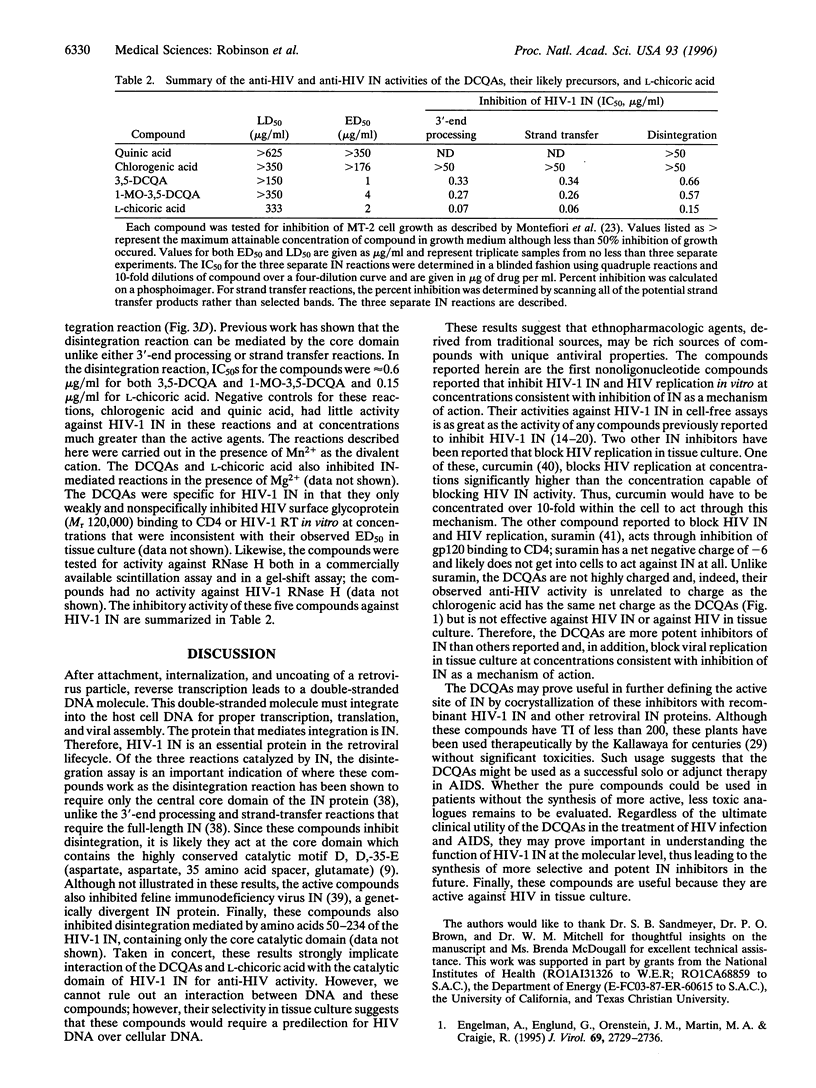
Abstract
HIV-1 replication depends on the viral enzyme integrase that mediates integration of a DNA copy of the virus into the host cell genome. This enzyme represents a novel target to which antiviral agents might be directed. Three compounds, 3,5-dicaffeoylquinic acid, 1-methoxyoxalyl-3,5-dicaffeoylquinic acid, and L-chicoric acid, inhibit HIV-1 integrase in biochemical assays at concentrations ranging from 0.06-0.66 microgram/ml; furthermore, these compounds inhibit HIV-1 replication in tissue culture at 1-4 microgram/ml. The toxic concentrations of these compounds are fully 100-fold greater than their antiviral concentrations. These compounds represent a potentially important new class of antiviral agents that may contribute to our understanding of the molecular mechanisms of viral integration. Thus, the dicaffeoylquinic acids are promising leads to new anti-HIV therapeutics and offer a significant advance in the search for new HIV enzyme targets as they are both specific for HIV-1 integrase and active against HIV-1 in tissue culture.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowerman B., Brown P. O., Bishop J. M., Varmus H. E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989 Apr;3(4):469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Retroviral integration: structure of the initial covalent product and its precursor, and a role for the viral IN protein. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2525–2529. doi: 10.1073/pnas.86.8.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. I., Sharova N., McDonald T. L., Pushkarskaya T., Tarpley W. G., Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman F. D., Engelman A., Palmer I., Wingfield P., Craigie R. Domains of the integrase protein of human immunodeficiency virus type 1 responsible for polynucleotidyl transfer and zinc binding. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3428–3432. doi: 10.1073/pnas.90.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon P. M., Wilson W., Byles E., Kingsman S. M., Kingsman A. J. Human immunodeficiency virus type 1 integrase: effect on viral replication of mutations at highly conserved residues. J Virol. 1994 Aug;68(8):4768–4775. doi: 10.1128/jvi.68.8.4768-4775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardellina J. H., 2nd, Munro M. H., Fuller R. W., Manfredi K. P., McKee T. C., Tischler M., Bokesch H. R., Gustafson K. R., Beutler J. A., Boyd M. R. A chemical screening strategy for the dereplication and prioritization of HIV-inhibitory aqueous natural products extracts. J Nat Prod. 1993 Jul;56(7):1123–1129. doi: 10.1021/np50097a016. [DOI] [PubMed] [Google Scholar]
- Carteau S., Mouscadet J. F., Goulaouic H., Subra F., Auclair C. Inhibition of the in vitro integration of Moloney murine leukemia virus DNA by the DNA minor groove binder netropsin. Biochem Pharmacol. 1994 May 18;47(10):1821–1826. doi: 10.1016/0006-2952(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Carteau S., Mouscadet J. F., Goulaouic H., Subra F., Auclair C. Inhibitory effect of the polyanionic drug suramin on the in vitro HIV DNA integration reaction. Arch Biochem Biophys. 1993 Sep;305(2):606–610. doi: 10.1006/abbi.1993.1468. [DOI] [PubMed] [Google Scholar]
- Chow S. A., Vincent K. A., Ellison V., Brown P. O. Reversal of integration and DNA splicing mediated by integrase of human immunodeficiency virus. Science. 1992 Feb 7;255(5045):723–726. doi: 10.1126/science.1738845. [DOI] [PubMed] [Google Scholar]
