.05 after correction for Tanner stage ≤2; or
P≤0.05 after correction for waist/height ratio, using multivariate linear regression analysis.
P<0.10 and
P≤0.05 between PCOSr and Controls when considering familial clustering using mixed-model ANOVA.
Nine Caucasians subjects were recruited from 7 PCOS probands, of which 6 were mothers and one was an older sister. One PCOS proband had two daughters recruited in the study and one had two younger sisters recruited. The median BMI of the probands at diagnosis was 33.5 (30.7–35.4) kg/m2, and during their clinical evolution, two probands (25%) developed IGT and none developed type 2 diabetes. Control group included 10 age-matched Caucasians girls, among which there were 3 pairs of sisters. As expected, both groups had very similar age, but PCOSr girls were more advanced in puberty. Birth weight and length [51.0 (49.1–51.4) cm for controls and 49.5 (49.3–53.2) cm for PCOSr, P=0.97] were very similar between groups. Gestational age at birth was significantly higher in PCOSr.
Anthropometric and endocrine characteristics (Table 1)
PCOSr girls had significantly higher measures of adiposity than controls. Accordingly, 44.4% of PCOSr and 10.0% of controls were obese (P=0.14), as defined by a BMI above the 95th percentile for age. No girl met the 2007 IDF criteria for metabolic syndrome in children (30). All measured androgens were higher in PCOSr as compared to controls, approximately twice as high in general, but only group difference in the adrenal androgen 17OHPg reached statistical significance. This difference persisted after adjustment for Tanner stage (≤2 vs >2; P=0.017) and waist/height ratio (P=0.030), but not after BMI-z adjustment. SHBG was significantly reduced by 33% in PCOSr girls but this difference was no longer significant after adjustment for adiposity (BMI-z or waist/height ratio).
Metabolic measures and calculations (Tables 2 and 3, and Figure 1)
Table 2.
Metabolic measures during fasting and 2-h OGTT, after 75g glucose load
| Controls (n = 10) | PCOSr (n = 9) | |||
|---|---|---|---|---|
| Fasting | 2h | Fasting | 2h | |
| Glucose (mg/dL) | 85 (79–88) | 110(86–128) | 86 (83–90) | 112(97–124) |
| Insulin (µU/mL) | 4.8 (3.5–9.8) |
(15.1–38.9)22.5 |
10.1 (7.6–12.7)a,e,j |
47.9 (31.8–107.5)a,j |
| C-peptide (µg/L) | 1.2(1.1–1.8) | 5.6 (4.6–7.0) | 2.3 (1.3–2.6) | 7.0 (5.8–8.5) |
| NEFA (µEq/L) | 222 (159–281) | 5 (4–22) | 269 (194–358) | 16 (8–29) |
| Triglycerides (mg/dL) | 64 (45–96) | - | 59 (39–73) | – |
| HDL-Cholesterol | 0.35 (0.28–0.47) | - | 0.38 (0.29–0.44) | - |
| Ghrelin (ng/L) | 1058 (834–1453) |
887 (682–1115) |
951 (746–1119) |
702 (626–937) |
| Total adiponectin (mg/L) | 3.8 (2.9–5.4) | 3.9(3.2–6.1) | 6.6 (4.0–9.7) | 6.7 (4.2–7.6) |
| HMW/total adiponectin ratio | 0.06 (0.05–0.09) | 0.06 (0.04–0.08) | 0.05 (0.04–0.06) | 0.04 (0.03–0.05) |
| Leptin (µg/L) | 3.0(1.7–4.6) | 2.6 (1.4–4.8) | 8.2 (3.3–23.6)a,e,j | 10.8 (4.7–21.5)a,e,k |
Values are expressed as median (25th –75th percentiles). To convert values for glucose to mmol/L, multiply by 0.0555; for insulin to pmol/L, multiply by 6.945; for AUC insulin to nmol/L, multiply by 6.945; for NEFA to mmol/L, multiply by 1.0 and for triglycerides to mmol/L, multiply by 0.0113. HDL = High density lipoprotein; HMW/total adiponectin = High molecular weight out of total adiponectin; and NEFA = Non-esterified fatty acids.
