Abstract
This study examined the distribution of γ-aminobutyric acid (GABA)B receptors on immunohistochemically identified neurons, and levels of GABAB(1) and GABAB(2) mRNA, in the L4 and L5 dorsal root ganglia (DRG) of the rat in the absence of injury and 2 weeks after L5 spinal nerve ligation. In uninjured DRG, GABAB(1) immunoreactivity colocalized exclusively with the neuronal marker (NeuN) and did not colocalize with the satellite cell marker S-100. The GABAB(1) subunit colocalized to >97% of DRG neurons immunoreactive (IR) for neurofilament 200 (N52) or calcitonin gene-related peptide (CGRP), or labeled by isolectin B4 (IB4). Immunoreactivity for GABAB(2) was not detectable. L5 spinal nerve ligation did not alter the number of GABAB(1)-IR neurons or its colocalization pattern in the L4 DRG. However, ligation reduced the number of GABAB(1)-IR neurons in the L5 DRG by ≈38% compared with sham-operated and naïve rats. Specifically, ligation decreased the number of CGRP-IR neurons in the L5 DRG by 75%, but did not decrease the percent colocalization of GABAB(1) in those that remained. In the few IB4-positive neurons that remained in the L5 DRG, colocalization of GABAB(1)-IR decreased to 75%. Ligation also decreased levels of GABAB(1) and GABAB(2) mRNA in the L5, but not the L4 DRG compared with sham-operated or naïve rats. These findings indicate that the GABAB receptor is positioned to presynaptically modulate afferent transmission by myelinated, unmyelinated, and peptidergic afferents in the dorsal horn. Loss of GABAB receptors on primary afferent neurons may contribute to the development of mechanical allodynia after L5 spinal nerve ligation.
INDEXING TERMS: neuropathic pain, mechanical allodynia, primary afferent neuron
Loss of γ-aminobutyric acid (GABA)-mediated inhibition in the dorsal horn of the spinal cord has long been proposed to contribute to the development or maintenance of spontaneous pain behaviors, mechanical allodynia, and thermal hyperalgesia after peripheral nerve injury (reviewed by Hammond, 1997; Sandkuhler, 2009; Todd, 2010). Studies of the disposition and release of GABA in the dorsal horn have yielded contradictory neuroanatomical (Ibuki et al., 1997; Eaton et al., 1998; Polgar et al., 2003; Scholz et al., 2005) and neurochemical findings (Stiller et al., 1996; Somers and Clemente, 2002; Lever et al., 2003). However, loss of GABA-mediated inhibition can occur independently of changes in neurotransmitter release, e.g., by a decrease in the affinity, number, or function of the receptors at which GABA acts. Of these, the heterodimeric GABAB receptor (Bettler and Tiao, 2006; Padgett and Slesinger, 2010; Pinard et al., 2010) is particularly well situated to modulate synaptic transmission in the dorsal horn. Activation of postsynaptic GABAB receptors hyperpolarizes dorsal horn neurons (Kangrga et al., 1991; Yang et al., 2001) and inhibits their responses to noxious stimuli (Willcockson et al., 1984), while activation of presynaptic GABAB receptors (Price et al., 1987; Yang et al., 2002) inhibits the release of peptide and excitatory amino acid neurotransmitters from the central terminals of primary afferents in the dorsal horn (Go and Yaksh, 1987; Kangrga et al., 1991; Malcangio and Bowery, 1993; Ataka et al., 2000; Iyadomi et al., 2000; Wang et al., 2007).
Several observations support the proposal that a decrease in the number, affinity, or coupling of the GABAB receptor in the dorsal horn may underlie the allodynia and hyperalgesia that occur after peripheral nerve injury. Pharmacological antagonism of spinal GABAB receptors (Hao et al., 1994) produces mechanical allodynia. Deletion of the gene for either subunit of the GABAB receptor (Schuler et al., 2001; Gassmann et al., 2004) produces hyperalgesia. Conditional deletion of the GABAB(1) subunit in C and Aδ primary afferent fibers results in exacerbated responses of Aδ fibers to mechanical stimuli, although this finding does not translate to enhanced mechanical allodynia or hyperalgesia after nerve injury (Gangadharan et al., 2009). Finally, streptozocin-induced diabetic neuropathy is associated with a loss of mRNA and protein for the GABAB(1) subunit in the dorsal horn (Wang et al., 2011), and a diminution of the presynaptic inhibitory actions of baclofen on glutamatergic, but not GABAergic or glycinergic afferents (Wang et al., 2007).
The present study therefore examined whether the number of primary afferent neurons that express the GABAB receptor is decreased after L5 spinal nerve ligation (SNL), a rodent model of nerve injury that results in our hands in mechanical allodynia and spontaneous pain behaviors (Engle et al., 2006). Indirect immunofluorescence methods were used to examine the distribution of GABAB(1) subunit immunoreactivity among three different classes of primary afferent neuron in the dorsal root ganglion (DRG) of naïve rats and rats that underwent sham surgery or L5 SNL. Myelinated afferents were identified by their immunoreactivity for neurofilament 200 using the N52 monoclonal antibody (Robertson et al., 1991), while unmyelinated afferents were identified by their labeling with isolectin B4 (IB4) from Griffonia simplicifonia (Kitchener et al., 1993; Wang et al., 1998) and peptidergic afferents were identified by their immunoreactivity for calcitonin gene-related peptide (CGRP). The optical fractionator method was used to obtain an unbiased estimate of the number of GABAB(1)-immunoreactive (IR) neurons, while area measurements of GABAB(1)-IR profiles identified possible changes in the distribution of cell size among treatment groups. The immunohistochemical analysis was restricted to the GABAB(1) subunit because levels of GABAB(2) protein were below detectable limits in the DRG (McCarson and Enna, 1999; Engle et al., 2006). Finally, quantitative real-time polymerase chain reaction (RT-PCR) was used to determine levels of GABAB(1) and GABAB(2) subunit mRNA in the L4 and L5 DRG of naïve, sham-operated and ligated rats.
MATERIALS AND METHODS
These experiments were approved by the University of Iowa Animal Care and Use Committee and were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health and the ethical guidelines of the International Association for the Study of Pain. Every effort was made to reduce the number and suffering of animals used in this study.
Animals and surgical preparation
Male Sprague–Dawley rats (Charles River, Portage, IN) were housed in pairs on a reverse 12-hour light cycle (lights off at 9:00 AM) with water and soy-free food (Harlan Teklad 1026; Madison, WI) provided ad libitum. The rats were maintained for at least 7 days on the new diet and light conditions before they were used. Tight ligation of the L5 spinal nerve was performed as described by Kim and Chung (Kim and Chung, 1992; Choi et al., 1994). Briefly, under halothane anesthesia the L6 transverse process was exposed and removed using aseptic surgical technique. The L4 and L5 spinal nerves were gently separated using a blunt probe and the L5 spinal nerve was tightly ligated with 6-0 silk. The muscle and fascia were closed in layers using Vicryl sutures and the skin was closed with surgical staples. Sham surgeries were performed in an identical manner, including placement of the glass hook under the L5 spinal nerve, except that the L5 spinal nerve was not ligated. Naïve rats did not undergo surgery, but were housed, transported, and handled in an identical fashion. For the stereological analysis, eight rats underwent SNL, seven rats underwent sham surgery, and six rats were allocated to the naïve group. For the RT-PCR experiments, seven rats underwent SNL, seven rats underwent sham surgery, and six rats were allocated to the naïve group.
Behavioral testing
Two weeks after surgery, rats were transported to the behavioral testing facility and were allowed to acclimate for 30 minutes. Rats were then placed in elevated Plexiglas testing chambers with mesh flooring and allowed to acclimate for a further 30 minutes. The mechanical withdrawal threshold was then determined for both hindpaws of each rat using calibrated von Frey monofilaments (Smith & Nephew, Germantown, WI) and the up-and-down method of Dixon as described by Chaplan et al. (1994). The von Frey monofilaments were applied to the webbing between the third and fourth digit of the rat hindpaw. The maximum filament used was 28.8 g. Rats that did not respond to this filament were assigned this value. The tester was blinded to treatment condition except for naïve rats, which did not have a surgical scar. Measurements of thermal hyperalgesia were not made because a previous study failed to demonstrate a consistent reduction in thermal response latencies (Engle et al., 2006). This finding concurs with another report (Hogan et al., 2004) using the same strain of rat, similar stimulus intensity, and observers who were also unaware of the surgical status of the rats.
Immunohistochemistry
One day after behavioral testing, rats were deeply anesthetized with sodium pentobarbital (75 mg/kg intra-peritoneally). A catheter was then inserted into the proximal ascending aorta and the rat was perfused with 150 ml of 0.05 M phosphate buffer pH 7.4 at 37°C followed by 500 ml of ice-cold 4% paraformaldehyde in phosphate buffer, pH 7.4. The ipsilateral L4 and L5 DRG were removed and placed in 30% sucrose phosphate buffer overnight at 4°C for cryoprotection.
