Abstract
Objective
After endothelial keratoplasty for Fuchs’ endothelial dystrophy, vision is degraded by scattered light because of structural changes in the retained host stroma. In this study, we determined if keratocyte populations were different in corneas with Fuchs’ dystrophy compared to normal corneas.
Methods
Eleven corneas excised at penetrating keratoplasty for Fuchs’ dystrophy, and five normal corneas of eyes enucleated for choroidal melanoma, were examined by using light microscopy. Twenty normal corneas, age-matched to the corneas with Fuchs’ dystrophy, were examined by using confocal microscopy in vivo. The number of keratocytes in a full thickness column of central stroma with frontal area of 1 mm2 (referred to as a “column”), determined by using histologic and confocal methods, was compared between Fuchs’ dystrophy and normal.
Results
By histology, the number of cells in a full-thickness column of stroma in Fuchs’ dystrophy (12,215 ± 1,394, [mean ± standard deviation]) was less than in normal corneas (15,628 ± 710, p<0.001). The number of keratocytes in the anterior 10% of the stroma of corneas with Fuchs’ dystrophy (682 ± 274 cells) was less than that in the normal corneas measured by using histology (1,858 ± 404 cells, p<0.001) and by using confocal microscopy (1,481 ± 397 cells, p<0.001).
Conclusions
Keratocytes are depleted by 54–63% in the anterior 10% of the stroma of corneas requiring penetrating keratoplasty for Fuchs’ dystrophy. Keratocyte loss might contribute to anterior stromal changes that persist and degrade vision after endothelial keratoplasty.
INTRODUCTION
Fuchs’ endothelial dystrophy is a bilateral, slowly progressive corneal endothelial disorder characterized by the formation of collagenous excrescences (guttae) on Descemet membrane, and corneal edema from endothelial cell loss or dysfunction. Surgical intervention for Fuchs’ dystrophy involves endothelial cell replacement by corneal transplantation. Previously, penetrating keratoplasty (PK) was the transplantation procedure of choice for Fuchs’ dystrophy, with replacement of all layers of the host cornea, but in the last decade, endothelial keratoplasty (EK) has surpassed PK as the preferred transplantation technique.1–3 After EK for Fuchs’ dystrophy, the host corneal stroma is retained and scatters more light than the stroma of normal corneas, presumably contributing to visual degradation.4,5 The scatter, or haze, is highest from the anterior cornea, and does not return to normal throughout the first year after EK. The origin of increased scatter has been hypothesized to be from cellular or extracellular ultrastructural changes in the stroma in response to chronic edema.4
A few studies have examined the stroma of corneas with Fuchs’ dystrophy, and have demonstrated subepithelial fibrosis6 with alterations in the collagenous extracellular matrix,7,8 but little is known about the keratocytes.9 Keratocytes maintain the stroma and presumably its transparency, and are also involved in wound healing and collagen formation. Activation of keratocytes in response to injury has also been suggested to cause corneal haze,10,11 and thus it is possible that keratocyte dysfunction contributes to corneal haze in Fuchs’ dystrophy. Confocal microscopy in vivo has demonstrated structural alterations in keratocytes in Fuchs’ dystrophy,12 but quantification of keratocytes by confocal microscopy is potentially inaccurate because of increased image reflectivity caused by corneal haze.
Given the current era of EK, the cells and structure of the retained host cornea are becoming important for understanding visual function after EK (Seery et al. IOVS 2009;50:ARVO E-Abstract #614). Thus, the main goal of this study was to compare keratocyte populations in corneas with advanced Fuchs’ endothelial dystrophy to populations in normal corneas by using a histologic method. In addition, we attempted to validate our automated program for estimating keratocyte density from confocal microscopy images13 in corneas with Fuchs’ dystrophy by comparing its estimates to histologic estimates of keratocyte density in the same corneas.
