Abstract
How the left ventricular (LV) remodels in response to a high volume stimulus is important in evaluating the endurance athlete’s heart. Marathoners and patients with isolated, moderate chronic compensated mitral regurgitation (MR) represent physiologic and pathologic forms of eccentric LV remodeling in response to intermittent and chronic volume overload, respectively. We therefore performed magnetic resonance imaging with tissue tagging and three-dimensional data analysis at rest in 19 marathoners (39±10 years, 47% female), 17 patients with isolated MR without coronary artery disease or medical therapy (46±5 years, 53% female), and 24 controls (45±8 years, 50% female). Marathoners and MR patients had ~35% greater LV end-diastolic (ED) volume index, ~ 50% greater end-systolic (ES) volume index and ~ 34% great LV stroke volume index (P<0.0001) vs. controls. However, marathoners’ hearts had increased long axis length, while MR hearts did not differ from controls. MR hearts had greater LV global and apex sphericity vs. marathoners and controls (P<0.0001). Marathoners had normal LV mass/volume ratio and wall thickness while these were significantly decreased in the MR group. In marathoners, LV baseline work rate was similar to controls and higher in MR vs. controls. In conclusion, marathoners’ hearts achieve elevated resting stroke volume with adherence to an elliptical shape defined by 3D geometry and mass/volume. Thus, a comprehensive evaluation of LV geometry and mass to volume may be important in the evaluation of the athlete’s heart.
Keywords: marathoner, mitral regurgitation, sphericity, MRI
INTRODUCTION
Marathoners experience cardiac remodeling that is characterized by commensurate increases in left ventricle (LV) volume and mass that result in increased stroke volume (SV).1 This eccentric cardiac hypertrophy is a putative adaptation to endurance training that is generally thought to enhance physiologic reserve capacity. However, there is controversy regarding the potential pathological consequences of cardiac enlargement2–3. Chronic compensated mitral regurgitation (MR) represents another form of cardiac enlargement. Although both conditions are associated with an increase in LVSV, MR is facilitated by regurgitation through a secondary ejection pathway into left atrium that preserves LV shortening and ejection fraction (EF). Importantly, the MR hearts do not achieve such a similar large increase in LV volumes and stroke volume due to functional MR that is usually a result of primary myocardial disease, but rather due to primary degenerative mitral valve disease with a normal LV ejection fraction. Although not nearly the same facilitation of ejection, the marathon heart ejects into a relatively compliant vascular bed.4–5 Previous study has compared these two conditions by their biochemical and molecular signaling mechanisms.6 However, how changes in LV geometry and mechanics in the marathoners differ from a pathologic form of volume overload which still maintains LV systolic shortening in a normal range remains to be elucidated. The question of the “appropriateness or suitability” of cardiac enlargement in the athlete’s heart is frequently raised. One major factor in defining a physiologic response to a chronic hemodynamic stress is the adherence to an elliptical LV shape, as opposed to an increase in LV sphericity, and the maintenance of a normal LV mass/volume. Deviations from a normal match of geometry and muscle mass can cause increases in wall stress and myocardial oxygen demand, resulting in decreased LV function or sudden death.7 Therefore, in the current study, we compare marathon runners’ hearts to that of patients with isolated MR with similar LV volumes and SV utilizing magnetic resonance imaging (MRI) with tissue tagging and 3-dimensional (3D) analysis.
METHODS
Nineteen marathoners, 17 degenerative isolated MR patients and 24 controls comprised the study population. The control subjects and the marathoners had no history of cardiovascular disease and were not using any prescription medication. Control subjects were not engaged in any aerobic training, with only varying degrees of recreational activities. Marathoner designation was based upon having run 4 full marathons over the prior two years and running an average of 50 miles per week. Chronic isolated MR was defined as at least moderate severity with LV EF>60% based on echocardiographic/Doppler examination in the absence of symptoms or obstructive coronary artery diseases determined by exercise testing with nuclear perfusion. No MR patient had a history of hypertension or was taking any medication at the time of study. The study protocol was approved by the University of Alabama at Birmingham Institutional Review Board and all participants gave written informed content.