DRG from three to six rats from each treatment group were selected for inclusion in this study. The length of each DRG was measured and the number of transverse 80-μm sections that could be obtained through the DRG was determined. The total number of possible slices was then divided by the number of desired disectors (8–10) to establish the sampling interval (k). The start number for the first disector was assigned using a table of random integers between the number 1 and k. Each disector comprised three serial sections. The first section in the set was processed for GABAB(1)-IR and N52-IR, the second section for GABAB(1)-IR and labeling by IB4, and the third section for GABAB(1)-IR and CGRP-IR. Hence, sections were obtained through the DRG in accordance with stereological principles (Coggeshall, 1992; Coggeshall and Lekan, 1996; West, 1999).
All immunohistochemistry was performed using free-floating techniques. The DRGs were frozen in OCT mounting media, cut transversely at 80 μm on a cryostat, and collected into 0.1 M phosphate-buffered saline (PBS) pH 7.4. Sections were first incubated in 50% methanol to increase tissue permeability and rinsed four times in 0.1 M PBS. Sections were then incubated for 1 hour in 6% normal donkey serum (Lampire, Pipersville, PA) with 0.3% Triton X-100 prepared in 0.1 M PBS pH 7.4, which was also used as the diluent for all antibody solutions. The sections were then incubated in primary antibody solutions for 3 days at 4°C on an orbital shaker. The first section of each set of disectors was labeled with guinea pig anti-GABAB(1) (1:2,000; Chemicon AB1531; Temecula, CA) and mouse anti-N52 (1:4,000; Sigma N0142; St. Louis, MO). The second section of each set was labeled with guinea pig anti-GABAB(1) and biotinylated IB4 (1:4,000; Vector B-1105; Burlingame, CA). The third section of each set was labeled with guinea pig anti-GABAB(1) and rabbit anti-CGRP (1:4,000; Peninsula T-4032, San Carlos, CA). After six washes in 0.1 M PBS, the sections were incubated in secondary antibody solutions for 1 hour at room temperature. Secondary antibodies used were donkey antiguinea pig Cy3, donkey antimouse Cy2 and donkey antirabbit Cy2. IB4 labeling was visualized with streptavidin-Cy2. All secondary antibodies were highly cross-absorbed for minimal species crossreactivity (Jackson ImmunoResearch, West Grove, PA) and used at a dilution of 1:200. Following incubation in secondary antibodies, the sections were washed with 0.1 M phosphate buffer six times, mounted from distilled water onto slides, and allowed to dry overnight at room temperature. Sections were cleared in xylenes for 1 minute and cover-slipped with DPX.
An ancillary experiment ascertained whether GABAB(1) subunit expression was restricted to neurons, or was also expressed by satellite cells in the DRG. The GABAB(1) subunit was visualized as described above. Neurons were identified by their labeling with mouse anti-neuronal nuclei (NeuN) (1:2,000; Chemicon MAB377), while satellite cells were identified by their labeling with mouse anti-S100 (1:1,000; Chemicon MAB079-1) using the procedures outlined above.
Antibody characterization
Table 1 provides details about the antibodies and lectin used in this study. The antibody against the GABAB(1) subunit was raised against a sequence common to both the 1a and 1b isoforms of the receptor. As expected, this antibody labeled two bands at ≈100 and ≈130 kDa in western blots of rat brain (Martin et al., 2004). Its specificity was further confirmed by loss of staining in the central nervous system (CNS) of GABAB(1) null mice (Fritschy et al., 2004) and medulla of rats after preabsorption with an excess of antigen (Pinto et al., 2008). Its presence in the DRG is consistent with both in situ hybridization (Poorkhalkali et al., 2000) and RT-qPCR analyses (this study).
TABLE 1.
Antibody Information
| Antibody or lectin | Immunogen | Source and type |
|---|---|---|
| Antineuronal nuclei α-Calcitonin gene-related peptide | Purified cell nuclei SCNTATCVTHRLAGLLSRSGGVVKDNFVPTNVGSQAFNH2 (disulfide bond) |
Chemicon, MAB377, lot 0511015365, monoclonal, raised in mouse Peninsula, T-4032, lot 040826-1, polyclonal raised in rabbit |
| Biotinylated Griffonia (Bandeiraea) simplicifolia lectin I | – | Vector Laboratories, B-1105, lots M1022 and S0102 |
| Neurofilament 200 S-100 protein | Carboxyterminal tail of enzymatically dephosphorylated pig neurofilament H subunit Purified bovine S-100 protein | Sigma, Clone N52, N0142, lot 90K4843, monoclonal, raised in mouse Chemicon, MAB079-1, clone 15E2E2; monoclonal, raised in mouse; recognizes S100α and S100β |
| GABAB(1) | PSEPPDRLSCDGSRVHLLYK | Chemicon, AB1531, lots 25030396 and 25050730, polyclonal, raised in guinea pig |
The N52 antibody is derived from a hybridoma produced by fusion of mouse myeloma cells and splenocytes obtained from a mouse immunized with the carboxyterminal tail segment of enzymatically dephosphorylated pig neurofilament H subunit. This clone recognizes both phosphorylated and dephosphorylated forms of the 200-kDa neurofilament protein (Perry et al., 1991) as demonstrated by labeling of a band of appropriate molecular weight in immunoblots of untreated and alkaline phosphatase-treated lysates of rat spinal cord (manufacturer’s data sheet). As expected, the N52 antibody labeled medium and large-diameter DRG neurons (this study; Hammond et al., 2004).
The antibody against α-CGRP was raised using an antigen that corresponds to α-CGRP in the rat and mouse (amino acids 83–119 of the CGRP precursor protein). This antibody labeled a band of appropriate molecular weight (≈4 kDa) on a western blot of commercial α-CGRP, as well as a band at 4 kDa and a higher molecular weight band that corresponds to the 128 amino acid precursor of CGRP in lysates of mouse trigeminal ganglia. In both instances the bands were absent when the primary antiserum was preabsorbed with an excess of the peptide antigen (Kosaras et al., 2009). The specificity of this antiserum was also demonstrated by a loss of staining in the rat DRG (Hammond et al., 2004) when the primary antiserum was preabsorbed with an excess of the peptide antigen.
The A60 clone of the NeuN antibody has been used extensively to identify neurons (Mullen et al., 1992). This clone labeled a triplet of bands of 46–48 kDa in western blots of mouse brain nuclear protein (Mullen et al., 1992). In agreement with Wolf (1996), it labeled the nuclei and less intensely the cytoplasm of DRG neurons; no labeling of satellite cells was observed.
At the high dilutions used in this study, the antibody to S-100 labeled satellite cells exclusively in agreement with others (Cocchia and Michetti, 1981; Stefansson et al., 1982; Yan and Keast, 2008). This antibody recognizes intact alpha and beta subunits of S-100. On western blots of PC12 cells it labeled a single band of ≈10 kDa (manufacturer’s data sheet). It also gave the same pattern of labeling as a polyclonal antibody raised in rabbit against bovine S-100 protein (Yan and Keast, 2008).
The lectin IB4 binds to α-D-galactose residues in unmyelinated axons (Streit et al., 1986). As expected, it produced the characteristic labeling of Golgi apparatus in soma of small-diameter DRG neurons (this study; Hammond et al., 2004). Additional specificity controls in this study included loss of labeling after omission of the primary antibodies or lectin and upon sequential serial dilution of primary antibodies. In colocalization studies, labeling was absent when secondary antibodies were swapped between the primary antibodies.
Quantitation
All antibodies were established to uniformly penetrate the full thickness of each section. A Nikon E800 epifluorescence microscope and Stereoinvestigator software (MicroBrightField, Colchester, VT) were used to estimate the number of GABAB(1)-IR neurons in each DRG. The microscope was equipped with a Nikon 60× oil Planapoachromat lens (N.A. 1.4), which afforded sufficient resolution to identify tops of individual neurons as they came into focus as the focal plane descended through the tissue. The average tissue thickness was 20.9 ± 0.4 μm in the z-axis after processing. The optical fractionator method of neuronal estimation was used with a minimum disector height of 14.5 μm for each section in addition to 2 μm guard zones on both the top and bottom of the disector. A counting frame of 75 × 75 μm on a grid of 125 × 125 μm was used because it consistently yielded a Gundersen coefficient of error that was less than 6%. On average, 750 ± 43 neurons were sampled per DRG. Individual DRG were not included in the study if more than two sections were lost in processing, or if more than one central section was missing. On average, 0.5 sections were lost per DRG, and most of those were either the first or last section that rarely contained neuronal profiles. The number of GABAB(1)-IR neurons reported is based on weighted total estimates, which accommodates variation in the thickness of individual sections.
Size measurements of neuronal profiles were performed using Neurolucida software (MicroBrightField). For each DRG, the first disector in the series with >200 neuronal profiles was chosen for measurement of cell area. At 60× magnification, the perimeters of all neurons in the section were traced at the z-axis plane where the nucleus was present. Intense staining occasionally obscured the nucleus, in which case the neuron was traced at the z-axis depth where its diameter was maximal.
