Abstract
In a recent high-throughput screen against specific Candida albicans drug targets, several compounds that exhibited non-specific antifungal activity were identified, including the non-steroidal anti-inflammatory drug flufenamic acid (FFA). This study sought to determine the effect of different doses of FFA, alone or in combination with fixed concentrations of the standard antifungal agents amphotericin B (AmB), caspofungin (CAS) or fluconazole (FLU), for the prevention and treatment of C. albicans biofilms. Biofilms were formed in a 96-well microplate followed by evaluation of antifungal activity using the XTT assay. FFA concentrations of ≥512 mg/L demonstrated >80% prevention of biofilm formation. FFA concentrations of 1024 mg/L demonstrated >85% reduction of mature biofilms. When FFA (≥8 mg/L) was used in combination with FLU (32 mg/L), antifungal activity increased to 99% for the prevention of biofilm formation. Similarly, when a FFA concentration of ≥8 mg/L was used in combination with either AmB (0.25 mg/L) or CAS (0.125 mg/L), antifungal activity also increased up to 99% for the prevention of biofilm formation. The inhibitory effect of FFA on C. albicans biofilms has not been reported previously, therefore these findings suggest that FFA in combination with traditional antifungals might be useful for the treatment and prevention of C. albicans biofilms.
Keywords: Candida albicans, Biofilm, Flufenamic acid, Antifungal
1. Introduction
The opportunistic fungal pathogen Candida albicans has the ability to form biofilms, a characteristic that enhances resistance to antifungal agents and permits colonisation of mucosal surfaces with the potential for subsequent dissemination [1]. Candida albicans also forms biofilms on catheters and medical devices, which may be impossible to cure unless the device is removed [1,2]. These challenges have driven the search for novel therapies for biofilm-related Candida infections. Antimicrobial lock therapy has been proposed as an alternative strategy for the prevention and treatment of microbial biofilms, particularly for catheter-related bloodstream infections [2,3]. Furthermore, there has been increasing attention to drugs belonging to different pharmacological classes for possible antimicrobial activity, including a number of non-steroidal anti-inflammatory drugs (NSAIDs), which have been discovered to possess potent antibacterial activities [4,5]. The antimicrobial activity of NSAIDs is thought to be due to inhibition of prostaglandin biosynthesis [5]; interestingly, prostaglandin biosynthesis contributes to fungal hyphal formation and biofilm development [5].
In a recent high-throughput screening study performed at the Centre for Molecular Discovery at the University of New Mexico (Albuquerque, NM) (unpublished data), the NSAID flufenamic acid (FFA) was identified as having antifungal activity against C. albicans. FFA is a NSAID that has been specifically used to treat rheumatoid arthritis [6], although its potential as an antifungal agent has not been previously defined. We therefore sought to determine the effect of increasing concentrations of FFA, alone or in combination with standard concentrations of the commonly used antifungals amphotericin B (AmB), caspofungin (CAS) and fluconazole (FLU), for the prevention and treatment of C. albicans biofilms.
2. Materials and methods
2.1. Strains and reagents
Four wild-type C. albicans strains were selected (ATCC 10231, ATCC 14053, ATCC 24433 and the widely used laboratory strain SC5314) as well as two clinically derived echinocandin-resistant strains (42379 and 53264) [7]. Strains were grown and maintained at 30 °C in YPD (1% yeast extract, 2% peptone, 2% glucose; BD Diagnostic Systems, Franklin Lakes, NJ). For biofilm formation and susceptibility studies, overnight cultures were re-suspended at a density of 1 × 106 cells/mL in RPMI 1640 supplemented with L-glutamine (US Biologicals, Swampscott, MA) and buffered to pH 7.0 with 165 mM morpholinepropanesulfonic acid (MOPS) (Sigma Chemical Co., St Lois, MO).