All participants underwent MRI on a 1.5T scanner (Signa, GE Healthcare, Milwaukee, Wisconsin) optimized for cardiac imaging. Electrocardiographically gated, breath-hold, segmented k-space steady state free precession technique was used to obtain cine images with standard (2-, 3- and 4- Chamber, and Short-axis) views using the following typical parameters: field of view 40×40 cm, image matrix 256 ×128, flip angle 45°, repetition/echo times 4/1.8 ms, cardiac phases 20, slice thickness 8 mm without any slice gap. Tagged MRI was acquired on the same scanner using the following typical parameters: repetition/echo times 8/4.2 ms, tag spacing 7 mm. Tag lines were tracked8 and edited, if necessary, by expert users. Endocardial and epicardial contours were manually traced on cine MRI acquired near end-diastole (ED) and end-systole (ES) by blinded assignment. Volumes were calculated from summated serial short axis volumes and indexed to BSA.9 LV 3D geometric parameters were measured based on the contours using the in-house developed software9. LV two-dimensional (2D) apex curvatures were computed from endocardial contours drawn on 4-chamber view using standard formula.10 Sphericity index was defined as the ratio of LV long-axis length to inner diameter.11 LVES maximum shortening was computed at all wall segments12 (excluding the apex) using an in-house developed software13 and averaged. LVES 3D twist was computed as described by Russel IK et al14. LV 2D twist was calculated in each timeframe using improved HARP tracking15 and 2D twist-time curve was constructed and differentiated with respect to time to compute LV peak early diastolic untwist velocity (°/beat) which was normalized to heart rate (HR).
LV work rate (mmHg×L/min) was defined as16, 17, LV work rate = LVSV×LV-Pes×HR/1000, where LV-Pes is the LVES pressure, which can be approximated by the mean LV systolic blood pressure Pmean, defined as, . Both systolic and diastolic BPs were measured by sphygmomanometry with patients in the supine position immediately before and after MRI scanning. The reported systolic and diastolic BPs were the average of pre- and post-MRI scanning values.
One-way analysis of variance (ANOVA) and generalized Wald test were used to compare groups for each continuous and categorical variables, respectively. Homogeneity of variance was tested using Levene’s test. Appropriate Data transformation was conducted if the homogeneity assumption was violated. Tukey-Kramer procedure was performed to control the pairwise comparisons among the groups. Data are presented here as mean±SD. A P<0.05 was considered statistically significant. All statistical analysis was performed using SAS version 9.2.
RESULTS
Controls, marathoners and MR groups had matched age, gender and BSA (Table 1). Marathoners had a lower resting HR vs. controls and MR. Both diastolic and systolic BPs did not differ among the three groups. LV work rate did not differ in the marathoners vs. controls while it was significantly higher in MR. Marathoners and MR had greater LVEDV, LVESV and LVSV indices vs. controls (Table 2). LVEF did not differ among the groups. Marathoners had significantly greater LV mass index vs. controls but LV mass index did not differ between MR and controls. LV mass/volume ratio was significantly lower in MR group vs. control and marathoners. Marathoners had commensurate increases in RVEDV, RVESV and RVSV indices vs. controls; while MR RV volumes did not differ from controls. RVEF was similar in all groups.
Table 1.
Baseline Characteristics in Controls, Marathoners and Mitral regurgitation
| Variable | Control (N=24) | Marathoner (N=19) | Mitral regurgitation (N=17) |
|---|---|---|---|
| Age (years) | 45±8 | 39±10 | 46±5 |
| Women (%) | 50 | 47 | 53 |
| Body Surface Area (m2) | 1.91±0.17 | 1.78±0.23 | 1.90±0.20 |
| Heart Rate (beats/min) | 67±11 | 55±9* | 64±11† |
| Diastolic BP (mm Hg) | 77±9 | 73±10 | 75±9 |
| Systolic BP (mm Hg) | 123 ±13 | 114±15 | 118±15 |
| LV Work Rate (mm Hg × L/min) | 613±174 | 544±177 | 789±215*† |
Values are n or mean ± std. BP: Blood Pressure; LV: Left Ventricle;
P<0.05 vs. Control;
P <0.05 vs. Marathoner
Table 2.
