Dear Sirs
The interest in circulating microparticles (MP) shown by the scientific community is increasing. These MP emitted from membranes by activated or apoptotic blood cells have been considered for a long time merely as “cell debris” without biological activities. However, MP are now considered as critical effectors involved in numerous biological processes (e.g., coagulation, angiogenesis, immunomodulation) (1–5).
Two major complementary approaches on the boundary between cellular hematology and haemostasis are used to monitor circulating MP in clinical settings: a quantitative and descriptive approach which uses flow cytometry and a qualitative/function alone assessing MP biological activities, involving phospholipid-dependent procoagulant activities in MP. This is explored using haemostasis techniques such as fluorometry or chronometry. Few studies have compared both approaches (6). Here, we report a correlation between platelet-derived MP (PMP) enumeration by cytometry and assessment of their procoagulant activity by fluorometry (thrombin generation [TG] measurement) and chronometry assays (factor X[FX] activity). Two different centrifugation protocols were compared.
The study population consists of 40 platelet donors. Samples were taken at the French Blood Agency (EFS Bourgogne-Franche-Comté, Besançon, France) after signature of the informed consent (Approval from the local research ethics committee, CCP Est II, Besançon). After a venepuncture without a tourniquet as proposed (7), samples were collected after the elimination of the first millimeters, which are rich in tissue factor (TF)(7), in Vacutainer tubes (Becton-Dickinson, Le Pont de Claix, France) containing Citrate-Theophylline-Adenosine-Dipyridamole (CTAD) anticoagulant, in order to limit in vitro platelet activation (7) and therefore the non-specific MP production. Samples were centrifuged (less than an hour after venepuncture). The 40 samples were centrifuged in order to obtain platelet-poor plasma (PPP) with the following centrifugation protocol: 1500g for 15 minutes, decantation and then 13000g for 2 minutes (centrifugation protocol #1, Ref.8). The last 20 samples collected were also centrifuged at the same time with the following protocol: 2500g for 15 minutes, decantation and then 2500g for 15 minutes again (centrifugation protocol #2, according to International Society on Thrombosis and Haemostasis[ISTH] recommendations). Residual platelet removal assessed by cytometry was similar between the two centrifugation protocols (number of platelets before centrifugation: 121 000 to 234 000/μL; number of platelets obtained after centrifugation: 6 – 32/μL using protocol #1vs 6 – 21/μL using protocol #2). All the samples were frozen and stored at −80°C in 1.5ml polypropylene microtubes (TreffLab, Degersheim Switzerland) and thawed quickly at 37°C for 5 minutes before analysis.
The later generation NAVIOS cytometer (Beckman Coulter, Miami, FL) was used together with Megamix™ beads (Biocytex, Marseille, France) to define a standard-sized area and Flow-Set™ fluorosphere beads (Beckmann Coulter) to enumerate PMP as we previously described (9). Inter-, intra-assay coefficient of variations (CV), lower limits of detection and raw cytometry data are given in (9) for protocol #1. To compare the data obtained by cytometry with haemostasis techniques, PMP were assessed by triple staining using Annexin V (a phosphatidylserine ligand) and two monoclonal antibodies directed against platelets (CD31 and CD41). We assessed externalized phosphatidylserines in PMP, since phospholipid-dependent procoagulant activitiesare limited to the PMP subsets exposing phosphatidylserines (6).
Thrombin generation (TG) was measured by means of Calibrated Automated Thrombography (CAT, chronometric method) (Diagnostica Stago, Asnières-sur-Seine, France) using the platelet-rich plasma (PRP) Reagent (containing only of 1 pMTF). The aim of this reagent is to assess procoagulant phospholipid-dependent activities of MP and the thrombin peak (assessed by Cmax parameter) is the critical parameter.
The chronometric method used was the STA Procoag PPL® (Diagnostica Stago) performed on the STA-Revolution® (STA-R®) instrument (Diagnostica Stago). This method explores phospholipid-dependent procoagulant activity component of MP and is not sensitive to the TF(10). After an incubation (120 seconds,37°C) of PPP with a reagent consisting of procoagulant phospholipid-depleted human plasma, activated FX and calcium were added and clotting time was measured using the STA-R® instrument.