2.2. Biofilm formation and susceptibility assays
The antifungal activities of FFA (Sigma Chemical Co.) against pre-formed biofilms and for the prevention of biofilm formation were assessed using the XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] (Sigma-Aldrich, St Louis, MO) reduction assay in a standard 96-well static microplate model as previously described [8]. In brief, cells were incubated in RPMI 1640 medium in a microplate (Corning Inc., Corning, NY) to allow formation of mature biofilms (24 h at 37 °C). The wells were gently washed and biofilms were incubated for another 24 h at 37 °C with increasing concentrations of FFA (2, 4, 8, 16, 32, 64, 128, 256, 512 and 1024 mg/L in RPMI 1640 medium). The resulting metabolic activity was measured using the XTT reduction assay [8]. For studies on the prevention of biofilm formation, cells from an overnight culture were re-suspended at a final density of 1 × 106 cells/mL in RPMI 1640 medium containing increasing concentrations of FFA and were incubated for 24 h at 37 °C. Metabolic activity was measured using the XTT reduction assay. Experiments were performed three times independently (biological replicates), each in quadruplicate (technical replicates).
Next, the effects of FFA in combination with different antifungals on biofilms of the clinically derived C. albicans strain SC5314 were measured. Antifungal agents selected were FLU (32 mg/L) (Sigma Chemical Co.), AmB (0.25 mg/mL) (Sigma Chemical Co.) and CAS (0.125 mg/mL) (Merck, Whitehouse Station, NJ).
2.3. Light microscopy
Light micrographs of biofilms were acquired using an inverted microscope (Micromaster™ Inverted Digital Microscope with Infinity Optics; Fisher Scientific, Waltham, MA) and data acquisition software (Micron 2.0.0; Westover Scientific, Mill Creek, WA).
2.4. Statistical analyses
The metabolic activities of treatment groups and controls were compared using one-way analysis of variance (ANOVA) followed by the post-hoc or Tukey comparison post-test. Differences between groups were considered to be significant at a P-value of <0.05. Statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA).
2.5. Definitions
Enhanced activity was present if FFA in combination with FLU, AmB or CAS resulted in a statistically significant reduction in metabolic activity compared with when any of the respective antifungals were used alone (P < 0.05). The combination was considered to have an indifferent effect if the combination did not have a significant difference in reducing metabolic activity compared with the antifungals used alone. A paradoxical effect was present when there was greater metabolic activity of C. albicans biofilms (i.e. less killing) when a higher concentration of an agent was used compared with the effect of a lower concentration of the same agent [8–10].
3. Results
3.1. Effects of flufenamic acid on Candida albicans biofilms
FFA was tested for activity against pre-formed biofilms as well as against biofilm formation of six C. albicans strains. Sessile minimum inhibitory concentrations (sMICs) were also determined. For the prevention of biofilm formation, sMIC50 values (sMIC that inhibits 50% of biofilm growth) varied from 128 mg/L to 512 mg/L; for treatment of mature biofilms, sMICs values were higher than those observed for the prevention of biofilm formation, with sMIC50 values of >256 mg/L. Interestingly, the echinocandin-resistant strains 42379 and 53264 showed an sMIC50 of 1024 mg/L (the highest concentration of FFA tested) for treatment of mature biofilms.
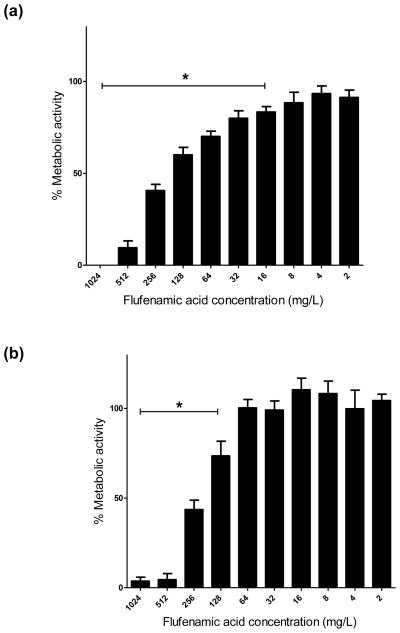
To study the antifungal activity of FFA in detail, C. albicans reference strain SC5314 was selected because it is widely used as a standard laboratory strain for pathogenesis and translational studies and it forms robust biofilms. For the prevention of biofilm formation, FFA concentrations of ≥16 mg/L caused a significant decrease (P < 0.05) in biofilm metabolic activity compared with the untreated control. A dramatic reduction (≥80%) in biofilm metabolic activity was achieved at concentrations of 512 mg/L and 1024 mg/L (Fig. 1a). For the treatment of mature biofilms, a significant reduction in metabolic activity was observed at concentrations of 128 mg/L, and FFA concentrations of 512 mg/L and 1024 mg/L markedly reduced biofilm metabolic activity (≥85%) compared with lower concentrations (Fig. 1b). Compared with the untreated control, no significant differences in metabolic activity were observed using lower concentrations of FFA (8, 4 and 2 mg/L).
