Abstract
Objective
To determine if tobacco smoke (TS), a risk factor for cancers of the aerodigestive tract, may contribute to oral carcinogenesis, in part, by suppressing local immunity.
Methods
Mice were placed in plexiglass holders in which they breathed TS through the nose and mouth for 1 hour daily for 21 days. Control mice breathed room air in the same manner. One day after the last exposure, mice were immunized by application of oxazolone to each buccal mucosa. Control mice were mock-immunized by application of vehicle alone. Five days later, all mice were challenged on the ears with oxazolone and 24 hour ear swelling assessed as contact hypersensitivity (CHS).
Results
Mice exposed to TS had a significantly smaller CHS response compared to controls. When subsequently re-immunized on the glabrous skin, mice originally primed through TS-exposed mucosa could not be fully immunized, indicating induction of immunologic tolerance by exposure to hapten through TS-perturbed mucosa. Immunocompetent mice exposed to TS in this manner and challenged by submucosal placement of a syngeneic malignant tumor had significantly increased tumor growth over time compared to controls. No difference in growth rate was observed when the experiment was performed with NK cell-deficient, SCID mice. Additionally, exposure of epidermal Langerhans cells in vitro to an aqueous extract of TS impaired their ability to undergo maturation and to present antigen to responsive T cells.
Conclusions
Immunologic changes induced in the oral cavity by exposure to TS may play a role in the development of oral cancers.
Keywords: tobacco, mucosa, cancer, immune suppression, mouse
Introduction
Exposure to tobacco smoke (TS) from cigarettes and other tobacco products is responsible for approximately 30% of all cancer-related deaths in the United States [1]. More than sixty carcinogens are found in TS and smoking is a major risk factor for development of malignancies of the aerodigestive tract including the oral cavity [2,3]. Oral squamous cell carcinoma is the most common head and neck malignancy and the sixth most common cancer in the world [4]. Tobacco exposure is believed to be responsible for up to 90% of cases of oral squamous cell carcinoma [5,6]. Carcinogens in tobacco smoke induce DNA mutations leading to oral squamous cell carcinoma [7].
There is existing evidence that the immune system plays a role in regulating the appearance of squamous cell carcinomas of the upper aerodigestive tract. The incidence of cancers of the upper aerodigestive tract appears to be increased in HIV-infected individuals [8]. Squamous cell carcinomas of the head and neck also occur at a younger age in HIV-infected individuals and present at a higher tumor stage [9]. There is also a significantly increased risk of cancer, including lung and oral cancers, in patients immunosuppressed due to allotransplanted organs [10-12]. As a whole, these results suggest that immune suppression may predispose to squamous cell carcinomas of the upper aerodigestive tract. However, a confounding variable in these studies is that smoking is more common in patients infected with the human immunodeficiency virus than in the general population [13,14].
We wished to test the hypothesis that exposure to TS suppresses immunity locally. We theorize that, if true, this would lead to inappropriate presentation of putative tumor antigens on transformed cells leading to failure of generation of effective immune responses against incipient tumors. These immunologic changes may contribute to the development of oral, TS-associated, squamous cell carcinomas. Furthermore, we have recently found that Langerhans cells (LCs), epithelial dendritic antigen presenting cells, are increased in the buccal mucosa of human smokers [15]. Although classically these cells were thought to be potent antigen presenting cells, more recent data have demonstrated that in the steady-state they serve to limit or downregulate immunity [16,17], perhaps to prevent unwarranted immune reactivity against commensal organisms. We have utilized mouse models of oral induction of contact hypersensitivity to demonstrate that exposure of the oral mucous membranes to TS inhibited induction of hapten-specific immunity associated with the development of immunologic tolerance. Furthermore, submucosal inoculation of a transplantable syngeneic malignant tumor results in increased growth in TS-exposed mice compared to controls due to immunologic changes in the TS-treated mice. In accordance with these findings, an aqueous extract of TS was found to inhibit LC maturation and antigen presenting capability.
Materials and Methods
Mice
C3H/HeNCrl (H-2k) mice were obtained from Charles River Laboratories (Willimantic, CT). BALB/c (H-2d) and A/J (H-2a) mice, mutant nonobese diabetes (NOD) severe combined immunodeficiency (SCID) gamma (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, g7) mice and transgenic DO11.10 (C.Cg-Tg(DO11.10) 10Dlo/J, H-2d) mice (BALB/c background) were purchased from The Jackson Laboratory (Bar Harbor, ME). Six to 8 week old female mice were used for all in vivo experiments. BALB/c and DO11.10 mice employed for in vitro experiments ranged in age from 7 to 10 weeks of age. NOD SCID mice are deficient in mature lymphocytes, serum immunoglobulin is not detectable and natural killer (NK) cell activity is extremely low [18]. DO11.10 mice express T cell α and β receptor transgenes, which spontaneously recognize a fragment of chicken ovalbumin (cOVA323-339) [19]. All mice were housed in the Weill Cornell Medical College research animal facility under specific pathogen-free conditions with a 12 hour photoperiod. All machinery and experimental sites were cleaned with a disinfectant daily and sterilized with UVC radiation overnight. Approval for all experiments was obtained from the Institutional Animal Care and Use Committee at Weill Cornell Medical College.
Media and cells
The S1509a fibrosarcoma cell line was kindly provided by Mark I. Greene, University of Pennsylvania (Philadelphia, PA) [20]. This tumor cell line was originally induced by methylcholanthrene in A/J mice and was passaged through A/J mice in our lab.
S1509a cells, LCs and CD4+ T cells were cultured in complete medium (CM): RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, 0.1 mmol/L non essential amino acids, 0.1 mmol/L essential amino acids, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate and 10 mmol/L HEPES buffer (Mediatech, Manassas, VA).
