Abstract
The molecular mechanisms leading to asexuality remain little understood despite their substantial bearing on why sexual reproduction is dominant in nature. Here we examine the role of hybridization in the origin and spread of obligate asexuality in Daphnia pulex, arguably the best-documented case of contagious asexuality. Obligately parthenogenetic (OP) clones of D. pulex have traditionally been separated into “hybrid” (Ldh SF) and “non-hybrid” (Ldh SS) forms because the lactase dehydrogenase (Ldh) locus distinguishes the cyclically parthenogenetic (CP) lake dwelling Daphnia pulicaria (Ldh FF) from its ephemeral pond dwelling sister species D. pulex (Ldh SS). The results of our population genetic analyses based on microsatellite loci suggest that both Ldh SS and SF OP individuals can originate from the crossing of CP female F1 (D. pulex × D. pulicaria) and backcrosses with males from OP lineages carrying genes that suppress meiosis specifically in female offspring. In previous studies, a suite of diagnostic markers was found to be associated with OP in Ldh SS D. pulex lineages. Our association mapping supports a similar genetic mechanism for the spread of obligate parthenogenesis in Ldh SF OP individuals. Interestingly, our study shows that CP D. pulicaria carry many of the diagnostic microsatellite alleles associated with obligate parthenogenesis. We argue that the assemblage of mutations that suppress meiosis and underlie obligate parthenogenesis in D. pulex originated due to a unique historical hybridization and introgression event between D. pulex and D. pulicaria.
Keywords: parthenogenesis, hybridization, meiosis suppression, Daphnia pulex, Daphnia pulicaria, asexuality
Introduction
The maintenance of sex is one of the most puzzling biological questions (Maynard Smith 1978; Bell 1982; Otto 2009). The phylogenetic distribution of reproductive strategies across eukaryotes shows that sexual reproduction is predominant, whereas asexual taxa are rare and usually occupy the tips on the tree of life (Maynard Smith 1978), suggesting their evolutionarily short persistence. However, relative to their sexual counterparts, asexually reproducing individuals have a two-fold demographic advantage, do not need to allocate energy to seeking mates, and are free from the risk of sexually transmitted diseases (Otto 2009). Thus, extensive research has focused on the long-term and immediate costs and benefits of sex, providing insights into how sexual individuals can overcome their demographic disadvantages and outcompete asexuals (Kondrashov 1993; Otto 2009). Despite some illuminating findings such as that the persistence of asexual lineages can be severely compromised by the accumulation of deleterious mutations due to the lack of genetic recombination (e.g., Lynch et al. 1993), theoretical studies generally assume that mutations are the only source of genetic variation in asexuals. This assumption ignores the fact that parthenogenesis (i.e., offspring hatching from unfertilized eggs) has evolved in plants and animals through distinct biological processes and underlying molecular mechanisms, which may have great impact on the genetic diversity of asexual lineages.
Previous investigations have used crossing experiments and association studies to understand the genetics of the elements (e.g., dominance effects and number of loci) responsible for meiosis suppression in plants including Hieracium, Erigeron annuus, and Taraxacum (Catanach et al. 2006; Noyes et al. 2007; Van Dijk et al. 2009) and animals such as the parasitoid wasp Lysiphlebus fabarum (Sandrock & Vorburger 2011), and the honey bee Apis mellifera capensis (Lattorff et al. 2005). However, we still have little insight into the molecular mechanisms underlying the conversion of ancestrally sexual reproduction to a derived state of parthenogenesis (Lynch et al. 2008).
Parthenogenesis in animals can arise by different mechanisms such as spontaneous origin, hybrid origin, and contagious origin (Simon et al. 2003). Spontaneous origin may occur through mutations in the genes involved in sexual reproduction and meiosis, e.g., the loss of sex in monogonont rotifers (Serra & Snell 2009). Parthenogenesis may also occur when normal meiosis is disrupted due to genomic divergences (e.g., chromosomal rearrangements) between hybridizing parental species (Shimizu et al. 2000; Kearney et al. 2009). Although phylogenetic studies have revealed that parthenogenetic lineages in vertebrates, snails, crustaceans, and insects such as aphids, weevils, and grasshoppers have a hybrid origin (reviewed in Simon et al. 2003), only a few attempts to produce parthenogens by hybridization in the laboratory have succeeded, e.g., in the marsh frog Rana ridibunda (Hotz et al. 1985), the fish Poeciliopsis (Schultz 1973), the grasshopper Warramaba (White et al. 1977), and planthoppers of the genus Muellerianella (Drosopoulos 1978), leaving the question open about the role of hybridization in the origin of parthenogenesis (Kearney et al. 2009). Contagious parthenogenesis can result from the transmission of genetic elements that confer asexuality as a consequence of mating between males produced by obligately parthenogenetic lineages and sexual females, e.g., Daphnia (Innes & Hebert 1988).
The Daphnia pulex species complex (Crustacea, Anomopoda), which contains multiple obligately parthenogenetic (OP) lineages derived from cyclically parthenogenetic (CP) ancestors, is perhaps one of the best documented systems of contagious asexuality in animals (Innes & Hebert 1988; Simon et al. 2003; Lynch et al. 2008) and is also an ideal system for examining the molecular mechanisms responsible for the conversion to asexual reproduction (Crease et al. 1989; Hebert et al. 1989; Paland et al. 2005). Daphnia species typically reproduce by cyclical parthenogenesis, i.e., alternation of asexual and sexual reproduction. Under favorable environmental conditions, females apomictically produce diploid eggs that directly develop into daughters. Environmental cues signalling deteriorating conditions (e.g., food shortage, high population density, and/or photoperiod changes) trigger the production of males and haploid eggs by females (Innes & Hebert 1988). Fertilized eggs that are deposited in a protective structure (ephippium) can hatch after extensive periods of dormancy (Decaestecker et al. 2007).
Interestingly, some lineages in the D. pulex complex have lost the ability to engage in sexual reproduction as females, reproducing strictly by obligate parthenogenesis. However, a subset of OP lineages can still produce males capable of haploid sperm production (Hebert 1981; Hebert et al. 1989). It has been assumed that an important mechanism responsible for the origin of new OP D. pulex lineages involves the transmission of meiosis suppression genes via this type of male mating with CP females (Innes & Hebert 1988). Assuming the sex-limited meiosis suppression genes are at least partially dominant, some OP offspring will arise from such crosses (Innes & Hebert 1988). Obligate parthenogenesis spreading in this contagious fashion probably accounts for the independent origin of multiple OP lineages documented for D. pulex (Hebert et al. 1989; Paland et al. 2005).
Initially, it was hypothesized that a single, dominant gene is responsible for meiosis suppression/obligate parthenogenesis in Daphnia (Innes & Hebert 1988). However, a recent genome-wide association study using a collection of D. pulex lineages across North America shows that at least four genomic regions are associated with meiosis suppression and other critical features for the persistence of obligate parthenogenetic lineages, e.g., production of males and hatching of unfertilized eggs (Lynch et al. 2008). This study suggests that different genomic regions may be responsible for different features of obligate parthenogenesis. Moreover, the recent work of Eads et al. (2012) shows that an insertion at the Rec8 B locus is associated with the spread of OP D. pulex in North America.
Despite these recent advances in pinpointing the genomic regions involved, the origin of the suite of meiosis suppression genes in D. pulex has not been investigated closely. Because mutations are rare events, the spontaneous and simultaneous occurrence of mutations in four different genomic regions or different loci on the same chromosome that specifically convert meiosis to apomixis is unlikely. However, as suggested by studies in other species (Vrijenhoek 1998), hybridization and/or backcrossing are potentially capable of accounting for the origin of polygenic meiosis suppression. For example, if multiple genomic regions with chromosome rearrangements exist between hybridizing species, meiosis can be disrupted, setting up the stage for the emergence of mutations conferring the capability of direct egg development without fertilization.