METHODS
Subjects
Eleven corneas of 10 patients scheduled for PK for Fuchs’ dystrophy were recruited from the cornea service at Mayo Clinic, Rochester, Minnesota; mean age was 70 ± 7 years (± standard deviation; range, 62–78 years). Corneas were examined by slit lamp biomicroscopy, and central thickness was measured by an ultrasonic pachymeter (DGH 1000, DGH Technologies, Inc., Frazer, PA). Best-corrected visual acuity (BCVA) was measured by using the electronic Early Treatment of Diabetic Retinopathy Study protocol14 and expressed as logarithm of the minimum angle of resolution (log MAR). Patients with a history of contact lens wear, glaucoma, previous ocular trauma or surgery (except cataract surgery), diabetes mellitus, or use of medications known to affect the cornea were excluded. Five normal corneas of 5 patients who were scheduled for enucleation for choroidal melanoma were also recruited to serve as normal controls with histologic data; mean age was 63 ± 15 years (range, 40–80 years). Twenty normal corneas of 20 subjects enrolled in a previous study15 were used as additional controls for estimates of keratocyte density by confocal microscopy in vivo. This group was age-matched to the subjects with Fuchs’ dystrophy and had a mean age of 70 ± 5 years (range, 61–80 years). This study adhered to the tenets of the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board. Written informed consent was obtained from all subjects after explanation of the study.
Confocal Microscopy In Vivo
The central cornea was examined in vivo by using a Tandem Scanning confocal microscope (Tandem Scanning Corporation, Reston, Virginia); eyes scheduled for surgery were examined prior to surgery (except for 2 normal corneas of 2 subjects with choroidal melanoma). The examination procedure has been described in detail previously.13,15,16
Histology
After removal of the corneas with Fuchs’ dystrophy for PK, and from enucleated eyes with choroidal melanoma, the central cornea was fixed in 10% buffered formalin. The corneal button was embedded in paraffin and 4 μm-thick serial sagittal sections were cut and stained with 4′,6-diamidino-2-phenylindole (DAPI). Fifteen serial sections spaced 8 μm apart were photographed at a magnification of 20x (Olympus DP70 camera operating on a BX60 transmitted microscope, Olympus America Inc., Center Valley, Pennsylvania). Images of a reticle were captured at the same magnification for calibration of horizontal and vertical dimensions.
Additional sections were stained with hematoxylin and eosin (H&E) for examination by light microscopy. The diagnosis of Fuchs’ dystrophy was confirmed by an ophthalmic pathologist based on the presence of guttae, thickening of Descemet membrane, and endothelial cell loss.
Keratocyte Density and Stereology
From confocal images, cell nuclei, which appeared as bright objects, were identified by using a custom automated program.13 From histologic images, DAPI-stained nuclei in the corneal stroma were identified by one observer using a point and click method (Figure 1). Volumetric cell density was calculated by using stereologic methods from the number of cells in a predefined area of the confocal and histologic images, as described previously.15,17 The sum of confocal depth of field and thickness of a keratocyte nucleus was 11.9 μm.18 In histologic images of all corneas, cell densities were calculated by using stromal thickness measured by confocal microscopy in vivo15,16 to avoid shrinkage artifacts caused by fixation; the other two dimensions were corrected for tissue shrinkage of 5.7% as described previously in normal corneas.15,17 We assumed that the frontal diameter of a keratocyte nucleus was 16 μm in histologic samples.15,19
Figure 1. Identification of keratocyte nuclei from histologic sections.
Upper Left, Sagittal section of a normal cornea stained with DAPI. Upper Right, The same sagittal section as in A, marked for counting. Keratocyte nuclei were counted by using a manual point and click method. By convention, nuclei were not counted if they crossed the right boundary of the area defined by the yellow box. Lower Left, Sagittal section of a cornea with Fuchs’ endothelial dystrophy. The cornea was edematous with a thickened Descemet membrane. Keratocyte loss was visible in the anterior stroma. Scale bar, 100 μm.
Keratocyte density was estimated in 5 layers of stroma, the anterior 10%, 10 to 33%, 33 to 66%, 66 to 90%, and the posterior 10% of stromal thickness (Figure 2).15 By confocal microscopy, cell density was estimated from two manually selected frames from each layer. Each histologic section was divided into the same layers of stroma based on the stromal thickness in the section.
Figure 2. Division of full-thickness stroma into 5 layers.
By confocal microscopy, mean keratocyte densities were determined from two manually selected frames (frontal section) from each of the 5 layers. By histology, each sagittal section was divided into the same 5 layers of stroma and mean cell density in each layer was determined from 15 full-thickness sections that were each spaced by 8 μm.
Absolute number of keratocytes
Keratocyte density decreases in edematous corneas because cells are distributed throughout a larger volume of tissue. To eliminate this effect of corneal edema, we estimated the number of cells in a full-thickness column of stroma with frontal area of 1 mm2 (referred to as “column”) by calculating the product of full-thickness weighted mean keratocyte density and central stromal thickness (measured in vivo by confocal microscopy).15,20
Histologic data for keratocyte density were not available for 2 normal corneas of 2 subjects because confocal microscopy was not performed preoperatively, and thus stromal thickness in vivo was not measured. Nevertheless, the absolute number of keratocytes in a column could be determined because this number was independent of stromal thickness.