Tobacco smoke (TS) exposure
After arrival, mice were rested for 1 week and then randomized so that each cage contained mice that were matched for weight. For TS exposure, mice were gently secured in fitted polycarbonate chambers prior to placement into snout-only exposure chambers (CH Technologies, Westwood, NJ) [21,22] and then exposed to TS for 1 hour per day on 21 consecutive days. All smoking procedures were performed in the morning. 3R4F Research Cigarettes (Kentucky Tobacco Research Institute, Lexington, KY) were smoked according to a modified Federal Trade Commission protocol (duration: 2 seconds/puff, frequency: 2 puffs/minute, volume: 40 ml/puff). We have previously demonstrated that exposure of mice to TS by this method results in enhanced gene expression in the upper aerodigestive tract including the mouth (21). Cigarettes were stored at −20°C until the day before use. Twenty-four hours prior to each smoking session, cigarettes were put into a humidifier to ensure a constant saturation of 50-60%. Smoke exposure was quantified by measurement of total suspended particulate matter (TSP) by aspirating TS at a fixed rate through a glass microfiber filter (Whatman, Piscataway, NJ) attached to one manifold port. The weight of smoke product collected on the filter divided by the total aspirated volume was calculated as the TSP in mg/m3 (average TSP per experiment 452.8 ± 68.3 mg/m3). Matched control mice were treated identically except that room air rather than TS was delivered. Under these conditions, there was a less than 5% difference in the mean weights of groups of TS-exposed mice compared to groups of mock-smoked mice.
Induction and elicitation of contact hypersensitivity (CHS)
The chemical hapten oxazolone (4-ethoxymethylene-2-phenyl-2-oxazolin-5-one) (OXA) was obtained from Sigma-Aldrich (St. Louis, MO). Twenty-four hours after the last exposure to TS, C3H/HeN mice were immunized by painting 10 μl of 1% OXA in acetone/olive oil (1:20) onto the buccal mucosa of each cheek. Negative control mice were mock immunized with vehicle alone. Vaseline ointment was applied periorally to prevent hapten solution reaching glabrous skin.
For elicitation of the CHS response, mice were challenged on each side of each ear with 5 μl of a 1% solution of OXA in acetone/olive oil (1:4) five days after immunization. Twenty-four hour ear swelling was assessed as a measure of CHS by the use of a spring-loaded micrometer (Mitutoyo USA, Aurora, IL).
Induction of immunologic tolerance
In some experiments, mice were re-immunized 16 days after challenge with immunization and mock-immunization of additional groups of naïve mice. Seventy μl of 3% OXA in acetone/ethanol (1:4) were painted on dorsal skin that had been shaved with electric clippers 1 day earlier. Elicitation and assessment of secondary CHS was performed 5 days later as above.
In vivo tumor growth assay
A/J mice were exposed to TS or mock-exposed for 21 days as above. Twenty-four hours after the last exposure, mice were injected with 2×106 viable S1509a fibrosarcoma cells suspended in 50 μl PBS into the left buccal lamina propria (the loose connective tissue below the epithelium). It is likely that some cells were deposited below this level. Tumor growth was evaluated daily in a coded fashion by 2-dimensional orthogonal measurement of the widest tumor margins with a vernier caliper. Eight days after tumor cell implantation, mice were sacrificed and tumors carefully excised in a coded fashion. Each tumor was then weighed.
Preparation of tobacco smoke extract (TSE)
Cigarettes (2R4F, Kentucky Tobacco Research Institute) were smoked in a Borgwaldt piston-controlled apparatus (model RG-1) using a Federal Trade Commission standard protocol [21]. Cigarettes were smoked one at a time in the apparatus and the smoke was drawn under sterile conditions into premeasured amounts of sterile PBS (pH 7.4). The material in PBS represents whole trapped mainstream smoke. TS content is expressed in puffs/ml of PBS with one cigarette yielding about 8 puffs drawn into a 5 ml volume.
LC isolation
LCs were obtained from BALB/c mice as previously reported [23]. First, the mice were shaved and chemically depilated. Skin was dissected and subcutaneous fat and panniculus carnosus removed by blunt dissection. The skin was then incubated dermis-side down in PBS containing 0.5 U/ml of dispase and 0.25% trypsin (Mediatech) for 45 minutes at 37°C. Epidermal sheets were peeled off gently, collected, washed, and dissociated by continuous mild agitation in HBSS (Mediatech) supplemented with 2% FBS. The resulting epidermal cell suspension was then filtered through a 40 μm cell strainer (BD Biosciences, San Jose, CA). LCs were enriched by incubation with mouse anti-mouse I-Ad antibody (BD Biosciences) followed by incubation with goat anti-mouse IgG antibody conjugated to magnetic microspheres (Dynabeads, Invitrogen, Grand Island, NY). LCs were isolated with a magnetic field. This procedure yields a cell population of ∼95% LCs, verified by flow cytometry. Throughout all procedures, LCs were kept at 4°C to prevent them from undergoing early maturation.
CD4+ T cell isolation
CD4+ T cells were isolated from the spleens of DO11.10 transgenic mice following a standardized protocol [23]. Briefly, spleens were harvested and mechanically disrupted to obtain a single cell suspension in PBS. Erythrocytes were removed by subsequent exposure to hypotonic medium. The splenocyte suspension was filtered through a 70 μm cell strainer (BD Falcon) and extensively washed prior to negative deletion of NKT cells with anti-CD49b (DX5) and the MACS magnetic bead system (Miltenyi Biotec, Auburn, CA). CD4+ T cells were then purified from the resulting cell suspension with the Dynal Mouse CD4 Cell Negative Isolation Kit (Invitrogen) following the manufacturer's manual. The procedure yields a highly purified, untouched suspension of CD4+ T cells with a viability >95%.