In Daphnia, hybridization between closely related species is common (Taylor & Hebert 1992; Dufresne & Hebert 1994; Taylor et al. 1996; Taylor et al. 2005), especially between D. pulex and its sister species D. pulicaria (Hebert et al. 1989; Heier & Dudycha 2009; Cristescu et al. 2012). Although morphologically indistinguishable, D. pulex and D. pulicaria occupy largely distinct habitats and can be identified by diagnostic alleles of a few allozyme loci, such as the lactate dehydrogenase (Ldh) locus and leucyl-alanine peptidase (Pep), and by microsatellite markers (Cristescu et al. 2012). D. pulex live mainly in ephemeral ponds and can be occasionally found in some permanent fishless habitats, whereas the primary habitats for D. pulicaria are permanent, stratified lakes with high fish predation (Crease et al. 1997; Pfrender et al. 2000). Cyclically parthenogenetic D. pulex are homozygous for the slow allele (S) of Ldh, while D. pulicaria are homozygous for the fast allele (F) (Table 1; Hebert et al. 1989; Pfrender et al. 2000). Across the North American distribution, D. pulex populations in the northeast part of the continent harbor exclusively OP individuals, whereas CP populations are found mainly in the mid-west and western US (Hebert & Finston 2001). Interestingly, the Great Lakes watershed represents a contact zone between these two reproductive strategies with many ephemeral ponds containing a mixture of OP (both Ldh SS and SF genotypes) and CP individuals (Hebert & Finston 2001). D. pulicaria is distributed across North America, and no OP lineages have been documented based on direct tests of breeding system (Heier & Dudycha 2009). Hybrids of D. pulex and D. pulicaria are characterized by a heterozygous (SF) genotype at the Ldh locus and are common in temperate regions of North America (Table 1). These SF individuals invariably appear to be OP in the field and are found predominately in ponds in disturbed areas where forests have been cleared (Hebert & Crease 1983). Furthermore, these Ldh SF OP individuals have a mitochondrial genotype from D. pulex (Cristescu et al. 2012), suggesting that their maternal genealogy is derived from D. pulex. However, the genetic basis of obligate parthenogenesis has not been investigated in these OP individuals.
Table 1.
Description of the association between species, Ldh genotype, reproduction mode (CP, cyclical parthenogenesis; OP, obligate parthenogenesis), presence of males, and the presence of diagnostic alleles associated with OP (see Table 3). The presence of OP diagnostic alleles in Ldh SF OP Daphnia pulex and Daphnia pulicaria is a result from the present study, whereas all other information is from pre-existing literature.
| Species | Ldh | Reproduction | Presence of males | Presence of OP diagnostic alleles | References |
|---|---|---|---|---|---|
| D. pulex | SS | CP | Yes | No | Lynch et al. (2008) |
| SS | OP | Yes (some lineages) | Yes | Lynch et al. (2008) | |
| SF | OP (in field) | Yes (some lineages) | Yes | Heier and Dudycha (2009), this study | |
| D. pulicaria | FF | CP | Yes | Yes (at some diagnostic loci) | This study |
Heier and Dudycha (2009) performed crosses between females of CP D. pulex and males of CP D. pulicaria and showed that the F1 (Ldh SF) progeny are consistently cyclical parthenogens, rather than obligate parthenogens. This finding contrasts with field observations in which the Ldh SF genotype is always OP. Thus, Heier and Dudycha (2009) suggested that the natural OP individuals might originate from the crossing of sexual female F1 hybrids (resulting from D. pulex × D. pulicaria) with males from OP D. pulex that can transmit meiosis suppressing genes. This hypothesis also raises the possibility that the Ldh SS OP individuals observed in temporary ponds may also be a consequence of crossing of F1 hybrid females with OP males in these pond environments (Fig. 1). Thus, some of the Ldh SS OP individuals are expected to retain D. pulicaria alleles (i.e., 25% or less) and to appear distinct from the Ldh SS OP D. pulex individuals arising due to the transmission of meiosis suppression genes within non-hybrid D. pulex populations (i.e., 100% genome from D. pulex, Heier & Dudycha 2009). Furthermore, Ldh SF OP individuals should show the highly diagnostic alleles for obligate parthenogenesis in Ldh SS individuals, and the OP offspring from the envisioned crossing (F1s × males from OP clones) could carry either Ldh SS or SF genotypes. Therefore, the Ldh SS OP D. pulex individuals could be derived from the crossing between males from OP lineages and pure CP D. pulex females as the current literature assumes, or from crosses of OP males with F1 (D. pulex × D. pulicaria) or backcross females, meaning that the Ldh SS genotype is not a reliable indicator of the origin of the OP lineages.
Figure 1.
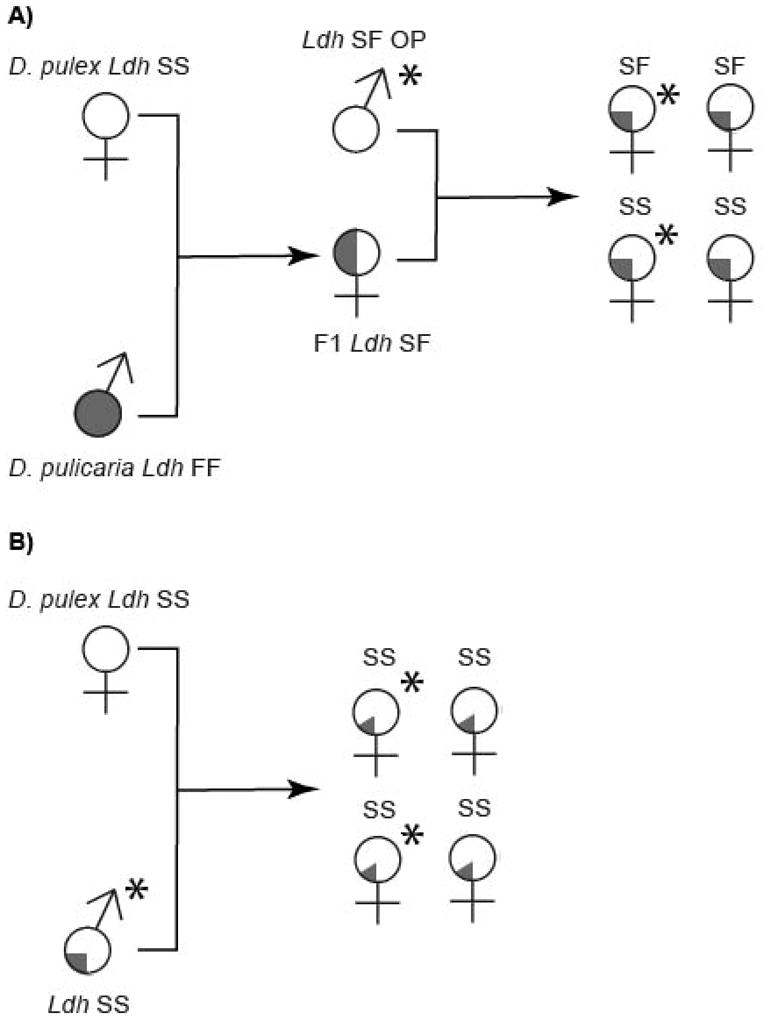
The transmission of meiosis suppression in Daphnia pulex. (A) The Ldh SF and SS obligately parthenogenetic (OP) D. pulex hybrids can originate from backcrossing between F1 cyclically parthenogenetic (CP) hybrids of Daphnia pulex (white) × Daphnia pulicaria (grey) with D. pulex males carrying meiosis-suppression genes. Stars indicate obligately parthenogenetic individuals. (B) Females of pure D. pulex can mate with Ldh SS OP males with some D. pulicaria genes, giving rise to both CP and OP offspring with some D. pulicaria genes (grey).
The underlying assumption of the hypothesis of Heier and Dudycha (2009) is that meiosis suppression initially evolved in D. pulex. However, considering the possibility that CP hybrids can facilitate the introgression of D. pulicaria genes into a D. pulex genomic background through continuous backcrossing, we hypothesize that the origin of meiosis suppression resulted from an ancestral hybridization event between D. pulex and D. pulicaria followed by backcrossing to D. pulex, with the prediction that some of the diagnostic alleles associated with obligate parthenogenesis in OP D. pulex should be traceable to the parental species (e.g., D. pulicaria).
