Abstract
Therapeutic vaccination is regarded as a promising strategy against multiple hematological malignancies including lymphoma. However, this approach alone possesses limited potential for the treatment of established tumors. As several anticancer regimens relies on the combination of multiple drugs, it is reasonable to predict that also cancer vaccination will be most effective in the context of multimodal approaches. In particular, low-dose or metronomic chemotherapy could be coupled to anticancer vaccines to improve the efficacy of this immunotherapeutic interventions. This review summarizes recent findings in support of the use of anticancer vaccines combined with low-dose or metronomic chemotherapy for the treatment and management of lymphoid malignancies.
Keywords: anticancer vaccines, immunotherapy, metronomic chemotherapy, low-dose chemotherapy, lymphoma
Introduction
Lymphomas are cancers of hematological origin that can be broadly classified into a Hodgkin’s and non-Hodgkin’s variant. Although most forms of Hodgkin’s lymphoma (HL) are curable with currently available treatment modality, aggressive forms of non-Hodgkin’s lymphoma (NHL) such as diffuse large B-cell lymphoma, follicular lymphoma, marginal zone B-cell lymphoma, and mantle cell lymphoma are considered as incurable in advanced stages of the disease, and patients affected by these neoplasms undergo repeated remissions and relapses.1,2 Regardless of the subtype and stage of the disease, the treatment currently employed for most lymphomas relies on radiation therapy or combinatorial chemotherapeutic regimens that include prednisone plus the cytotoxic agents cyclophosphamide, doxorubicin, and vincristine as well as a tumor-targeting monoclonal antibody, such as the anti-CD20 agent rituximab. Such an immunotherapeutic regimen is commonly referred to as R-CHOP. Despite the enhanced overall survival exhibited by patients treated with R-CHOP, this regimen exerts substantial undesirable effects and may promote chemoresistance.3,4 Hence, less toxic and more effective modalities for the treatment of lymphoma patients are clearly needed.
Conventional chemotherapeutic agents were developed based on their capacity to directly kill malignant cells at a maximal tolerated dose (MTD), the highest amount of the drug associated with tolerable toxicity and manageable side effects. In this context, patients must go through long periods off therapy, allowing for their full recovery from the adverse effects of chemotherapy. These rest periods, however, also allow cancer cells to become resistant to chemotherapy and hence to drive a disease relapse.5 A new therapeutic paradigm has recently been established based on the administration of low-dose chemotherapeutics at short intervals (so-called, metronomic chemotherapy), in the absence of prolonged drug-free periods.6, 7 Metronomic chemotherapy not only causes relatively mild and short-lived toxic effects, but also exerts an antiangiogenic activity had have been associated with robust antineoplastic effects in preclinical models of chemoresistant tumors.8 Accumulating evidence also indicates that some chemotherapeutic drugs given at a low dose are able to promote disease eradication by stimulating anticancer immune and selectively eliminating immunosuppressive cells (Table 1).9
Table 1. Use of low-dose chemotherapy to overcome immunosuppression and immune evasion by lymphomas.
| Escape mechanism | Effects of low-dose chemotherapeutic agents |
|---|---|
| Reduced immunogenicity10,11 | Cyclophosphamide, gemcitabine, doxorubicin - Promote the expression of MHC class I molecules on cancer cells12 - Stimulate the exposure of calreticulin on the cell surface,13 as well as the release of HMGB113 and ATP14 |
| Apoptosis resistance upon the downregulation of FAS and TRAILRs16,17 |
Etoposide, doxorubicin, cyclophosphamide - Promote the upregulation of FAS18 and TRAILRs19,21,22 |
| Impaired cytotoxicity of NK cells resulting from the downregulation of NKG2D ligands24,25 | Dacarbazine, doxorubicin, cisplatin - Induce the expression of NKG2D29 and CD226 ligands30 |
| Increased frequency of immunosuppressive Tregs41,42 and MDSCs55,56 | Cyclophosphamide, doxorubicin, paclitaxel, gemcitabine, 5-fluorouracil - Selectively kill Tregs43-45 and MDSCs57-59 |
| Stimulation of angiogenesis61 | Cyclophosphamide, doxorubicin, cisplatin - Promote the apoptotic demise of endothelial cells8 - Inhibit the emigration of circulating endothelial precursor cells from bone marrow65 - Reduce the expression of VEGF66,67 |
Abbreviations: HMGB1, high mobility group box 1; MDSC, myeloid-derived suppressor cell; NK, natural killer; Treg, regulatory T cell; VEGF, vascular endothelial growth factor; TRAILR, tumor necrosis factor α-related apoptosis-inducing ligand receptor.
These studies provided a rationale for using low-dose or metronomic chemotherapy as a means to counteract the numerous mechanisms of immunological tolerance that are set in place by malignant lesions, and hence improve the antitumor efficacy of therapeutic anticancer vaccination. Here, we review preclinical and clinical studies involving a panel of cytotoxic agents used in low doses or according to a metronomic schedule, either as standalone interventions or in combination with therapeutic anticancer vaccines. While focusing our attention on lymphoma-related studies, we describe how low-dose or metronomic chemotherapy may improve the potential of therapeutic anticancer vaccination (Fig. 1).