In vitro antigen presentation assay
To evaluate the impact of TSE on LC antigen presentation capability in vitro, 1×104 LCs were plated in CM in a 96 well round-bottom plate and pretreated with TSE at a range of concentrations for 3 hours at 37°C, then carefully washed with CM 4-times and co-cultured with 2×105 DO11.10 CD4+ T cells and 10 mmolL cOVA323-339 per well in fresh CM for another 48 hours. Supernatants were then collected and analyzed by sandwich ELISA for IL-4, IL-17 (DuoSet, R&D Systems, Minneapolis, MN) and IFNγ (BD Biosciences) content according to the manufacturer instructions as a measure of T cell activation.
FACS analyses of TSE-treated epidermal cells
Epidermal cells from BALB/c mice were cultured for 15 hours in TSE (1:80, 1:40, and 1:20 diluted in PBS) or medium alone. Then cells were labeled with PE-anti I-Ad, FITC-anti-CD86, FITC-anti-CD80 and/or FITC-anti-CD40. FACS analyses were performed with gating on I-A+ cells.
Biostatistics
All in vivo experiments were performed at least twice in their entirety. In vitro experiments were conducted as separate experiments three times. Difference in the average change in ear thickness and average tumor size in mice in the smoking and non-smoking groups at various time points under pairs of experimental conditions of interest was evaluated by the linear mixed-effects model which takes into account both within- and between-mouse variations for each experimental condition followed by simultaneous tests for general linear hypotheses [24]. P-values were adjusted for multiple comparisons with Bonferroni's method. At the time of sacrifice, the average weight of tumors removed from smokers vs. mock-smokers was compared using Student's t-test. Differences in average biomarker (IFNγ, IL-4, IL17) levels in supernatants under pairs of experimental conditions of interest were evaluated using the linear mixed-effects model, which takes into account both within and between experiment variations for each experimental condition, followed by simultaneous tests for general linear hypotheses [24].
Results
Exposure to TS inhibits the acquisition of immunity by application of a hapten to the buccal mucosa
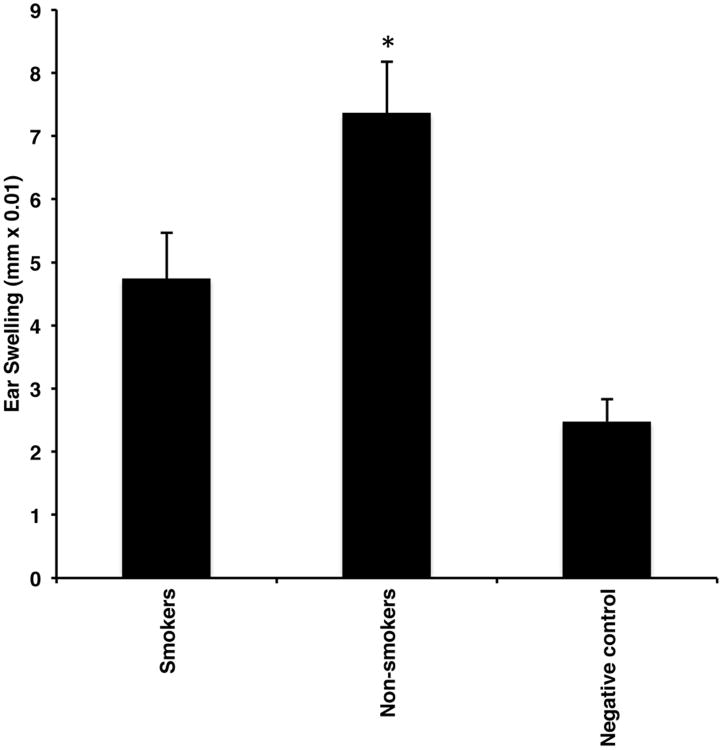
We tested the hypothesis that TS exposure inhibits the acquisition of immunity via hapten exposure to the buccal mucosa. Groups of mice were exposed to TS or room air for 21 days as described in Materials and Methods. Twenty-four hours after the last TS exposure, TS-exposed and mock-smoked mice were immunized by application of oxazolone to each buccal mucosa. Negative control mice received vehicle only. Five days later, all mice were challenged on the ears with oxazolone and 24-hour ear swelling assessed 24 as a measure of CHS. Mice exposed to TS exhibited a significant decrease in the CHS response after challenge compared to mock-smoked control mice (Fig. 1).
Figure 1.
Exposure to TS inhibits acquisition of immunity to a hapten applied to the buccal mucosa. Groups of mice were exposed to TS or room air delivered to the nose and mouth over 21 days as described in Materials and Methods. Twenty-four hours after the last exposure, TS-exposed and mock-smoked mice were immunized by application of oxazolone to the buccal mucosa and all mice were challenged on the ears with oxazolone for a CHS response 5 days later. Negative control mice were immunized with vehicle alone but were otherwise treated identically. Ear swelling was measured 24 hours later. *p<0.05. N = 30/group from 3 experiments.