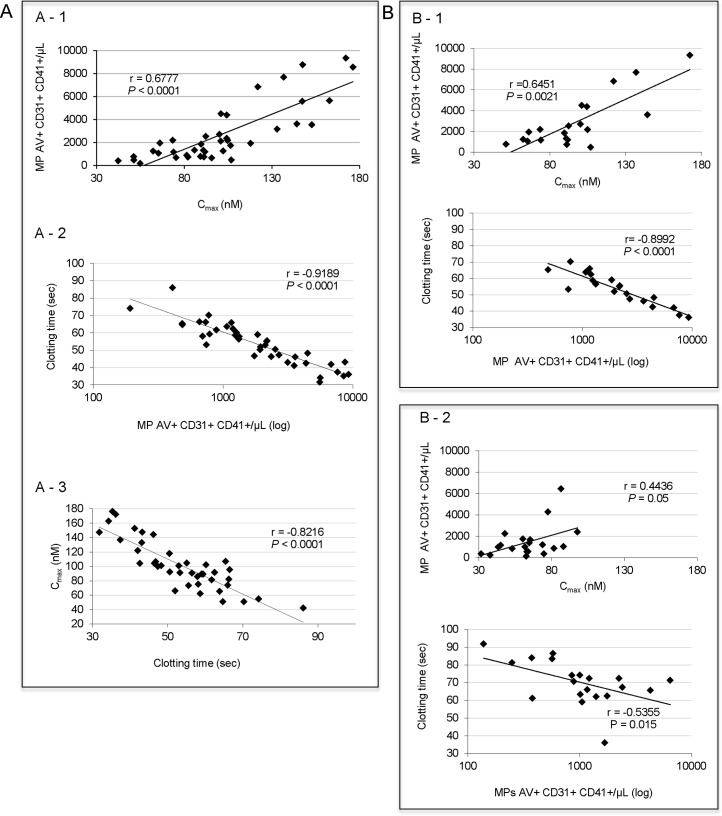
Correlation analyses were performed by Spearman’s rank correlation analysis. The inter-technique correlation curves obtained are shown in Figure 1.The strong correlations obtained between the 3 techniques demonstrate that with a good control of the pre-analytic variables, MP monitoring can be evaluated by two different approaches. An increase in the PMP numbers quantified by cytometry demonstrates an increase in procoagulant activity measured by haemostasis techniques (characterized by an increase in the Cmax of TG measurement and by shortening clotting time measured using the STA Procoag PPL® method).
Figure 1. The correlation between the enumeration of PMP assessed by cytometry and phospholipid-dependent procoagulant activity assessed by haemostasis methods.
A- The study was performed in 40 healthy donors and samples were centrifuged according to protocol #1. A1- Comparison of the enumeration of PMP assessed by cytometry and phospholipid-dependent procoagulant activity assessed by CAT method. A2- Comparison of the enumeration of PMP assessed by cytometry and phospholipid-dependent procoagulant activity assessed by STA Procoag PPL® method. A3- Comparison of the two haemostasis methods. B- Study of the effect of a pre-analytical variable, the centrifugation on the comparison of the enumeration of PMP assessed by cytometry and phospholipid-dependent procoagulant activity. Samples from 20 healthy donors were analyzed. B1- Data obtained for centrifugation protocol #1 (6) are similar to data obtained in A1 and A2, respectively with 40 samples. B2- Data obtained for centrifugation protocol #2 (ISTH) do not show correlation between either the enumeration of PMP assessed by cytometry and phospholipid-dependent procoagulant activity assessed by CAT (upper panel) or the enumeration of PMP assessed by cytometry and phospholipid-dependent procoagulant activity assessed by STA Procoag PPL® method (lower panel). P values and r correlation coefficients are given on each histogram. Intra- and inter-assay CV values are less than 3% and less than 14% for STA Procoag PPL® and CAT method, respectively. Intra- and inter-assay CV’s for cytometry using protocol #2 are 7.8 and 9.4%, respectively compared with 6.7% and 5.7% using protocol #1 (7).