- Craigie R., Fujiwara T., Bushman F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell. 1990 Aug 24;62(4):829–837. doi: 10.1016/0092-8674(90)90126-y. [DOI] [PubMed] [Google Scholar]
- Cushman M., Golebiewski W. M., Pommier Y., Mazumder A., Reymen D., De Clercq E., Graham L., Rice W. G. Cosalane analogues with enhanced potencies as inhibitors of HIV-1 protease and integrase. J Med Chem. 1995 Feb 3;38(3):443–452. doi: 10.1021/jm00003a007. [DOI] [PubMed] [Google Scholar]
- Cushman M., Sherman P. Inhibition of HIV-1 integration protein by aurintricarboxylic acid monomers, monomer analogs, and polymer fractions. Biochem Biophys Res Commun. 1992 May 29;185(1):85–90. doi: 10.1016/s0006-291x(05)80958-1. [DOI] [PubMed] [Google Scholar]
- Dyda F., Hickman A. B., Jenkins T. M., Engelman A., Craigie R., Davies D. R. Crystal structure of the catalytic domain of HIV-1 integrase: similarity to other polynucleotidyl transferases. Science. 1994 Dec 23;266(5193):1981–1986. doi: 10.1126/science.7801124. [DOI] [PubMed] [Google Scholar]
- Ellison V., Abrams H., Roe T., Lifson J., Brown P. Human immunodeficiency virus integration in a cell-free system. J Virol. 1990 Jun;64(6):2711–2715. doi: 10.1128/jvi.64.6.2711-2715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A., Englund G., Orenstein J. M., Martin M. A., Craigie R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J Virol. 1995 May;69(5):2729–2736. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesen M. R., Kohn K. W., Leteurtre F., Pommier Y. Inhibitors of human immunodeficiency virus integrase. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2399–2403. doi: 10.1073/pnas.90.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesen M. R., Pommier Y., Leteurtre F., Hiroguchi S., Yung J., Kohn K. W. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem Pharmacol. 1994 Aug 3;48(3):595–608. doi: 10.1016/0006-2952(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Mizuuchi K. Retroviral DNA integration: structure of an integration intermediate. Cell. 1988 Aug 12;54(4):497–504. doi: 10.1016/0092-8674(88)90071-2. [DOI] [PubMed] [Google Scholar]
- Goff S. P. Genetics of retroviral integration. Annu Rev Genet. 1992;26:527–544. doi: 10.1146/annurev.ge.26.120192.002523. [DOI] [PubMed] [Google Scholar]
- Heinzinger N. K., Bukrinsky M. I., Haggerty S. A., Ragland A. M., Kewalramani V., Lee M. A., Gendelman H. E., Ratner L., Stevenson M., Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashman Y., Gustafson K. R., Fuller R. W., Cardellina J. H., 2nd, McMahon J. B., Currens M. J., Buckheit R. W., Jr, Hughes S. H., Cragg G. M., Boyd M. R. The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J Med Chem. 1992 Jul 24;35(15):2735–2743. doi: 10.1021/jm00093a004. [DOI] [PubMed] [Google Scholar]
- Kulkosky J., Jones K. S., Katz R. A., Mack J. P., Skalka A. M. Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol Cell Biol. 1992 May;12(5):2331–2338. doi: 10.1128/mcb.12.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFemina R. L., Graham P. L., LeGrow K., Hastings J. C., Wolfe A., Young S. D., Emini E. A., Hazuda D. J. Inhibition of human immunodeficiency virus integrase by bis-catechols. Antimicrob Agents Chemother. 1995 Feb;39(2):320–324. doi: 10.1128/aac.39.