The percentage of N52-IR, IB4-positive, and CGRP-IR neurons that colocalized GABAB(1) was determined by systematically sampling a single disector through the middle of the L4 and L5 DRG in each rat. The total number of N52-IR, IB4-positive, or CGRP-IR neurons in the visual field was determined. By manually toggling back and forth between Cy3 and Cy2 filters it was possible to determine if the neuron was also immunoreactive for GABAB(1). The Cy3 filter (Chroma Technology, Rockingham, VT) has a very narrow bandwidth that eliminates spectral bleedthrough of Cy2.
The individual who performed the counting, measurements of cell area, and percent colocalization studies was blinded to the treatment group and identity of each DRG. The number of GABAB(1)-IR neurons was determined in disectors colabeled for N52 because the number of N52-IR neurons was only subtly changed after spinal nerve ligation. Disector sets colabeled for IB4 were only viewed during calculation of the percent colocalization of GABAB(1)-IR on IB4-positive neurons. IB4 staining is greatly decreased in the L5 DRG after spinal nerve ligation (Hammond et al., 2004) and its prior visualization would have effectively unblinded the investigator.
Confocal image acquisition
A Zeiss 510 laser confocal microscope with sequential single line scanning was used to acquire digital images. For each fluorescent label, image acquisition was performed with the pinhole set to 1 airy unit and the gain set to 1. Digital images were then transferred to Adobe Photoshop CS2 (San Jose, CA) to generate figures. Only minor changes in contrast and levels were made.
Real-time PCR
Another set of rats underwent L5 SNL or sham surgery and were housed with a cohort of naïve rats for 2 weeks. After determination of paw withdrawal threshold (PWT), the rats were euthanized by CO2 inhalation followed by thoracotomy and the L4 and L5 DRG rapidly removed and stored in RNAlater (Ambion, Austin, TX).
Individual DRG were transferred to Trizol reagent (Ambion) and homogenized with a Tissue Tearor for isolation of total RNA according to the manufacturer’s directions. Genomic DNA was a concern due to anticipated low levels of RNA in the DRG. Samples were therefore first treated with DNAse I (TURBO DNAfree; Ambion) as directed by the manufacturer, after which the pellet was reconstituted with Buffer RTL and 100% ethanol and further purified using a RNAeasy spin column (Qiagen, Valencia, CA) as directed by the manufacturer. The concentration and quality of the RNA were determined by spectrometry.
Reverse transcription was performed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) with 0.6 μg RNA, 1× reverse transcriptase buffer, 1× dNTP, 1× random primers, and 210 units of MultiScribe reverse transcriptase 50 U/μl in 20.5 μl at 25°C for 10 minutes, 37°C for 120 minutes, and 85°C for 5 seconds. The samples were then divided into three aliquots and TaqMan gene expression assays (Applied Biosystems) were used to quantitate GABAB(1) (Rn00578911_m1), GABAB(2) (Rn01486490_m1), and GAPDH (Rn99999916_sl) mRNA. For each reaction, 50 ng cDNA was mixed with 10 μl of TaqMan gene expression master mix and 0.1 μl of each primer in a 20 μl reaction volume. The cycle conditions were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, and annealing/extension at 60°C for 1 minute, 95°C for 1 minute, and 55°C for 1 minute using an AB 7000 Real Time PCR System (Applied Biosystems). Reactions were performed in triplicate and included control reactions with no reverse transcriptase, no template, or no primers. The PCR products were run on an agarose gel to verify the presence of a single band. In addition, the PCR products were cloned into a pSC-A-amp/kan plasmid using the Strata PCR cloning kit (Agilent Technologies, Santa Clara, CA) according to the manufacturer’s directions and sequenced to confirm amplification of gabbr1 and gabbr2.
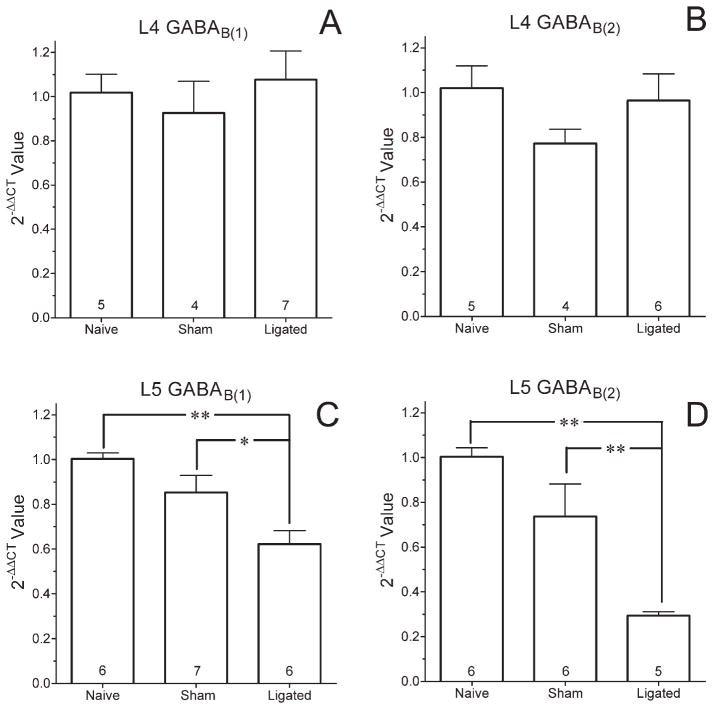
Levels of GAPDH mRNA in the different surgical conditions were compared for the L4 and the L5 DRG using the 2−ΔCT method (Livak and Schmittgen, 2001). Values were 1.03 ± 0.13 (n = 5), 1.19 ± 0.2 (n = 4), and 0.90 ± 0.10 (n = 7) in the L4 DRG and 1.04 ± 0.14 (n = 6), 1.18 ± 0.11 (n = 7), and 1.10 ± 0.10 (n = 6) in the L5 DRG of naïve, sham-operated and L5 ligated rats, respectively. These values did not differ among surgical conditions or DRG (P > 0.3). Therefore, threshold cycle (CT) values for GABAB(1) and GABAB(2) subunits were normalized to GAPDH as a housekeeping gene. To compare the relative amounts of mRNA for the two subunits, CT values were transformed to linear values using the equation 2−CT (Livak and Schmittgen, 2001). To compare values among the different surgical groups, the data were expressed as a fold-change relative to levels in naïve rats using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Statistical analyses
The mechanical withdrawal thresholds were not normally distributed and were analyzed by a Kruskal–Wallis one-way analysis of variance (ANOVA) with Dunn’s post-hoc test. However, to facilitate comparison with the literature the data are presented as mean and standard error of the mean (SEM). The number of GABAB(1)-IR neurons was normally distributed and was analyzed using a one-way ANOVA with Student Newman-Keul’s post-hoc test. Measurements of profile area were not normally distributed and were analyzed using a Kruskal–Wallis one-way ANOVA with Dunn’s post-hoc test. A two-sample Kolmogorov–Smirnov test was also applied to the cumulative distribution of profile measurements to identify differences in neuron size among treatment groups. All statistical tests compared ipsilateral data among treatment groups rather than comparing ipsilateral to contralateral within a treatment group because the contralateral side is not necessarily an appropriate control (Koltzenburg et al., 1999).
For the RT-PCR experiments, Student’s t-test was used to compare relative amounts of mRNA for the two subunits. One-way ANOVA was used to compare 2−ΔΔCT values among surgical conditions. Post-hoc comparisons of mean values for the different treatments were made by Newman–Keuls test. P < 0.05 was considered significant.
RESULTS
GABAB(1)-IR is predominantly expressed by neurons, and not satellite cells
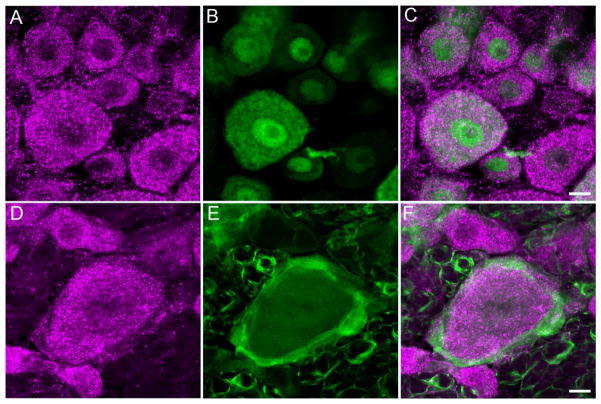
GABAB(1)-IR profiles in the DRG exhibited a strong punctate labeling of the cytoplasm (Fig. 1A,D). The antibody to NeuN produced strong labeling of nuclei with no labeling of the nucleoli and only faint cytoplasmic labeling of DRG neurons (Fig. 1B). Consistent with its predominant labeling of satellite cells (Gonzalez-Martinez et al., 2003; Rezajooi et al., 2004), S100-IR was confined to small profiles that ringed larger, presumably neuronal profiles in the DRG (Fig 1E). The GABAB(1) subunit colocalized extensively with NeuN-IR profiles (Fig. 1C), but rarely colocalized with S100-IR profiles in the DRG (Fig. 1F). Of a total of 316 NeuN-IR profiles sampled in single sections in the DRG of three different rats, 314 or 99.4% were immunoreactive for the GABAB(1) subunit. These data suggest that virtually every DRG neuron expresses the GABAB(1) receptor and that GABAB(1)-IR profiles in the DRG are principally, if not exclusively, neuronal.