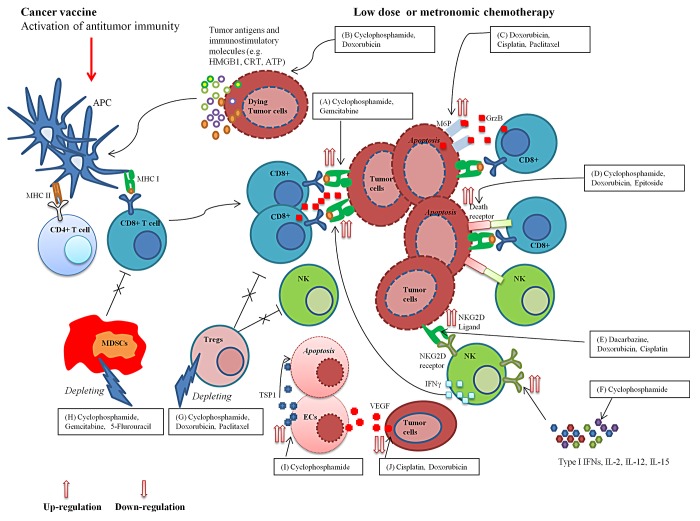
Figure 1. Immunomodulatory properties of low-dose or metronomic chemotherapy and its potential for generating potent anticancer immune responses in combination with therapeutic anticancer vaccines. Multiple chemotherapeutic agents given at low doses can promote the initiation and/or persistence of tumor-targeting immune responses by upregulating the expression of MHC class I molecules on the surface of cancer cells (A); promoting the exposure or release of endogenous immunostimulatory calreticulin (CRT), ATP and high mobility group box 1 (HMGB1) (B); favoring the apoptotic demise of neoplastic cells by upregulating mannose-6-phosphate (M6P) receptors and death receptors on their surface (C,D); increasing the sensitivity of malignant cells to natural killer (NK) cells by triggering the expression of NKG2D ligands (E); enhancing the secretion of immunostimulatory cytokines that stimulate NKG2D expression on NK cells (F); selectively eradicate immunosuppressive regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) (G,H); and by exerting anti-angiogenic effects (I,J). Combining low-dose or metronomic chemotherapy with therapeutic anticancer vaccines can thus result in the elicitation of potent innate and adaptive immune responses against malignant cells. APC, antigen-presenting cells; EC, endothelial cell; GZMB, granzyme B; IL, interleukin; IFN, interferon; THBS1, thrombospondin 1; VEGF, vascular endothelial growth factor.
Preclinical Studies on the Immunomodulatory Effects of Low-Dose Chemotherapy
The therapeutic outcome of immunotherapy depends on the interplay between malignant cells and components of the immune system, and it is well recognized that tumors can evade immune responses by a multitude of mechanisms. Low-dose chemotherapy is likely to overcome these obstacles and hence promote the (re)establishment of antitumor immunity (Table 1). Generally, lymphomas are weakly immunogenic due to a decreased expression of MHC class I molecules on the surface of malignant cells, which prevent the recognition of tumor-associated antigens (TAAs) by cytotoxic T lymphocytes (CTLs).10,11 Low-dose cyclophosphamide and gemcitabine have been shown to promote the expression of MHC class I molecules on the surface of cancer cells and thus to facilitate their antigen-dependent CTL-triggered demise.12 In the same study, the culture supernatants of cancer cells treated with low-dose gemcitabine were shown to promote the maturation and activation of dendritic cells (DCs) in vitro, in turn stimulating the proliferation of CD4+ and CD8+ T cells along with an increased production of interferon (IFN)γ.12 These findings suggest that low-dose chemotherapy can trigger the immunogenic demise of cancer cells by stimulating them to release immunostimulatory factors as they die. In this regard, Schiavoni and coworkers13 found that a low concentration of the active cyclophosphamide analog mafosfamide stimulates dying tumor cells to expose calreticulin (CRT) on the cell surface and to release the nuclear protein high mobility group box 1 (HMGB1). These events were deemed as signals for the induction of innate immune responses along with the maturation and expansion of conventional CD8α+ DCs.13 In a follow-up in vivo study, DCs from cyclophosphamide-treated mice were observed to infiltrate neoplastic lesions and to engulf apoptotic cancer cells before migrating to tumor-draining lymph nodes and eliciting a tumor-specific CTL responses through cross-priming.13 Doxorubicin has also been shown to trigger immunogenic cell death by facilitating the release of ATP from dying tumor cells, resulting in the activation of DCs and in the priming of anticancer immune responses.14
It has recently been discovered that low-dose chemotherapy can increase the susceptibility of cancer to the cytotoxic activity of CTLs by allowing granzymes to enter target cells independently of perforin. In fact, low-dose paclitaxel, doxorubicin, and cisplatin were found to increase the permeability of cancer cells to granzyme B (GZMB) released by CTLs in vitro.15 This effect was solely attributed to the ability of low-dose chemotherapy to upregulate mannose-6-phosphate (M6P) receptors on malignant cells, hence facilitating the delivery of cytotoxic GZMB molecules.15 CTLs and natural killer (NK) cells also trigger the apoptotic demise of cancer cells upon the engagement of death receptors including FAS (CD95) and various members of the tumor necrosis factor α (TNFα)-related apoptosis-inducing ligand receptor (TRAILR) superfamily, with the respective ligands (e.g., FASL and TRAIL). However, several reports have revealed a marked downregulation or loss of FAS and TRAILRs in certain lymphomas, allowing malignant cells to evade the immunosurveillance mediated by CTLs and NK cells.16,17 Several chemotherapeutic drugs employed at low doses have been shown to restore the expression of these death receptors. For example, a low dose of etoposide reportedly upregulates the expression of FAS on the surface of human monocytic leukemia U937 cells, resulting in an increased FAS-dependent apoptotic responses.18 Low concentrations of doxorubicin or etoposide significantly increased TRAILR2 (also known as DR5) expression on human solid cancer cells, sensitizing them to TRAIL-mediated killing.19,20 Along similar lines, low-dose doxorubicin restored the sensitivity to TRAIL of an originally TRAIL-resistant variant of human prostatic cancer LNCaP cells upon the upregulation of TRAILR1 (also known as DR4).21 Furthermore, a single, low dose of cyclophosphamide sensitized malignant mesotheliomas to TRAIL-dependent CD8+ T cell- and NK cell-mediated cytotoxicity in vivo, resulting in the suppression of tumor growth.22