Fig. 1.
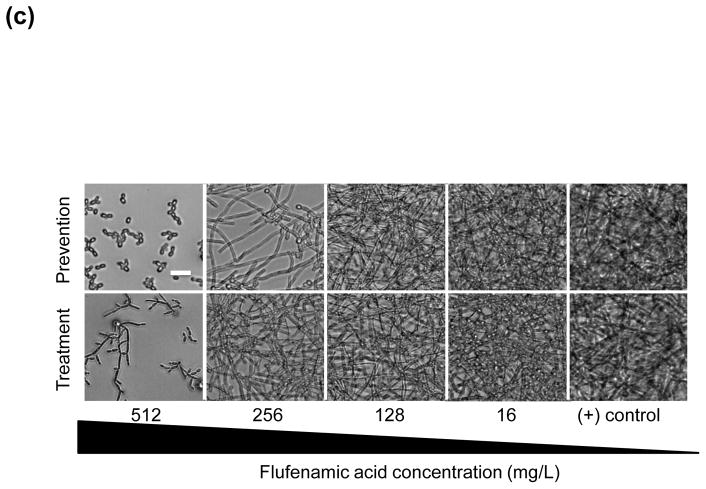
(a) In vitro effect of flufenamic acid (FFA) against formation (prevention) of Candida albicans biofilms. Planktonic cells were incubated in RPMI 1640 containing FFA at different concentrations (2–1024 mg/L) and biofilms were allowed to form for 24 h. Metabolic activity was then assessed using the XTT assay. (b) In vitro effect of FFA against mature C. albicans biofilms (treatment). Mature (pre-formed) biofilms were treated with FFA at different concentrations (2–1024 mg/L) and metabolic activity was assessed using the XTT assay. Experiments were performed three times independently (biological replicates), each time in quadruplicate (technical replicates). * Significant difference (P < 0.05) in reduction of biofilm metabolic activity with FFA compared with the growth control (cells containing only media and biofilms). (c) Representative light microscopy images of C. albicans strain SC5314 biofilms. FFA was added at different concentrations for prevention of biofilm formation or for the treatment of mature biofilms. Visual differences in biofilm architecture are apparent at four different FFA concentrations (16, 128, 256 and 512 mg/L). Images were taken using a 40× power field. Scale bar = 10 μm.