Mice immunized through TS-exposed buccal mucosa exhibit immunologic tolerance after subsequent re-immunization at a distant site
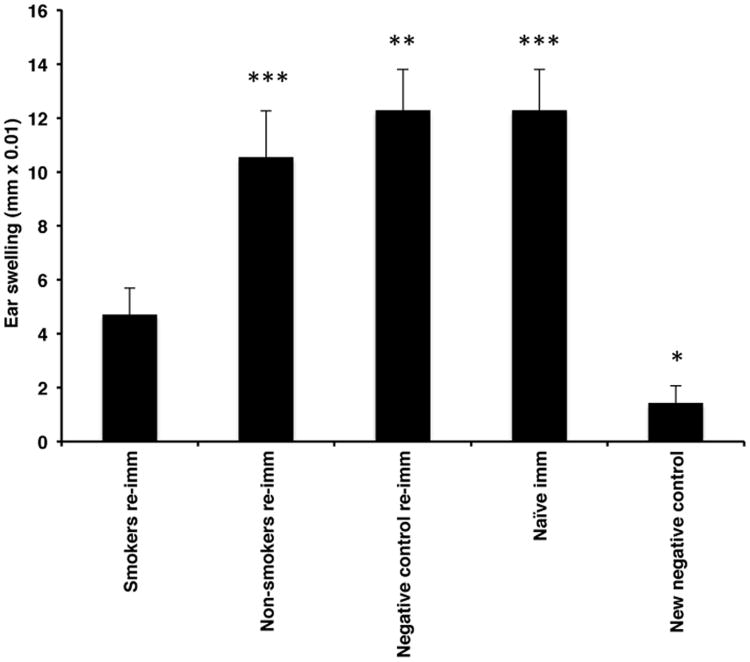
We next asked whether the decreased CHS response observed in mice immunized through TS-exposed buccal mucosa was associated with immunologic tolerance. Each group of mice from an individual experiment demonstrating that TS exposure inhibited the induction of CHS were re-immunized 16 days after challenge along with a group of naïve mice (additional positive control) by application of oxazolone to the shaved dorsal skin. A group of naïve mice was also mock-immunized by application of vehicle alone to the shaved dorsum. Five days later, all mice were challenged on the ears and 24-hour ear swelling assessed. Mice originally immunized via TS-exposed buccal mucosa showed a depressed CHS response compared to the other immunized groups (Fig. 2).
Figure 2.
Mice immunized through TS-exposed buccal mucosa exhibit immunologic tolerance. Each group of mice from an individual experiment demonstrating that TS exposure inhibited induction of CHS was re-immunized 16 days after challenge along with a group of naïve mice (additional positive control) by application of oxazolone to the shaved dorsal skin. A new negative control group consisted of a group of naïve mice mock-immunized by application of vehicle alone to the shaved dorsum. All mice were challenged 5 days later for a CHS response. *p<0.05, **p<0.01, ***p<0.001. N = 20/group from 2 experiments.
Exposure to TS enhances S1509a tumor growth in the buccal tissues of immunocompetent mice
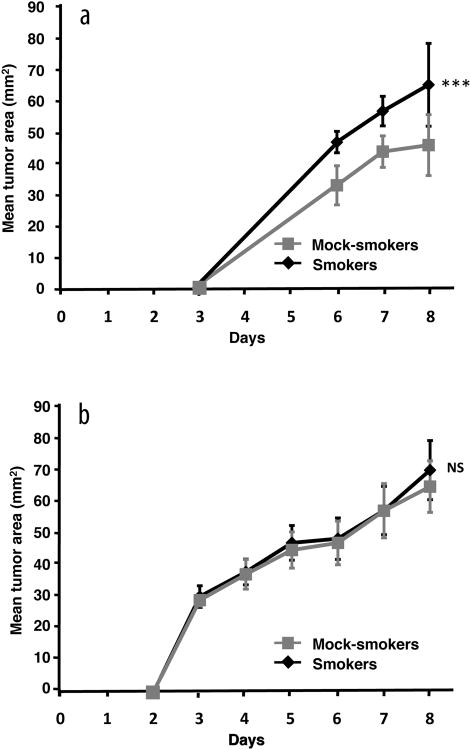
To test the hypothesis that exposure to TS alters the immune environment in the buccal mucosa in a manner that would suppress immunity against putative malignancies, a group of mice was exposed to TS for 21 days while a control group was exposed to room air in the same manner. Twenty-four hours later, all mice were injected with live S1509a tumor cells into the lamina propria mucosae of the left buccal tissue. Tumors grew significantly larger over time in the TS-exposed group compared to the control group (Fig. 3a). At the end of the 8-day period of tumor growth, mice were euthanized and tumors carefully dissected out from surrounding tissue in a blinded fashion. Consistent with the differences in growth rate, the mean tumor weight was larger in the smoking group compared to the mock-smoking group (weight: 106.7 +/− 13.6 (SD) mg vs. 83.7 +/−14.8 mg, p<0.01). In order to be certain that the enhanced tumor growth in TS-exposed mice was due to immunologic changes and not to other effects, this experiment was repeated in SCID mice with impaired natural killer (NK) cell activity. As shown by the data in Fig. 3b, tumor growth rates between TS-exposed and control mice were identical. Tumor weights were also similar in smokers and mock-smokers (weight: 199.9 +/− 34.8 (SD) mg vs. 201.0 +/− 48.0 mg, NS).
Figure 3.
Exposure to TS enhances the growth of an immunogenic tumor in the buccal tissue. Groups of A/J (a) and SCID mice with impaired natural killer cell activity (b) were exposed to TS or room air (mock-smokers) delivered to the nose and mouth over 21 days as described in Materials and Methods. Twenty-four hours later, all mice were injected with viable S1509a tumor cells in the submucosa of the left buccal tissue. Tumor growth was assessed over time by taking the product of two perpendicular diameters as a measure of tumor area. ***p<0.001; NS, not significant. N = 7/group for A/J experiment and N = 11/group for SCID experiment. These experiments are representative of two of each type showing similar results.