NK cells play an important role in the recognition and elimination of (pre)malignant cells. Activating killer cell lectin-like receptor subfamily K, member 1 (KLRK1, best known as NKG2D) receptors can unleash the cytotoxic functions of NK cells, yet only when these signals overcome inhibitory signals delivered by MHC class I molecules or killer inhibitory receptor (KIR) ligands.23 NKG2D ligands are generally absent from normal cells, but are expressed on the surface of stress, infected or malignant cells.23 Lymphoma patients often exhibit decreased NK-cell activity, resulting in impaired cytotoxicity against malignant cells.24,25 It has been reported that malignant lymphomas are characterized by a significant downregulation of the NKG2D ligand MHC class I polypeptide-related sequence A (MICA) as this sheds from the plasma membrane, and patients with elevated circulating levels of MICA and similar molecules exhibit impaired NK-cell function.25,26 Once shed, these molecules are known to circumvent NK-dependent immunosurveillance mechanisms by blocking NKG2D receptors and promoting their downregulation.27,28 To elicit productive immune responses, lymphomas must be visible to NK cells and susceptible to their killing. Recently, dacarbazine was shown to trigger the expression of NKG2D ligands on the surface of cancer cells, leading to the activation of NK-cell cytotoxic functions and to IFNγ secretion in mice with melanoma.29 IFNγ boosts the immunogenicity of cancer cells by increasing the levels of MHC class I molecules on their surface and hence promoting their killing by CTLs.29 Interestingly, upon exposure to low-dose doxorubicin and cisplatin, multiple myeloma cells exhibit an increase susceptibility to NK-cell cytotoxicity, at least in part due to the upregulation of ligands for NKG2D and CD226, which is also involved in NK cell-mediated tumor cell killing.30
Several cytokines including IFNα, interleukin (IL)-2, IL-12, and IL-15 have been shown to upregulate NKG2D receptors, a process that is associated with an amplification of NKG2D-mediated antitumor response.31-34 Interestingly, low-dose cyclophosphamide has been reported to stimulate the expression of type 1 IFNs, i.e., IFNα and IFNβ, which are important stimuli for the proliferation and persistence of CD8+ T cells.35 Mice pretreated with low-dose cyclophosphamide were also found to produce immunostimulatory cytokines including IL-1β, IL-2, IL-7, IL-15, IL-21, IFNγ, and granulocyte macrophage colony-stimulating factor (GM-CSF), while secreting decreased amounts of immunosuppressive cytokines, such as IL-4 and IL-10. These immunological effects of low-dose cyclophosphamide exacerbated the antineoplastic potential of adoptively transferred immune cells against melanoma.36 Taken together, these observations indicate that some cytotoxic drugs such as cyclophosphamide at low doses can enhance the secretion of immunostimulatory cytokines, which in turn promote the expression of NKG2D receptors on the surface of NK cells to ultimately boost antitumor immunity.
Selective Eradication of Immunosuppressive Cell Populations by Low-Dose Chemotherapy
Regulatory T cells
Regulatory T cells (Tregs), which constitute about 5% of peripheral CD4+ T cells in both mice and humans, play a key role in the maintenance of peripheral tolerance and prevent the emergence of pathological autoimmune reactions.37 In tumor-bearing hosts, Tregs facilitate disease progression by curtailing NK and T-cell immunity through various mechanisms, including the expression of the inhibitory checkpoint regulator cytotoxic T lymphocyte-associated protein 4 (CTLA4) on their surface and the production of immunosuppressive mediators such as IL-10 and transforming growth factor β1 (TGFβ1).38-40 Notably, lymphoma patients exhibit elevated levels of Tregs, and this contributes to the resistance of malignant cells to immunosurveillance.41,42 Interventions aimed at enhancing antitumor immune responses based on the depletion or neutralization of Tregs by chemotherapy have been intensively studied. Thus, low-dose cyclophosphamide was found to selectively kill Tregs in vivo, in a murine model of lymphoma.43 In this setting, effector T cells were activated along with Treg depletion and increased expression of IL-2.43 When mice with colon cancer were treated with low-dose cyclophosphamide plus doxorubicin, a significant suppression of tumor growth was associated with Tregs depletion and decreased secretion of TGFβ1 within the tumor microenvironment.44 Another study has demonstrated that low-dose paclitaxel decreases the viability of Tregs and their capacity to produce immunosuppressive cytokine in vivo, in a murine model of lung cancer, while leaving conventional T cells untouched.45
Myeloid-derived suppressor cells
Under steady-state conditions, highly plastic immature myeloid cells arise from multipotent hematopoietic stem cells (HSCs) and develop into mature macrophages, dendritic cells, and granulocytes with diverse functions, through sequential steps of differentiation.46 Conversely, factors produced in the tumor microenvironment interfere with normal myelopoiesis, resulting in the accumulation of immature myeloid cells that exert immunosuppressive functions, which are cumulatively known as myeloid-derived suppressor cells (MDSCs), in the circulation, lymphoid tissues as well as within neoplastic lesions.47 In tumor bearing mice, co-expression of surface markers Gr-1+ and CD11b+ are commonly found on MDSCs.47 In humans, 2 major MDSC subsets have been characterized: CD11b+D14+HLA-DRloCD33+CD124+ monocytic MDSCs (M-MDSCs) and, CD11b+CD14−CD66b+CD15+CD124+ granulocytic/polymorphonuclear MDSCs (G-MDSCs/PMN-MDSCs).48,49 The accumulation of these MDSCs in the peripheral blood and within neoplastic lesions has been reported in a large number of cancer patients.49-51 MDSCs suppress innate and adaptive antitumor immune response by favoring the recruitment and expansion of Tregs, as well as by producing high levels of L-arginase, reactive oxygen species (ROS), inducible nitrogen oxide synthase (iNOS), and various immunosuppressive cytokines. Overall, these enzymes and soluble mediators promote neoangiogenesis and allow for the escape of immune cells from immunosurveillance, hence promoting disease progression.48,52-54 Of note, in lymphoma patients, an increased frequency of circulating MDSCs has been reported to correlate with aggressive disease and the inhibition of antitumor immunity.55,56