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFemina R. L., Schneider C. L., Robbins H. L., Callahan P. L., LeGrow K., Roth E., Schleif W. A., Emini E. A. Requirement of active human immunodeficiency virus type 1 integrase enzyme for productive infection of human T-lymphoid cells. J Virol. 1992 Dec;66(12):7414–7419. doi: 10.1128/jvi.66.12.7414-7419.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Raghavan K., Weinstein J., Kohn K. W., Pommier Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem Pharmacol. 1995 Apr 18;49(8):1165–1170. doi: 10.1016/0006-2952(95)98514-a. [DOI] [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Mitchell W. M. In vitro evaluation of mismatched double-stranded RNA (ampligen) for combination therapy in the treatment of acquired immunodeficiency syndrome. AIDS Res Hum Retroviruses. 1989 Apr;5(2):193–203. doi: 10.1089/aid.1989.5.193. [DOI] [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Schuffman S. S., Mitchell W. M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988 Feb;26(2):231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Montefiori D. C., Gillespie D. H., Mitchell W. M. Complement-mediated, antibody-dependent enhancement of HIV-1 infection in vitro is characterized by increased protein and RNA syntheses and infectious virus release. J Acquir Immune Defic Syndr. 1989;2(1):33–42. [PubMed] [Google Scholar]
- Roe T., Reynolds T. C., Yu G., Brown P. O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993 May;12(5):2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Kawamura M., Sakuragi J., Sakuragi S., Shibata R., Ishimoto A., Ono N., Ueda S., Adachi A. Integration is essential for efficient gene expression of human immunodeficiency virus type 1. J Virol. 1993 Mar;67(3):1169–1174. doi: 10.1128/jvi.67.3.1169-1174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. A., Fyfe J. A. Human immunodeficiency virus integration protein expressed in Escherichia coli possesses selective DNA cleaving activity. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5119–5123. doi: 10.1073/pnas.87.13.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Haggerty S., Lamonica C. A., Meier C. M., Welch S. K., Wasiak A. J. Integration is not necessary for expression of human immunodeficiency virus type 1 protein products. J Virol. 1990 May;64(5):2421–2425. doi: 10.1128/jvi.64.5.2421-2425.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddeo B., Haseltine W. A., Farnet C. M. Integrase mutants of human immunodeficiency virus type 1 with a specific defect in integration. J Virol. 1994 Dec;68(12):8401–8405. doi: 10.1128/jvi.68.12.8401-8405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K. A., Ellison V., Chow S. A., Brown P. O. Characterization of human immunodeficiency virus type 1 integrase expressed in Escherichia coli and analysis of variants with amino-terminal mutations. J Virol. 1993 Jan;67(1):425–437. doi: 10.1128/jvi.67.1.425-437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink C., van der Linden K. H., Plasterk R. H. Activities of the feline immunodeficiency virus integrase protein produced in Escherichia coli. J Virol. 1994 Mar;68(3):1468–1474. doi: 10.1128/jvi.68.3.1468-1474.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga T., Fujiwara T. Different roles of bases within the integration signal sequence of human immunodeficiency virus type 1 in vitro. J Virol. 1995 May;69(5):3233–3236. doi: 10.1128/jvi.69.5.3233-3236.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]