The inhibitory effect of FFA on biofilms was next examined using light microscopy. A clear visual difference in biofilm density was observed at concentrations of 16, 128, 256 and 512 mg/L for the prevention and treatment of biofilms (Fig. 1c). Interestingly, when biofilms were treated with high concentrations (512 mg/L) of FFA, many of the biofilm cells appeared to be predominantly pseudohyphal (after treatment of mature biofilms) or yeast form (for prevention of biofilm formation).
3.2. Effects of flufenamic acid in combination with antifungals
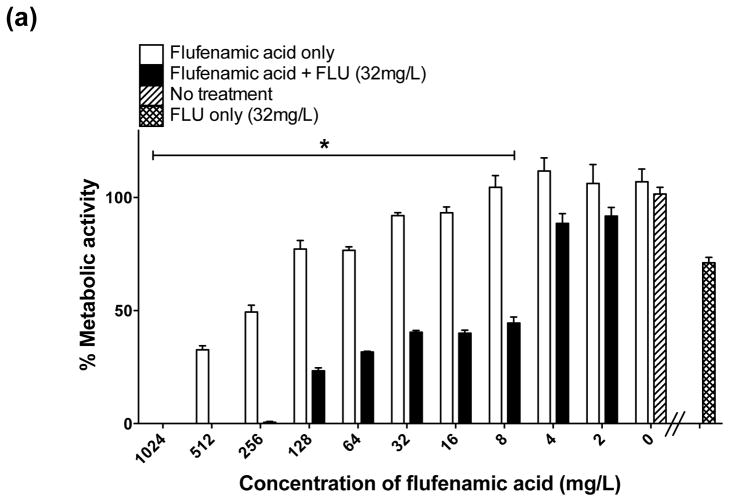
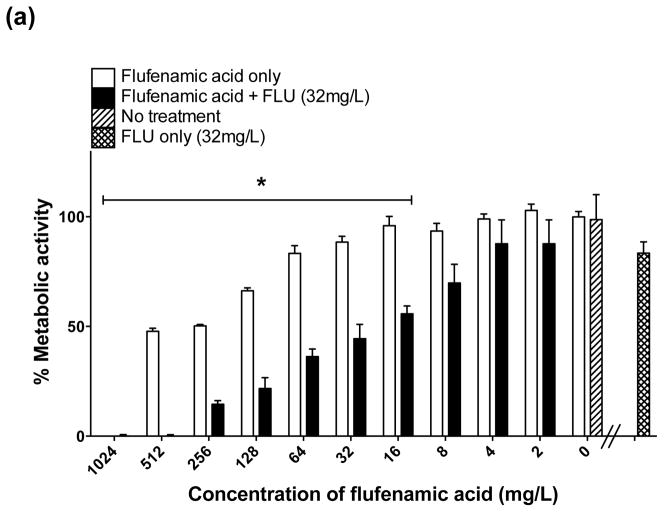
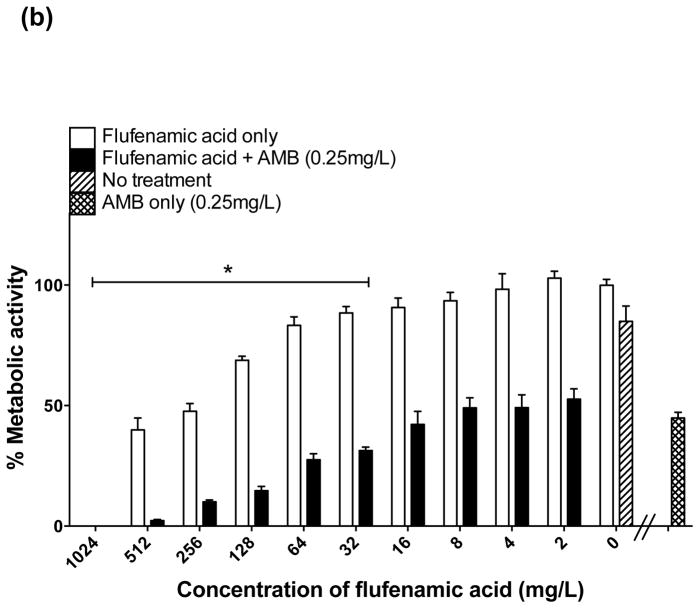
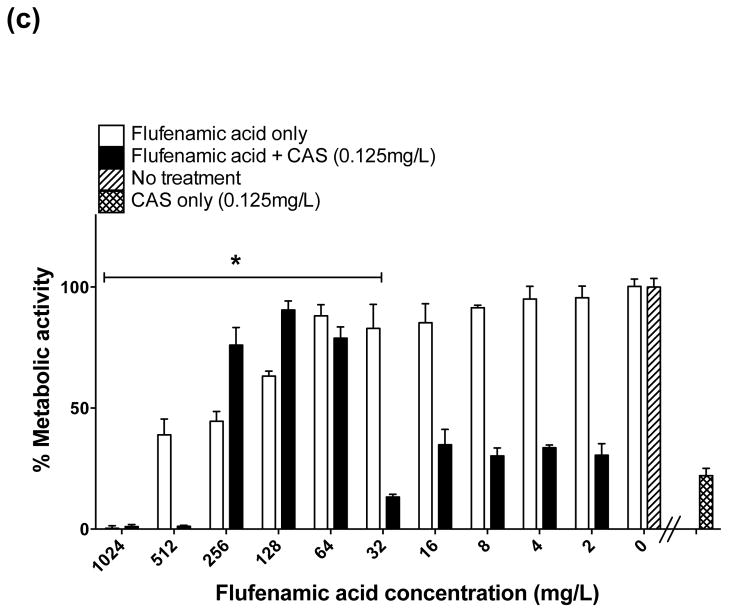
There was substantial inhibition of biofilm formation (50–99% decrease in biofilm metabolic activity) when FLU (32 mg/L) was used alone against formation of biofilms (prevention) (Fig. 2a); however, as expected, FLU alone showed minimal activity against mature biofilms (Fig. 3a). In distinct contrast, the combination of FFA with FLU significantly enhanced the activity of either agent against biofilm formation and mature biofilms. Statistically significant inhibition of biofilm formation began when FLU was combined with ≥8 mg/L of FFA (Fig. 2a). For treatment of mature biofilms, a statistically significant reduction was observed when FLU was combined with FFA at concentrations of ≥16 mg/mL (Fig. 3a). Thus, the combination of FLU with FFA had superior antifungal activity compared with FFA or FLU alone, meeting the criteria for enhanced activity.