Clearly, both these immunosuppressive cell populations must be inhibited or eliminated in order to restore therapeutically-relevant antitumor immune responses. Some low-dose or metronomic chemotherapeutic regimens have been shown to selectively kill immunosuppressive cell populations. For example, low-dose gemcitabine appears to selectively inhibit tumor-associated MDSCs in mice bearing 4T1 mammary carcinomas, favoring the expansion of tumor-targeting T cells.57 Along similar lines, low-dose 5-flurouracil has been shown to provoke a major decrease in splenic and tumor-infiltrating MDSCs in mice bearing EL4 thymomas while leaving T cells, NK cells, dendritic cells, and B cells unaffected.58 Interestingly, the elimination of MDSCs by 5-flurouracil was associated with a boost in antitumor immunity, manifesting with an increment in IFNγ production by tumor-specific CD8+ T cells.58 Of note, metronomic chemotherapy with low-dose cyclophosphamide and gemcitabine has been demonstrated to deplete tumor-associated Tregs and MDSCs in mice bearing CT26 colon carcinomas, leading to antitumor T-cell response and tumor eradication, at least in some animals.59 As previously mentioned, doxorubicin is known for its ability to induce immunogenic cell death by allowing for the release of ATP from dying cancer cells and hence favoring the elicitation of antitumor immune responses.14 The release of ATP from malignant cells succumbing to doxorubicin was found to induce the differentiation of tumor-infiltrating myeloid cells toward CD11c+CD11b+Ly6Chi cells sharing some features with DCs, including the ability to efficiently engulf TAAs in situ and to elicit cellular immune responses against cancer cells.60 Taken together, these observations suggest that low-dose chemotherapy is a promising approach to restore antitumor immunity by eliminating or inhibiting immunosuppressive Tregs and MDSCs, thus promoting the differentiation of antigen-presenting cells and the elicitation of tumor-targeting CTL responses.
Anti-Angiogenic Effects of Low-Dose or Metronomic Chemotherapy
The expansion of the tumor vasculature is vital for the growth of hematological malignancies and increased angiogenesis is often correlated with poor prognosis in lymphoma patients.61,62 The major mediator of the angiogenic switch is vascular endothelial growth factor (VEGF), which is produced by lymphoma cells and binds VEGF receptors expressed on endothelial cells from pre-existing capillaries as well as on circulating endothelial precursor cells of bone marrow derivation.61 Metronomic chemotherapy has been shown to mediate beneficial anti-angiogenic effects. For example, Browder et al.8 administered cyclophosphamide on a metronomic schedule to mice affected by Lewis lung carcinoma or L1210 leukemia, and observed a substantial apoptotic response among endothelial cells of the growing tumor vasculature, resulting in the inhibition of angiogenesis. This effect was lost when the drug was given at the MTD. In addition, metronomic cyclophosphamide was 3 times more effective than the same drug given as a standard regimen in treating neoplastic lesions developing from cancer cells that had been made cyclophosphamide-resistant before inoculation.8
Adopting the same metronomic schedule, Hamano et al.63 found that the anti-angiogenic effect of low-dose cyclophosphamide is mediated by the selective upregulation of thrombospondin 1 (THBS1) in malignant cells and tumor-associated endothelial cells. THBS1 is an endogenous inhibitor of angiogenesis that arrests the proliferation and triggers the apoptotic demise of endothelial cells. This finding was supported by results from Bocci and collegues,64 who observed that the plasma of SCID mice bearing human tumors contains higher levels of THBS1 upon the administration of metronomic low-dose cyclophosphamide. Most importantly, the anti-angiogenic and antitumor effects of metronomic cyclophosphamide were diminished in Thbs1−/− mice, further corroborating the notion that THBS1 is a pivotal mediator of the therapeutic activity of low-dose cyclophosphamide. In addition, Bertolini et al.65 reported that the administration of metronomic cyclophosphamide to mice harboring human lymphomas decreases the abundance and viability of endothelial precursor cells, resulting in a significant reduction of tumor growth as compared with cyclophosphamide given at its MTD. Furthermore, metronomic doxorubicin as well as metronomic cisplatin have been shown to reduce the expression of VEGF, leading to a significant inhibition of tumor growth in murine models of adenocarcinoma66 and hepatocarcinoma.67
Preclinical and Clinical Studies of Low-Dose Chemotherapy Alone and in Combination with Anticancer Vaccines
The efficacy of low-dose chemotherapy against lymphoma has been demonstrated in several preclinical studies. Mice bearing A20 lymphomas exhibted a prolonged survival and reduced rates of tumor growth upon treatment with low-dose cyclophosphamide (20 mg/kg).43 Bertolini et al.65 also observed a significant inhibition of tumor growth in mice bearing human mantle lymphomas upon the administration of cyclophosphamide in drinking water as a continuous low-dose of 20 mg/kg/day. In rat models of lymphoma, Rozados et al.68 showed that the metronomic administration of low-dose cyclophosphamide (10 mg/kg) i.p. results in tumor eradication in the absence of overt toxicity. This finding indicates that metronomic chemotherapy is an effective and safe alternative to conventional anticancer regimens.