In the present study, we focus on a small geographic region in the Great Lakes watershed where the coexistence of CP (Ldh SS) and OP D. pulex lineages (both Ldh SS and SF) presents an opportunity to investigate the evolution of meiosis suppression in D. pulex. First, we use microsatellite data to test the origin of the Ldh SF OP D. pulex individuals and to evaluate whether there are distinct groups of Ldh SS OP individuals that vary in the amounts of D. pulicaria genes in their genomes. Second, we consider whether the mechanisms responsible for OP in Ldh SF individuals are similar to those involved in the meiosis suppression in Ldh SS D. pulex by examining whether the diagnostic markers associated with Ldh SS OP lineages are the same as those in association with Ldh SF OP individuals. Lastly, we investigate the origin of meiosis suppression in D. pulex by examining whether some of the diagnostic alleles on each of the four genomic regions associated with obligate parthenogenesis in D. pulex are predominant in D. pulicaria.
Materials and methods
Sampling and sexuality test
D. pulex and D. pulex/pulicaria “hybrids” were collected from four ephemeral ponds (Canard 1, 1B, 2, and 3, see Table 2) located within 5 km of Windsor, Ontario, Canada. These ponds are usually filled with water by snow melt in early spring, dry up by early summer, and are unique in containing both CP (Ldh SS) and OP (Ldh SS and SF) D. pulex individuals. This is in contrast to the ponds in northeast Canada and USA that contain only Ldh SS OP individuals. Such mixed populations have been described in Southwestern Ontario and Michigan (Hebert & Finston 2001). To investigate the relationship of Ldh SF OP individuals with CP D. pulex and D. pulicaria, we incorporated into our dataset 101 CP individuals of D. pulex from the Disputed Pond (near the Canard ponds) and Solomon pond in Michigan, USA and 217 D. pulicaria individuals from three lakes (Three Lakes II, Lawrence, and Warner) from Michigan (Cristescu et al. 2012). Disputed Pond and Solomon Pond do not contain OP individuals. The Canard ponds were not included in previous studies by Lynch et al. (2008) and Eads et al. (2012) because these studies did not involve Ldh SF OP D. pulex individuals. It should be noted that in this study we refer to F1s and F2s between CP D. pulex and D. pulicaria as hybrids and the cross between F1s and the parental CP D. pulex as backcross.
Table 2.
Numbers of individuals sampled (N), mean numbers of alleles/locus (Na), expected heterozygosity (He), observed heterozygosity (Ho), and the P value of the exact test of Hardy-Weinberg equilibrium (HWE) for the sampled sites based on 54 microsatellite loci. Asterisks indicate significant P values for HWE tests.
| Site | Latitude | Longitude | Sexuality | N | Na | He | Ho | P |
|---|---|---|---|---|---|---|---|---|
| Can 1 | 42.16292 | -83.02453 | OP | 19 | 3.91 | 0.62 | 0.51 | <0.0001* |
| CP | 6 | 1.13 | 0.19 | 0.09 | 0.0199 | |||
| Can 1B | 42.16292 | -83.02453 | OP | 21 | 3.37 | 0.54 | 0.70 | <0.0001* |
| Can 2 | 42.12953 | -82.98713 | CP | 10 | 3.30 | 0.54 | 0.26 | <0.0001* |
| Can 3 | 42.12375 | -82.98031 | OP | 58 | 7.07 | 0.71 | 0.58 | <0.0001* |
| CP | 70 | 6.19 | 0.61 | 0.45 | <0.0001* |
The collected individuals were brought to the laboratory, isolated, and maintained in 250-ml beakers with filtered river water at 20 °C. These individuals (both CP and OP) were maintained under benign laboratory conditions so that they can reproduce parthenogenetically essentially indefinitely. Clonal lines were fed ad libitum with a suspension of Scenedesmus obliquus. Three to five parthenogenetic offspring descended from each individual were used for sexuality tests. The sexuality tests involved examining whether, in the absence of males, diapausing embryos were present in ephippia. Consistent results from at least three consecutive rounds of production of ephippial embryos for each individual were used to determine the reproductive mode. The presence of embryos in ephippia indicates obligate parthenogenesis, whereas the absence of embryos suggests that individuals are cyclical parthenogens in which fertilisation by sperm is necessary for the production of diapausing embryos (Innes & Hebert 1988). In total, 98 OP and 86 CP isolates were identified and used in the subsequent genetic analyses.
Molecular protocols and data analyses
DNA of 5-10 parthenogenetic offspring of each individual was extracted using a cetyltrimethylammonium bromide method (Doyle & Doyle 1987). The Ldh genotype for each OP individual was examined using allele-specific PCR. The Ldh slow allele was amplified using the forward primer 5’-GAGCGATTTAACGTTGCGCCC-3’ and the reverse primer 5’-GGACGACTTGTGTGTGAATTTG-3’, whereas the fast allele was amplified with the forward primer 5’-GAGCGATTTAACGTTGCGCCT-3’ and the reverse primer 5’-GGACGACTTGTGTGTGAATTTC-3’ (Cristescu et al. 2012). To determine the genotype of an individual, two PCR reactions were run to amplify the slow and fast alleles, respectively. For each PCR reaction, individuals with known Ldh genotypes (SS or FF) initially determined by allozyme screening (from genetically identical siblings) were used as control samples to monitor whether there was cross-amplification between the slow and fast alleles and to determine whether the absence of amplicon is truly due to allelic absence. The PCR cycling regime consisted of 2 min denaturing at 94 °C and 35 cycles of 30 sec denaturing at 94 °C, 30 sec annealing at 58 °C, and 30 sec extension at 72 °C. The PCR product for each reaction was checked on a 1.5% agarose gel to determine the Ldh genotype. The presence of amplicon for both slow and fast reactions indicates SF heterozygotes, whereas the presence of a band for either slow or fast PCR reaction is recognized as SS or FF homozygotes. The Ldh genotypes for a subset of samples (n = 30) examined using this allele-specific PCR approach were verified by allozyme screening, confirming the robustness of this method.
To investigate the origin of “hybrid” (Ldh SF) OP lineages, we used nine unlinked microsatellite markers from nine linkage groups (a subset of the total 54 markers, supplementary File S1) and 101 CP D. pulex and 167 D. pulicaria isolates to construct a neighbour-joining tree based on pairwise allele-shared distances (DAS) between all genotypes in the software Populations 1.2 (http://bioinformatics.org/~tryphon/populations/). The allele-shared distance was chosen because of the possibility of observing shared alleles between parental populations and offspring if backcrossing occurs. Although DAS asymptotes with large divergence time, its lower variance makes it a reliable metric for population assignment (Goldstein et al. 1995). We also performed a Bayesian assignment test in the software Structure (Pritchard et al. 2000) to assign a relative probability that each OP individual is associated with either non-hybrid CP D. pulex (Ldh SS) or D. pulicaria (Ldh FF). Although Structure assumes Hardy-Weinberg equilibrium and no strong linkage among markers, our test aimed to reveal the parental (sexual) sources of microsatellite alleles. The genotypes observed in OP lineages are likely a result of mating between males from OP clones that still undergo meiosis to produce sperm and females from CP lineages. In this process, gametes are still produced through meiosis and there is no reason to exclude random mating at the point of fertilization. All OP individuals were analyzed as unknowns under an admixture ancestry model. The ancestry analysis was run with each of the nine markers individually and the average ancestry estimates from nine runs were used as the final result. Burn-in and run lengths were set to 100,000 and 1,000,000, respectively. We also used the R software package INTROGRESS that does not assume Hardy-Weinberg equilibrium to estimate the hybrid index of the OP individuals (Gompert & Buerkle 2009, 2010). INTROGRESS is a regression-based method and provides maximum likelihood estimates of hybrid index using multi-locus genotype data. With CP D. pulex and D. pulicaria as fixed parental sources, a hybrid index (H index) of 0 represents pure D. pulex genomic background, whereas a H index of 1 represents pure D. pulicaria background. It should be noted that a low H index value corresponds to a low D. pulicaria and high D. pulex Bayesian ancestry estimate. Furthermore, in the R software package adegenet (Jombart 2008; Jombart & Ahmed 2011), we used the genotypes of pond CP D. pulex and lake D. pulicaria to simulate the genotypes for 9 microsatellite markers that are not associated with obligate parthenogenesis for their F1 and the crossing of F1 with OP males that carry meiosis suppression genes. These markers should have the same segregation pattern in both CP and OP offspring. We also assume that half of the offspring from crosses with OP males are obligately parthenogenetic based on previous crossing experiments (Innes & Hebert 1988). We then used Structure to analyze the ancestry of these simulated genotypes and used the ancestry profiles to test whether the Ldh SF and SS OP individuals are descendants of OP males crossing with F1s or advanced backcrosses. We also used INTROGRESS to analyze the hybrid index of the simulated F1 crosses. Furthermore, to understand what genes may be involved in meiosis suppression, we searched the 200 kb flanking region (wfleabase.org) centered at each of the microsatellite markers associated with OP to identify possible genes that are involved in OP.