Fig. 2.
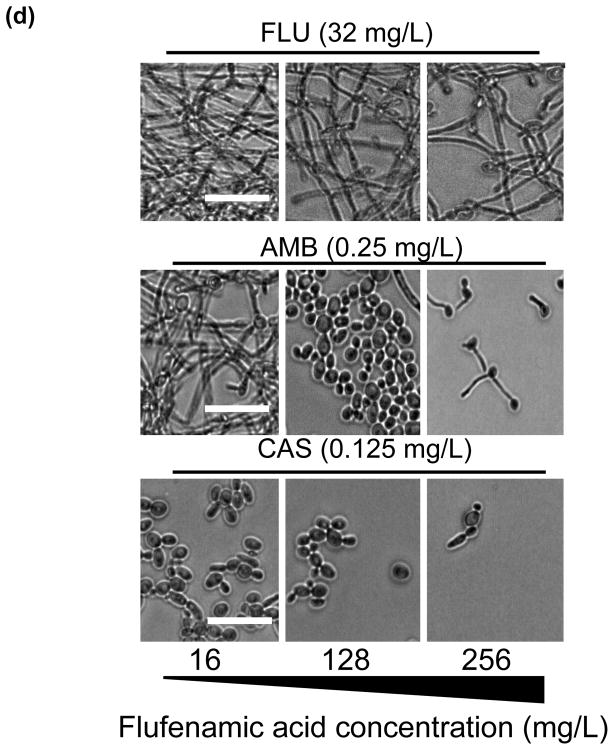
In vitro effect of different concentrations of flufenamic acid (FFA) (2–1024 mg/L) combined with a constant concentration of (a) fluconazole (FLU) at 32 mg/L, (b) amphotericin B (AmB) at 0.25 mg/L and (c) caspofungin (CAS) at 0.125 mg/L for prevention of biofilm formation. The metabolic activity of drug-free biofilms (no treatment) is shown as a positive control. Graphs represent the average of two experiments performed independently, each time in triplicate. * P < 0.05. (d) Representative light microscopy images of Candida albicans strain SC5314 biofilms. FFA was added at different concentrations combined with constant concentrations of FLU (32 mg/L), AmB (0.25 mg/L) or CAS (0.125 mg/L) for prevention of biofilm formation. Visual differences in biofilm architecture are apparent at three different FFA concentrations (16, 128 and 256 mg/L). Images were taken using a 40× power field. Scale bar = 10 μm.
Fig. 3.
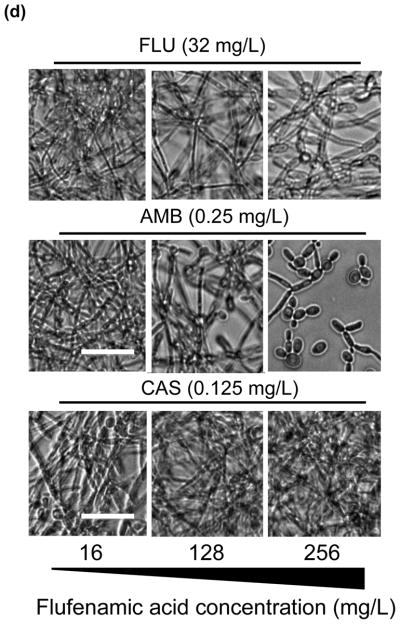
In vitro effect of different concentrations of flufenamic acid (FFA) (2–1024 mg/L) combined with a constant concentration of (a) fluconazole (FLU) at 32 mg/L, (b) amphotericin B (AmB) at 0.25 mg/L and (c) caspofungin (CAS) at 0.125 mg/L for the treatment of mature biofilms. The metabolic activity of drug-free biofilms (no treatment) is shown as a positive control. Graphs represent the average of two experiments performed independently, each time in triplicate. * P < 0.05. (d) Representative light microscopy images of Candida albicans strain SC5314 biofilms. FFA was added at different concentrations combined with constant concentrations of FLU (32 mg/L), AmB (0.25 mg/L) or CAS (0.125 mg/L) for treatment of mature biofilms. Visual differences in biofilm architecture are apparent at three different FFA concentrations (16, 128 and 256