P≤0.05 between PCOSr and Controls for the same time-point, using Wilcoxon test.
P≤0.05 after correction for Tanner stage ≤2, using multivariate linear regression analysis.
P<0.10 and
P≤0.05 between PCOSr and Controls when considering familial clustering using mixed-model ANOVA.
Table 3.
Calculated metabolic parameters based on fasting, OGTT and FSivGTT measures
| Conditions |
Metabolic parameter |
Calculated parameter |
Controls (n = 10) |
PCOSr (n = 9) |
pa |
|---|---|---|---|---|---|
| OGTT | Integrated insulin levels |
AUC insulin (mU-min/mL) |
5.88 (5.08–8.61) | 7.33 (5.73–9.46) | 0.14 |
| Integrated C- peptide levels |
AUC C-peptide (ng-min/mL) |
686 (600–748) | 763(731–834) | 0.051 | |
| Integrated ghrelin levels |
AUC ghrelin (µg-min/L) |
107.7(88.1– 136.7) |
90.8(75.9–110.8) | 0.21 | |
| Integrated NEFA levels |
AUC NEFA (mEq-min/L) |
8.2(5.1–11.3) | 13.0 (7.5–16.0) | 0.12 | |
| Insulin sensitivity |
ISIMatsuda | 11.0(7.9–12.9) | 4.3 (2.7–7.4) | 0.018e, k | |
| Insulin secretion |
CIR30 | 0.8(0.5–1.2) | 0.7(0.3–1.3) | 0.54 | |
| β-cell function | DIoGTT | 7.1 (4.4–10.4) | 3.2(1.1–6.8) | 0.028e,j | |
| NEFA suppressibility |
Ln(NEFA) Slope | −0.045 (−0.055– −0.030) |
−0.031 (−0.035– −0.025) |
0.10 | |
| Ln(NEFA) Slope/ AUC insulin |
−1.10 (−1.47–−0.52) |
−0.61 (−0.64– −0.58) |
0.08 | ||
| TSOnefa (min) | 15.1 (15.1–29.9) | 30.1(29.9–30.2) | 0.027b | ||
| FSivGTT | Insulin sensitivity | ISfSivGTT | 11.4(7.1–13.6) | 4.1 (2.5–5.5) | 0.001e,f,g,h,i,l |
| Insulin secretion |
AIRg | 261 (136–401) | 256(158–772) | 0.57 | |
| β-cell function | DIfSivGTT | 2644 (1658–3405) |
1289 (815–2132) |
0.022e,f,g,k | |
| NEFA suppressibility |
Ln(NEFA) Slope | −0.051 (−0.071– −0.038) |
−0.025 (−0.035– −0.020) |
0.004e,f,g,h,i,j | |
| Ln(NEFA) Slope/ AUCinsulin |
−89.2 (−141.8– −39.8) |
−32.6 (−68.2– −11.9) |
0.021e,j | ||
| T50NEFA (min) | 9.5 (6.3–18.2) | 18.8 (12.1–20.4) | 0.041j |
Values are expressed as median (25th–75th percentiles). AIRg = Acute insulin response to glucose; AUC = Area under the curve; CIR30 = Corrected insulin response at 30 min; FSivGTT = Frequent sampling intra-venous glucose tolerance test; DI = Glucose disposition index; DIOGTT = ISIMatsuda × CIR30; DIFSivGTT = ISFSivGTT × AIRg; ISFSivGTT = Insulin sensitivity determined during the FSivGTT; ISI = Insulin sensitivity index; Ln(NEFA) Slope = Slope of Ln of NEFA concentrations (between time 0 to 20 min for the FSivGTT); Ln(NEFA) Slope/AUCInsulin = Ratio of the Slope of Ln of NEFA concentrations to the area under the insulin curve ×1000 (between time 0 to 20 min for the FSivGTT); NEFA = Non-esterified fatty acid; OGTT = Oral glucose tolerance test; and T50NEFA = Time to suppress 50% of NEFA baseline levels.
Comparisons between PCOSr and Controls using Wilcoxon tests.
Data not normally distributed even after log-transformation, such that this result was not adjusted with linear regression analysis.