To test whether the Ldh SF OP individuals are likely to have the same mechanism for meiosis suppression as Ldh SS OP D. pulex individuals, 34 microsatellite markers that were shown to be significantly associated with obligate parthenogenesis in Ldh SS D. pulex (Lynch et al. 2008) were genotyped in both CP and OP (including both Ldh SF and SS) individuals. These 34 microsatellite markers reside in four linkage groups V, VIII, IX, and X. A subset of these markers (Table 3) was also genotyped in 48 D. pulicaria individuals from Three Lakes II, Lawrence Lake, and Warner Lake (16 individuals from each lake). In addition to these diagnostic markers, 20 markers mapped in linkage group I were genotyped. Previous studies suggested that this linkage group is involved in the production of males, an essential feature for the contagious spread of parthenogenesis (Colbourne, unpublished data). PCR reactions and genotyping followed methods in Cristescu et al. (2006). The OP individuals were divided into two groups based on their Ldh genotypes (SS and SF). GST (Nei 1973) between CP (Ldh SS) and OP individuals (Ldh SS or SF genotypes) was calculated for all markers in the software DISPAN (https://homes.bio.psu.edu/people/faculty/nei/software.htm).
Table 3.
Highly diagnostic microsatellite markers associated with obligate parthenogenesis, including the length (bp) of the diagnostic allele, the number of individuals that carry the diagnostic allele out of the total number of individuals that are Ldh SF OP (obligate parthenogenetic), Ldh pure and hybrid SS OP, CP (cyclically parthenogenetic) D. pulex, and CP D. pulicaria, based on the neighbour joining tree. Asterisks indicate the markers identified by Lynch et al. (2008) and confirmed in this study. N/A indicates markers not genotyped for Daphnia pulicaria.
| Marker | Allele size (bp) | SF OP | “Pure” SS OP |
“Hybrid” SS OP |
CP | D. pulicaria |
|---|---|---|---|---|---|---|
| Linkage group I | ||||||
| d039 | 252 | 43/50 | 5/8 | 6/9 | 0/55 | N/A |
| d063 | 152 | 53/59 | 5/12 | 4/9 | 1/72 | 42/48 |
| d091 | 279 | 31/38 | 2/7 | 2/5 | 0/65 | 12/48 |
| Linkage group V | ||||||
| d160* | 166 | 39/45 | 2/10 | 3/8 | 0/43 | 43/48 |
| Linkage group VIII | ||||||
| d117 | 190 | 48/52 | 5/7 | 3/10 | 0/66 | 44/48 |
| d077 | 235 | 28/29 | 2/3 | 3/5 | 0/60 | 40/48 |
| Linkage group IX | ||||||
| d043* | 164 | 44/52 | 7/8 | 4/9 | 0/67 | 43/48 |
| 9-8* | 130 | 51/63 | 11/15 | 7/13 | 0/76 | N/A |
| 13-5* | 200 | 36/49 | 5/8 | 4/6 | 8/57 | N/A |
| 13-14* | 113 | 59/62 | 9/10 | 14/15 | 1/68 | N/A |
| 99-50* | 163 | 46/59 | 8/8 | 7/11 | 2/68 | N/A |
| 99-52* | 163 | 25/31 | 4/5 | 4/7 | 5/61 | N/A |
| d118 | 120 | 59/60 | 10/12 | 12/14 | 6/69 | 12/48 |
| d145 | 264 | 26/34 | 4/9 | 2/5 | 0/53 | 5/48 |
| Linkage group X | ||||||
| d005* | 302 | 33/36 | 3/4 | 3/6 | 0/57 | 40/48 |
Results
Overall patterns of microsatellite diversity
Two of the Canard ponds (1 and 3) contained both CP and OP individuals, whereas pond 1B was purely OP and pond 2 was purely CP. Past studies revealed that the frequency of CP and OP lineages in the ephemeral Canard ponds can change dramatically from year to year mainly due to their high interconnectivity during wet seasons (Innes, unpublished data). Allelic diversity at the 54 microsatellite loci ranged from 4 to 17 alleles per locus, with 571 alleles identified across all loci. The mean number of alleles per locus for the ponds ranged from 1.13 to 7.07 (Table 2). All Canard populations analysed deviated significantly from Hardy-Weinberg equilibrium over all microsatellite loci (Table 2). Because all these samples were collected in the middle of the season, the deviation from Hardy-Weinberg equilibrium could be due to differential clonal growth.
Microsatellite neighbour-joining tree and Bayesian estimate of ancestry
The neighbour-joining tree based on a subset of nine, unlinked microsatellite markers that are not diagnostic for OP showed that the CP D. pulex and D. pulicaria formed distinct clusters (Fig. 2A). The majority of OP Daphnia including all Ldh SF and some SS Ldh genotypes formed a distinct clade between the pond (D. pulex) and lake (D. pulicaria) clade. However, many Ldh SS OP individuals clustered within the D. pulex clade. The results of the Bayesian assignment test were largely consistent with those of the phylogenetic analysis (Fig. 2B). Ldh SF OP individuals showed on average 61.1% (SD = 7.2%) probability of ancestry from D. pulex. These Ldh SF OP individuals showed significantly higher D. pulex ancestry (t-test, P < 0.0001) than the simulated dataset of 100 F1 individuals between CP D. pulex and D. pulicaria (average D. pulex ancestry 48.7%, SD = 6.6%). Moreover, the Ldh SS OP individuals grouped with CP D. pulex on the NJ tree (average D. pulex ancestry 84.5%, SD = 7.1%) had significantly higher D. pulex ancestry compared to the Ldh SF OP individuals (D. pulex ancestry 61.1%, SD = 7.2%, t-test, P < 0.0001) and the simulated backcrosses with D. pulex (n = 100, average D. pulex ancestry = 71.6%, SD = 10.9%, t-test, P < 0.0001). These Ldh SS OP individuals were also significantly different from the 100% pure D. pulex (t-test, P < 0.0001) that they group with. On the other hand, the Ldh SS OP individuals that clustered with Ldh SF OP individuals (average D. pulex ancestry = 65.4%, SD = 6.6%) in the NJ tree had no significant difference in terms of the D. pulex ancestry when compared to the Ldh SF OP individuals (t-test, P = 0.09) and the simulated backcrosses (t-test, P = 0.11). Furthermore, the two groups of Ldh SS OP individuals had significantly different amount of D. pulicaria and D. pulex ancestry (t-test, P < 0.0001).
Figure 2.
