Abstract
Inflammatory cytokines and chemokines released by macrophages in the prostate cancer microenvironment may signal via the androgen receptor (AR) to influence tumor progression. In particular, macrophages appear to promote tumorigenesis by altering the chemokine (C-C motif) ligand 4 (CCL4)/AR signaling axis. This process can be blocked by ASC-J9®, an enhancer of AR degradation. ASC-J9® also inhibits the CCL2-dependent, signal transducer and activator of transcription 3 (STAT3)-mediated pro-metastatic signaling cascade that is generally activated by androgen deprivation therapy. Thus, targeting inflammatory cytokines signaling via the AR, with ASC-J9®, represents a promising therapeutic approach against prostate cancer progression.
Keywords: inflammatory cytokines, androgen receptor, prostate cancer, macrophage, tumorigenesis, metastasis
Introduction
Prostate cancer is the most frequently diagnosed cancer in men and the second-leading cause of cancer-related death in the United States.1 Although androgen deprivation therapy (ADT) is beneficial for patients with advanced prostate cancer, its efficacy is limited because malignant cells change to a castration-resistant phenotype over 1–2 years of therapy. Early studies demonstrated that systemic ADT based on anti-androgens, which prevent androgens from binding to the androgen receptor (AR), promotes the development of invasive prostate cancer.2 This suggests that the therapeutic suppression of androgen/AR binding may elicit unwanted signals that may favor the progression of surviving prostate cancer cells.2 In another study, targeting AR-dependent signaling suppressed the wound-healing process by altering macrophage infiltration as well as their cytokine expression profile.3 Since gene signatures associated with wound healing are similar to those linked to highly invasive breast cancer,4 we studied the potential relationship between AR signaling and inflammatory responses mediated by tumor-infiltrating macrophages as well as the impact of these cells of the innate immune system on disease outcome.
Tumor-Infiltrating Macrophages Promote Prostatic Tumorigenesis
Tumor-infiltrating macrophages are a key component of the inflammatory process that often accompany prostatic tumorigenesis. Fang et al. first demonstrated that co-culturing immortalized prostate epithelial cells with macrophages renders them tumorigenic.5 Clinical studies indicate that the number of macrophages is significantly increased within high-grade prostatic intraepithelial neoplasms (PINs) or prostate cancer lesions as compared with benign prostate tissues. Macrophage-induced prostatic tumorigenesis appears to involve alterations in the AR-dependent signaling pathway elicited by chemokine (C-C motif) ligand 4 (CCL4) and transduced by signal transducer and activator of transcription 3 (STAT3), the loss of 2 major oncosuppressors, namely p53 and phosphatase and tensing homolog (PTEN), and the epithelial-to-mesenchymal transition (EMT). Pten+/− mice lacking the AR in macrophages developed far fewer PINs than their Pten+/−, macrophage AR-positive counterparts. Moreover, CCL4-neutralizing antibodies have been shown to effectively block macrophage-induced prostatic oncogenesis. Targeting the AR by means of a newly identified enhancer of its degradation, namely, ASC-J9®, resulted in reduced CCL4 expression and limited the growth of prostate cancer xenografts in mice. Importantly, the upregulation of CCL4 was associated with increased expression levels of snail family zinc finger 1 (Snai1) as well as with the downregulation of p53 and PTEN in high-grade PINs and prostate cancers. Taken together, these results demonstrate that the CCL4/AR signaling axis may represent a therapeutic target to effectively inhibit inflammation-associated prostatic tumorigenesis.