P≤0.05 after correction for Tanner stage ≤2;
P≤0.05 after correction for waist/height ratio,
P≤0.05 after correction for BMI Z-score,
P≤0.05 after correction for 17-hydroxyprogesterone, or
P≤0.05 after correction for BMI Z-score plus 17-hydroxyprogesterone, using multivariate linear regression analyses.
P<0.10,
P≤0.05 and
P=0.002 between PCOSr and Controls when considering familial clustering using mixed-model ANOVA.
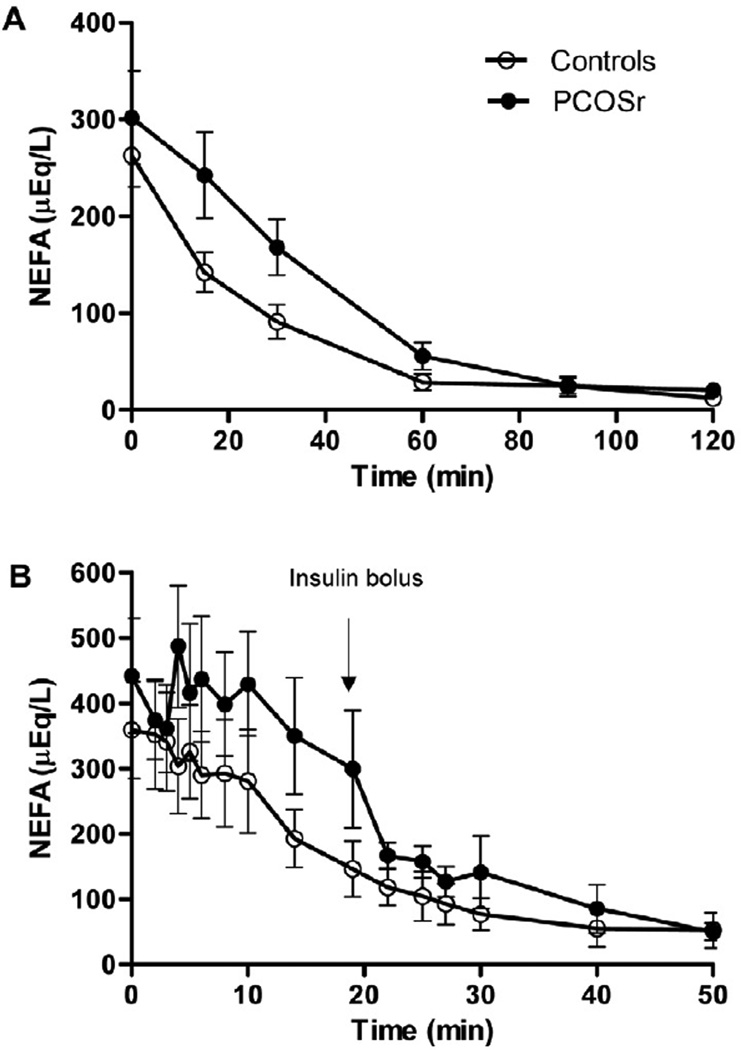
FIGURE 1.
Insulin-induced suppression of non-esterified fatty acids (NEFA) during OGTT (A) and FSivGTT (B) in girls related to polycystic ovary syndrome (PCOSr, black circles) and control girls (Controls, white circles). Data are means ± SD.
Table 2 summarizes results of metabolic measures obtained during fasting and 2h after glucose load (OGTT). As compared to the control group, fasting and 2-h insulin levels were doubled in PCOSr girls, (P=0.030 and 0.050, respectively), fasting leptin levels were increased 2.7 folds (P=0.041) and 2-h leptin levels were increased 4 folds (P=0.013). Group differences in fasting insulin levels, and fasting and 2-h leptin levels remained significant even after adjustment for Tanner stage (P=0.044, 0.042 and 0.008, respectively), but not after correction for adiposity or 17OHPg.