Based on the results of these and other preclinical studies, numerous clinical trials have assessed the antineoplastic profile of metronomic chemotherapy in lymphoma patients. For instance, a regimen consisting of daily low-dose lomustine and chlorambucil, daily subcutaneous bleomycin and vincristine, plus methotrexate and dexamethasone given on an eight-week cycle (LBCMVD-56) has been evaluated in patients with refractory/relapsed lymphoma. The overall response rate (ORR) of these patients was 67%, encompassing 21% of complete responses (CRs), with a median overall survival of 13 mo.69 In a similar approach, Coleman et al.70 reported the clinical efficacy of continuous, low-dose oral prednisone (20 mg), etoposide (50 mg), procarbazine (50 mg), and cyclophosphamide (50 mg) (PEP-C) administered either daily, on alternate days, or on a fractionated basis, in heavily pretreated patients with refractory/relapsed lymphoma. In this study the ORR was 75%, 38% of patients achieved a CR, and minimal toxicity was reported. These clinical findings demonstrate that continuous low-dose chemotherapy is effective and well-tolerated by lymphoma patients. Objective responses and prolonged progression-free survival have also been reported among Hodgkin’s lymphoma patients treated with a combination of low-dose daily oral cyclophosphamide, weekly vinblastine and rofecoxib.71 As demonstrated in both preclinical and clinical settings, the efficacy of metronomic chemotherapy mainly relies on anti-angiogenic effects8,61,63-65 coupled to the selective eradication of Tregs, enabling the restoration of antitumor T-cell and NK-cell activity.72
Therapeutic vaccines, given their specificity, low toxicity and capability to harness the patient’s immune system to fight cancer, are being recognized as a potential complementary approach against solid and hematopoietic malignancies. Therapeutic vaccination is an active for of immunotherapy that can promote the elicitation of tumor-specific cellular immune responses, the production of tumor-targeting polyclonal antibodies as well as the establishment of memory immune responses against multiple TAAs, hence limiting the emergence of tumor immune escape variants. In a pilot study, Di Nicola et al.73 vaccinated indolent NHL patients undergoing disease relapse with autologous dendritic cells loaded with tumor cells. At a median follow up of 50.5 mo, 6 out of 18 patients manifested objective clinical responses including 3 CRs and 3 partial responses (PRs). Impressively, clinical responses were associated with a reduction in the levels of Tregs coupled to the induction of NK, T, and B-cell responses. BiovaxID, a patient-specific therapeutic anti-idiotype (ID) lymphoma vaccine conjugated to keyhole limpet hemocyanin (KLH), has recently been shown to provide clinical benefits to patients in a phase III study. At a median follow-up of 56.6 mo, the administration of ID-KLH together with GM-CSF to follicular lymphoma patients achieving a CR upon chemotherapy was associated with an impressive disease-free survival of 44.2 mo, compared with 30.6 mo for the control arm.74 Nevertheless, it is becoming apparent that anticancer vaccines administered as standalone therapeutic interventions have limited potential for the treatment of established tumors, owing to the intrinsic and/or acquired ability of neoplastic lesions to suppress the antitumor immune responses that are elicited by vaccination. Hence, low-dose or metronomic chemotherapy might be used in conjunction with anticancer vaccines in order to promote beneficial immunomodulatory effects and abrogate tumor-induced immunosuppression, thereby boosting vaccine-induced immune responses and achieving therapeutically-relevant antineoplastic effects.
The proof-of-principle in support of the use of this combinatorial immunochemotherapeutic strategy in lymphoma patients has been provided in a study in which a dendritic cell-based vaccine coupled to low-dose cyclophosphamide for 2 consecutive days was given to mice bearing A20 lymphomas, resulting in a significant improvement of survival and complete, long-term tumor regressions.75 Moreover, most of the mice in which the primary tumor was eradicated by combinatorial immunochemotherapy were resistant to a secondary tumor challenge, pointing to the development of long-lasting memory T-cell responses in response to vaccination.75 This approach was also shown to be effective at eradicating tumors that recurred after a period of regression following the initial vaccination.75 Along similar lines, a combination of low-dose cyclophosphamide and oligodeoxynucleotides containing unmethylated CG sequences administered intratumorally cured widely disseminated B-cell lymphomas in mice.76 The authors of this study found that CpG oligodeoxynucleotides enhance the presentation of TAAs by local DCs and hence induce tumor-specific CD8+ T-cell responses that are able to eradicate large and systemic lymphomas.76
Our group has investigated the efficacy of combining metronomic cyclophosphamide with an NKT cell adjuvant-based vaccine against Eµ-myc-driven B-cell lymphomas in mice. In this setting, a single therapeutic vaccination with irradiated, α-galactosylceramide-loaded autologous tumor cells induced a large systemic spike of IFNγ coupled to a transient expansion of peripheral NKT and NK cells, globally leading to the suppression of tumor growth and to a prolonged protection against Eµ-myc-driven lymphoma.77 However, the therapeutic response to the vaccine was not durable and most mice succumbed to delayed disease relapse. In our latest study, cyclophosphamide was given to mice bearing Eµ-myc-driven lymphoma according to metronomic low-dose regimen (5 mg/kg, on alternative days for 2 weeks) prior to vaccination, in order to investigate the benefits of such a combinatorial immunochemotherapeutic approach. In preliminary experiments, mice that received cyclophosphamide followed by the NKT cell adjuvant-based vaccine were cured from disease, with no evidence of relapse. Conversely, mice treated with either the vaccine or metronomic chemotherapy alone succumbed to the lymphoma (Heng Sheng Sow, personal communication). These findings suggest that metronomic chemotherapy can enhance the efficacy of NKT adjuvants against B-cell lymphoma. The mechanisms underlying this effects, however, remain to be determined.