Depth of hypocellular zone
The thickness of the anterior stromal hypocellular zone in corneas with Fuchs’ dystrophy was determined from the depth of key structures in confocal scans.15,16 Images were reviewed by two experienced observers (JWM, SVP) to identify the surface epithelium, the subbasal nerve plexus, and the first image with normal-appearing countable keratocyte nuclei. In normal corneas, the Bowman layer is not visible but the image of the most anterior and highest density of keratocyte nuclei corresponds to the boundary between the Bowman layer and the cellular stroma (Figure 3). In corneas with Fuchs’ dystrophy, we identified the boundary between the Bowman layer and stroma by the presence of sparse bright objects that were reminiscent of keratocyte nuclei but were morphologically abnormal and possibly represented degenerate keratocytes (Figure 3). This image was always deep to the subbasal nerves, and the distance between this image and the first keratocyte nuclei with normal morphology was defined as the hypocellular zone. Images were also examined to identify activated keratocytes, which morphologically had visible cell bodies and processes.
Figure 3. Confocal images of the anterior stroma in Fuchs’ dystrophy and normal corneas.
Few keratocytes were visible at the anterior boundary of the stroma in Fuchs’ dystrophy (Upper Left), although in normal corneas this region of stroma has the highest density of stromal cells (Upper Right). The stromal boundary was approximately 15 μm posterior to subbasal nerves in both eyes. In patients with Fuchs’ dystrophy, this boundary was identified by the appearance of remnants of cells and cellular debris, whereas in normal corneas it was identified by the high concentration of cell nuclei. Cell density was also reduced 25 μm posterior to the stromal boundary in Fuchs’ dystrophy (Lower Left) as compared to normal (Lower Right).
Statistical Methods
Keratocyte density and the number of keratocytes determined by histology in corneas with Fuchs’ dystrophy were compared to those determined by histology in the normal corneas after enucleation, and to those determined by confocal microscopy in vivo in the 20 age-matched, normal corneas. Densities were compared between groups by using generalized estimating equation (GEE) models to account for possible correlation between fellow eyes of the same subject.21 P-values were adjusted for multiple comparisons by using the Bonferroni technique, and p<0.05 was considered significant.
Keratocyte density and the number of keratocytes determined by using confocal microscopy were compared to those determined by using histology; for corneas with Fuchs’ dystrophy, we used GEE models, and for normal corneas, we used paired t-tests. Correlations were assessed by using the Pearson correlation coefficient, with significances calculated by using GEE models.
RESULTS
Clinical and histopathologic findings
By slit-lamp examination, all 11 eyes with Fuchs’ dystrophy had central confluent guttae with variable amounts of stromal edema; epithelial edema was clinically noted in only 1 eye. Best-corrected visual acuity was 0.49 ± 0.10 log MAR (Snellen equivalent, 20/62; range, 0.34 to 0.66 log MAR). Central corneal thickness by ultrasonic pachymetry was 642 ± 54 μm (range, 575 to 766 μm). Seven eyes had a cataract and 4 eyes were pseudophakic with posterior chamber intraocular lenses. Examination of H&E-stained sections of the excised corneas showed guttata, thickening of Descemet membrane, and endothelial cell loss in all 11 eyes; subepithelial bullae were noted in 4 eyes, and mild subepithelial fibrosis was present in only 1 eye.