Figure 1.
Colocalization of GABAB(1)-IR with NeuN-IR, but not S100-IR profiles in the DRG. The top row illustrates that GABA B(1)-IR profiles in the DRG (A) colocalize extensively with profiles immunoreactive for the neuronal marker, NeuN (B). The merged image is shown in panel (C). Images are the composite projections of two sections obtained taken at 0.45 μm intervals. The bottom row illustrates that GABA B(1)-IR profiles (D) do not colocalize with profiles immunoreactive for the satellite cell marker, S-100 (E). Images are the composite projection of four sections obtained at 0.45 μm intervals. The merged image is shown in panel (F). Scale bars = 10 μm.
Size distribution of GABAB(1)-IR profiles and colocalization with N52-IR, IB4-positive, and CGRP-IR neurons in the DRG
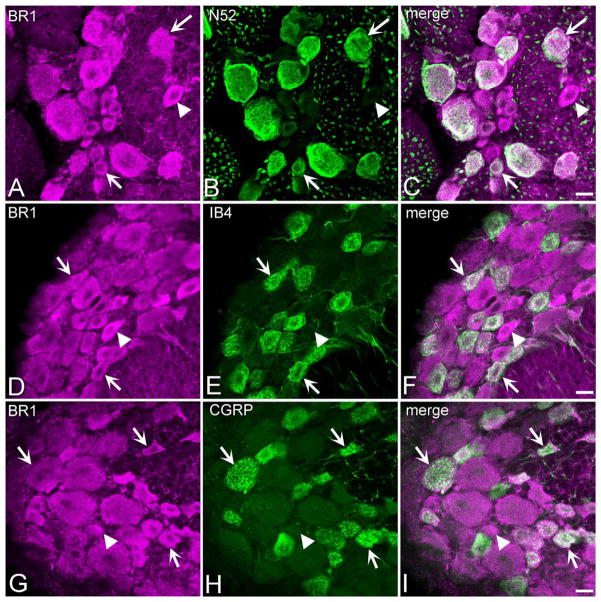
Figure 2 illustrates the size distribution of GABAB(1)-IR profiles in the L4 and L5 DRG. Small-, medium-, and large-sized DRG neurons were immunoreactive for the GABAB(1) subunit. The median size of GABAB(1)-IR profiles in the L4 and L5 DRG of naïve rats was 594 μm2 and 575 μm2, respectively. Thus, the majority of GABAB(1)-IR profiles corresponded to small- and medium-sized neurons. Figure 3 illustrates the colocalization of GABAB(1)-IR with immunohistochemical markers for myelinated, unmyelinated, and peptidergic afferents in the L4 and L5 DRG. As previously reported (Goldstein et al., 1987; Hammond et al., 2004), N52-IR was largely confined to medium- and large-sized DRG neurons (Fig. 3B) that give rise to myelinated Aδ and Aβ fibers, respectively. Nearly every (>98%) N52-IR profile in the DRG of naïve rats also exhibited GABAB(1)-IR (Fig. 3C; Table 2). Neurons positive for IB4, a marker for unmyelinated fibers, exhibited the characteristic staining of the Golgi apparatus (Fig. 3E) (Streit et al., 1986). More than 98% of all IB4-positive neurons in the DRG of naïve rats were also immunoreactive for the GABAB(1) subunit (Fig. 3F; Table 2). Consistent with previous reports, CGRP-IR was predominantly confined to small- or medium-sized neurons in the DRG; a smaller proportion of large-sized neurons was also labeled less strongly (Fig. 3H) (Hammond et al., 2004). GABAB(1)-IR was present in >99% of all CGRP-IR neurons in the DRG of naïve rats (Fig. 3I; Table 2). Together, these results indicate that the GABAB(1) subunit is extensively expressed by all major classes of neurons in the DRG as characterized by immunohistochemical properties or cell size.
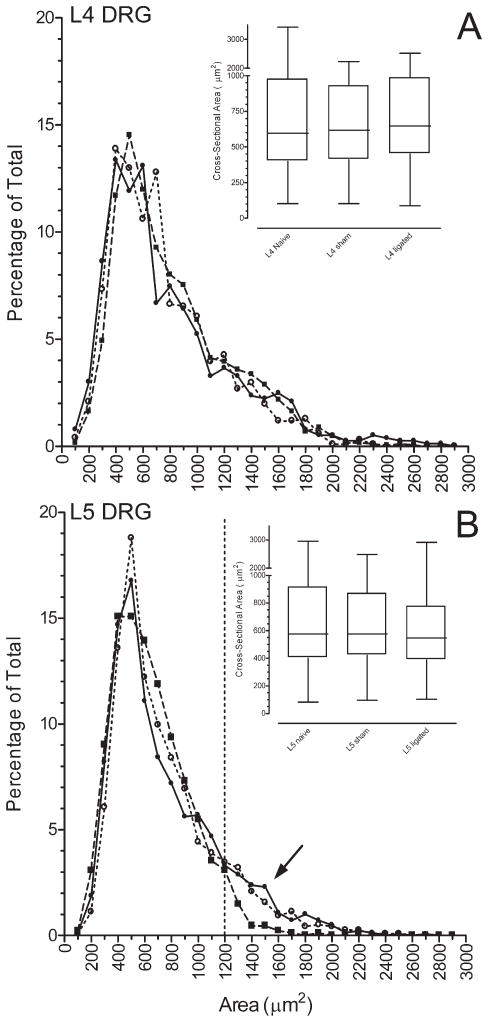
Figure 2.
Size distribution of GABAB(1)-IR profiles in the (A) L4 or (B) L5 DRG of naïve (solid circle), sham-operated (open circle), or L5 spinal nerve ligated (solid squares) rats. The histogram was generated in bins of 100 μm2. Ordinate: percentage of the total number of profiles. Abscissa: Cross-sectional area of the soma in μm2. Dashed vertical line identifies profiles ≥ 1,200 μm2 in size. Insets are box-and-whisker plots of the data. The box defines the 25th and 75th percentiles; horizontal line within the box identifies the median. Vertical error bars indicate the 5th and 95th percentiles. Number of profiles measured in the L4 DRG ranged from 764 to 1,846 and in the L5 DRG from 876 to 1,289 and were sampled from single sections taken from the ipsilateral L4 or L5 DRG of six naive rats, seven sham-operated, and six ligated rats.
Figure 3.
Immunoreactivity for the GABAB(1) subunit (A,D,G) colocalizes to several different populations of neurons in the rat dorsal root ganglion. N52 immunoreactivity (B), a marker for myelinated afferents, was predominantly confined to medium- and large-sized neurons. IB4-positive neurons (E) exhibited characteristic labeling of the Golgi apparatus and were uniformly of small size as expected of unmyelinated afferents. CGRP-immunoreactivity (H), indicative of peptidergic afferents, was predominantly localized to small- or medium-sized, with some labeling of large neurons. Arrows identify examples of double-labeled neurons. Arrowheads identify examples of GABAB(1)-IR profiles that did not colocalize the designated marker. C,F,I: Merged images. Each image is the composite projection of 3–4 sections obtained at intervals of 0.44 μm. Scale bars = 20 μm.
TABLE 2.
Percent Colocalization of GABAB(1)-IR in Three Different Populations of Immunohistochemically Identified Dorsal Root Ganglion (DRG) Neurons in Naïve Rats and in Rats 2 Weeks After Sham Surgery or Tight Ligation of the L5 Spinal Nerve
| N52-IR neurons
|
IB4-positive neurons
|
CGRP-IR neurons
|
||||
|---|---|---|---|---|---|---|
| L4 DRG | L5 DRG | L4 DRG | L5 DRG | L4 DRG | L5 DRG | |
| Naïve (n=3) | 98.1 ± 0.4 (354 ) | 98.3 ± 0.9 (484) | 98.1 ± 0.1 (263) | 99.5 ± 0.5 (318) | 99.5 ± 0.5 (264) | 99.0 ± 0.6 (389) |
| Sham (n=3) | 99.1 ± 0.7 (466) | 96.9 ± 1.2 (392) | 99.4 ± 0.1 (507) | 99.4 ± 0.3 (363) | 99.4 ± 0.3 (475) | 97.9 ± 0.6 (413) |
| Ligated (n=6) | 97.5 ± 1.6 (1021) | 98.7 ± 0.3 (636) | 99.5 ± 0.5 (784) | 74.9 ± 4.1 *† (99) | 99.1 ± 0.4 (793) | 98.5 ± 0.9 (208) |
Values are the mean ± SEM. In naïve and sham-operated rats, profiles were sampled in a single DRG section taken from each of three rats. In ligated rats, profiles were sampled in a single DRG section taken from each of six rats. This was necessitated by the paucity of IB4-positive neurons in the L5 DRG of ligated rats; measurements of colocalization with CGRP-IR and N52-IR were made in these rats as well. Total number of profiles examined is indicated in parentheses.
P < 0.05 significantly different from the corresponding DRG of naïve rats.
P < 0.05 significantly different from the corresponding DRG of sham-operated rats, one-way ANOVA.