Ventricular Volume and Mass
| Variable | Control (N=24) | Marathoner (N=19) | Mitral regurgitation (N=17) |
|---|---|---|---|
| LV ED volume index (ml/m2) | 69±10 | 92±15* | 98±18* |
| LV ES volume index (ml/m2) | 25±7 | 37±8* | 36±8* |
| LV stroke volume index (ml/m2) | 44±7 | 55±7* | 62±14* |
| LV ejection fraction (%) | 65±7 | 60±4 | 63±6 |
| LV mass index (gm/m2) | 52±12 | 62±9* | 58±10† |
| LV mass/volume (gm/ml) | 0.76±0.18 | 0.76±0.12 | 0.60±0.07*† |
| RV ED volume index (ml/m2) | 72±11 | 104±13* | 78±15† |
| RV ES volume index (ml/m2) | 34±8 | 47±8* | 35±8† |
| RV stroke volume index (ml/m2) | 39±8 | 58±8* | 43±10† |
| RV ejection fraction (%) | 54±8 | 55±5 | 55±7 |
Values are mean ± std. LV: Left Ventricle; RV: Right Ventricle; ED: end-diastolic; ES: end-systolic; BSA: Body surface area;
P<0.05 vs. Control;
P <0.05 vs. Marathoner.
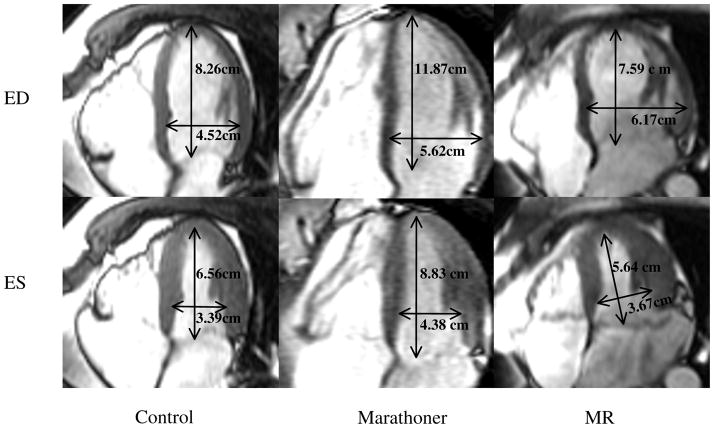
As shown in Table 3, LV dimension was significantly increased in MR and marathoners vs. controls, while LVES inner diameter did not differ in marathoners vs. controls. However, marathoners had significantly longer LV length vs. MR. Therefore, marathoners had normal sphericity indices, while MR hearts were more spherical. MR hearts also had significantly lower apex curvatures vs. marathoners and controls, as demonstrated in Figures 1 and 2.
Table 3.
Left Ventricular Global Geometry
| Variable | Control (N=24) | Marathoner (N=19) | Mitral regurgitation (N=17) |
|---|---|---|---|
| LV ED diameter (mm) | 51±4 | 54±4* | 59±4*† |
| LV ES diameter (mm) | 36±4 | 38±4 | 39±3* |
| LV ED length (mm) | 90±8 | 98±10* | 89±10† |
| LV ES length (mm) | 71±8 | 76±9 | 67±10† |
| LV ED sphericity index | 1.77±0.19 | 1.80±0.18 | 1.53±0.16*† |
| LV ES sphericity index | 1.96±0.29 | 1.96±0.17 | 1.71±0.20*† |
| LV ED apex curvature (1/cm) | 1.40±0.29 | 1.36±0.26 | 1.00±0.31*† |
| LV ES apex curvature (1/cm) | 2.84±1.21 | 2.20±0.53 | 1.70±0.43*† |
Values are mean ± std. MR: mitral regurgitation; LV: Left Ventricle; ED: End-Diastole; ES: End-Systole;
P<0.05 vs. controls;
P <0.05 vs. Marathoner.