Published preclinical observations and our preliminary findings provide a rationale for combining low-dose or metronomic chemotherapy with anticancer vaccination for treating lymphoma. However, to the best of our knowledge, no clinical studies published so far have evaluated this strategy in lymphoma patients. Conversely, the therapeutic efficacy of this combinatorial immunochemotherapeutic approach has been explored in patients bearing solid tumors. Enhanced vaccine-induced immune responses have been observed in breast carcinoma patients treated with an allogeneic GM-CSF-secreting vaccine along with low doses of cyclophosphamide, given one day prior to vaccination, and doxorubicin, adminitered 1 week later.78 Moreover, the combination of metronomic cyclophosphamide with methotrexate and a 1E10 anti-idiotype vaccine has been reported to increase the clinical responses achieved by patients with metastatic breast cancer.79 Kandalaft et al.80 reported that the combination of a dendritic cell-based autologous vaccine with bevacizumab and metronomic cyclophosphamide provides clinical benefits to advanced recurrent ovarian cancer patients. In light of the feasibility and potential clinical activity demonstrated by this approach in patients with solid tumors, the possible therapeutic benefits of active immunotherapy and low-dose or metronomic chemotherapy for lymphoma patients are worthy further investigation.
Concluding Remarks
As demonstrated in multiple preclinical and clinical studies, including those discussed in this review, the multipronged immunostimulatory effects and good safety profile of low-dose or metronomic chemotherapy have gradually led to its use in cancer patients as an alternative to chemotherapy based on conventional doses and administration schedules. We speculate that low-dose or metronomic chemotherapy used in combination with therapeutic anticancer vaccines or other forms of immunotherapy will ameliorate disease outcomes as compared with standard, high-dose chemotherapy or immunotherapy administered as a standalone intervention. Additional studies are required to determine the repertoire of immunostimulatory effects mediated by various cytotoxic agents given at low doses or according to a metronomic schedule as well as the optimal dosage, delivery route, and schedule at which such effects can be obtained for combining these drugs with anticancer vaccines. The combination of anticancer vaccines with low-dose chemotherapy nowadays stands out as a promising therapeutic approach against a range of neoplasms, including lymphomas.
Glossary
Abbreviations:
- CTL
cytotoxic T lymphocytes
- DC
dendritic cell
- NHL
non-Hodgkin’s lymphoma
- MDSC
myeloid-derived suppressor cell
- MTD
maximal tolerated dose
- THBS1
thrombospondin 1
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
Citation: Sheng Sow H, Mattarollo S. Combining low-dose or metronomic chemotherapy with anticancer vaccines: A therapeutic opportunity for lymphomas. OncoImmunology 2013; 2:e27058; 10.4161/onci.27058
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27058
References
- 1.Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380:848–57. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi MK, Marcus RE. Follicular lymphoma: time for a re-think? Blood Rev. 2005;19:165–78. doi: 10.1016/j.blre.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H, Hegewisch-Becker S, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–32. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 4.Krause SW, Gerken M, Andreesen R, Hofstädter F, Klinkhammer-Schalke M. Treatment of B cell lymphoma with chemotherapy plus rituximab: a survival benefit can be demonstrated in the routine data of a regional cancer registry. Ann Hematol. 2012;91:561–70. doi: 10.1007/s00277-011-1361-6. [DOI] [PubMed] [Google Scholar]
- 5.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5:516–25. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 6.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–40. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 7.Mross K, Steinbild S. Metronomic anti-cancer therapy – an ongoing treatment option for advanced cancer patients. journal of Cancer Therapeutics and Research. 2012:1. [Google Scholar]
- 8.Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86. [PubMed] [Google Scholar]
- 9.Ménard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57:1579–87. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poppema S, Visser L. Absence of HLA class I expression by Reed-Sternberg cells. Am J Pathol. 1994;145:37–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Drénou B, Le Friec G, Bernard M, Pangault C, Grosset JM, Lamy T, Fauchet R, Amiot L. Major histocompatibility complex abnormalities in non-Hodgkin lymphomas. Br J Haematol. 2002;119:417–24. doi: 10.1046/j.1365-2141.2002.03814.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115–23. doi: 10.1038/sj.bjc.6605465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D’Urso MT, Belardelli F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71:768–78. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- 14.Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, Smyth MJ, Zitvogel L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–8. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 15.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–24. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoi-Toli O, Vermeer MH, De Vries E, Van Beek P, Meijer CJ, Willemze R. Expression of Fas and Fas-ligand in primary cutaneous T-cell lymphoma (CTCL): association between lack of Fas expression and aggressive types of CTCL. Br J Dermatol. 2000;143:313–9. doi: 10.1046/j.1365-2133.2000.03656.x. [DOI] [PubMed] [Google Scholar]