Effect of spinal nerve ligation on the number of GABAB(1)-IR neurons in the L4 and L5 DRG
Tight ligation of the L5 spinal nerve significantly decreased the mechanical threshold of the ipsilateral hindpaw (5.5 ± 0.5 g; n = 8) compared with thresholds in the ipsilateral hindpaw of either sham-operated (27.9 ± 1.0 g; n = 7) or naïve (25.9 ± 1.9 g; n = 6) rats (P < 0.001). Mechanical thresholds for the ipsilateral hindpaw did not differ between sham and naïve rats (P > 0.2). Mechanical thresholds for the contralateral hindpaw were 24.8 ± 1.9, 26.4 ± 1.7, and 23.2 ± 2.5 g for the naïve, sham, and ligated treatment groups, respectively (P > 0.8).
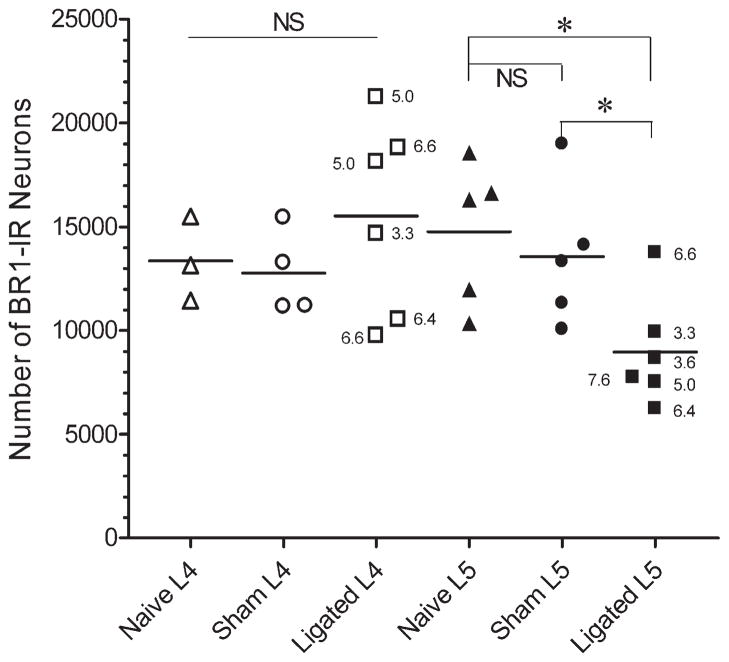
Two weeks after tight ligation of the L5 spinal nerve, the number of GABAB(1)-IR neurons in the ipsilateral L4 DRG was unchanged relative to either sham-operated or naïve rats (P > 0.4; Fig 4). In contrast, the number of GABAB(1)-IR neurons in the ipsilateral L5 DRG was significantly decreased in ligated rats compared with sham-operated (P < 0.03) and naiive (P < 0.03) rats. Figure 5 illustrates the intensity of GABAB(1)-IR in DRG neurons from naïve, sham-operated, and ligated rats. The intensity and density of GABAB(1)-IR in neurons of the L5 DRG of ligated rats was often less than that in naïve or sham-operated rats. The number of GABAB(1)-IR neurons in the ipsilateral L5 DRG of naïve and sham-operated rats did not differ (P = 0.5). In the L5 spinal nerve ligation group, a single DRG had a larger number of GABAB(1)-IR neurons than others in that treatment group. However, the mechanical threshold of this rat was consistent with its cohort (Fig. 4), as was the reduction in IB4-positive neurons in the L5 DRG (data not shown). Similarly, in the L5 sham-operated group, a single DRG had a larger number of GABAB(1)-IR neurons than others in that treatment group. The mechanical threshold of this rat was also consistent with its cohort. Further, neither data point fulfilled Grubb’s test for detection of an outlier and therefore did not warrant exclusion from the analysis. If these two data points were excluded from the analysis, the statistical significance of the differences was even more robust. These data support the contention that a reduction in GABA(B) receptors on L5 DRG neurons mediates mechanical allodynia after nerve injury.
Figure 4.
Scatterplot of the number of GABAB(1)-IR neurons in the ipsilateral L4 or L5 DRG of naïve rats or rats that underwent sham surgery or L5 spinal nerve ligation. Spinal nerve ligation caused a significant decrease in the number of GABAB(1)-IR neurons in the L5 DRG of both sham-operated and ligated rats compared with values in naïve rats. The numbers of GABAB(1)-IR neurons were unchanged in the adjacent L4 DRG. Each symbol is the number of neurons estimated from 8 or 10 optical disectors taken through the DRG of a single rat. Numbers adjacent to the symbol show the mechanical threshold of the ipsilateral hindpaw. Horizontal lines indicate the mean value. Ordinate: number of GABAB(1)-IR neurons. Abscissa: Treatment condition. NS: not significant. *P < 0.05; one-way ANOVA.
Figure 5.
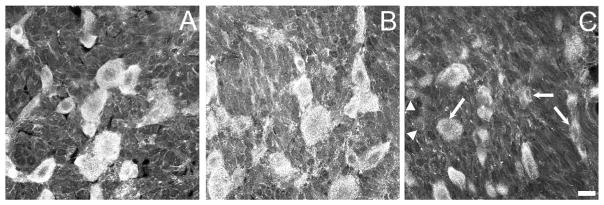
Representative photomicrographs of the intensity of GABAB(1)-IR in the L5 DRG of (A) naïve, (B) sham-operated, and (C) ligated rats. Images were obtained by first setting optimal gain and contrast for the naïve tissue. Identical setting were then used to image tissue from sham-operated and ligated rats. Note that the intensity of labeling of many (arrows), but not all, neurons in the tissue from ligated rats is reduced relative to that in sham-operated or ligated rats. Arrowheads identify two examples of neurons with marginal staining intensity. Scale bar = 20 μm for all images.
L5 spinal nerve ligation changed the size distribution of GABAB(1)-IR neurons in both the L4 and L5 DRG
The size distribution of GABAB(1)-IR profiles in the L4 DRG of naïve (median: 594 μm2) and sham-operated (median: 616 μm2) rats did not differ. The median size of GABAB(1)-IR profiles in the L4 DRG of spinal nerve ligated rats (646 μm2) was slightly larger compared with either sham-operated or naive rats (Fig. 2; P < 0.05 both comparisons). The biological relevance of this increase is unclear, and statistical significance is most likely due to the large numbers sampled.
The size distribution of GABAB(1)-IR profiles in the L5 DRG of naïve (median: 575 μm2) and sham-operated (median: 575 μm2) rats also did not differ. In contrast, the size distribution of GABAB(1)-IR neurons in the L5 DRG after spinal nerve ligation (median: 547 μm2) was significantly different from that of either sham-operated or naïve rats (Fig. 2 and inset; P < 0.05 both comparisons). This shift could be attributed to an apparent loss of large-sized (>1,200 μm2) GABAB(1)-IR neurons (Fig. 2), which comprised about 10% of the total population, and an increase in the percentage of medium-sized neurons (600–900 μm2) that were immunoreactive for GABAB(1) (P < 0.05, Kolmogorov–Smirnoff test).
Changes in colocalization of GABAB(1)-IR after spinal nerve ligation
Counts of the numbers of N52-IR, CGRP-IR, and IB4-positive profiles in single sections taken through the L4 and L5 DRG yielded findings consistent with an earlier stereological analysis (Hammond et al., 2004). Specifically, L5 spinal nerve ligation did not alter the number of N52-IR, CGRP-IR, or IB4-positive profiles in the L4 DRG compared with sham-operated or naïve rats. It also did not alter the percentage of N52-IR, CGRP-IR, or IB4-positive profiles in the L4 DRG that colocalized GABAB(1)-IR compared with sham-operated or naïve rats (Table 2). In contrast, L5 spinal nerve ligation decreased the number of CGRP-IR profiles by 75% and those of IB4-positive neurons by 87% in the L5 DRG. Of the CGRP-IR neurons that remained, >97% continued to express GABAB(1)-IR. Of the few IB4-positive profiles that remained, only 74.9 ± 4.1% were immunoreactive for the GABAB(1) subunit (Table 2). This percentage was significantly less than the percentage determined for the L5 DRG in sham-operated and naïve rats (P < 0.05 both comparisons). Neither the numbers of N52-IR neurons nor the percentage of N52-IR neurons that colocalized GABAB(1)-IR in the L5 DRG were altered by L5 spinal nerve ligation.
Effect of SNL on levels of GABAB(1) and GABAB(2) mRNA in the L4 and L5 DRG
Levels of GABAB(1) mRNA, normalized to GAPDH, were ≈2-fold higher than GABAB(2) mRNA in both the L4 (0.097 ± 0.008; n = 5 vs. 0.052 ± 0.005; n = 5) and L5 (0.096 ± 0.003; n = 6 vs. 0.046 ± 0.002; n = 6) DRG of naïve rats. Levels of GABAB(1) or GABAB(2) mRNA in the L4 DRG did not differ among naïve, sham-operated, or ligated rats (P > 0.25 both transcripts; Fig. 6A). However, levels of GABAB(1) and GABAB(2) subunit mRNA were significantly decreased in the L5 DRG of ligated rats compared with sham-operated or naïve rats (P < 0.01 both transcripts; Fig. 6B). The reduction in the GABAB(2) subunit was proportionately greater than the loss of GABAB(1) subunit.