Fuchs’ dystrophy versus normal
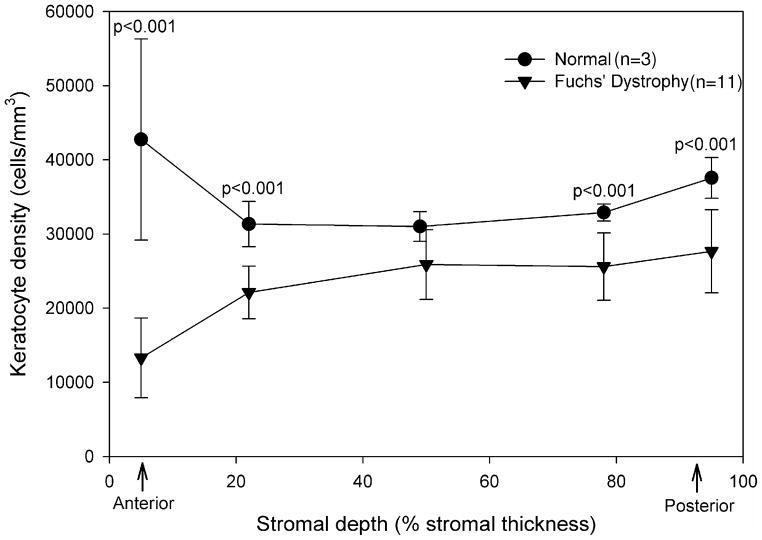
By histology, full-thickness keratocyte density in corneas with Fuchs’ dystrophy (23,872 ± 3,340 cells/mm3, n=11) was lower than that in normal corneas (33,376 ± 2,430 cells/mm3, n=3, p<0.001). Keratocyte densities were lower in all layers of stroma in corneas with Fuchs’ dystrophy than in corresponding layers of normal corneas (p<0.001) with the exception of the middle third of the stroma (p=0.99, Table 1, Figure 4). By histology, the number of keratocytes in a full-thickness column of stroma of corneas with Fuchs’ dystrophy (12,215 ± 1,394 cells, n=11) was lower than that of normal corneas (15,628 ± 710 cells, n=5, p<0.001, Table 1). The number of keratocytes in the anterior 10% of the stroma of corneas with Fuchs’ dystrophy (682 ± 274 cells, n=11) was lower than that in the normal corneas (1,858 ± 404 cells, n=5, p<0.001, Figure 1, Table 1).
Table 1.
Density and number of keratocytes determined by histologic methods in corneas with Fuchs’ dystrophy and normal corneas.
| Stromal Depth (%stromal thickness | Keratocyte Density (cells/mm3)
|
Number of Keratocytes*
|
||||
|---|---|---|---|---|---|---|
| Fuchs’ (11 eyes) | Normal (3 eyes) | P‡ | Fuchs’ (11 eyes) | Normal (5 eyes) | P‡ | |
|
|
|
|
||||
| Full-thickness stroma | 23,872 ± 3,340 | 33,376 ± 2,430 | <0.001 | 12,215 ± 1,394 | 15,628 ± 710 | <0.001 |
| By stromal layer: | ||||||
| 0–10% (anterior) | 13,295 ± 5,359 | 42,747 ± 13,555 | <0.001 | 682 ± 274 | 1,858 ± 404 | <0.001 |
| 11–33% | 22,121 ± 3,546 | 31,345 ± 3,050 | <0.001 | 2,608 ± 402 | 3,297 ± 357 | 0.03 |
| 34–66% | 25,886 ± 4,707 | 31,026 ± 2,006 | 0.99 | 4,361 ± 612 | 5,082 ± 431 | 0.17 |
| 67–90% | 25,611 ± 4,542 | 32,906 ± 1,155 | <0.001 | 3,143 ± 479 | 3,636 ± 420 | 0.34 |
| 91–100% (posterior) | 27,655 ± 5,608 | 37,562 ± 2,735 | <0.001 | 1,421 ± 300 | 1,752 ± 163 | 0.19 |
Mean ± standard deviation.
Absolute number of cells in a section of stroma with frontal area of 1 mm2
Figure 4. Keratocyte density determined by histologic methods in 5 stromal layers (mean ± SD).
Keratocyte density was lower in corneas with Fuchs’ dystrophy (n=11) than in normal corneas (n=3) in all layers of stroma except for the middle third.
The full-thickness cell density determined by histology in the 11 corneas with Fuchs’ dystrophy was not different from full thickness cell density determined by confocal microscopy in the 20 age-matched, normal corneas (21,463 ± 4,429 cells/mm3, p=0.13). In corneas with Fuchs’ dystrophy, the number of keratocytes was decreased only in the anterior 10% of the stroma, as compared to the number of keratocytes in the same layer of the age-matched, normal corneas (1,481 ± 397 cells, n=20, p<0.001).
In eyes with Fuchs’ dystrophy, BCVA correlated with the absolute number of keratocytes in a full-thickness column of stroma (r= −0.76, p=0.007, n=11), but not with the number of keratocytes in the anterior 10% of the stroma (r= −0.47, p=0.14, n=11). There were no correlations between central corneal thickness and the absolute number of keratocytes in a full-thickness column of stroma (r= 0.28, p=0.40, n=11) or the number of keratocytes in the anterior 10% of the stroma (r= 0.13, p=0.71, n=11). With a sample size of 11, the minimum detectable correlation was ±0.69 (or r2=0.48; α= 0.05, β=0.20).