(Leroyer et al., 2010, Tsimerman et al., 2011)
1. Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, Jourde N, Brunet P, Camoin-Jau L, Sampol J, Dignat-George F. Endothelial-derived microparticles: Biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost. 2010 Sep;104(3):456–63.
2. Tsimerman G, Roguin A, Bachar A, Melamed E, Brenner B, Aharon A. Involvement of microparticles in diabetic vascular complications. Thromb Haemost. 2011 Aug;106(2):310–21.
Furthermore, we observed that with a more intense centrifugation protocol (protocol #2), the number and procoagulant activity of the PMPs were reduced and that for lower values (< 2000 MP/μl, clotting time > 70 sec, Cmax< 60 nM) correlations between techniques were not as good (Figure 1B). Today, we cannot explain such observation. The advantages of cytometry are the detection of lineage markers which are useful in determining the cellular origin of MPs, but the complexity in detecting MPs < 0.5μm in a standardized manner is currently its main limitation. The advantages of haemostasis techniques are their low cost and the fact that they can be carried out quickly, and therefore easy to perform in routine practice. These techniques assess a functional parameter and do not rely on size determination, unlike cytometry.
In conclusion, a statistically significant correlation exists between the three approaches used to analyze phospholipid-dependent procoagulant activities in MPs by two distinct haemostasis methods and enumeration of circulating PMP expressing phosphatidylserines. Each method exhibits advantages and restrictions. One may suggest that the most automated method (i.e., the STA Procoag PPL®) can be used as first-line method to monitor MP. Moreover, our data support that the less intense centrifugation protocol (protocol #1) gives a better correlation between the different methods. Further studies are urgently needed to confirm these observations.
Acknowledgments
This work is supported by a grant from the Etablissement Français du Sang (French Blood Agency depending from the French Ministry of Health, APR 2011, grant #2011-11 to PS).
We would like to thank Frances Mary Sheppard and Sarah Odrion for editorial assistance.
Footnotes
Conflict of interest: The authors reported no potential conflicts of interest.
References
- 1.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101:439–51. [PubMed] [Google Scholar]
- 2.Angelot F, Seilles E, Biichle S, Berda Y, Gaugler B, Plumas J, Chaperot L, Dignat-George F, Tiberghien P, Saas P, Garnache-Ottou F. Endothelial cell-derived microparticles induce plasmacytoid dendritic cell maturation: potential implications in inflammatory diseases. Haematologica. 2009;94:1502–12. doi: 10.3324/haematol.2009.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 4.Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, Jourde N, Brunet P, Camoin-Jau L, Sampol J, Dignat-George F. Endothelial-derived microparticles: Biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost. 2010;104:456–63. doi: 10.1160/TH10-02-0111. [DOI] [PubMed] [Google Scholar]
- 5.Tsimerman G, Roguin A, Bachar A, Melamed E, Brenner B, Aharon A. Involvement of microparticles in diabetic vascular complications. Thromb Haemost. 2011;106:310–21. doi: 10.1160/TH10-11-0712. [DOI] [PubMed] [Google Scholar]
- 6.Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044–52. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 7.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011;105:396–408. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- 8.Robert S, Poncelet P, Lacroix R, Arnaud L, Giraudo L, Hauchard A, Sampol J, Dignat-George F. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190–7. doi: 10.1111/j.1538-7836.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 9.Pelletier F, Garnache-Ottou F, Angelot F, Biichle S, Vidal C, Humbert P, Saas P, Seilles E, Aubin F. Increased levels of circulating endothelial-derived microparticles and small-size platelet-derived microparticles in psoriasis. J Invest Dermatol. 2011;131:1573–6. doi: 10.1038/jid.2011.57. [DOI] [PubMed] [Google Scholar]
- 10.Connor DE, Exner T, Ma DD, Joseph JE. Detection of the procoagulant activity of microparticle-associated phosphatidylserine using XACT. Blood Coagul Fibrinolysis. 2009;20:558–64. doi: 10.1097/MBC.0b013e32832ee915. [DOI] [PubMed] [Google Scholar]