TSE impairs LC maturation and function as measured by their antigen presentation capability to CD4+ T cells in vitro
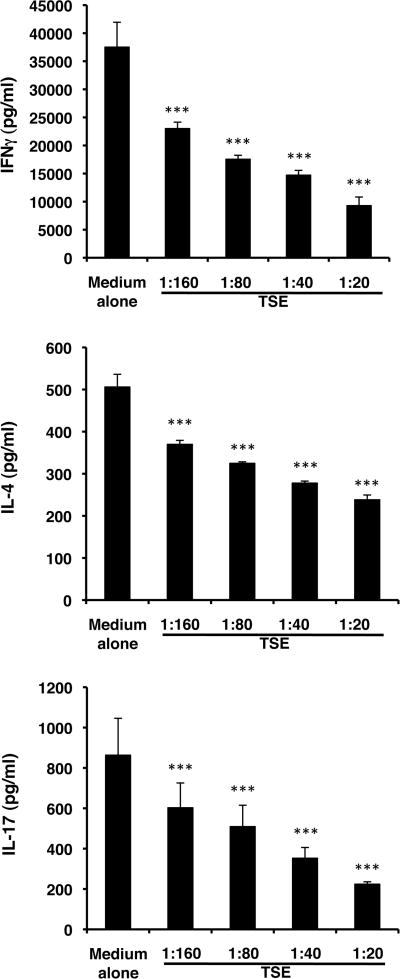
To test the hypothesis that TS alters the function of LCs, we utilized an aqueous extract of TS (TSE). LCs were incubated in medium containing various dilutions of TSE for 3 hours and then co-cultured with DO11.10 Tg CD4+ T cells and cOVA323-339. Exposure to TSE induced a dose-dependent decrease in the effectiveness of antigen presentation as assessed by production of interferon gamma (IFNγ), IL-4, and IL-17 as measures of Th1, Th2 and Th17-type immunity, respectively (Fig. 4). Exposure of LCs to TSE for 3 hours failed to induce cellular toxicity as measured by lactic dehydrogenase release after 24 and 48 hours (data not shown).
Figure 4.
TSE treatment of LCs prior to use for antigen presentation inhibits LC presentation to CD4+ T cells for IFNγ, IL-4 and IL-17 responses in vitro. BALB/c LCs were exposed in vitro to various dilutions of TSE for 3 hours and then co-cultured with DO11.10 transgenic CD4+ T cells with cOVA323-339. After 48 hours, supernatants were harvested and assessed for content of IFNγ, IL-4 and IL-17. *p<0.001. N = 6/group from 2 experiments.
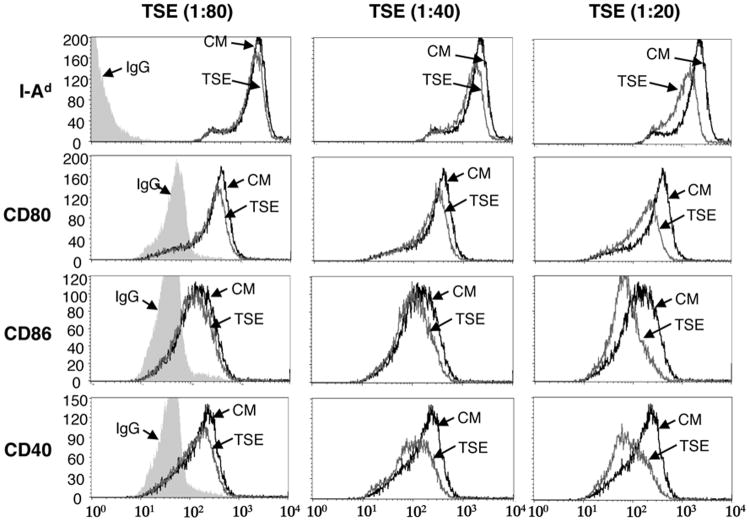
Epidermal LCs exposed to TSE have decreased expression of I-A, CD80, CD86 and CD40
LCs mature when cultured in the presence of keratinocytes due to the release of cytokines, primarily GM-CSF, by keratinocytes [25,26] We took advantage of this phenomenon to examine TSE effects on LC maturation. Unseparated epidermal cells were cultured in dilutions of TSE or medium alone for 15 hours and then examined by FACS. TSE exposed I-A+ cells exhibited decreased expression of I-A and the APC co-stimulatory surface markers CD80, CD86 and CD40, in a dose-dependent manner compared to cells cultured in medium alone (Fig. 5).
Figure 5.
LC upregulation of of I-Ad, CD80, CD86 and CD40 by cultured epidermal cells is inhibited by TSE. BALB/c epidermal cells were cultured in dilutions of TSE or medium alone for 15 hours and then cells were stained with chromophore-labeled antibodies to I-Ad, CD80, CD86 and CD40. After gating on I-A+ cells, expression of I-A, CD80, CD86 and CD40 was examined by FACS.
Discussion
Although carcinogens in TS play a critical role in the formation of cancers of the upper aerodigestive tract, immune status may also be important for the development and/or progression of epithelial malignancies. In the skin, there is ample evidence in animal models and circumstantial evidence in humans that immune suppression induced by exposure to ultraviolet radiation plays an important role in the development of non-melanoma skin cancers [27]. Epidemiologic evidence suggests that the immune system plays a role in the development of cancers of the upper aerodigestive tract. As discussed above, cancers of the upper aerodigestive tract including the oral cavity are increased in incidence in HIV-infected individuals [8]. Moreover, in patients immunosuppressed due to an allotransplanted organ, there is a significantly increased risk of cancer including lung and oral cancers [10-12]. Patients with an index head and neck cancer who continue to smoke are at greater risk of a second primary tumor than individuals who quit smoking [28]. However, former cigarette smokers still have an increased risk of cancer compared to never smokers including an increased risk of aerodigestive tract malignancies [29,30]. Also, former smokers with a history of upper aerodigestive tract malignancies have a higher risk of a second primary tumor than never smokers [30]. Taken together, it's possible that smoking induced immune suppression contributes to the development of malignancies in the upper aerodigestive tract.