Confocal microscopy versus histology
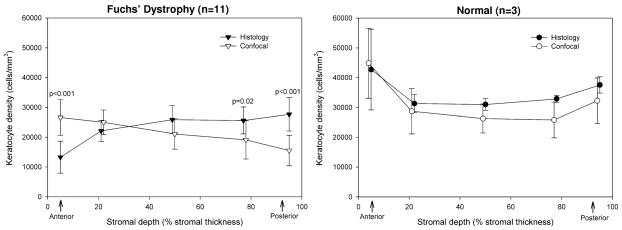
In corneas with Fuchs’ dystrophy, the weighted mean keratocyte density for the full-thickness stroma determined by automated assessment of confocal images was 21,507 ± 3,915 cells/mm3 and did not differ from that determined by histology (23,872 ± 3,340 cells/mm3, p=0.11). By the confocal method, keratocyte density was overestimated in the anterior 10% of the stroma (p<0.001) and was underestimated in the posterior third of the stroma (p≤0.02) compared to the histologic method (Figure 5).
Figure 5. Keratocyte density determined by histologic and confocal methods (mean ± SD).
Left, In corneas with Fuchs’ dystrophy (n=11), keratocyte density was overestimated by automated assessment of confocal images in the anterior 10% of the stroma, and underestimated by the confocal method in the posterior third of the stroma. Right, In normal corneas (n=3), keratocyte density determined by automated assessment of confocal images did not differ from that determined by the histologic method. Data points are offset slightly for clarity.
In normal corneas, the weighted mean keratocyte density for the full-thickness stroma determined by automated assessment of confocal images was 29,200 ± 6,534 cells/mm3 and did not differ from that determined by histology (33,376 ± 2,430 cells/mm3, p=0.25), although our sample size was small (n=3). There were no differences in keratocyte density between the confocal and histologic methods within the different layers of stroma (p>0.50, Figure 5).
Hypocellular Zone
The thickness of the anterior stromal hypocellular zone in corneas with Fuchs’ dystrophy was 15.6 ± 8.5 μm (range 4.9 to 32.0 μm). The first morphologically normal keratocyte nuclei were 27.4 ± 9.6 μm (range 9.6 to 41.8 μm) deep to the subbasal nerves, which were identified in all eyes. None of the images of corneas with Fuchs’ dystrophy contained activated keratocytes.
DISCUSSION
The major finding in this study was that keratocytes were depleted from the anterior stroma of corneas with advanced Fuchs’ endothelial dystrophy, forming an anterior hypocellular zone. Decreased stromal cellularity was strongly associated with worse visual acuity in eyes with Fuchs’ dystrophy; however, this finding should be interpreted with caution because 7 of the 11 eyes had cataracts, which would confound visual acuity. We also confirmed our suspicion that under the degraded confocal image conditions in corneas with Fuchs’ dystrophy, the automated estimation of keratocyte density by our software is not accurate.
Keratocyte Loss in Fuchs’ Dystrophy
Fuchs’ endothelial dystrophy is generally accepted to be a primary disorder of the corneal endothelium, although the exact pathophysiology of the condition is unknown. Changes in the stroma of corneas with Fuchs’ dystrophy are typically assumed to be secondary to endothelial dysfunction. To our knowledge, keratocyte populations in Fuchs’ dystrophy have not been studied by using histologic methods. We found absolute keratocyte loss, and not simply redistribution from corneal edema, in Fuchs’ dystrophy compared to normal. By histology, the number of keratocytes in the anterior 10% of the stroma of 11 corneas with Fuchs’ dystrophy was reduced by 63% compared to the normally high-density anterior layer in the 5 normal corneas. This loss might be somewhat underestimated because we assumed that swelling was uniform whereas, in fact, the anterior stroma swells less than the posterior stroma.22,23
Normal histologic data was only available in a small number of corneas in this study, and these corneas were not age-matched. To overcome these limitations, we compared keratocyte density determined by the histologic method in the corneas with Fuchs’ dystrophy to keratocyte density determined by the confocal method in 20 age-matched normal corneas; the number of keratocytes in the anterior 10% of the stroma was reduced by 54% in Fuchs’ dystrophy, confirming that anterior keratocytes were indeed depleted in corneas with advanced Fuchs’ dystrophy. The difference in keratocyte density between the 3 normal corneas examined by histology and the 20 normal corneas examined by confocal microscopy could be explained by differences in age15 and by the large variability of keratocyte density in the normal population.20,24
Mechanism of Cell Loss