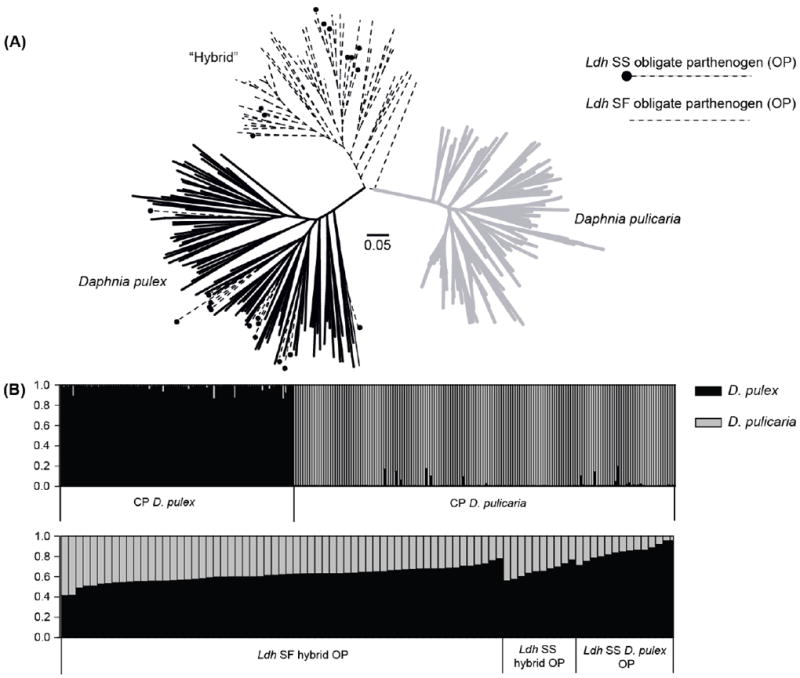
Neighbor-joining (NJ) tree (A) and Bayesian estimates of ancestry (B) for cyclically parthenogenetic Daphnia pulex, Daphnia pulicaria, and obligately parthenogenetic D. pulex individuals based on nine microsatellite markers. Dashed lines in the NJ tree designate obligate parthenogens (OP, both Ldh SS and SF), with the black circles designating Ldh SS OP individuals, whereas grey lines and black lines represent cyclically parthenogenetic D. pulicaria and D. pulex individuals, respectively. Each vertical bar of the Bayesian estimates of ancestry represents a genotype, with the black and grey colour representing the probability that an individual is derived from a D. pulex and D. pulicaria genetic background, respectively (ordered by increasing probability of D. pulicaria). Ldh SF hybrid OP and Ldh SS OP represent lineages in the hybrid clade (panel A), whereas Ldh SS D. pulex OP are lineages from the D. pulex clade (panel A).
The hybrid index analyses by the software INTROGRESS corroborate the results of Structure analyses. A subset of Ldh SS OP individuals have an H index of zero, meaning they have retained almost no D. pulicaria genes, whereas the rest have an H index ranging from 0.12 to 0.46, suggesting that a substantial number of D. pulicaria genes are retained in their genomes. The Ldh SF OP individuals have an average H index of 0.29 (SD = 0.15), with two individuals showing an H index of 0. The simulated F1 hybrids have an average hybrid index of 0.50 (SD = 0.06), whereas the simulated offspring of the F1 crossing with D. pulex show an average hybrid index of 0.25 (SD = 0.11). The distribution of the hybrid index of Ldh SF OP is significantly different from the simulated F1 hybrids (t-test, P < 0.0001) and almost statistically different from the backcrosses (t-test, P = 0.05), suggesting the Ldh SF OP individuals in our dataset are derived from more advanced backcrosses rather than F1 hybrids.
Meiosis suppression loci and diagnostic alleles
We estimated population subdivision between the Ldh SS/SF OP individuals and CP D. pulex individuals in the ponds using GST with 54 microsatellite markers (supplementary Tables 1 and 2). The average GST between OP (Ldh SS) D. pulex and CP D. pulex individuals was 0.085 (SE = 0.009), which is consistent with the previous GST estimate based on genome-wide microsatellite markers in a comparison of SS OP and CP D. pulex (Lynch et al. 2008), whereas the average GST when comparing Ldh SF OP with D. pulex CP individuals was 0.156 (SE = 0.012). Individual markers (15 out of 54) with GST values higher than the average GST (supplementary Fig. 1) are considered candidates associated with OP (supplementary Fig. 2). The alleles of these candidate markers were compared between OP and CP D. pulex individuals to determine diagnostic alleles for obligate parthenogenesis (Table 3). Our results showed that all regions on linkage groups V, VIII, IX and X that appeared to be associated with parthenogenesis in a previous study (Lynch et al. 2008) contain microsatellite markers that are highly differentiated between OP (either Ldh SS or SF genotypes) and CP individuals (Table 3) in this study.
For all identified diagnostic alleles, the association with OP (both Ldh SF and SS) and D. pulicaria compared to CP D. pulex is statistically significant (chi-square test, in all cases P value < 0.01), although the proportion of Ldh SS OP D. pulex individuals carrying diagnostic alleles is lower than that of Ldh SF OP D. pulex. The lower percentage of Ldh SS OP individuals carrying the diagnostic alleles does not imply that these alleles are not associated with obligate parthenogenesis because these alleles have already been shown to have nearly perfect correlation with Ldh SS OP clones (Lynch et al. 2008). Most OP individuals carried a single copy of the diagnostic allele at these loci and an allele of a different size on the other chromosome. Furthermore, the markers d039, d063, and d091 on linkage group I appeared to be highly diagnostic in both comparisons (i.e., Ldh SF OP vs. CP D. pulex and Ldh SS OP vs. CP D. pulex, see Table 3), indicating the likely involvement of these genomic regions in obligate parthenogenesis. However, a few markers on linkage group I (i.e., d076, d001, d148, d188, and d138) and d113 on linkage group VIII only appeared to be diagnostic when contrasting Ldh SF OP and D. pulex CP individuals, suggesting that these alleles likely arose in a D. pulicaria genetic background but are not necessarily involved in obligate parthenogenesis. The genotyping of diagnostic microsatellite markers in D. pulicaria revealed that in each of the four linkage groups (V, VIII, IX, and X) associated with OP, the diagnostic alleles for OP at some of the loci were nearly fixed in D. pulicaria, whereas at other loci D. pulicaria show an array of different alleles including the diagnostic allele.
Meiosis suppression genes
The search in the 200-kb genomic regions centered at the identified diagnostic markers revealed many genes involved in DNA replication, repair, recombination, cell cycle, spindle formation, and chromatin assembly (supplementary Table 3). For example, the newly identified diagnostic marker (d039) on linkage group I is located close to Transcription factor IIIC, which has functional roles in DNA replication, repair, and recombination. Linkage group IX, a large part of which is associated with meiosis suppression, contains many genes that are responsible for meiotic cell division, recombination, and sister chromatid cohesion (supplementary Table 3).
Discussion
In this study, we build on previous knowledge of the contagious spread of asexuality in Daphnia pulex to further explore the origin of meiosis-suppression genes. It is well known that parthenogenesis in animals and plants is often associated with hybridization. For example, almost all asexual vertebrates have a hybrid origin (Kearney et al. 2009; but see Sinclair et al. 2010) and asexual polyploid plants often arise from hybridization events (e.g., allopolyploidy) (Coyne & Orr 2004; Bengtsson 2009). Nonetheless, the link between hybridization and the origin of the genetic elements responsible for meiosis suppression has not been explored. Here, we investigated whether asexual D. pulex-pulicaria hybrids (Ldh SF) originated from crosses between males from asexual clones and cyclically parthenogenetic F1 hybrids between D. pulex and D. pulicaria. The results of population genetic analyses were consistent with this hypothesis and prompted us to perform an association study to determine whether Ldh SF OP individuals and Ldh SS OP D. pulex share similar genetic mechanisms for meiosis suppression and to detect the origin of the alleles that are associated with meiosis suppression.
The origin of meiosis suppression in D. pulex
A previous population-genetic study (Cristescu et al. 2012) has shown clear genetic divergence across the nuclear genome (Fst ranges between 0.434 and 0.521) between populations of the two ecological species (D. pulex and D. pulicaria) examined in the present study. Given the significant genetic divergence between these two species, it is striking that D. pulicaria carries diagnostic alleles of several loci on each of the linkage groups involved in meiosis suppression/obligate parthenogenesis in D. pulex. This finding is consistent with some alleles in genomic regions involved in meiosis suppression/obligate parthenogenesis originated from D. pulicaria. These observations further suggest that meiosis suppression likely originated via hybridization followed by backcrossing and introgression of alleles from D. pulicaria into a D. pulex genomic background. Although we have little understanding about the origin of the diagnostic alleles at other microsatellite loci (see Table 3), it is clear that these alleles do not have an origin from CP D. pulex because of their near absence from CP D. pulex (Lynch et al. 2008). Furthermore, the insertion at the Rec8 B locus, found exclusively in OP D. pulex, is not found in D. pulicaria (Eads et al. 2012). Laboratory hybridization and backcrossing experiments between CP D. pulex and CP D. pulicaria did not produce OP offspring (Heier & Dudycha 2009). Thus, we can trace the origin of only some diagnostic alleles to D. pulicaria, whereas the origin of the other diagnostic alleles remains unclear. It is plausible that meiosis suppression originated through the ancestral hybridization/backcrossing that occurred between D. pulicaria and an unknown or extinct lineage of D. pulex.