Figure 1. Left ventricular (LV) and right ventricular (RV) MRI short-axis images from control, Marathoner and MR at their basal slice just above the tip of the papillary muscle at end-diastole.
The marathoner and the MR patient have increased LV dimension vs. control. However, the RV size was proportionately increased in the marathoner but did not differ from control in the MR.
Figure 2. LV and RV MRI long-axis images from control, Marathoner and MR patient at ED and end-systole (ES).
As opposed to the MR heart, the marathoner’s heart has a proportionate increase in both LV and RV lengths and transverse dimensions.
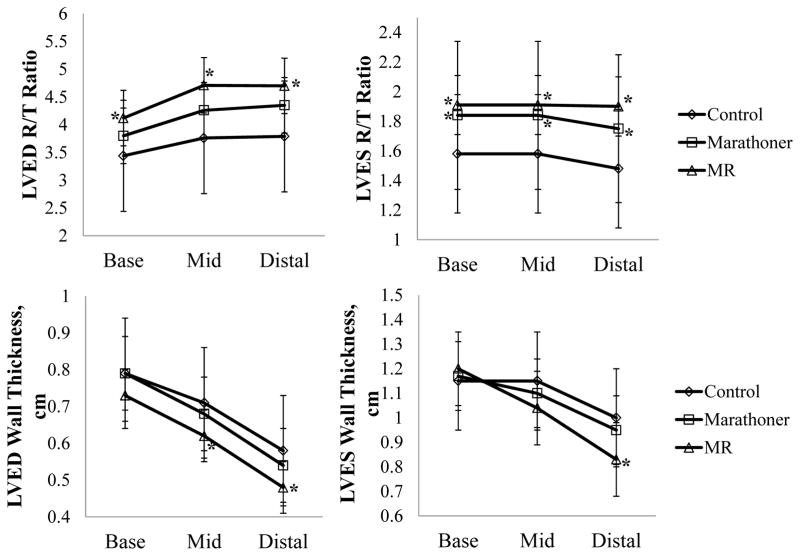
The two most important factors that determine a physiologic remodeling response are the adherence to an elliptical geometry and an appropriate match of LV mass to volume. In an attempt to provide a clinically useful parameter to define an appropriate match of geometry and mass, control group’ mean-1 SD of the product of LVED sphericity index and LVED mass/volume ratio (0.9269) was used to differentiate pathologic vs. physiologic LV enlargement in marathoners (Figure 3). Using this cutoff, only one marathoner was categorized having pathologic volume overload while the others were considered physiologic LV enlargement. Figure 4 shows LV R/T ratios were increased in MR vs. control while LVES R/T ratio was increased in marathoners vs. controls. LV wall thickness remained normal in the marathoner group; while it was significantly decreased in MR from mid to distal LV vs. controls.
Figure 3. Classification of marathoners with pathologic versus physiologic volume overload using the product of LVED sphericity and LV mass/volume ratio.
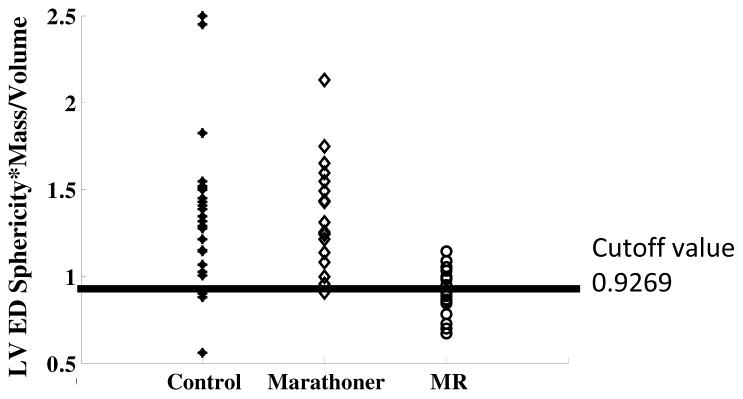
Black line: cutoff value.