Results of calculated metabolic indices determined during fasting, OGTT or FSivGTT are presented in Table 3. Lower results of HOMA-S%, ISIMatsuda and DIOGTT in PCOSr girls remained significant after correction for Tanner stage (Ps=0.017, 0.033 and 0.023, respectively), but not after adjustment for adiposity or 17OHPg. Regarding FSivGTT results, ISFSivGTT was significantly reduced by almost 3 folds in PCOSr (vs. controls) and remained significantly reduced even after correction for Tanner stage (P=0.0005), waist/height ratio (P=0.004), BMI-z (P=0.006), 17OHPg (P=0.012) or both BMI-z and 17OHPg (P=0.050). This group difference was also independent of Ln(NEFA) Slope during the FSivGTT (see below) (P=0.019). Since AIRg was similar between groups, DIFSivGTT was also significantly decreased by half in PCOSr compared to controls, which was independent of Tanner stage (P=0.040), waist/height ratio (P=0.0004) and BMI-z (P=0.004), but not 17OHPg.
Table 3 also presents estimates of insulin-induced NEFA suppression. During the OGTT, T50NEFA was significantly prolonged two folds in PCOSr compared to control girls, but no correction was applied to this variable because it was not normally distributed even after log-transformation. During the FSivGTT, the log-linear slope of NEFA levels during the first 20 min of FSivGTT (Ln(NEFA) Slope) was half less steep in PCOSr compared to controls, which was independent of Tanner stage (P=0.011), waist/height ratio (P=0.009), BMI-z (P=0.004), 17OHPg (P=0.028) or both BMI-z and 17OHPg (P=0.037). However, this group difference was not significant after adjustment for ISFSivGTT (P=0.13). Ln(NEFA) Slope/AUCinsulin, which corrects the slope for circulating amount of insulin, was also significantly less steep by 2.7-fold, which remained after correction for Tanner stage (P=0.029), but not after adjustments for the other covariates. T50NEFA during FSivGTT was 2-fold longer in PCOSr, which was no longer significant after adjustments. Figure 1 depicts insulin-induced suppression of NEFA levels with time during OGTT and FSivGTT. As shown, NEFA curves are right-shifted in PCOSr as compared to controls, both during OGTT and FSivGTT. This shift is representative of the delay in insulin-induced NEFA suppression characterizing PCOSr girls.
Correlations
Including all women of both groups, we performed correlations between selected metabolic parameters (ISFSivGTT, 17OHPg, Leptin during OGTT, Ln(NEFA) Slope (FSivGTT) or Ln(NEFA) Slope/AUCinsulin (FSivGTT)) and either BMI-z score, waist/height ratio, insulin sensitivity (ISFSivGTT) or 17OHPg. After correction for Tanner stage or 17OHPg, adiposity only correlated with reduced ISFSivGTT (adjusted Ps≤0.03 for BMI-z and ≤0.03 for waist/height ratio), a less negative Ln(NEFA) slope/AUCinsulin ratio (adjusted Ps≤0.02 for BMI-z and Ps≤0.05 for waist/height ratio) and even more strongly with elevated fasting and 2-h leptin levels (adjusted Ps<0.0001 for BMI-z and ≤0.0002 for waist/height ratio). After adjustment for Tanner stage, waist/height ratio or BMI-z, reduced ISFSivGTT correlated with a less negative Ln(NEFA) slope (adjusted Ps≤0.04) and Ln(NEFA) slope/AUCinsulin ratio (adjusted Ps≤0.05, and P=0.03 after adjustment for 17OHPg), but was more strongly associated with high 17OHPg levels (adjusted Ps=0.0003). 17OHPg was also associated with leptin at time 120 min (P=0.02), which was independent of Tanner stage (adjusted P=0.02).
DISCUSSION
This study reports on the comparison between peripubertal girls with a genetic predisposition to PCOS and age-matched controls who were comprehensively assessed for metabolic and endocrine anomalies using the more accurate LC-MS/MS technique for androgen assays, the direct and robust FSivGTT method to determine insulin sensitivity, and for the first time a detailed in vivo assessment of insulin-induced NEFA suppression. Accordingly, this study found that peripubertal girls at risk for PCOS display increased levels of the adrenal androgen 17OHPg that are strongly correlated with insulin resistance, and that both these associations are independent of Tanner stage and adiposity. Furthermore, this study is the first to report that PCOS at-risk girls are characterized by an important decrease in insulin sensitivity and in insulin-induced suppression of NEFA levels, which are independent of adiposity and androgenemia, and correlate one with the other.