The possibility that TS has immunologic consequences has been examined in the past. Studies have examined human smokers for numbers of circulating CD4+ and CD8+ lymphocytes, NK cell activity [31-35], and serum immunoglobulin levels [31,32]. Recall skin allergy testing has been examined in smokers and non-smokers [24,36,37]. In vitro experiments have studied the effects of TS extracts on release of cytokines by stimulated human peripheral blood T cell38 and alveolar macrophages from smokers and non-smokers [39]. Due to the different types, amounts and periods of exposure to smoking, or exposure of cells to TS extracts in vitro, drawing firm conclusions from these various studies is difficult. Indeed, in many cases differing results were obtained, presumably because of the use of varied experimental conditions.
Animal studies have also examined the immunologic effects of inhaling TS. As with human studies, there are conflicting reports. Reports of moderate exposure to TS have indicated enhancement, suppression or no effect on humoral immunity [40-42]. After long-term TS exposure (15 to 84 weeks) some studies found suppressed cell-mediated immunity [32,39]. In others, murine peripheral blood and splenic lymphocytes or regional lymph node lymphocytes showed decreased responsiveness to the mitogen phytohemagglutinin [40,43]. In another study, lymphocyte-mediated cytotoxicity to inoculated tumor cells in TS-exposed mice was compromised compared to controls [44]; however, in this study control mice were not subjected to the stress of the smoking device. In this regard, previous work from our group has shown an increased growth rate of a transplanted immunogenic tumor in stressed mice compared to non-stressed controls [45]. In one study TS exposure of mice for 1 to 2 months was found to inhibit NK activity against intravenously administered melanoma cells [46] while a different study reported that mice exposed to TS for periods of up to 15 weeks had an enhanced cell-mediated immune response [47]. A recent study found that rats exposed to dilute TS for up to 30 months or to nicotine from implanted osmotic pumps for 4 weeks showed decreased antigen-mediated T cell proliferation [48]. We elected to expose mice to TS over 3 weeks, approximately 3 percent of the mouse lifespan thus corresponding to 2 to 3 years of human smoking. The choice of 3 weeks was somewhat arbitrary. A detailed time course study to determine the minimum period of TS exposure necessary to observe the effects we have described is beyond the scope of the current report but is something that should be done in the future.
Interestingly and of potential relevance to the findings in the current study, when TS condensate was painted on the skin of BALB/c mice, it increased the density and changed the morphology of LCs and induction of contact hypersensitivity was suppressed at the site of TS condensate application [49]. Continued application of TS condensate resulted in the development of skin cancers.
Our results suggest that TS may contribute to immune suppression in the oral cavity through effects on LCs. Notably, we employed an experimental apparatus in which TS is delivered directly to the nose and mouth of mice, mimicking the human state rather than employing a smoking chamber, which was commonly used in some of the previous studies. LCs have classically been thought to be potent antigen-presenting cells important in the initiation of immunity [50]. However, recent work has challenged this paradigm. Genetically engineered mice lacking epidermal LCs because of expression of diphtheria toxin on a human langerin promoter, but retaining langerin positive cells in the dermis and secondary lymphoid organs exhibit increased contact hypersensitivity responses upon sensitization epicutaneously to a hapten [16, 17]. This finding suggests that, in the steady state, LCs may serve to limit or downregulate immunity. Indeed, there is some evidence suggesting that LCs contribute to the development of T regulatory cells. Of course, in the presence of a danger signal, LCs may mature in situ into potent antigen-presenting cells. If LCs in the buccal mucosa of mice are increased in number by exposure to TS, as occurs in humans, more of an immunologic down-regulatory pathway may be present at that site [15]. Additionally, our data suggest that exposure to TS inhibits the maturation of LCs, preventing them from being capable of properly inducing an immune response. Although our data were from in vitro experiments (interestingly, showing an effect on a generation of cytokines associated with Th1, Th2 and Th17 cells), we hypothesize that similar mechanisms occur in situ after exposure of the oral mucosa to TS. Thus, even in the presence of a danger signal TS may prevent LCs from becoming more potent antigen-presenting cells and, thus, prevent induction of immunity.
Using a chemical hapten as a surrogate for a tumor antigen, our data show that immunization via the buccal mucosa in smokers results in a decreased contact hypersensitivity response. Most interestingly, it appears that immunization in this manner does not result in simply less robust immunization but, rather, the induction of immunologic tolerance. If such tolerance occurs to incipient epithelial tumor antigens, it may explain, at least in part, the increased risk of epithelial malignancies of the aerodigestive tract in former smokers. To test the relevance of these immunologic changes to a tumor, we injected syngeneic immunogenic tumor cells into the buccal mucosa of mice previously exposed to TS or room air. Tumors grew larger in animals exposed to TS. The difference in tumor growth over time between the smoking and mock-smoking groups was definitively shown to be due to immunologic changes as this effect was lost in SCID mice with impaired NK cell activity. Of course, the immunologic locus of action of the TS in this model cannot be definitively ascertained. As a whole, these results strongly suggest that immunologic changes induced by exposure to TS may have profound consequences for the overt development of malignancies in the oral cavity. We hypothesize that a robust immune response may control or eliminate incipient oral cancers under normal circumstances; however, this mechanism is degraded by exposure to TS resulting in escape of incipient tumors from immune control with the development of overt cancers. Our data are consistent with this model. We recognize that the overwhelming majority of oral cancers are squamous cell carcinomas. The decision to utilize the S1509a fibrosarcoma was made because the tumor is immunogenic and there is extensive literature on the host immune response to the tumor [20]. The histologic type of the tumor is unlikely to be important to answering the question of whether TS alters the immune response to an immunogenic tumor.