Figure 6.
Levels of mRNA for GABAB(1) and GABAB(2) in the ipsilateral L4 (A,B) and L5 (C,D) DRG of rats that underwent sham surgery or L5 spinal nerve ligation 2 weeks earlier and a cohort of time- and age-matched naïve rats. Cycle thresholds (CT) were normalized to GAPDH in the same DRG and expressed relative to values in naïve rats using the equation 2−ΔΔCT. Numbers in the columns are the number of rats. Values are the mean ± SEM. *P < 0.05, **P < 0.01.
DISCUSSION
GABAB(1) subunit is expressed by virtually every neuron in the DRG
The functional GABAB receptor is a heterodimer composed of a GABAB(1) subunit that contains the ligand binding site and a GABAB(2) subunit that couples to Gi/o (Couve et al., 2000; Bettler and Tiao, 2006; Padgett and Slesinger, 2010). The GABAB(2) subunit is largely undetectable in the DRG by western blotting (Engle et al., 2006) or immunohistochemical methods (data not shown), consistent with the low levels of GABAB(2) mRNA present in the DRG (this study; Durkin et al., 1999; McCarson and Enna, 1999; Towers et al., 2000). This study therefore used an antibody that recognizes the GABAB(1) subunit to identify the neuronal populations that express the GABAB receptor. The number of GABAB(1)-IR neurons in the L5 DRG of naïve rats ranged between 10,358 and 18,571, similar to the range of cresyl-violet stained DRG neurons reported by Carlton and Hargett (2002) and other estimates of 14,000 to 18,000 neurons (Schmalbruch, 1987; Tandrup, 1993; Popken and Farel, 1997; Vestergaard et al., 1997; Schionning et al., 1998). Moreover, every NeuN-IR profile colocalized GABAB(1)-IR. These data suggest that >98% of DRG neurons that give rise to myelinated, unmyelinated, and peptidergic afferents express the GABAB receptor, consistent with the ability of baclofen to activate GABAB receptors on all major classes of primary afferent neurons (Schlichter et al., 1987). This receptor is therefore well situated to presynaptically regulate the transmission of both innocuous and noxious sensory information in the spinal cord dorsal horn.
Loss of GABAB(1) receptors on primary afferent neurons is likely to contribute to mechanical allodynia after spinal nerve ligation
Tight ligation of the L5 spinal nerve resulted in a 38% decrease in the number of GABAB(1)-IR neurons in the L5 DRG. It also resulted in an ≈40% decrease in levels of GABAB(1) mRNA and a 70% decrease in levels of GABAB(2) mRNA. These results support the conclusion that a loss of GABAB(1) receptors on primary afferent neurons in the injured spinal nerve contributes to the occurrence of mechanical allodynia after spinal nerve ligation. Afferents in the L5 DRG are not rendered quiescent as a result of the ligation or transection of their peripheral processes. Rather, A-fiber afferents in the L5 DRG begin to discharge spontaneously (Liu et al., 2000a,b; Ma et al., 2003; Djouhri et al., 2006). This activity can be measured in the corresponding dorsal root and is presumably the source of continuous input that contributes to an increased excitability of dorsal horn neurons and the facilitation of (normally) subliminal inputs from intact afferents in the L4 DRG. Indeed, tactile allodynia produced by L5 SNL can be eliminated or obtunded by subsequent rhizotomy of the L5 dorsal root or application of low concentrations of local anesthetic to the L5 DRG (Sukhotinsky et al., 2004, and references therein). These data point to a key role of the injured L5 DRG neurons in the generation of tactile allodynia. In this context, a loss of presynaptic inhibition mediated by GABAB receptors on the central terminals of L5 afferents may enhance the synaptic efficacy of this spontaneous drive and contribute to the allodynia or spontaneous pain behaviors that arise after injury.
L5 spinal nerve ligation and the number of GABAB(1)-IR neurons in the L4 DRG
Tight ligation of the L5 spinal nerve alters the expression of a number of channels, receptors, growth factors, and neurotransmitters in neurons of the adjacent L4 DRG (Fukuoka et al., 1998, 2001, 2002; Hudson et al., 2002; Ma et al., 2003; Nagano et al., 2003; Schäfers et al., 2003; Zhang et al., 2004). These changes are attributed to exposure of the axons of L4 DRG neurons to substances released within the sciatic nerve from the axons of injured L5 DRG neurons that undergo Wallerian degeneration. In contrast, ligation of the L5 spinal nerve did not alter the number or expression pattern of GABAB(1)-IR neurons or the levels of GABAB(1) or GABAB(2) mRNA in the L4 DRG. However, the variance of these measurements was greatly increased in contrast to the uniformity of neuron counts in the naïve and sham-operated rats, with some rats showing an increase and others a decrease in number of GABAB(1)-IR neurons. The mechanism and importance of this increase in variance is unknown. However, it provides further evidence that afferents in the adjacent L4 DRG are not immune to injury of the L5 spinal nerve.
GABAB receptor downregulation in the L5 DRG occurs predominantly in peptidergic and unmyelinated afferents
Analysis of the size distribution of GABAB(1)-IR neurons in the L5 DRG after spinal nerve ligation provides additional insight. Spinal nerve ligation was previously reported to cause a decrease in the percentage of large-sized N52-IR neurons with a concomitant increase in the percentage of medium-sized N52-IR neurons in the L5 DRG in the absence of a change in the total number of N52-IR neurons (Hammond et al., 2004). In this study, spinal nerve ligation caused a similar shift in the size distribution of GABAB(1)-IR profiles such that the percentage of large-sized (>1,200 μm2) profiles was decreased while the percentage of medium-sized (600–900 μm2) profiles was increased. Given that virtually every N52-IR neuron colocalized the GABAB(1) subunit, a similar change in the distribution of medium- and large-sized GABAB(1)-IR neurons was not unexpected. Neither the number of N52-IR profiles nor the percent colocalization of GABAB(1)-IR with N52-IR neurons changed in the L5 DRG after spinal nerve ligation. The shift in cell size distribution may be an artifact of cell shrinkage, which has been documented for Type A DRG neurons after nerve injury (Vestergaard et al., 1997; Degn et al., 1999; Sapunar et al., 2005). However, an alternate interpretation is that there is small subpopulation of injured large myelinated fibers (≈11%) that express few GABAB receptors after ligation (Fig. 2, arrow). It is notable that a similar percentage of Aβ fibers in the injured L5 DRG (6–8%) exhibit increased excitability and spontaneous activity after spinal nerve ligation (Liu et al., 2000a,b; Ma et al., 2003; Djouhri et al., 2006). Loss of GABAB receptor-mediated inhibition on these fibers could enhance excitatory transmission in the spinal cord.
In line with a previous stereological analysis (Hammond et al., 2004), the numbers of CGRP-IR profiles and IB4-positive profiles decreased by 75% and 87% in the L5 DRG after spinal nerve ligation, respectively. IB4-positive neurons represent about 40% of the total population in the DRG. If there was a concomitant complete loss of GABAB(1) subunit on these neurons, a 35% loss (87% of 40%) in the number of GABAB(1)-IR neurons in the L5 DRG would be expected. Similarly, CGRP-IR neurons represent about 30% of the total population. If there was a complete loss of GABAB(1) subunit on these neurons, a 22% (75% of 30%) decrease in the number of GABAB(1)-IR neurons in the L5 DRG would be expected. Of note, the distribution of small-sized GABAB(1)-IR neurons was unchanged, consistent with a generalized downregulation of the receptor on the soma of both unmyelinated and peptidergic afferents rather than complete loss in a subset of afferents. Given that C fibers in the L5 DRG do not exhibit ectopic or spontaneous firing after spinal nerve ligation (Liu et al., 2000a,b; Boucher et al., 2000; Ma et al., 2003; Djouhri et al., 2006), the loss of GABAB(1)-IR on IB4-positive neurons in the L5 DRG after spinal nerve ligation is unlikely to be biologically significant. Interestingly, conditional deletion of GABAB(1) subunit in C fibers does not result in an exacerbated response to mechanical stimuli, whereas it does exacerbate the responsiveness of Aδ fibers (Gangadharan et al., 2009).