Figure 4. Comparisons of LV ED and ES geometric remodeling in controls, marathoners and MR patients.
The MR group has significantly increased LVED and LVES R/T ratio, while the marathoners’ R/T ratio is significantly increased only at ES. The MR group also demonstrates significantly decreased wall thickness at both ED and ES, while the wall thickness remains normal in the marathoners’ group. *: P<0.05 vs. controls.
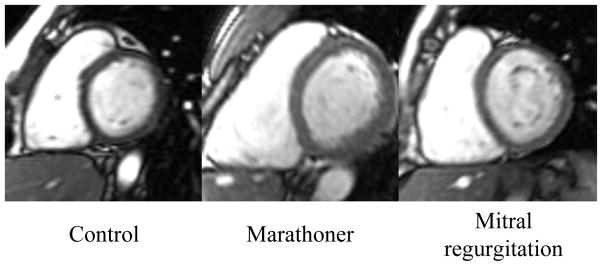
LVES maximum shortening strain did not differ among all groups. LVES twist angle was significantly decreased in marathoner vs. MR and controls (Table 4). Figure 5 illustrates the differences in LVES twist among three groups using a 3D color map, an overall brighter color (larger twist angle) in the MR vs. dimmer color (smaller angle) in the marathoner. LV peak early diastolic untwist velocity normalized to HR was not significantly different among all groups.
Table 4.
Left Ventricular Strain Parameters
| Variable | Control (N=24) | Marathoner (N=19) | Mitral regurgitation (N=17) |
|---|---|---|---|
| LV ejection fraction (%) | 65±7 | 60±4 | 63±6 |
| LV ES maximum shortening (%) | 20.66±2.19 | 19.48±1.46 | 21.08±2.42 |
| LV ES twist (°) | 4.49±1.01 | 3.67±1.04* | 4.65±0.93† |
| Peak early diastolic untwist velocity (°/beat) | 32±9 | 27±9 | 30±13 |
Values are mean ± std. LV: Left Ventricle; ES: End-Systole; E: mitral valve peak velocity in early diastole; A: mitral valve peak velocity in late diastole;
P<0.05 vs. controls;
P <0.05 vs. Marathoners.
Figure 5. LVES twist of three examples of control, marathoner and MR.
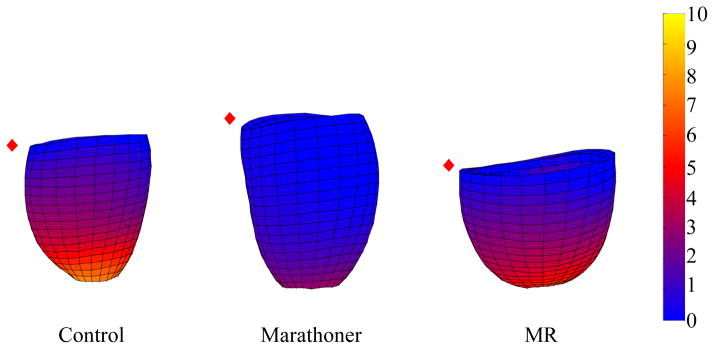
The graphic displays LVES twist angle using a color map (range from 0° to 10°). Twist angle in the marathoner’s heart is significantly less compared with both control and MR. (red diamond: mid septum)
DISCUSSION
This work is the first study to compare 3D LV geometric remodeling and mechanics in marathoners at rest vs. chronic compensated MR using MRI. In the setting of similar increases in LV volumes and SV, marathoners’ hearts maintain an elliptical shape and normal wall thickness with lower LV twist, while there is a greater global and regional LV sphericity in the MR hearts.