As a whole, the current results underscore the potential need for immunomodulatory approaches to reduce the risk of tobacco smoke-related cancers. Indeed, measures to reverse immunologic tolerance to putative incipient tumor antigens or enhance immune status may prove to be of great benefit to reduce the risks of aerodigestive cancers in the millions of former smokers.
Acknowledgments
Supported by a gift from the Jacob L. and Lillian Holtzmann Foundation (RDG), contributions from the Carl and Fay Simons Family Trust (RDG) and the Seth Sprague Educational and Charitable Foundation (RDG), and grants from the Flight Attendants Medical Research Institute (AJD), the Edith C. Blum Foundation (RDG), the Lewis B. and Dorothy Cullman Foundation (RDG), NIDCD T32 000027 (AK) and NIH UL1-RR024996 (XKZ).
Footnotes
Drs. Schierl and Patel contributed equally to this work.
References
- 1.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14:2287–2293. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 2.Mayne ST, Lippman SM. Cigarettes: a smoking gun in cancer chemoprevention. J Natl Cancer Inst. 2005;97:1319–1321. doi: 10.1093/jnci/dji306. [DOI] [PubMed] [Google Scholar]
- 3.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;30:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 4.Nagler R, Dayan D. The dual role of saliva in oral carcinogenesis. Oncology. 2006;71:10–17. doi: 10.1159/000100445. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.Piyathilake CJ, Macaluso M, Hine RJ, et al. Cigarette smoking, intracellular vitamin deficiency, and occurrence of micronuclei in epithelial cells of the buccal mucosa. Cancer Epidemiol Biomarkers Prev. 1995;4:751–758. [PubMed] [Google Scholar]
- 7.Lacko M, Oude Ophuis MB, Peters WH, et al. Genetic polymorphisms of smoking-related carcinogen detoxifying enzymes and head and neck cancer susceptibility. Anticancer Res. 2009;29:753–761. [PubMed] [Google Scholar]
- 8.Haigentz M., Jr Aerodigestive cancers in HIV infection. Curr Opin Oncol. 2005;17:474–478. doi: 10.1097/01.cco.0000174036.46785.8f. [DOI] [PubMed] [Google Scholar]
- 9.Singh B, Balwally AN, Shaha AR, et al. Upper aerodigestive tract squamous cell carcinoma. The human immunodeficiency virus connection. Arch Otolaryngol Head Neck Surg. 1996;122:639–643. doi: 10.1001/archotol.1996.01890180047012. [DOI] [PubMed] [Google Scholar]
- 10.Curtil A, Robin J, Tronc F, et al. Malignant neoplasms following cardiac transplantation. Eur J Cardiothorac Surg. 1997;12:101–106. doi: 10.1016/s1010-7940(97)00114-0. [DOI] [PubMed] [Google Scholar]
- 11.Dantal J, Pohanka E. Malignancies in renal transplantation: an unmet medical need. Nephrol Dial Transplant. 2007;22(1):i4–i10. doi: 10.1093/ndt/gfm085. [DOI] [PubMed] [Google Scholar]
- 12.Danpanich E, Kasiske BL. Risk factors for cancer in renal transplant recipients. Transplantation. 1999;68:1859–1864. doi: 10.1097/00007890-199912270-00008. [DOI] [PubMed] [Google Scholar]
- 13.Saves M, Chene G, Ducimetiere P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. 2003;37:292–298. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 14.Stall RD, Greenwood GL, Acree M, et al. Cigarette smoking among gay and bisexual men. Am J Public Health. 1999;89:1875–1878. doi: 10.2105/ajph.89.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyle JO, Gumus ZH, Kacker A, et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev Res (Phila) 2010;3:266–278. doi: 10.1158/1940-6207.CAPR-09-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan DH, Jenison MC, Saeland S, et al. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 18.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 19.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 20.Schatten S, Granstein RD, Drebin JA, et al. Suppressor T cells and the immune response to tumors. Crit Rev Immunol. 2004;4:335–379. [PubMed] [Google Scholar]
- 21.Port JL, Yamaguchi K, Du B, et al. Tobacco smoke induces CYP1B1 in the aerodigestive tract. Carcinogenesis. 2004;25:2275–2281. doi: 10.1093/carcin/bgh243. [DOI] [PubMed] [Google Scholar]
- 22.Catanzaro DF, Zhou Y, Chen R, et al. Potentially reduced exposure cigarettes accelerate atherosclerosis: evidence for the role of nicotine. Cardiovasc Toxicol. 2007;7:192–201. doi: 10.1007/s12012-007-0027-z. [DOI] [PubMed] [Google Scholar]
- 23.Ding W, Stohl LL, Wagner JA, et al. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J Immunol. 2008;181:6020–6026. doi: 10.4049/jimmunol.181.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 25.Ozawa H, Nakagawa S, Tagami H, et al. Interleukin-1 beta and granulocyte-macrophage colony-stimulating factor mediate Langerhans cell maturation differently. J Invest Dermatol. 1996;106:441–445. doi: 10.1111/1523-1747.ep12343589. [DOI] [PubMed] [Google Scholar]
- 26.Romani N, Schuler G. The immunologic properties of epidermal Langerhans cells as a part of the dendritic cell system. Springer Semin Immunopathol. 1992;13:265–279. doi: 10.1007/BF00200527. [DOI] [PubMed] [Google Scholar]