- 17.Pospisilova J, Vit O, Lorkova L, Klanova M, Zivny J, Klener P, Petrak J. Resistance to TRAIL in mantle cell lymphoma cells is associated with the decreased expression of purine metabolism enzymes. Int J Mol Med. 2013;31:1273–9. doi: 10.3892/ijmm.2013.1302. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama H, Ino T, Tokunaga E, Katsuda I, Ezaki K. A synergistic increase of apoptosis utilizing Fas antigen expression induced by low doses of anticancer drug. Rinsho Byori. 2003;51:733–9. [PubMed] [Google Scholar]
- 19.Wu XX, Jin XH, Zeng Y, El Hamed AM, Kakehi Y. Low concentrations of doxorubicin sensitizes human solid cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-receptor (R) 2-mediated apoptosis by inducing TRAIL-R2 expression. Cancer Sci. 2007;98:1969–76. doi: 10.1111/j.1349-7006.2007.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy pretreatment sensitizes solid tumor-derived cell lines to V alpha 24+ NKT cell-mediated cytotoxicity. Int J Cancer. 2006;119:1630–7. doi: 10.1002/ijc.22019. [DOI] [PubMed] [Google Scholar]
- 21.Kang J, Bu J, Hao Y, Chen F. Subtoxic concentration of doxorubicin enhances TRAIL-induced apoptosis in human prostate cancer cell line LNCaP. Prostate Cancer Prostatic Dis. 2005;8:274–9. doi: 10.1038/sj.pcan.4500798. [DOI] [PubMed] [Google Scholar]
- 22.van der Most RG, Currie AJ, Cleaver AL, Salmons J, Nowak AK, Mahendran S, Larma I, Prosser A, Robinson BWS, Smyth MJ, et al. Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth. PLoS One. 2009;4:e6982. doi: 10.1371/journal.pone.0006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–43. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 24.Konjević G, Jurisić V, Banićevic B, Spuzić I. The difference in NK-cell activity between ptients with non-Hodgkin’s lymphomas and Hodgkin’s disease. Br J Haematol. 1999;104:144–51. doi: 10.1046/j.1365-2141.1999.01129.x. [DOI] [PubMed] [Google Scholar]
- 25.Reiners KS, Kessler J, Sauer M, Rothe A, Hansen HP, Reusch U, Hucke C, Kohl U, Durkop H, Engert A, et al. Rescue of impaired NK cell activity in hodgkin lymphoma with bispecific antibodies in vitro and in patients. Molecular therapy. the journal of the American Society of Gene Therapy. 2013;21:895–903. doi: 10.1038/mt.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zocchi MR, Catellani S, Canevali P, Tavella S, Garuti A, Villaggio B, Zunino A, Gobbi M, Fraternali-Orcioni G, Kunkl A, et al. High ERp5/ADAM10 expression in lymph node microenvironment and impaired NKG2D ligands recognition in Hodgkin lymphomas. Blood. 2012;119:1479–89. doi: 10.1182/blood-2011-07-370841. [DOI] [PubMed] [Google Scholar]
- 27.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 28.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 29.Hervieu A, Rébé C, Végran F, Chalmin F, Bruchard M, Vabres P, Apetoh L, Ghiringhelli F, Mignot G. Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth. J Invest Dermatol. 2013;133:499–508. doi: 10.1038/jid.2012.273. [DOI] [PubMed] [Google Scholar]
- 30.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–11. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Zhang J, Sun R, Feng J, Wei H, Tian Z. Opposing effect of IFNgamma and IFNalpha on expression of NKG2 receptors: negative regulation of IFNgamma on NK cells. Int Immunopharmacol. 2005;5:1057–67. doi: 10.1016/j.intimp.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Zhang J, Niu J, Zhang J, Tian Z. Interleukin-15 improves cytotoxicity of natural killer cells via up-regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine. 2008;42:128–36. doi: 10.1016/j.cyto.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhang C, Zhang J, Niu J, Zhou Z, Zhang J, Tian Z. Interleukin-12 improves cytotoxicity of natural killer cells via upregulated expression of NKG2D. Hum Immunol. 2008;69:490–500. doi: 10.1016/j.humimm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Park YP, Choi SC, Kiesler P, Gil-Krzewska A, Borrego F, Weck J, Krzewski K, Coligan JE. Complex regulation of human NKG2D-DAP10 cell surface expression: opposing roles of the γc cytokines and TGF-β1. Blood. 2011;118:3019–27. doi: 10.1182/blood-2011-04-346825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–30. [PubMed] [Google Scholar]
- 36.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644–53. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 38.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 39.Ohkusu-Tsukada K, Toda M, Udono H, Kawakami Y, Takahashi K. Targeted inhibition of IL-10-secreting CD25- Treg via p38 MAPK suppression in cancer immunotherapy. Eur J Immunol. 2010;40:1011–21. doi: 10.1002/eji.200939513. [DOI] [PubMed] [Google Scholar]
- 40.Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–85. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–62. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 42.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111:5359–70. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 43.Dou AX, Feng LL, Liu XQ, Wang X. Cyclic adenosine monophosphate involvement in low-dose cyclophosphamide-reversed immune evasion in a mouse lymphoma model. Cell Mol Immunol. 2012;9:482–8. doi: 10.1038/cmi.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tongu M, Harashima N, Yamada T, Harada T, Harada M. Immunogenic chemotherapy with cyclophosphamide and doxorubicin against established murine carcinoma. Cancer Immunol Immunother. 2010;59:769–77. doi: 10.1007/s00262-009-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y, Liu N, Xiong SD, Zheng YJ, Chu YW. CD4+Foxp3+ regulatory T-cell impairment by paclitaxel is independent of toll-like receptor 4. Scand J Immunol. 2011;73:301–8. doi: 10.1111/j.1365-3083.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- 46.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 48.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–20. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–7. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89:311–7. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 51.Verma C, Eremin JM, Robins A, Bennett AJ, Cowley GP, El-Sheemy MA, Jibril JA, Eremin O. Abnormal T regulatory cells (Tregs: FOXP3+, CTLA-4+), myeloid-derived suppressor cells (MDSCs: monocytic, granulocytic) and polarised T helper cell profiles (Th1, Th2, Th17) in women with large and locally advanced breast cancers undergoing neoadjuvant chemotherapy (NAC) and surgery: failure of abolition of abnormal treg profile with treatment and correlation of treg levels with pathological response to NAC. J Transl Med. 2013;11:16. doi: 10.1186/1479-5876-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602–11. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 54.Schmid MC, Varner JA. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J Oncol. 2010;2010:201026. doi: 10.1155/2010/201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117:872–81. doi: 10.1182/blood-2010-05-283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parrinello N, La Cava P, Tibullo D, Giallongo C, Di Bartolo O, Spina P, Fiumara P, Schinocca L, Conticello C, Chiarenza A, et al. Myeloid-Derived Suppressor Cells in Patients with Hodgkin Lymphoma. ASH Annual Meeting Abstracts 2009; 114:3662-. [Google Scholar]
- 57.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–9. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 59.Tongu M, Harashima N, Monma H, Inao T, Yamada T, Kawauchi H, Harada M. Metronomic chemotherapy with low-dose cyclophosphamide plus gemcitabine can induce anti-tumor T cell immunity in vivo. Cancer Immunol Immunother. 2013;62:383–91. doi: 10.1007/s00262-012-1343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–41. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Ribatti D, Nico B, Ranieri G, Specchia G, Vacca A. The role of angiogenesis in human non-Hodgkin lymphomas. Neoplasia. 2013;15:231–8. doi: 10.1593/neo.121962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Passam FH, Alexandrakis MG, Moschandrea J, Sfiridaki A, Roussou PA, Siafakas NM. Angiogenic molecules in Hodgkin’s disease: results from sequential serum analysis. Int J Immunopathol Pharmacol. 2006;19:161–70. [PubMed] [Google Scholar]
- 63.Hamano Y, Sugimoto H, Soubasakos MA, Kieran M, Olsen BR, Lawler J, Sudhakar A, Kalluri R. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004;64:1570–4. doi: 10.1158/0008-5472.CAN-03-3126. [DOI] [PubMed] [Google Scholar]
- 64.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci U S A. 2003;100:12917–22. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–6. [PubMed] [Google Scholar]
- 66.Mainetti LE, Rico MJ, Fernández-Zenobi MV, Perroud HA, Roggero EA, Rozados VR, Scharovsky OG. Therapeutic efficacy of metronomic chemotherapy with cyclophosphamide and doxorubicin on murine mammary adenocarcinomas. Ann Oncol. 2013;24:2310–6. doi: 10.1093/annonc/mdt164. [DOI] [PubMed] [Google Scholar]
- 67.Shen FZ, Wang J, Liang J, Mu K, Hou JY, Wang YT. Low-dose metronomic chemotherapy with cisplatin: can it suppress angiogenesis in H22 hepatocarcinoma cells? Int J Exp Pathol. 2010;91:10–6. doi: 10.1111/j.1365-2613.2009.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rozados VR, Sánchez AM, Gervasoni SI, Berra HH, Matar P, Graciela Scharovsky O. Metronomic therapy with cyclophosphamide induces rat lymphoma and sarcoma regression, and is devoid of toxicity. Ann Oncol. 2004;15:1543–50. doi: 10.1093/annonc/mdh384. [DOI] [PubMed] [Google Scholar]
- 69.Shamash J, Walewski J, Apostolidis J, Wilson AM, Foran JM, Gupta RK, Rohatiner AZ, Kelsey SM, Lister TA. Low-dose continuous chemotherapy (LBCMVD-56) for refractory and relapsing lymphoma. Ann Oncol. 2000;11:857–60. doi: 10.1023/A:1008355417445. [DOI] [PubMed] [Google Scholar]
- 70.Coleman M, Ruan G, Elstrom RL, Martin P, Leonard JP. Metronomic therapy for refractory/relapsed lymphoma: the PEP-C low-dose oral combination chemotherapy regimen. Hematology. 2012;17(Suppl 1):S90–2. doi: 10.1179/102453312X13336169155970. [DOI] [PubMed] [Google Scholar]
- 71.Young SD, Whissell M, Noble JC, Cano PO, Lopez PG, Germond CJ. Phase II clinical trial results involving treatment with low-dose daily oral cyclophosphamide, weekly vinblastine, and rofecoxib in patients with advanced solid tumors. Clin Cancer Res. 2006;12:3092–8. doi: 10.1158/1078-0432.CCR-05-2255. [DOI] [PubMed] [Google Scholar]
- 72.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Nicola M, Zappasodi R, Carlo-Stella C, Mortarini R, Pupa SM, Magni M, Devizzi L, Matteucci P, Baldassari P, Ravagnani F, et al. Vaccination with autologous tumor-loaded dendritic cells induces clinical and immunologic responses in indolent B-cell lymphoma patients with relapsed and measurable disease: a pilot study. Blood. 2009;113:18–27. doi: 10.1182/blood-2008-06-165654. [DOI] [PubMed] [Google Scholar]
- 74.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–94. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song W, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of naive dendritic cells. Cancer Res. 2005;65:5958–64. doi: 10.1158/0008-5472.CAN-05-0406. [DOI] [PubMed] [Google Scholar]
- 76.Li J, Song W, Czerwinski DK, Varghese B, Uematsu S, Akira S, Krieg AM, Levy R. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol. 2007;179:2493–500. doi: 10.4049/jimmunol.179.4.2493. [DOI] [PubMed] [Google Scholar]
- 77.Mattarollo SR, West AC, Steegh K, Duret H, Paget C, Martin B, Matthews GM, Shortt J, Chesi M, Bergsagel PL, et al. NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma. Blood. 2012;120:3019–29. doi: 10.1182/blood-2012-04-426643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soriano JL, Batista N, Santiesteban E, Lima M, González J, García R, Zarza Y, López MV, Rodríguez M, Loys JL, et al. Metronomic Cyclophosphamide and Methotrexate Chemotherapy Combined with 1E10 Anti-Idiotype Vaccine in Metastatic Breast Cancer. Int J Breast Cancer. 2011;2011:710292. doi: 10.4061/2011/710292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kandalaft LE, Powell DJ, Jr., Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]