Marathoner hearts have normal LV wall thickness and mass/volume, which is decreased in MR, along with greater LV sphericity and smaller LV apex curvatures. The increase in LV length is of particular importance in distinguishing marathoners’ from MR hearts in the face of a similar LVESD measured at the tips of the paplillary muscles in both groups. Using MRI with 3D analysis, we have recently demonstrated extensive LV spherical remodeling in MR, beyond the base of the heart and tips of the papillary muscles9. This extensive amount of LV remodeling is not addressed by the standard LVESD but contributes to a greater LVES volume. Taken together, marathoners maintain the normal elliptical shape with a proportionately longer LV and higher LV mass, while the MR heart undergoes a more spherical eccentric remodeling that has been reported to be associated with increased adrenergic drive18,19 and abnormal extracellular matrix loss, along with cardiomyocyte thinning and elongation.19,20 Furthermore, the marathoner’s heart undergoes parallel increases in RV volume indices. Thus, the entire marathoner’s heart is enlarged and elongated with an appropriate compensatory increase in LV mass. Using the LV sphericity index and mass to volume product at one standard deviation from the mean control, all but one marathoner is classified with a physiologic LV enlargement.
Marathoners have lower resting HR, which is attributed to a higher vagal tone.21 Despite the smaller ES twist at rest, marathoners achieve normal LVEF and peak early diastolic untwist velocity through normal maximum shortening. It is of interest that a recent report shows that endurance exercise training is associated with a significant increase in LV twist mechanics during exercise.22 Taken together, the physiological and architectural findings herein support the concept that by adapting to high volume endurance training, marathoners meet resting cardiac output requirements by operating at a slightly lower percentage of maximum twist capacity vs. controls. This low-demand resting state may be a result of higher resting vagal tone and thus may allow for a greater physiological reserve during the demand of extreme endurance exercise.
By utilizing cMRI, all measurements of the study are performed in supine position. In addition to the intermittent vs. chronic volume overload stress, the comparison of marathoners to MR is limited in that the marathoner heart ejects into the high pressure aorta, while MR ejects into the low pressure left atrium. In aortic regurgitation patients, in which the excess LV volume ejected into the high pressure aorta, LV maintains a more elliptical even conical shape compared to MR and normal subjects.23 Nevertheless, the comparison of MR and marathoners’ hearts with similar LV dimensions and volumes underscores the potential importance of an assessment of LV 3D geometry and mass in determining physiologic vs. pathologic cardiac enlargement in the evaluation of an “enlarged heart” of an athlete.
Acknowledgments
This study was funded by NHLBI Specialized Center for Clinically Oriented Research (SCCOR) in Cardiac Dysfunction P50HL077100.
References
- 1.Levine BD. V̇O2, max: what do we know, and what do we still need to know? J Physiol. 2008;586:25–34. doi: 10.1113/jphysiol.2007.147629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauschke J, Maisch B. Athlete’s heart or hypertrophic cardiomyopathy? Clin Res Cardiol. 2009;98:80–88. doi: 10.1007/s00392-008-0721-2. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PD. Historical concepts of the athlete’s heart. Med Sci Sports Exerc. 2004;36:363–370. doi: 10.1249/01.mss.0000117117.67849.f6. [DOI] [PubMed] [Google Scholar]
- 4.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 6.Weeks KL, McMullen JR. The Athlete’s Heart vs. the Failing Heart: Can Signaling Explain the Two Distinct Outcomes? Physiology. 2011;26:97–105. doi: 10.1152/physiol.00043.2010. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S, Denney T, Jr, Powell P, McGiffin DC, Dell’Italia LJ. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction> 60% J Am Coll Cardiol. 2010;55:671–679. doi: 10.1016/j.jacc.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Denney TS. Left ventricular motion reconstruction with a prolate spheroidal B-spline model. Phys Med Biol. 2006;51:517–537. doi: 10.1088/0031-9155/51/3/004. [DOI] [PubMed] [Google Scholar]