- 27.Granstein RD, Matsui MS. UV radiation-induced immunosuppression and skin cancer. Cutis. 2004;74:4–9. [PubMed] [Google Scholar]
- 28.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 29.Khuri FR, Kim ES, Lee JJ, et al. The impact of smoking status, disease stage, and index tumor site on second primary tumor incidence and tumor recurrence in the head and neck retinoid chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2001;10:823–829. [PubMed] [Google Scholar]
- 30.Bosetti C, Gallus S, Peto R, et al. Tobacco smoking, smoking cessation, and cumulative risk of upper aerodigestive tract cancers. Am J Epidemiol. 2008;167:468–473. doi: 10.1093/aje/kwm318. [DOI] [PubMed] [Google Scholar]
- 31.Ginns LC, Goldenheim PD, Miller LG, et al. T-lymphocyte subsets in smoking and lung cancer: Analysis of monoclonal antibodies and flow cytometry. Am Rev Respir Dis. 1982;126:265–269. doi: 10.1164/arrd.1982.126.2.265. [DOI] [PubMed] [Google Scholar]
- 32.Johnson JD, Houchens DP, Kluwe WM, et al. Effects of mainstream and environmental tobacco smoke on the immune system in animals and humans: a review. Crit Rev Toxicol. 1990;20:369–395. doi: 10.3109/10408449009089870. [DOI] [PubMed] [Google Scholar]
- 33.Hughes DA, Haslam PL, Townsend PJ, et al. Numerical and functional alterations in circulatory lymphocytes in cigarette smokers. Clin Exp Immunol. 1985;61:459–466. [PMC free article] [PubMed] [Google Scholar]
- 34.Hersey P, Prendergast D, Edwards A. Effects of cigarette smoking on the immune system. Follow-up studies in normal subjects after cessation of smoking. Med J Aust. 1983;2:425–429. [PubMed] [Google Scholar]
- 35.Corberand J, Nguyen F, Do AH, et al. Effect of tobacco smoking on the functions of polymorphonuclear leukocytes. Infect Immun. 1979;23:577–581. doi: 10.1128/iai.23.3.577-581.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehrer SB, Wilson MR, Salvaggio JE. Immunogenic properties of tobacco smoke. J Allergy Clin Immunol. 1978;62:368–370. doi: 10.1016/0091-6749(78)90138-0. [DOI] [PubMed] [Google Scholar]
- 37.Zetterstrom O, Osterman K, Machado L, et al. Another smoking hazard: raised serum IgE concentration and increased risk of occupational allergy. Br Med J (Clin Res Ed) 1981;283:1215–1217. doi: 10.1136/bmj.283.6301.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouyang Y, Virasch N, Hao P, et al. Suppression of human IL-1beta, IL-2, IFN-gamma, and TNF-alpha production by cigarette smoke extracts. J Allergy Clin Immunol. 2000;106:280–287. doi: 10.1067/mai.2000.107751. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Cowan MJ, Hasday JD, et al. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J Immunol. 2007;179:6097–6106. doi: 10.4049/jimmunol.179.9.6097. [DOI] [PubMed] [Google Scholar]
- 40.Thomas WR, Holt PG, Keast D. Humoral immune response of mice with long-term exposure to cigarette smoke. Arch Environ Health. 1975;30:78–80. doi: 10.1080/00039896.1975.10666647. [DOI] [PubMed] [Google Scholar]
- 41.Esber HJ, Menninger FF, Jr, Bogden AE, et al. Immunological deficiency associated with cigarette smoke inhalation by mice. Primary and secondary hemagglutinin response. Arch Environ Health. 1973;27:99–104. doi: 10.1080/00039896.1973.10666328. [DOI] [PubMed] [Google Scholar]
- 42.Zetterström O, Nordvall SL, Björkstén B, et al. Increased IgE antibody responses in rats exposed to tobacco smoke. J Allergy Clin Immunol. 1985;75:594–598. doi: 10.1016/0091-6749(85)90035-1. [DOI] [PubMed] [Google Scholar]
- 43.Keast D, Ayre DJ. Effects of chronic tobacco smoke exposure on immune responses in aged mice. Arch Environ Health. 1981;36:201–207. doi: 10.1080/00039896.1981.10667626. [DOI] [PubMed] [Google Scholar]
- 44.Chalmer J, Holt PG, Keast D. Cell-mediated immune responses to transplanted tumors in mice chronically exposed to cigarette smoke. J Natl Cancer Inst. 1975;55:1129–3411. doi: 10.1093/jnci/55.5.1129. [DOI] [PubMed] [Google Scholar]
- 45.Lee EH, Ding W, Kulkarni AD, Granstein RD. Tumor growth and immune function in mice during hind-limb unloading. Aviat Space Environ Med. 2005;76:536–540. [PubMed] [Google Scholar]
- 46.Lu LM, Zavitz CC, Chen B, et al. Cigarette smoke impairs NK cell-dependent tumor immune surveillance. J Immunol. 2007;178:936–943. doi: 10.4049/jimmunol.178.2.936. [DOI] [PubMed] [Google Scholar]
- 47.Thomas WR, Holt PG, Keast D. Cellular immunity in mice chronically exposed to fresh cigarette smoke. Arch Environ Health. 1973;27:372–375. doi: 10.1080/00039896.1973.10666406. [DOI] [PubMed] [Google Scholar]
- 48.Kalra R, Singh SP, Savage SM, et al. Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive Ca(2+) stores. J Pharmacol Exp Ther. 2000;293:166–171. [PubMed] [Google Scholar]
- 49.Zeid NA, Muller HK. Tobacco smoke condensate cutaneous carcinogenesis: changes in Langerhans' cells and tumour regression. Int J Exp Pathol. 1995;76:75–83. [PMC free article] [PubMed] [Google Scholar]
- 50.Mathers AR, Larregina AT. Professional antigen-presenting cells of the skin. Immunol Res. 2006;36:127–136. doi: 10.1385/IR:36:1:127. [DOI] [PubMed] [Google Scholar]