The Downregulation of AR by RNA Interference Promotes CCL2 Expression and Favors the Metastatic Dissemination of Prostate Cancer
We next tested the hypothesis that suppressing the function of AR by RNA interference might trigger undesirable inflammatory signals that enhance macrophage infiltration and stimulate the progression of prostate cancers.6 Depleting AR from THP-1 macrophages enhances their migration toward prostate cancer cells. Similarly, the downregulation of AR in prostate cancer cells enhances the recruitment of THP-1 macrophages. Mechanistic studies revealed that AR silencing in either macrophages or prostate cancer cells induces the expression of CCL2 and the CCL2-dependent activation of STAT3, which in turn may favor the EMT and hence increase the invasiveness of malignant cells. In line with this notion, the pharmacological interruption of the CCL2/CCR2/STAT3 signaling axis suppressed EMT and prostate cancer cell invasiveness, pointing to a novel mechanism linking CCL2 to the EMT that may play a key role in prostate cancer progression. Knocking-out the AR from macrophages in TRAMP mice (a murine model of prostate cancer) promotes the metastatic dissemination of malignant cells as it enhances CCL2 expression and tumor infiltration by macrophages. Co-targeting the AR and the CCL2/CCR2 signaling axis results in improved antineoplastic effects against prostate cancer xenografts growing in mice as compared with inhibiting the AR alone. Tissue microarray analysis of human prostate cancer tissues reveal that patient outcomes may be negatively affected by elevated intratumoral levels of CCL2, suggesting that this chemokine may play a key role in stimulating the progression of prostate cancer. Altogether, our results may identify a novel therapeutic approach against castration-resistant, metastatic prostate cancer based on the combined inhibition of the AR and the CCL2/CCR2/STAT3 signaling axis.
ADT Enhances the Metastatic Dissemination of Prostate Cancer Cells by Promoting Tumor Infiltration by Macrophages and STAT3 Signaling
Previous meta-analyses demonstrated that ADT based on luteinizing hormone-releasing hormone (LH-RH) agonists plus anti-androgens improves the overall survival rate of prostate cancer patients as compared with ADT based on LH-RH agonists alone.7 Interestingly, we found that bicalutamide (also called Casodex), a currently used non-steroidal anti-androgen, as well as enzalutamide (also called MDV3100, a novel anti-androgen that potently inhibits castration-resistant prostate cancer),8 may promote the invasiveness of prostate cancer cells by stimulating the recruitment of macrophages.9 Mechanistic studies revealed that bicalutamide and enzalutamide reduced the AR-dependent expression of protein inhibitor of activated STAT3 (PIAS3), resulting in enhanced STAT3/CCL2 signaling. Suppressing the STAT3/CCL2/CCR2 signaling axis reversed the bicalutamide- or enzalutamide-induced migration of macrophages toward prostate cancer cells and limited the invasiveness of the latter. Importantly, ASC-J9® inhibited both macrophage migration and the subsequent invasion of prostate cancer cells by regulating the STAT3/CCL2/CCR2 signaling axis via an AR-independent mechanism that results in the direction suppression of STAT3 activation. These findings were confirmed in vivo, in orthotopic models of prostate cancer. Taken together, our results point to potential concerns related to currently-used ADT based on anti-androgens, as these may promote metastasis, and suggest that ASC-J9®, alone or combined with inhibitors of the STAT3/CCL2/CCR2 signaling axis, may represent an novel therapeutic paradigm against castration-resistant prostate cancer progression.