The origin of OP in Ldh SF D. pulex
An early hypothesis proposed by Hebert et al. (1989) suggested that obligate parthenogenesis in Ldh SF OP clones is due to the inheritance of a meiosis suppressor from a male D. pulex crossing with a female D. pulicaria. However, this hypothesis is inconsistent with the observation that the mitochondrial genotype of “hybrid” obligate asexuals is invariably D. pulex (Heier & Dudycha 2009; Cristescu et al. 2012). A more recent hypothesis suggests that Ldh SF OP lineages arise from the crossing of F1 CP hybrid females with males of Ldh SS lineages carrying meiosis suppressors (Heier & Dudycha 2009). Consistent with the prediction of this hypothesis, our neighbour-joining tree based on nine microsatellite markers reveals three distinct clades, the CP D. pulex with some obligate parthenogens (Ldh SS), D. pulicaria with no OP individuals, and the “hybrid” clade of obligate parthenogens with both Ldh SS and SF genotypes. These nine markers were selected from nine different chromosomes and from genomic regions that do not contain any of the diagnostic markers. The number of loci used to generate a tree is critical because high mutation rates at microsatellite loci may reduce divergence due to convergent evolution. However, this effect should be less severe for young species pairs capable of hybridization, such as D. pulex and D. pulicaria. In our phylogenetic tree based on these nine markers, the clear divergence between D. pulex and D. pulicaria is consistent with the results in Cristescu et al. (2012) based on 21 microsatellite markers.
The Bayesian estimates of ancestry and the results of hybrid index analyses support the mixed ancestry of individuals assigned to the “hybrid” clade as did our simulation results. The significantly higher D. pulex ancestry in the Ldh SF OP individuals compared to simulated F1 (D. pulex × D. pulicaria) offspring suggests that the former are not F1 hybrids and supports the model proposed by Heier and Dudycha (2009) (Fig. 1A). Moreover, the significantly higher D. pulex ancestry in the Ldh SS OP individuals assigned to the D. pulex CP clade relative to the simulated first round of backcrosses indicates that they are either offspring of males from OP lineages mating with more advanced backcross females or offspring of pure D. pulex females with males from OP lineages retaining some D. pulicaria genes (Fig. 1B). The D. pulex ancestry of the Ldh SS OP clones clustered with Ldh SF individuals and the simulated backcrosses was not significantly different, suggesting that both genotypes are generated by the same crossing process and that the Ldh genotype cannot be reliably used to distinguish between hybrids and non-hybrids. Furthermore, a large proportion of the OP individuals with the Ldh SF genotype (Table 3) carry the highly diagnostic alleles for meiosis suppression/obligate parthenogenesis identified by Lynch et al. (2008) in OP individuals with the Ldh SS genotype. Collectively, these results strongly suggest that the mechanism of meiosis suppression is similar in Ldh SF and Ldh SS OP individuals.
In our dataset, we observed two different groups of Ldh SS OP individuals. Nearly half of the Ldh SS OP individuals are contained within the “hybrid” clade, whereas the remaining Ldh SS obligate parthenogens cluster with the CP D. pulex individuals (Fig. 2). These two groups of Ldh SS OP clones are significantly different in terms of their D. pulex ancestry. The Ldh SS OP individuals that cluster with CP D. pulex could be the offspring of pure CP D. pulex mating with males from OP D. pulex of hybrid origin. On the other hand, the Ldh SS OP individuals that cluster with Ldh SF OP individuals are likely offspring of OP (Ldh SS) males and CP F1 (Ldh SF) hybrids. This observation is consistent with previous work showing that OP D. pulex lineages are derived from distinct genetic backgrounds/lineages (Crease et al. 1989). It is expected that the OP offspring derived from continuous crossing with D. pulex have 50% reduction of D. pulicaria genomic background each generation, i.e. 25% (first round), 12.5% (second round), 6.25% (third round), etc. The Bayesian estimates of ancestry show that some OP individuals with Ldh SS have ~25-40% similarity to the D. pulicaria genome (Fig. 2), which is higher than the expected D. pulicaria ancestry (25%). This might be because the parental male carrying the meiosis suppression genes (i.e., Ldh SS males from OP clones) have retained some D. pulicaria genes in their genomes.
Our results provide a unified framework for the origin of OP clones, i.e., both the Ldh SS and Ldh SF OP individuals can be generated by the same biological process (crossing of D. pulex males carrying meiosis suppressing genes with F1s or backcrosses between D. pulex and D. pulicaria) and the same molecular mechanism (anciently introgressed diagnostic alleles from D. pulicaria). The incomplete reproductive barrier between D. pulex and D. pulicaria and the possibility of advanced backcrossing with CP D. pulex (Heier and Dudycha 2009) can give rise to a constellation of CP offspring with dramatically different amounts of D. pulex ancestry. Similarly, males from different OP lineages could also carry different amount of D. pulex genes. The matings between CP females and males from OP lineages can generate offspring with different amount of D. pulex genes in their genomes. Therefore, OP individuals, regardless of their Ldh genotypes, may display different amounts of D. pulicaria ancestry.
Genes involved in meiosis suppression/obligate parthenogenesis
Our results confirm that the majority of diagnostic markers for parthenogenesis (Lynch et al. 2008) from linkage groups V, VIII, IX, and X are highly indicative of obligate parthenogenesis in both Ldh SF and SS OP individuals. Furthermore, we show that linkage group I likely carries genes that are associated with obligate parthenogenesis. Although these regions contain genes that are involved in cell cycle, cell division, transcription, DNA repair, and chromosome structure, some of them may not be directly responsible for meiosis suppression. Instead, some of these genomic regions likely contain genetic elements involved in other essential features associated with the transmission of obligate parthenogenesis such as the production of males and the activation of unfertilized diploid resting eggs (Eads et al. 2012).
The cell division of germ-line cells in OP Daphnia is apomictic, involving the suppression of meiosis I of a normal meiotic division and only a single maturation division (Schrader 1925; Zaffagnini & Sabelli 1972; Hiruta et al. 2010). However, this is distinct from mitotic cell division because a polar body, a distinguishing feature of meiosis, is emitted at the end of the maturation division (Schrader 1925; Zaffagnini & Sabelli 1972). CP Daphnia possess the genetic machinery for both apomictic division and meiotic division, with environmental cues triggering the switch between them. Meiosis suppression can be due to a dominant mutation in one of the key genes initiating meiosis, as indicated by a previous study in which nearly half of the F1 progeny between males from OP lineages and CP females was obligately parthenogenetic (Innes & Hebert 1988). In the plant literature, there have been reports of meiosis becoming ameiotic due to spontaneous mutations in major genes. In maize, mutants of the ameiotic 1 gene show phenotypes of equational division (Pawlowski et al. 2009). In Arabidopsis, a homologue of ameiotic 1, SWI1/DYAD, affects the key events for meiosis such as sister chromatid cohesion and recombination (Siddiqi et al. 2000; Mercier et al. 2001; Agashe et al. 2002; Mercier et al. 2003). The mutants of swi1/dyad genes show abnormal meiosis, with equational-like segregation of chromosomes (Agashe et al. 2002; Mercier et al. 2003).