The mechanism of cellular depletion from the anterior stroma of corneas with Fuchs’ dystrophy is unknown, but it is plausible that the keratocytes undergo apoptosis triggered by cytokine release from the epithelium, a known response to mechanical and viral epithelial injuries.25–27 Similarly, in corneas with advanced Fuchs’ dystrophy, it is conceivable that epithelial cell disruption or bullae formation result in cytokine release causing apoptosis of keratocytes. There is evidence of apoptosis in all cellular layers of the cornea in Fuchs’ dystrophy.9,28,29 Li et al. suggested that keratocytes were hypersensitive to apoptotic stimuli and that changes in keratocytes might precede endothelial and epithelial changes.9 Szentmary et al. found apoptotic cells in all layers of corneas with Fuchs’ dystrophy and pseudophakic corneal edema, indicating that keratocyte apoptosis might be a response to stromal edema from any cause.28 Indeed, if keratocyte loss were a result of apoptosis triggered by epithelial injury, one would expect to find anterior keratocyte depletion associated with any cause of chronic corneal edema, and not only Fuchs’ dystrophy. Alternative mechanisms of keratocyte loss in Fuchs’ dystrophy could include starvation of the stromal cells or cell death resulting from a chronically abnormal environment. The latter are less likely because we would expect increased cell death throughout the corneal stroma and not predominantly in the anterior stroma, and because endothelial permeability does not decrease in Fuchs’ dystrophy.30,31
Confocal Microscopy in Fuchs’ Dystrophy
Confocal microscopy enables visualization of keratocyte and other cell nuclei in vivo as bright objects. Our automated program13 for estimating keratocyte density is rapid and eliminates the subjectivity introduced by manual analysis, but requires keratocyte nuclei to have a consistent appearance and corneas to have normal or subnormal backscatter (haze).24,32 In the corneas with advanced Fuchs’ dystrophy, although automated assessment of confocal images indicated anterior keratocyte loss compared to normal, cell density was overestimated compared to histologic analysis. Manual review of the confocal images indicated that the program erroneously counted small bright objects and other diffuse localized areas of increased reflectivity (Figure 3). In the posterior stroma, the automated program underestimated keratocyte density compared to histologic methods, and we suspect that loss of image contrast caused by increased anterior stromal haze contributed to this underestimate. This program, and most likely other programs designed by using images of normal corneas, cannot be used in corneas with advanced Fuchs’ endothelial dystrophy without modification to overcome these limitations.
Qualitative examination of the confocal images confirmed a sparse population of keratocytes in the anterior stroma of corneas with Fuchs’ dystrophy; the high density of cell nuclei found in normal corneas was not visible in any of the corneas with Fuchs’ dystrophy (Figure 3), and the mean thickness of the anterior hypocellular stroma was 16 μm. Mustonen et al. found a range of appearances of the anterior stroma, including normal keratocyte nuclei, activated keratocytes, and diffuse haze in confocal images of corneas with different severities of Fuchs dystrophy.12 This suggests that the anterior stroma of corneas with Fuchs’ dystrophy undergoes different stages of cellular changes, or that some corneas behave differently compared to others. Further studies by confocal microscopy are needed to determine if a sequence of events in the anterior stroma is responsible for the changes that degrade vision.
Clinical Relevance
Endothelial keratoplasty has surpassed PK as the treatment of choice for Fuchs’ dystrophy, and although EK results in better uncorrected visual acuity compared to PK, many eyes fail to attain a best-corrected visual acuity of 20/20 or suffer from glare and poor contrast.3,4,33 Scatter or aberrations from the host cornea after EK may affect vision (Seery et al. IOVS 2009;50:ARVO E-Abstract #614) and thus understanding the changes and biology of the host cornea might provide insight into improving visual outcomes. Increased scatter in the anterior stroma of corneas with Fuchs’ dystrophy is not fully understood, although it is clinically recognized as subepithelial haze and increased forward scatter that affects vision.4 Increased scatter after keratorefractive surgery has been attributed to increased reflectivity from activated keratocytes,11 although our results suggest that increased scatter in Fuchs’ dystrophy was not directly from keratocytes; we found no evidence of keratocyte activation, and the number of keratocytes in the anterior stroma was decreased. The increased scatter possibly originates from the extracellular matrix and might be related to the deposition of abnormal proteins or changes in the proteoglycan properties of the anterior stroma7,34,35 Some have also suggested that increased scatter arises from fibril-free regions (“lakes”) of stroma caused by the death of keratocytes.23 The temporal relationship of matrix changes to keratocyte loss is unknown, but the loss of keratocytes may also impair the subsequent repair of the abnormal matrix.