Impaired suppression of NEFA levels by insulin in PCOS at-risk girls may result from an intrinsic defect in the ability of insulin to inhibit intracellular adipose tissue lipolysis and to increase storage of NEFA, which tends to occur when adiposity develops in individuals at higher risk for metabolic complication (31). We previously reported a blunted insulin-stimulated NEFA storage in healthy adults whose parents both demonstrated type 2 diabetes during intravenous lipid administration (32). Furthermore, there is evidence that adipose tissue in PCOS women may be dysfunctional, as suggested by reduced adiponectin levels and LPL activity (33;34) as well as enlarged adipocyte volume (33), in association with insulin resistance. These findings thus suggest that NEFA uptake and storage in adipocytes of PCOS women might be impaired. Since overexposure of lean tissues to NEFA has been associated with the development of lipotoxicity-mediated insulin resistance (31), and since impaired NEFA suppressibility was highly correlated with insulin resistance in our study, adipose tissue dysfunction may be the cause of early development of insulin resistance in girls predisposed to PCOS.
In addition to insulin resistance, PCOS at-risk girls also exhibited β-cell dysfunction evidenced by the failure to increase insulin secretion to compensate for reduced insulin sensitivity, as measured by the disposition index (DI) (24). Both insulin resistance and β-cell dysfunction have been previously observed in PCOS women (5–7;35) as well as in pre- and peripubertal daughters of PCOS women using OGTT (6;7). Importantly, prolonged exposure to high NEFA levels using heparin+Intralipid induces β-cell dysfunction in healthy subjects (36). Whether adipose tissue dysfunction may explain both the insulin resistance and β-cell dysfunction that were observed in this study remains to be determined.
Despite being very well matched for age, Tanner stage was more advanced in PCOSr than in control girls, a finding that might be due to earlier adrenarche in PCOS at-risk girls, as previously reported (5;37;38), although none of PCOSr were diagnosed with precocious adrenarche. This may explain why the adrenal androgen 17OHPg was more significantly and robustly increased in PCOSr girls than ovarian androgens. Interestingly, insulin resistance was closely correlated with high 17OHPg levels in the present study, which suggests that a factor associated with insulin resistance may cause hyperandrogenemia or, conversely, that hyperandrogenemia may have lead to insulin resistance. The latter is less likely based on our results, because group differences in insulin sensitivity were more striking and were independent of 17OHPg levels, whereas group differences in 17OHPg wre less robust and were not independent of insulin sensitivity.
Compensatory hyperinsulinemia developing with insulin resistance may in part explain higher levels of the androgen 17OHPg in PCOSr, as in PCOS women (39;40). However, insulin levels and secretion were loosely correlated with 17OHPg in our study, suggesting that other factors may be involved. A previous study found that healthy adult women infused with heparin plus Intralipid, which mimics NEFA spillover, increased their androgen production (14), suggesting that lipotoxicity may directly trigger hyperandrogenesis in vivo. This later finding was confirmed in vitro by our team using bovine adrenal cells exposed to the saturated NEFA palmitate (15). Interestingly, our study found a strong correlation between resistance to insulin-mediated NEFA suppression during OGTT and higher 17OHPg levels. Such an association suggests a link between adipose tissue insulin resistance, which results in higher exposure of lean tissues to NEFA, and higher androgen levels in our population. Together, these findings support the hypothesis that adipocyte dysfunction and resulting lipotoxicity may explain both the early development of insulin resistance and increased androgenemia in peripubertal girls at higher risk for PCOS.
Although our study has important strengths, its design also presents some limitations. First, the number of girls in each group is relatively small. Accordingly, non-significant results should be interpreted with caution, but significant results are valid because we used robust non-parametrical statistical tests. Furthermore, considering that we performed demanding and comprehensive OGTT and FSivGTT procedures in healthy young girls, we believe this limited sample size is justified. Secondly, adiposity and Tanner stage were not matched between cases and controls. It is not surprising that our sample of PCOS-related girls displayed higher adiposity than controls since PCOS women and their family have been found to have increased prevalence of central obesity (10). Earlier adrenarche and increased adiposity may thus be part of the phenotype of girls genetically predisposed to PCOS. Matching for these factors would have masked this phenotype and compared artificial groups, which are not naturally occurring. On the other hand, we performed statistical adjustments to determine PCOSr characteristics that were independent of adiposity or Tanner stage, one factor at a time in order to respect the power of our study. Finally, the design of the study does not allow to determine whether significant correlations reflect a causative link or only associations.