A recent study (Eads et al. 2012) shows that all OP individuals (Ldh SS) of D. pulex carry an allele of the Rec8 B locus, which encodes the meiotic cohesin REC8, that contains a transposable element insertion upstream and a frameshift mutation, both of which are completely absent from cyclically parthenogenetic lineages. It is thus hypothesized that obligate parthenogenesis in Daphnia is initiated by the abrogation or modification of REC8 function, likely due to the response triggered by the inserted transposable element at the post-transcriptional level (Eads et al. 2012).
Conclusions
Our current understanding of the role of hybridization in the evolution of asexuality in animals is based on a few experimental crosses and mostly phylogenetic analyses (reviewed in Simon et al. (2003)), but is severely short of the insights into how hybridization disrupts normal meiosis with the exception of some verbal arguments (Moritz et al. 1989). With the complex role of hybridization in the origin of asexuality in D. pulex becoming clear and the genomic regions conferring meiosis suppression identified (Lynch et al. 2008), this study has set the stage for future studies to further investigate the specific mechanisms underlying meiosis suppression.
Supplementary Material
Figure 3.
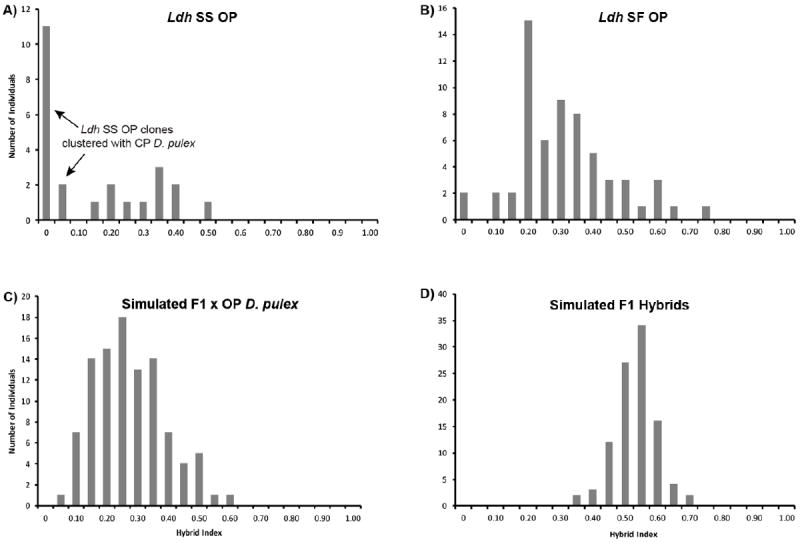
Hybrid index for A) Ldh SS OP, B) Ldh SF OP, C) simulated backcrosses between F1 and Daphnia pulex, and D) simulated F1. Samples were analyzed with the Introgress program (Gompert & Buerkle 2009, 2010) using the D. pulex (H index = 0.0) and Daphnia pulicaria (H index = 1.0) nine microsatellite loci as the reference parental genotypes.
Acknowledgments
We thank A. Constantin, S. Injic, and D. Persall for providing microsatellite data. K. Millette assisted with Ldh genotyping. This work benefited from numerous discussions with graduate students and postdoctoral fellows from Cristescu’s lab. A. Agrawal, T. Crease, A. Swan, K. Janko and three anonymous reviewers provided valuable comments that greatly improved this manuscript. This work is supported by a doctoral scholarship from the University of Windsor to SX, by NSF FIBR grant 0328516 and NIH grant GM101672 to ML, and by NSERC Canada grants to DJI and MEC.
Footnotes
Data accessibility
The microsatellite data (in genpop format) used for this study is uploaded as online supporting information.
References
- Agashe B, Prasad CK, Siddiqi I. Identification and analysis of DYAD: a gene required for meiotic chromosome organisation and female meiotic progression in Arabidopsis. Development. 2002;129:3935–3943. doi: 10.1242/dev.129.16.3935. [DOI] [PubMed] [Google Scholar]
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. Kluwer Academic Publishers; 1982. [Google Scholar]
- Bengtsson BO. Asex and evolution: a very large-scale overview. In: Schön I, Martens K, van Dijk P, editors. Lost Sex. Springer; Dordrecht: 2009. pp. 1–19. [Google Scholar]
- Catanach AS, Erasmuson SK, Podivinsky E, Jordan BR, Bicknell R. Deletion mapping of genetic regions associated with apomixis in Hieracium. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18650–18655. doi: 10.1073/pnas.0605588103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinauer Associates; Sunderland, Mass: 2004. [Google Scholar]
- Crease TJ, Lee SK, Yu SL, et al. Allozyme and mtDNA variation in populations of the Daphnia pulex complex from both sides of the Rocky Mountains. Heredity. 1997;79:242–251. [Google Scholar]
- Crease TJ, Stanton DJ, Hebert PDN. Polyphyletic origins of asexuality in Daphnia pulex. 2. mitochondrial DNA variation. Evolution. 1989;43:1016–1026. doi: 10.1111/j.1558-5646.1989.tb02547.x. [DOI] [PubMed] [Google Scholar]
- Cristescu ME, Constantin A, Bock DG, Caceres CE, Crease TJ. Speciation with gene flow and the genetics of habitat transitions. Molecular Ecology. 2012;21:1411–1422. doi: 10.1111/j.1365-294X.2011.05465.x. [DOI] [PubMed] [Google Scholar]
- Cristescu MEA, Colbourne JK, Radivojc J, Lynch M. A microsatellite-based genetic linkage map of the waterflea, Daphnia pulex: On the prospect of crustacean genomics. Genomics. 2006;88:415–430. doi: 10.1016/j.ygeno.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Decaestecker E, Gaba S, Raeymaekers JAM, et al. Host-parasite ‘Red Queen’ dynamics archived in pond sediment. Nature. 2007;450:870–873. doi: 10.1038/nature06291. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin. 1987;19:11–15. [Google Scholar]
- Drosopoulos S. Laboratory synthesis of a pseudogamous triploid species of the genus Muellerianella (Homoptera Delphacidae) Evolution. 1978;32:916–920. doi: 10.1111/j.1558-5646.1978.tb04645.x. [DOI] [PubMed] [Google Scholar]
- Dufresne F, Hebert PDN. Hybridization and origins of polyploidy. Proceedings of the Royal Society B-Biological Sciences. 1994;258:141–146. [Google Scholar]
- Eads BD, Tsuchiya D, Andrews J, Lynch M, Zolan ME. The spread of a transposon insertion in Rec8 is associated with obligate asexuality in Daphnia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:858–863. doi: 10.1073/pnas.1119667109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Ruiz Linares A, Cavalli-Sforza LL, Feldman MW. An evaluation of genetic distances for use with microsatellite loci. Genetics. 1995;139:463–471. doi: 10.1093/genetics/139.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompert Z, Buerkle CA. A powerful regression-based method for admixture mapping of isolation across the genome of hybrids. Molecular Ecology. 2009;18:1207–1224. doi: 10.1111/j.1365-294X.2009.04098.x. [DOI] [PubMed] [Google Scholar]
- Gompert Z, Buerkle CA. INTROGRESS: a software package for mapping components of isolation in hybrids. Molecular Ecology Resources. 2010;10:378–384. doi: 10.1111/j.1755-0998.2009.02733.x. [DOI] [PubMed] [Google Scholar]
- Hebert PDN. Obligate asexuality in Daphnia. American Naturalist. 1981;117:784–789. [Google Scholar]
- Hebert PDN, Beaton MJ, Schwartz SS, Stanton DJ. Polyphyletic origins of asexuality in Daphnia pulex. 1. Breeding system variation and levels of clonal diversity. Evolution. 1989;43:1004–1015. doi: 10.1111/j.1558-5646.1989.tb02546.x. [DOI] [PubMed] [Google Scholar]
- Hebert PDN, Crease T. Clonal diversity in populations of Daphnia pulex reproducing by obligate parthenogenesis. Heredity. 1983;51:353–369. [Google Scholar]
- Hebert PDN, Finston TL. Macrogeographic patterns of breeding system diversity in the Daphnia pulex group from the United States and Mexico. Heredity. 2001;87:153–161. doi: 10.1046/j.1365-2540.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- Heier CR, Dudycha JL. Ecological speciation in a cyclic parthenogen: sexual capability of experimental hybrids between Daphnia pulex and Daphnia pulicaria. Limnology and Oceanography. 2009;54:492–502. [Google Scholar]
- Hiruta C, Nishida C, Tochinai S. Abortive meiosis in the oogenesis of parthenogenetic Daphnia pulex. Chromosome Research. 2010;18:833–840. doi: 10.1007/s10577-010-9159-2. [DOI] [PubMed] [Google Scholar]