Conclusions
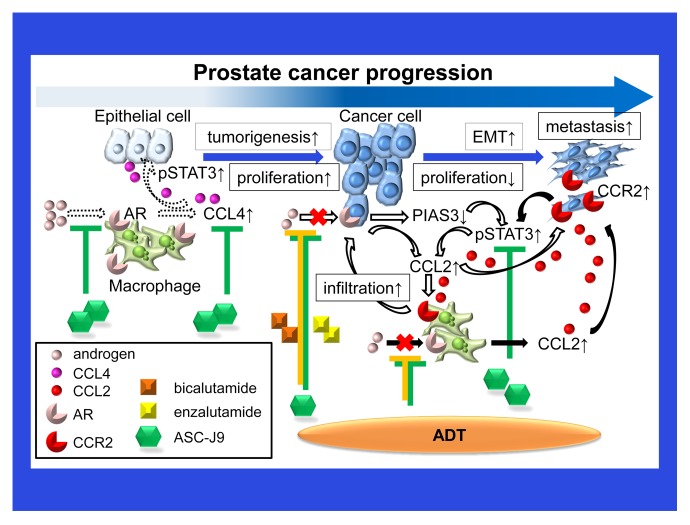
Macrophages appear to play a key role at multiple stages of prostatic tumorigenesis by regulating AR signaling and/or the AR-modulated secretion of inflammatory cytokines or chemokines (Fig. 1). ADT with anti-androgens may stimulate the release of inflammatory cytokines by macrophages, resulting in enhanced AR signaling and metastatic cancer cell dissemination. Combining ADT with agents that specifically target these macrophage-derived inflammatory cytokines may be needed to efficiently inhibit prostate cancer progression. Alternatively, a novel enhancer of AR degradation that simultaneously suppresses AR signaling and macrophage-derived inflammatory cytokines, as well as limited toxicity,10 namely, ASC-J9®, may represent a promising approach against castration-resistant prostate cancer progression.
Figure 1. Chemokines mediate a vicious cycle between prostate cancer cells and macrophages that drives disease progression. Tumor-infiltrating macrophages and inflammatory cytokines such as chemokine (C-C motif) ligand 4 (CCL4) play a key role in prostatic tumorigenesis via an androgen receptor (AR)-, and signal transducer and activation of transcription 3 (STAT3)-dependent signaling axis (dashed arrows). As bicalutamide or enzalutamide reduce the AR-mediated expression of protein inhibitor of activated STAT, 3 (PIAS3), and stimulate the chemokine (C-C motif) ligand 4 (CCL4)- and STAT3-dependent signaling pathway, they promote the migration of macrophages toward prostate cancer cells, thus enhancing the invasiveness of the latter (white arrows). Suppressing the function of the AR in macrophages simultaneously triggers undesirable inflammatory signals that prompt macrophage infiltration and stimulate prostate cancer progression through the activation of a CCL2/CCR2/STAT3 signaling axis (black arrows).
Disclosure of Potential Conflicts of Interest
ASC-J9® was patented by the University of Rochester, the University of North Carolina, and AndroScience Corp., and then licensed to AndroScience Corp. Both the University of Rochester and C. Chang own royalties and equity in AndroScience Corp.
Acknowledgments
This work was supported by NIH grant CA156700 and George Whipple Professorship Endowment, and Taiwan Department of Health Clinical Trial and Research Center of Excellence grant DOH99-TD-B-111–004.
Citation: Izumi K, Chang C. Targeting inflammatory cytokines-androgen receptor (AR) signaling with ASC-J9® to better battle prostate cancer progression. OncoImmunology 2013; 2:e26853; 10.4161/onci.26853
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26853
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, Yao J, Yeh S, Chang C. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci U S A. 2008;105:12182–7. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009;119:3739–51. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sørlie T, Dai H, He YD, van't Veer LJ, Bartelink H, van de Rijn M, Brown PO, van de Vijver MJ. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. 2005;;8:3738–43. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang LY, Izumi K, Lai KP, Liang L, Li L, Miyamoto H, Lin WJ, Chang C. Infiltrating Macrophages Promote Prostate Tumorigenesis via Modulating Androgen Receptor-Mediated CCL4-STAT3 Signaling. Cancer Res. 2013;73:5633–46. doi: 10.1158/0008-5472.CAN-12-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izumi K, Fang LY, Mizokami A, Namiki M, Li L, Lin WJ, Chang C. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol Med. 2013;5:1383–401. doi: 10.1002/emmm.201202367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists’ Collaborative Group. Lancet. 2000;355:1491–8. doi: 10.1016/S0140-6736(00)02163-2. [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et al. AFFIRM Investigators Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 9.Lin TH, Izumi K, Lee SO, Lin WJ, Yeh S, Chang C. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis. 2013;4:e764. doi: 10.1038/cddis.2013.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol. 2013;182:1942–9. doi: 10.1016/j.ajpath.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]