In conclusion, peripubertal girls at high risk for developing PCOS display increased adiposity and androgenemia, but are mainly characterized by global insulin resistance and resistance to insulin-mediated suppression of lipolysis that are independent of adiposity and androgenemia. We thus report the first evidence that genetic predisposition to PCOS may be related to adiposity, early adipocyte dysfunction and insulin resistance, which may be linked together through lipotoxicity and which may drive the higher androgen levels found in peripurbertal PCOS at-risk girls.
Acknowledgments
Authors would like to thank the research nurses Diane Lessard and Caroll-Lynn Thibodeau of the Centre de recherche Clinique Étienne-LeBel for their work with research participants.
Financial support: This work was funded by Foundation of Stars. J.P.B. is a Senior Clinical-Investigator of the Fonds de recherche en santé du Québec (FRSQ). D.H.G. is the principal investigator of a multi-center study on the insulin sensitivities of peripubertal girls at risk to develop PCOS. He is supported for this study by National Institutes of Health K23 HD40325 and M01-RR000425 (Cedars-Sinai General Clinical Research Center Grant from National Center for Research Resources). A.C.C. is the recipient of the Canadian Institutes of Health Research-GlaxoSmithKline Chair in diabetes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary: No conflict of interest to report.
Reference List
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2759. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 4.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2008;93:1662–1669. doi: 10.1210/jc.2007-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maliqueo M, Sir-Petermann T, Perez V, Echiburu B, de Guevara AL, Galvez C, et al. Adrenal function during childhood and puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:3282–3288. doi: 10.1210/jc.2009-0427. [DOI] [PubMed] [Google Scholar]
- 6.Sir-Petermann T, Maliqueo M, Codner E, Echiburu B, Crisosto N, Perez V, et al. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4637–4642. doi: 10.1210/jc.2007-1036. [DOI] [PubMed] [Google Scholar]
- 7.Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, Ladron dG, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75:53–58. doi: 10.1016/s0015-0282(00)01662-9. [DOI] [PubMed] [Google Scholar]
- 9.Legro RS, Driscoll D, Strauss JF, III, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yildiz BO, Yarali H, Oguz H, Bayraktar M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2031–2036. doi: 10.1210/jc.2002-021499. [DOI] [PubMed] [Google Scholar]
- 11.Baillargeon JP, Carpentier AC. Brothers of women with polycystic ovary syndrome are characterised by impaired glucose tolerance, reduced insulin sensitivity and related metabolic defects. Diabetologia. 2007;50:2424–2432. doi: 10.1007/s00125-007-0831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sir-Petermann T, Angel B, Maliqueo M, Carvajal F, Santos JL, Perez-Bravo F. Prevalence of Type II diabetes mellitus and insulin resistance in parents of women with polycystic ovary syndrome. Diabetologia. 2002;45:959–964. doi: 10.1007/s00125-002-0836-3. [DOI] [PubMed] [Google Scholar]
- 13.Gormsen LC, Nielsen C, Jessen N, Jorgensen JO, Moller N. Time-course effects of physiological free fatty acid surges on insulin sensitivity in humans. Acta Physiol (Oxf) 2011;201:349–356. doi: 10.1111/j.1748-1716.2010.02181.x. [DOI] [PubMed] [Google Scholar]
- 14.Mai K, Bobbert T, Reinecke F, Andres J, Maser-Gluth C, Wudy SA, et al. Intravenous lipid and heparin infusion-induced elevation in free fatty acids and triglycerides modifies circulating androgen levels in women: a randomized, controlled trial. J Clin Endocrinol Metab. 2008;93:3900–3906. doi: 10.1210/jc.2008-0714. [DOI] [PubMed] [Google Scholar]
- 15.Bellanger S, Battista MC, Fink GD, Baillargeon JP. Saturated fatty acid exposure induces androgen overproduction in bovine adrenal cells. Steroids. 2012;77:347–353. doi: 10.1016/j.steroids.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpentier A, Patterson BW, Leung N, Lewis GF. Sensitivity to acute insulin-mediated suppression of plasma free fatty acids is not a determinant of fasting VLDL triglyceride secretion in healthy humans. Diabetes. 2002;51:1867–1875. doi: 10.2337/diabetes.51.6.1867. [DOI] [PubMed] [Google Scholar]