The sequence of cellular and extracellular changes in the anterior stroma prior to EK, and whether or not keratocytes repopulate the anterior stroma after restoration of endothelial function, are unknown and warrant further investigation. Correlation of these changes to visual function might provide a better understanding of visual outcomes after EK, and the opportunity to modulate the stromal changes in the future could improve outcomes of this procedure.
Acknowledgments
The corresponding author has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by NIH EY 19339, Bethesda, MD (SVP); Research to Prevent Blindness, New York, NY (SVP as Olga Keith Wiess Special Scholar, and an unrestricted departmental grant); Mayo Foundation, Rochester, MN.
Footnotes
Presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting, Fort Lauderdale, FL, May 2-6, 2010.
Financial Disclosure: None (All Authors)
References
- 1.Patel SV. Keratoplasty for endothelial dysfunction. Ophthalmology. 2007;114(4):627–628. doi: 10.1016/j.ophtha.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Price MO, Price FW. Descemet’s stripping endothelial keratoplasty. Curr Opin Ophthalmol. 2007;18(4):290–294. doi: 10.1097/ICU.0b013e3281a4775b. [DOI] [PubMed] [Google Scholar]
- 3.Terry MA, Ousley PJ. Deep lamellar endothelial keratoplasty visual acuity, astigmatism, and endothelial survival in a large prospective series. Ophthalmology. 2005;112(9):1541–1548. doi: 10.1016/j.ophtha.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Patel SV, Baratz KH, Hodge DO, Maguire LJ, McLaren JW. The effect of corneal light scatter on vision after Descemet stripping with endothelial keratoplasty. Arch Ophthalmol. 2009;127(2):153–160. doi: 10.1001/archophthalmol.2008.581. [DOI] [PubMed] [Google Scholar]
- 5.Patel SV, McLaren JW, Hodge DO, Baratz KH. Scattered light and visual function in a randomized trial of deep lamellar endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2008;145(1):97–105. doi: 10.1016/j.ajo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Morishige N, Yamada N, Teranishi S, Chikama T-i, Nishida T, Takahara A. Detection of subepithelial fibrosis associated with corneal stromal edema by second harmonic generation imaging microscopy. Invest Ophthalmol Vis Sci. 2009;50(7):3145–3150. doi: 10.1167/iovs.08-3309. [DOI] [PubMed] [Google Scholar]
- 7.Calandra A, Chwa M, Kenney MC. Characterization of stroma from Fuchs’ endothelial dystrophy corneas. Cornea. 1989;8(2):90–97. [PubMed] [Google Scholar]
- 8.Iwamoto T, DeVoe AG. Electron microscopic studies on Fuchs’ combined dystrophy. II. Anterior portion of the cornea. Invest Ophthalmol. 1971;10(1):29–40. [PubMed] [Google Scholar]
- 9.Li QJ, Ashraf MF, Shen DF, et al. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch Ophthalmol. 2001;119(11):1597–1604. doi: 10.1001/archopht.119.11.1597. [DOI] [PubMed] [Google Scholar]
- 10.Moller-Pedersen T. On the structural origin of refractive instability and corneal haze after excimer laser keratectomy for myopia. Acta Ophthalmol Scand Suppl. 2003;237:1–20. [PubMed] [Google Scholar]
- 11.Moller-Pedersen T. Keratocyte reflectivity and corneal haze. Exp Eye Res. 2004;78(3):553–560. doi: 10.1016/s0014-4835(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 12.Mustonen RK, McDonald MB, Srivannaboon S, Tan AL, Doubrava MW, Kim CK. In Vivo Confocal Microscopy of Fuchs’ Endothelial Dystrophy. Cornea. 1998;17(5):493–503. doi: 10.1097/00003226-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 13.McLaren JW, Patel SV, Nau CB, Bourne WM. Automated assessment of keratocyte density in clinical confocal microscopy of the corneal stroma. J Microsc. 2008;229(1):21–31. doi: 10.1111/j.1365-2818.2007.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 15.Patel SV, McLaren JW, Hodge DO, Bourne WM. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42(2):333–339. [PubMed] [Google Scholar]