- Hotz H, Mancino G, Bucciinnocenti S, et al. Rana ridibunda varies geographically in inducing clonal gametogenesis in interspecies hybrids. Journal of Experimental Zoology. 1985;236:199–210. [Google Scholar]
- Innes DJ, Hebert PDN. The origin and genetic basis of obligate parthenogenesis in Daphnia pulex. Evolution. 1988;42:1024–1035. doi: 10.1111/j.1558-5646.1988.tb02521.x. [DOI] [PubMed] [Google Scholar]
- Jombart T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- Jombart T, Ahmed I. adegenet 1.3-1: new tools for the analysis of genome-wide SNP data. Bioinformatics. 2011;27:3070–3071. doi: 10.1093/bioinformatics/btr521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M, Fujita MK, Ridenour J. Lost sex in the reptiles: constraints and correlations. In: Schön I, Martens K, van Dijk P, editors. Lost Sex. Springer; Dordrecht: 2009. pp. 447–474. [Google Scholar]
- Kondrashov AS. Classification of hypotheses on the advantage of amphimixis. Journal of Heredity. 1993;84:372–387. doi: 10.1093/oxfordjournals.jhered.a111358. [DOI] [PubMed] [Google Scholar]
- Lattorff HM, Moritz RF, Fuchs S. A single locus determines thelytokous parthenogenesis of laying honeybee workers (Apis mellifera capensis) Heredity. 2005;94:533–537. doi: 10.1038/sj.hdy.6800654. [DOI] [PubMed] [Google Scholar]
- Lynch M, Burger R, Butcher D, Gabriel W. The mutational meltdown in asexual populations. Journal of Heredity. 1993;84:339–344. doi: 10.1093/oxfordjournals.jhered.a111354. [DOI] [PubMed] [Google Scholar]
- Lynch M, Seyfert A, Eads B, Williams E. Localization of the genetic determinants of meiosis suppression in Daphnia pulex. Genetics. 2008;180:317–327. doi: 10.1534/genetics.107.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J. The evolution of sex. Cambridge University Press; Cambridge; New York: 1978. [Google Scholar]
- Mercier R, Armstrong SJ, Horlow C, et al. The meiotic protein SWI1 is required for axial element formation and recombination initiation in Arabidopsis. Development. 2003;130:3309–3318. doi: 10.1242/dev.00550. [DOI] [PubMed] [Google Scholar]
- Mercier R, Vezon D, Bullier E, et al. SWITCH1 (SWI1): a novel protein required for the establishment of sister chromatid cohesion and for bivalent formation at meiosis. Genes & Development. 2001;15:1859–1871. doi: 10.1101/gad.203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz C, Brown W, Densmore L, et al. Genetic diversity and the dynamics of hybrid parthenogenesis in Cnemidophorus (Teiidae) and Heteronotia (Gekkonidae) In: Dawley R, JP B, editors. Evolution and ecology of unisexual vertebrates. University of the State of New York; Albany, New York: 1989. pp. 87–112. [Google Scholar]
- Nei M. Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Sciences of the United States of America. 1973;70:3321–3323. doi: 10.1073/pnas.70.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes RD, Baker R, Mai B. Mendelian segregation for two-factor apomixis in Erigeron annuus (Asteraceae) Heredity. 2007;98:92–98. doi: 10.1038/sj.hdy.6800907. [DOI] [PubMed] [Google Scholar]
- Otto SP. The evolutionary enigma of sex. American Naturalist. 2009;174:S1–S14. doi: 10.1086/599084. [DOI] [PubMed] [Google Scholar]
- Paland S, Colbourne JK, Lynch M. Evolutionary history of contagious asexuality in Daphnia pulex. Evolution. 2005;59:800–813. [PubMed] [Google Scholar]
- Pawlowski WP, Wang C-JR, Golubovskaya IN, et al. Maize AMEIOTIC1 is essential for multiple early meiotic processes and likely required for the initiation of meiosis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3603–3608. doi: 10.1073/pnas.0810115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrender ME, Spitze K, Lehman N. Multi-locus genetic evidence for rapid ecologically based speciation in Daphnia. Molecular Ecology. 2000;9:1717–1735. doi: 10.1046/j.1365-294x.2000.01062.x. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock C, Vorburger C. Single-locus recessive inheritance of asexual reproduction in a parasitoid wasp. Current Biology. 2011;21:433–437. doi: 10.1016/j.cub.2011.01.070. [DOI] [PubMed] [Google Scholar]
- Schrader F. The cytology of pseudosexual eggs in a species of Daphnia Z.indukt.Abstamm.- u. Vererb. -L. 1925;40:1–36. [Google Scholar]
- Schultz RJ. Unisexual fish: laboratory synthesis of a species. Science. 1973;179:180–181. doi: 10.1126/science.179.4069.180. [DOI] [PubMed] [Google Scholar]
- Serra M, Snell TW. Sex loss in monogonont rotifers. In: Schön I, Martens K, van Dijk P, editors. Lost Sex. Springer; Dordrecht: 2009. pp. 281–294. [Google Scholar]
- Shimizu Y, Shibata N, Sakaizumi M, Yamashita M. Production of diploid eggs through premeiotic endomitosis in the hybrid medaka between Oryzias latipes and O-curvinotus. Zoological Science. 2000;17:951–958. [Google Scholar]
- Siddiqi I, Ganesh G, Grossniklaus U, Subbiah V. The dyad gene is required for progression through female meiosis in Arabidopsis. Development. 2000;127:197–207. doi: 10.1242/dev.127.1.197. [DOI] [PubMed] [Google Scholar]
- Simon JC, Delmotte F, Rispe C, Crease T. Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biological Journal of the Linnean Society. 2003;79:151–163. [Google Scholar]
- Sinclair EA, Pramuk JB, Bezy RL, Crandall KA, Sites JW. DNA evidence for nonhybrid origins of parthenogenesis in natural populations of vertebrates. Evolution. 2010;64:1346–1357. doi: 10.1111/j.1558-5646.2009.00893.x. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Hebert PDN. Daphnia galeata mendotae as a cryptic species complex with interspecific hybrids. Limnology and Oceanography. 1992;37:658–665. [Google Scholar]
- Taylor DJ, Hebert PDN, Colbourne JK. Phylogenetics and evolution of the Daphnia longispina group (Crustacea) based on 12S rDNA sequence and allozyme variation. Molecular Phylogenetics and Evolution. 1996;5:495–510. doi: 10.1006/mpev.1996.0045. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Sprenger HL, Ishida S. Geographic and phylogenetic evidence for dispersed nuclear introgression in a daphniid with sexual propagules. Molecular Ecology. 2005;14:525–537. doi: 10.1111/j.1365-294X.2005.02415.x. [DOI] [PubMed] [Google Scholar]
- Van Dijk P, de Jong H, Vijverberg K, Biere A. An apomixis-gene’s view on Dandelions. In: Schön I, Martens K, van Dijk P, editors. Lost Sex. Springer; Dordrecht: 2009. pp. 475–493. [Google Scholar]
- Vrijenhoek RC. Animal clones and diversity. Bioscience. 1998;48:617–628. [Google Scholar]
- White MJD, Contreras N, Cheney J, Webb GC. Cytogenetics of parthenogenetic grasshopper Warramaba (formerly Moraba) virgo and its bisexual relatives. 2. hybridization studies. Chromosoma. 1977;61:127–148. doi: 10.1007/BF00327397. [DOI] [PubMed] [Google Scholar]
- Zaffagnini F, Sabelli B. Karyologic observations on the maturation of the summer and winter Eggs of Daphnia pulex and Daphnia middendorffiana. Chromosoma. 1972;36:193–203. doi: 10.1007/BF00285213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