- 17.Pesant MH, Desmarais G, Fink GD, Baillargeon JP. Reference ranges for total and calculated free and bioavailable testosterone in a young healthy women population with normal menstrual cycles or using oral contraception. Clin Biochem. 2012;45:148–150. doi: 10.1016/j.clinbiochem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Azziz R, Dewailly D, Owerbach D. Clinical review 56: Nonclassic adrenal hyperplasia: current concepts. J Clin Endocrinol Metab. 1994;78:810–815. doi: 10.1210/jcem.78.4.8157702. [DOI] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 20.Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 21.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FERRIMAN D, GALLWEY JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 23.Henderson M, Rabasa-Lhoret R, Bastard JP, Chiasson JL, Baillargeon JP, Hanley JA, et al. Measuring insulin sensitivity in youth: How do the different indices compare with the gold-standard method? Diabetes Metab. 2011;37:72–78. doi: 10.1016/j.diabet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 25.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Berkowitz K, Marroquin A, et al. Response of pancreatic beta-cells to improved insulin sensitivity in women at high risk for type 2 diabetes. Diabetes. 2000;49:782–788. doi: 10.2337/diabetes.49.5.782. [DOI] [PubMed] [Google Scholar]
- 26.Marcelli-Tourvieille S, Hubert T, Pattou F, Vantyghem MC. Acute insulin response (AIR): review of protocols and clinical interest in islet transplantation. Diabetes Metab. 2006;32:295–303. doi: 10.1016/s1262-3636(07)70283-5. [DOI] [PubMed] [Google Scholar]
- 27.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 28.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 29.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 30.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 31.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 32.Brassard P, Frisch F, Lavoie F, Cyr D, Bourbonnais A, Cunnane SC, et al. Impaired plasma nonesterified fatty acid tolerance is an early defect in the natural history of type 2 diabetes. J Clin Endocrinol Metab. 2008;93:837–844. doi: 10.1210/jc.2007-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Oden A, Holm G, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:E304–E311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 34.Villa J, Pratley RE. Adipose tissue dysfunction in polycystic ovary syndrome. Curr Diab Rep. 2011;11:179–184. doi: 10.1007/s11892-011-0189-8. [DOI] [PubMed] [Google Scholar]
- 35.Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Tapanainen JS. Insulin sensitivity, insulin secretion, and metabolic and hormonal parameters in healthy women and women with polycystic ovarian syndrome. Hum Reprod. 2000;15:1266–1274. doi: 10.1093/humrep/15.6.1266. [DOI] [PubMed] [Google Scholar]
- 36.Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF. Lipid-induced pancreatic beta-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab. 2011;300:E255–E262. doi: 10.1152/ajpendo.00416.2010. [DOI] [PubMed] [Google Scholar]
- 37.Ibanez L, Potau N, Carrascosa A. Insulin resistance, premature adrenarche, and a risk of the Polycystic Ovary Syndrome (PCOS) Trends Endocrinol Metab. 1998;9:72–77. doi: 10.1016/s1043-2760(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 38.Bronstein J, Tawdekar S, Liu Y, Pawelczak M, David R, Shah B. Age of onset of polycystic ovarian syndrome in girls may be earlier than previously thought. J Pediatr Adolesc Gynecol. 2011;24:15–20. doi: 10.1016/j.jpag.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83:2001–2005. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 40.Baillargeon JP, Carpentier A. Role of insulin in the hyperandrogenemia of lean women with polycystic ovary syndrome and normal insulin sensitivity. Fertil Steril. 2007;88:886–893. doi: 10.1016/j.fertnstert.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]