- 16.McLaren JW, Nau CB, Erie JC, Bourne WM. Corneal thickness measurement by confocal microscopy, ultrasound, and scanning slit methods. Am J Ophthalmol. 2004;137(6):1011–1020. doi: 10.1016/j.ajo.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 17.Patel SV, McLaren JW, Camp JJ, Nelson LR, Bourne WM. Automated quantification of keratocyte density by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 1999;40(2):320–326. [PubMed] [Google Scholar]
- 18.McLaren JW, Nau CB, Kitzmann AS, Bourne WM. Keratocyte density: comparison of two confocal microscopes. Eye Contact Lens. 2005;31(1):28–33. doi: 10.1097/01.icl.0000151948.92593.c3. [DOI] [PubMed] [Google Scholar]
- 19.Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36(13):2557–2567. [PubMed] [Google Scholar]
- 20.Patel SV, Erie JC, McLaren JW, Bourne WM. Keratocyte density and recovery of subbasal nerves after penetrating keratoplasty and in late endothelial failure. Arch Ophthalmol. 2007;125(12):1693–1698. doi: 10.1001/archopht.125.12.1693. [DOI] [PubMed] [Google Scholar]
- 21.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 22.Lee D, Wilson G. Non-uniform swelling properties of the corneal stroma. Curr Eye Res. 1981;1(8):457–461. doi: 10.3109/02713688109019986. [DOI] [PubMed] [Google Scholar]
- 23.Meek KM, Leonard DW, Connon CJ, Dennis S, Khan S. Transparency, swelling and scarring in the corneal stroma. Eye. 2003;17(8):927–936. doi: 10.1038/sj.eye.6700574. [DOI] [PubMed] [Google Scholar]
- 24.McLaren JW, Bourne WM, Patel SV. Automated assessment of keratocyte density in stromal images from the ConfoScan 4 confocal microscope. Invest Ophthalmol Vis Sci. 2010;51(4):1918–1926. doi: 10.1167/iovs.09-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39(2):276–283. [PubMed] [Google Scholar]
- 26.Wilson SE, Kim WJ. Keratocyte apoptosis: implications on corneal wound healing, tissue organization, and disease. Invest Ophthalmol Vis Sci. 1998;39(2):220–226. [PubMed] [Google Scholar]
- 27.Wilson SE, He YG, Weng J, et al. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62(4):325–327. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- 28.Szentmary N, Szende B, Suveges I. Epithelial cell, keratocyte, and endothelial cell apoptosis in Fuchs’ dystrophy and in pseudophakic bullous keratopathy. Eur J Ophthalmol. 2005;15(1):17–22. doi: 10.1177/112067210501500103. [DOI] [PubMed] [Google Scholar]
- 29.Borderie VM, Baudrimont M, Vallee A, Ereau TL, Gray F, Laroche L. Corneal endothelial cell apoptosis in patients with Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 2000;41(9):2501–2505. [PubMed] [Google Scholar]
- 30.Wilson SE, Bourne WM, O’Brien PC, Brubaker RF. Endothelial function and aqueous humor flow rate in patients with Fuchs’ dystrophy. Am J Ophthalmol. 1988;106(3):270–278. doi: 10.1016/0002-9394(88)90360-1. [DOI] [PubMed] [Google Scholar]
- 31.Wilson SE, Bourne WM, Brubaker RF. Effect of dexamethasone on corneal endothelial function in Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 1988;29(3):357–361. [PubMed] [Google Scholar]
- 32.McLaren JW, Bourne WM, Patel SV. Standardization of corneal haze measurement in confocal microscopy. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5614. Epub 2010 Jun 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen ES, Terry MA, Shamie N, Hoar KL, Friend DJ. Descemet-stripping automated endothelial keratoplasty: six-month results in a prospective study of 100 eyes. Cornea. 2008;27(5):514–520. doi: 10.1097/ICO.0b013e3181611c50. [DOI] [PubMed] [Google Scholar]
- 34.Castoro JA, Bettelheim AA, Bettelheim FA. Water gradients across bovine cornea. Invest Ophthalmol Vis Sci. 1988;29(6):963–968. [PubMed] [Google Scholar]
- 35.Ljubimov AV, Burgeson RE, Butkowski RJ, et al. Extracellular matrix alterations in human corneas with bullous keratopathy. Invest Ophthalmol Vis Sci. 1996;37(6):997–1007. [PubMed] [Google Scholar]