- 9.Schiros CG, Dell’Italia LJ, Gladden JD, Clark D, 3rd, Aban I, Gupta H, Lloyd SG, McGiffin DC, Perry G, Denney TS, Jr, Ahmed MI. Magnetic resonance imaging with three-dimensional analysis reveals important left ventricular remodeling in isolated mitral regurgitation: implications beyond dimensions. Circulation. 2012;125:2334–2342. doi: 10.1161/CIRCULATIONAHA.111.073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipschultz MM. Differential Geometry. 1. McGraw Hill; 1969. p. 61. [Google Scholar]
- 11.Di Donato M, Dabic P, Castelvecchio S, Santambrogio C, Brankovic J, Collarini L, Joussef T, Frigiola A, Buckberg G, Menicanti L RESTORE Group. Left ventricular geometry in normal and post-anterior myocardial infarction patients: sphericity index and ‘new’ conicity index comparisons. Eur J Cardiothorac Surg. 2006;29:S225–S230. doi: 10.1016/j.ejcts.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Denney TS. Left ventricular motion reconstruction with a prolate spheroidal B-spline model. Phys Med Biol. 2006;51:517–537. doi: 10.1088/0031-9155/51/3/004. [DOI] [PubMed] [Google Scholar]
- 14.Russel IK, Gotte MJ, Bronzwaer JG, Knaapen P, Paulus WJ, van Rossum AC. Left ventricular torsion: an expanding role in the analysis of myocardial dysfunction. JACC Cardiovasc Imaging. 2009;2:648–655. doi: 10.1016/j.jcmg.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Khalifa A, Youssef AB, Osman N. Improved harmonic phase (harp) method for motion tracking a tagged cardiac mr images. Conf Proc IEEE Eng Med Biol Soc. 2005;4:4298–4301. doi: 10.1109/IEMBS.2005.1615415. [DOI] [PubMed] [Google Scholar]
- 16.Knaapen P, Germans T, Knuuti J, Paulus WJ, Dijkmans PA, Allaart CP, Lammertsma AA, Visser FC. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation. 2007;115:918–927. doi: 10.1161/CIRCULATIONAHA.106.660639. [DOI] [PubMed] [Google Scholar]
- 17.Stewart RA, Raffel OC, Kerr AJ, Gabriel R, Zeng I, Young AA, Cowan BR. Pilot Study to Assess the Influence of β-Blockade on Mitral Regurgitant Volume and Left Ventricular Work in Degenerative Mitral Valve Disease. Circulation. 2008;118:1041–1046. doi: 10.1161/CIRCULATIONAHA.108.770438. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed MI, Aban I, Lloyd SG, Gupta H, Howard G, Inusah S, Peri K, Robinson J, Smith P, McGiffin D, Denney TS, Dell’Italia LJ. A randomized controlled phase IIb trial of Beta-1 receptor blockade in isolated degenerative mitral regurgitation. J Am Coll Cardiol. 2012;60:833–838. doi: 10.1016/j.jacc.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carabello BA. Beta-blockade for mitral regurgitation: could the management of valvular heart disease actually be moving into the 21st century? J Am Coll Cardiol. 2012;60:839–840. doi: 10.1016/j.jacc.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Pat B, Killingsworth C, Denney TS, et al. Dissociation between cardiomyocyte function and remodeling with -adrenergic receptor blockade in isolated canine mitral regurgitation. Am J Physiol Heart Circ Physiol. 2008;295:H2321–2327. doi: 10.1152/ajpheart.00746.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen Urstad K, Saltin B, Ericson M, Storck N, Jensen Urstad M. Pronounced resting bradycardia in male elite runners is associated with high heart rate variability. Scand J Med Sci Sports. 1997;7:274–278. doi: 10.1111/j.1600-0838.1997.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 22.Weiner RB, Hutter AM, Jr, Wang F, Kim J, Weyman AE, Wood MJ, Picard MH, Baggish AL. The impact of endurance exercise training on left ventricular torsion. JACC Cardiovasc Imaging. 2010;3:1001–1009. doi: 10.1016/j.jcmg.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Hiro T, Katayama K, Miura T, Kohno M, Fujii T, Hiro J, Matsuzaki M. Stroke volume generation of the left ventricle and its relation to chamber shape in normal subjects and patients with mitral or aortic regurgitation. Jpn Circ J. 1996;60:216–227. doi: 10.1253/jcj.60.216. [DOI] [PubMed] [Google Scholar]