Loss of GABAB(1)-IR and GABAB receptor mRNA in the L5 DRG
There are four possible explanations for the loss of GABAB(1)-IR neurons in the L5 DRG. Vestergaard et al. (1997) reported a 22% reduction in the number of neurons in the L5 DRG after L5 spinal nerve transection 2 weeks after injury compared with naïve rats. To ascertain whether neuronal death contributed to the decrease in GABAB(1)-IR neurons, disectors were stained for NeuN. However, marked alterations in the cellular localization and intensity of NeuN-IR in the DRG of ligated rats precludes its use for counting purposes. Nonetheless, we consider it unlikely that the loss is secondary to cell death in the DRG. Although the number of IB4-positive and CGRP-IR neurons is decreased 2 weeks after L5/6 spinal nerve ligation, by 20 weeks the number of these neurons does not differ from values in sham or naïve rats. Furthermore, the number of N52-IR neurons is unchanged between 2 and 20 weeks after injury (Hammond et al., 2004). Thus, in this model in which the spinal nerve is tightly ligated, but not transected, there is no clear evidence for neuronal death (Hammond et al., 2004). It is more likely that the decrease in number of GABAB(1)-IR neurons is the result of a decrease in the expression of the GABAB(1) subunit to levels below the limit of immunohistochemical detection. Although not quantitated, the intensity and density of GABAB(1)-IR in many, but not all, neurons in the L5 DRG of ligated rats often appeared less than that in neurons of sham-operated or naïve DRG that were processed concurrently with these tissues. Moreover, RT-PCR analysis revealed a 40% decrease in levels of GABAB(1) mRNA and 70% decrease in those of GABAB(2). A third possibility is that injury results in an increased trafficking of GABAB(1) subunit to the central or peripheral terminals of L5 afferents, which could masquerade as a downregulation of subunit in the soma. This possibility is unlikely given the decrease in mRNA for both subunits. Finally, it is possible that the loss is limited to a reserve of GABAB(1) subunits and of no consequence to functional receptors. Although difficult to exclude, the very large reduction in the GABAB(2) subunit mRNA, which couples the receptor to Gi/o proteins and traffics GABAB(1) subunits to the plasma membrane from the endoplasmic reticulum, makes this possibility unlikely. We previously concluded on the basis of western blotting of dorsal horn and DRG as well as densitometric measurements of GABAB receptor IR in the dorsal horn that spinal nerve ligation did not alter the levels of the GABAB receptor (Engle et al., 2006). In retrospect, these methods lacked the necessary sensitivity to detect the relatively modest reduction in number of GABAB-IR primary afferent neurons, which represent a subset of the neuronal elements in the dorsal horn that express the GABAB receptor (Yang et al., 2002).
CONCLUSION
This study provides the first direct demonstration that the GABAB(1) subunit is expressed by the vast majority (>98%) of myelinated, unmyelinated, and peptidergic primary afferent neurons. It further demonstrates that the number of GABAB(1)-IR neurons in the L5 DRG, but not the L4 DRG, is significantly decreased after L5 spinal nerve ligation. As there appears to be no permanent loss of neurons in this model (Hammond et al., 2004), it is most likely that this loss represents a decrease in levels of receptor protein in the soma of these neurons. These data are consistent with the hypothesis that a loss of GABAB(1) receptors in neurons of the L5 DRG contributes to the development of mechanical allodynia in this model of peripheral nerve injury. The proportionately greater loss of GABAB(2) mRNA is consistent with the loss of functional receptor, and further suggests that reliance on GABAB(1)-IR may have underestimated the magnitude of the loss. Together, these findings provide important anatomical evidence to complement the results of embryonic gene deletion (Schuler et al., 2001; Gassmann et al., 2004), intrathecal antagonist (Hao et al., 1994), and conditional deletion of subunit in Aδ fibers (Gangadharan et al., 2009) in which allodynia or hyperalgesia occur after antagonism or deletion of the GABAB receptor.
Acknowledgments
We thank Alan Domeyer and Dr. Tamie Takenami for expert technical assistance.
Grant sponsor: National Institutes of Health; Grant numbers: 1R01DA016430 (to D.L.H.) and F30DA017447 (to M.P.E.).
LITERATURE CITED
- Ataka T, Kumamoto E, Shimoji K, Yoshimura M. Baclofen inhibits more effectively C-afferent than Adelta-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain. 2000;86:273–282. doi: 10.1016/S0304-3959(00)00255-4. [DOI] [PubMed] [Google Scholar]
- Bettler B, Tiao JY. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol Ther. 2006;110:533–543. doi: 10.1016/j.pharmthera.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Stereological analysis of Ca2+/calmodulin-dependent protein kinase II a-containing dorsal root ganglion neurons in the rat: colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol. 2002;448:102–110. doi: 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Cocchia D, Michetti F. S-100 antigen in satellite cells of the adrenal medulla and the superior cervical ganglion of the rat. An immunochemical and immunocytochemical study. Cell Tissue Res. 1981;215:103–112. doi: 10.1007/BF00236252. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Degn J, Tandrup T, Jakobsen J. Effect of nerve crush on perikaryal number and volume of neurons in adult rat dorsal root ganglion. J Comp Neurol. 1999;412:186–192. doi: 10.1002/(sici)1096-9861(19990913)412:1<186::aid-cne14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MM, Gunwaldsen CA, Borowsky B, Jones KA, Branchek TA. An in situ hybridization study of the distribution of the GABAB2 protein mRNA in the rat CNS. Mol Brain Res. 1999;71:185–200. doi: 10.1016/s0169-328x(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- Engle MP, Gassman M, Sykes KT, Bettler B, Hammond DL. Spinal nerve ligation does not alter the expression or function of GABAB receptors in spinal cord and dorsal root ganglia of the rat. Neuroscience. 2006;138:1277–1287. doi: 10.1016/j.neuroscience.2005.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Sidler C, Parpan F, Gassmann M, Kaupmann K, Bettler B, Benke D. Independent maturation of the GABAB receptor subunits GABAB1 and GABAB2 during postnatal development in rodent brain. J Comp Neurol. 2004;477:235–252. doi: 10.1002/cne.20188. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABAA receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K. VR1, but not P2X3, increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/s0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Gangadharan V, Agarwal N, Brugger S, Tegeder I, Bettler B, Kuner R, Kurejova M. Conditional gene deletion reveals functional redundancy of GABAB receptors in peripheral nociceptors in vivo. Mol Pain. 2009;5:68. doi: 10.1186/1744-8069-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Shaban H, Vigot R, Sansig G, Haller C, Barbieri S, Humeau Y, Schuler V, Muller M, Kinzel B, Klebs K, Schmutz M, Froestl W, Heid J, Kelly PH, Gentry C, Jaton AL, Van der Putten H, Mombereau C, Lecourtier L, Mosbacher J, Cryan JF, Fritschy JM, Luthi A, Kaupmann K, Bettler B. Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J Neurosci. 2004;24:6086–6097. doi: 10.1523/JNEUROSCI.5635-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go VLW, Yaksh TL. Release of substance P from the cat spinal cord. J Physiol (Lond) 1987;391:141–167. doi: 10.1113/jphysiol.1987.sp016731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein ME, Cooper HS, Bruce J, Carden MJ, Lee VM, Schlaepfer WW. Phosphorylation of neurofilament proteins and chromatolysis following transection of rat sciatic nerve. J Neurosci. 1987;7:1586–1594. doi: 10.1523/JNEUROSCI.07-05-01586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Martinez T, Perez-Pinera P, Diaz-Esnal B, Vega JA. S-100 proteins in the human peripheral nervous system. Microsc Res Tech. 2003;60:633–638. doi: 10.1002/jemt.10304. [DOI] [PubMed] [Google Scholar]
- Hammond DL. Inhibitory neurotransmitters and nociception: role of GABA and glycine. In: Dickenson A, Besson JM, editors. The pharmacology of pain. Berlin: Springer; 1997. pp. 361–384. [Google Scholar]
- Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475:575–589. doi: 10.1002/cne.20209. [DOI] [PubMed] [Google Scholar]
- Hao J-X, Xu X-J, Wiesenfeld-Hallin Z. Intrathecal γ-aminobutyric acidB (GABAB) receptor antagonist CGP 35348 induces hypersensitivity to mechanical stimuli in the rat. Neurosci Lett. 1994;182:299–302. doi: 10.1016/0304-3940(94)90821-4. [DOI] [PubMed] [Google Scholar]
- Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- Hudson LJ, Bevan S, McNair K, Gentry C, Fox A, Kuhn R, Winter J. Metabotropic glutamate receptor 5 upregulation in A-fibers after spinal nerve injury: 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J Neurosci. 2002;22:2660–2668. doi: 10.1523/JNEUROSCI.22-07-02660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuki T, Hama AT, Wang X-T, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- Iyadomi M, Iyadomi I, Kumamoto E, Tomokuni K, Yoshimura M. Presynaptic inhibition by baclofen of miniature EPSCs and IPSCs in substantia gelatinosa neurons of the adult rat spinal dorsal horn. Pain. 2000;85:385–393. doi: 10.1016/S0304-3959(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Kangrga I, Jiang M, Randic M. Actions of (−) baclofen on rat dorsal horn neurons. Brain Res. 1991;562:265–275. doi: 10.1016/0006-8993(91)90630-e. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kitchener PD, Wilson P, Snow PJ. Selective labelling of primary sensory afferent terminals in lamina II of the dorsal horn by injection of Bandeiraea simplicifolia isolectin B4 into peripheral nerves. Neuroscience. 1993;54:545–551. doi: 10.1016/0306-4522(93)90274-j. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999;22:122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009;515:331–348. doi: 10.1002/cne.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever I, Cunningham J, Grist J, Yip PK, Malcangio M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur J Neurosci. 2003;18:1169–1174. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000a;85:503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- Liu X, Eschenfelder S, Blenk KH, Janig W, Habler H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain. 2000b;84:309–318. doi: 10.1016/s0304-3959(99)00211-0. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG. γ-Aminobutyric acidB, but not γ-aminobutyric acidA receptor activation, inhibits electrically evoked substance P-like immunoreactivity release from the rat spinal cord in vitro. J Pharmacol Exp Ther. 1993;266:1490–1496. [PubMed] [Google Scholar]
- Martin SC, Steiger JL, Gravielle MC, Lyons HR, Russek SJ, Farb DH. Differential expression of γ-aminobutyric acid type B receptor subunit mRNAs in the developing nervous system and receptor coupling to adenylyl cyclase in embryonic neurons. J Comp Neurol. 2004;473:16–29. doi: 10.1002/cne.20094. [DOI] [PubMed] [Google Scholar]
- McCarson KE, Enna SJ. Nociceptive regulation of GABAB receptor gene expression in rat spinal cord. Neuropharmacology. 1999;38:1767–1773. doi: 10.1016/s0028-3908(99)00121-5. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nagano M, Sakai A, Takahashi N, Umino M, Yoshioka K, Suzuki H. Decreased expression of glial cell line-derived neurotrophic factor signaling in rat models of neuropathic pain. Br J Pharmacol. 2003;140:1252–1260. doi: 10.1038/sj.bjp.0705550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol. 2010;58:123–147. doi: 10.1016/S1054-3589(10)58006-2. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Lawson SN, Robertson J. Neurofilament immunoreactivity in populations of rat primary afferent neurons: a quantitative study of phosphorylated and non-phosphorylated subunits. J Neurocytol. 1991;20:746–758. doi: 10.1007/BF01187848. [DOI] [PubMed] [Google Scholar]
- Pinard A, Seddik R, Bettler B. GABAB receptors: physiological functions and mechanisms of diversity. Adv Pharmacol. 2010;58:231–255. doi: 10.1016/S1054-3589(10)58010-4. [DOI] [PubMed] [Google Scholar]
- Pinto M, Sousa M, Lima D, Tavares I. Participation of mu-opioid, GABAB, and NK1 receptors of major pain control medullary areas in pathways targeting the rat spinal cord: implications for descending modulation of nociceptive transmission. J Comp Neurol. 2008;510:175–187. doi: 10.1002/cne.21793. [DOI] [PubMed] [Google Scholar]
- Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Poorkhalkali N, Juneblad K, Jonsson AC, Lindberg M, Karlsson O, Wallbrandt P, Ekstrand J, Lehmann A. Immunocytochemical distribution of the GABAB receptor splice variants GABAB R1a and R1b in the rat CNS and dorsal root ganglia. Anat Embryol (Berl) 2000;201:1–13. doi: 10.1007/pl00008224. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Farel PB. Sensory neuron number in neonatal and adult rats estimated by means of stereologic and profile-based methods. J Comp Neurol. 1997;386:8–15. [PubMed] [Google Scholar]
- Price GW, Kelly JS, Bowery NG. The location of GABAB binding sites in mammalian spinal cord. Synapse. 1987;1:530–538. doi: 10.1002/syn.890010605. [DOI] [PubMed] [Google Scholar]
- Rezajooi K, Pavlides M, Winterbottom J, Stallcup WB, Hamlyn PJ, Lieberman AR, Anderson PN. NG2 proteoglycan expression in the peripheral nervous system: upregulation following injury and comparison with CNS lesions. Mol Cell Neurosci. 2004;25:572–584. doi: 10.1016/j.mcn.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Robertson B, Perry MJ, Lawson SN. Populations of rat spinal primary afferent neurons with choleragenoid binding compared with those labelled by markers for neurofilament and carbohydrate groups: a quantitative immunocytochemical study. J Neurocytol. 1991;20:387–395. doi: 10.1007/BF01355535. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Sapunar D, Ljubkovic M, Lirk P, McCallum JB, Hogan QH. Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats. Anesthesiology. 2005;103:360–376. doi: 10.1097/00000542-200508000-00020. [DOI] [PubMed] [Google Scholar]
- Schäfers M, Sorkin LS, Geis C, Shubayev VI. Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett. 2003;347:179–182. doi: 10.1016/s0304-3940(03)00695-5. [DOI] [PubMed] [Google Scholar]
- Schionning JD, Larsen JO, Tandrup T, Braendgaard H. Selective degeneration of dorsal root ganglia and dorsal nerve roots in methyl mercury-intoxicated rats: a stereological study. Acta Neuropathol (Berl) 1998;96:191–201. doi: 10.1007/s004010050881. [DOI] [PubMed] [Google Scholar]
- Schlichter R, Desarmenien M, Li Volsi G, Desaulles E, Feltz P. Low concentrations of GABA reduce accommodation in primary afferent neurons by an action at GABAB receptors. Neuroscience. 1987;20:385–393. doi: 10.1016/0306-4522(87)90099-6. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. The number of neurons in dorsal root ganglia L4-L6 of the rat. Anat Rec. 1987;219:315–322. doi: 10.1002/ar.1092190313. [DOI] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABAB responses in mice lacking GABAB(1) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Somers DL, Clemente FR. Dorsal horn synaptosomal content of aspartate, glutamate, glycine and GABA are differentially altered following chronic constriction injury to the rat sciatic nerve. Neurosci Lett. 2002;323:171–174. doi: 10.1016/s0304-3940(02)00157-x. [DOI] [PubMed] [Google Scholar]
- Stefansson K, Wollmann RL, Moore BW. Distribution of S-100 protein outside the central nervous system. Brain Res. 1982;234:309–317. doi: 10.1016/0006-8993(82)90871-x. [DOI] [PubMed] [Google Scholar]
- Stiller CO, Cui JG, O’Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of γ-aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39:367–374. doi: 10.1097/00006123-199608000-00026. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Schulte BA, Balentine JD, Spicer SS. Evidence for glycoconjugate in nociceptive primary sensory neurons and its origin from the Golgi complex. Brain Res. 1986;377:1–17. doi: 10.1016/0006-8993(86)91185-6. [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I, Ben-Dor E, Raber P, Devor M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. Eur J Pain. 2004;8:135–143. doi: 10.1016/S1090-3801(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Tandrup T. A method for unbiased and efficient estimation of number and mean volume of specified neuron subtypes in rat dorsal root ganglion. J Comp Neurol. 1993;329:269–276. doi: 10.1002/cne.903290208. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers S, Princivalle A, Billinton A, Edmunds M, Bettler B, Urban L, Castro-Lopes J, Bowery NG. GABAB receptor protein and mRNA distribution in rat spinal cord and dorsal root ganglia. Eur J Neurosci. 2000;12:3201–3210. doi: 10.1046/j.1460-9568.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- Vestergaard S, Tandrup T, Jakobsen J. Effect of permanent axotomy on number and volume of dorsal root ganglion cell bodies. J Comp Neurol. 1997;388:307–312. [PubMed] [Google Scholar]
- Wang HF, Robertson B, Grant G. Anterograde transport of horseradish-peroxidase conjugated isolectin B4 from Griffonia simplicifolia I in spinal primary sensory neurons of the rat. Brain Res. 1998;811:34–39. doi: 10.1016/s0006-8993(98)00916-0. [DOI] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol (Lond) 2007;579:849–861. doi: 10.1113/jphysiol.2006.126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Zhang Q, Zhang YZ, Liu YT, Dong R, Wang QJ, Guo YX. Downregulation of GABAB receptors in the spinal cord dorsal horn in diabetic neuropathy. Neurosci Lett. 2011;490:112–115. doi: 10.1016/j.neulet.2010.12.038. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Willcockson WS, Chung JM, Hori Y, Lee KH, Willis WD. Effects of iontophoretically released amino acids and amines on primate spinothalamic tract cells. J Neurosci. 1984;4:732–740. doi: 10.1523/JNEUROSCI.04-03-00732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blumcke I. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Yan H, Keast JR. Neurturin regulates postnatal differentiation of parasympathetic pelvic ganglion neurons, initial axonal projections, and maintenance of terminal fields in male urogenital organs. J Comp Neurol. 2008;507:1169–1183. doi: 10.1002/cne.21593. [DOI] [PubMed] [Google Scholar]
- Yang K, Wang D, Li YQ. Distribution and depression of the GABAB receptor in the spinal dorsal horn of adult rat. Brain Res Bull. 2001;55:479–485. doi: 10.1016/s0361-9230(01)00546-9. [DOI] [PubMed] [Google Scholar]
- Yang K, Ma WL, Feng YP, Dong YX, Li YQ. Origins of GABAB receptor-like immunoreactive terminals in the rat spinal dorsal horn. Brain Res Bull. 2002;58:499–507. doi: 10.1016/s0361-9230(02)00824-9. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Zhu CZ, Thimmapaya R, Choi WS, Honore P, Scott VE, Kroeger PE, Sullivan JP, Faltynek CR, Gopalakrishnan M, Shieh CC. Differential action potentials and firing patterns in injured and uninjured small dorsal root ganglion neurons after nerve injury. Brain Res. 2004;1009:147–158. doi: 10.1016/j.brainres.2004.02.057. [DOI] [PubMed] [Google Scholar]