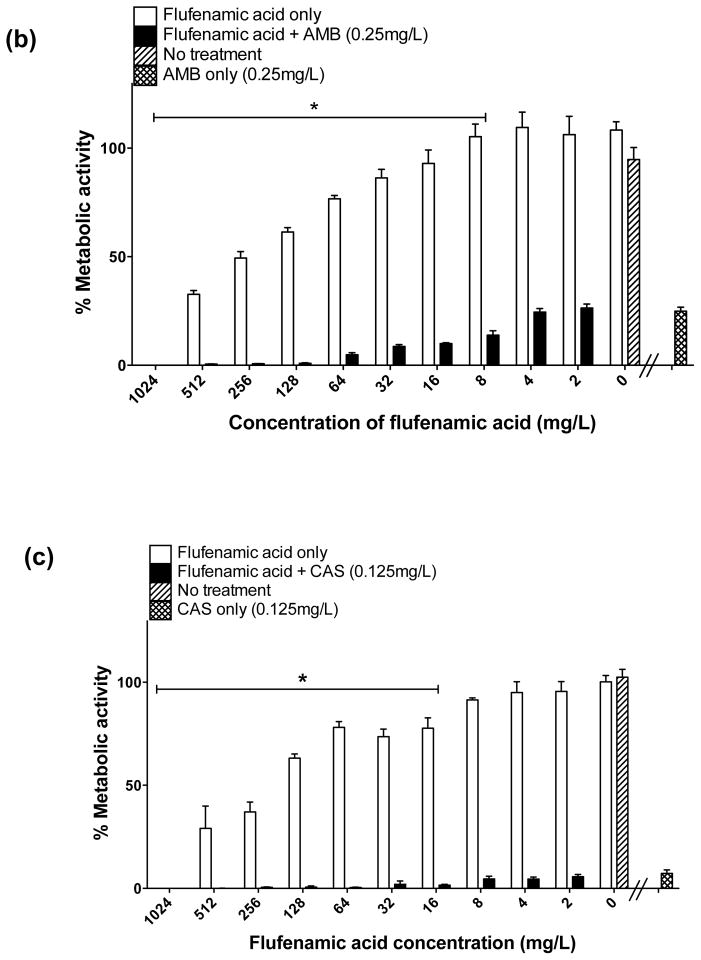
When AmB was used in combination with serially increasing concentrations of FFA, a progressive reduction of biofilm formation (i.e. prevention) was achieved at concentrations of 8–1024 mg/L of FFA (Fig. 2b). For treatment of mature biofilms, AmB combined with FFA at concentrations of 32 mg/L and 64 mg/L resulted in a 75% decrease, and a maximum effect (up to 99% reduction) was observed at FFA concentrations of 128–1024 mg/L (Fig. 3b), meeting the criteria of enhanced activity. For treatment of biofilms, the combination of lower concentrations of FFA (16, 8, 4 and 2 mg/L) with AmB did not result in statistically significant differences in antifungal activity compared with the effect of AmB alone, meeting the criteria of an indifferent effect (Fig. 3b).
When CAS was used alone at a concentration of 0.125 mg/L for prevention and treatment of biofilms, as expected a high inhibitory effect was observed (95% reduction in prevention and 80% reduction in treatment). When used in combination with FFA for prevention of biofilm formation, a maximum inhibitory effect (99.5% reduction in biofilm metabolic activity) was achieved at concentrations of FFA of ≥16 mg/L (Fig. 2c). For treatment of mature biofilms, enhanced activity was observed when CAS (0.125 mg/L) was combined with FFA at concentrations of 32, 512 and 1024 mg/L; surprisingly, a paradoxical effect was observed when biofilms were treated with concentrations of FFA of 64, 128 and 256 mg/L combined with low-dose CAS (Fig. 3c), suggesting that the in vitro paradoxical activity may not simply be a dose-dependent phenomenon related to CAS.
The inhibitory effect of the traditional antifungals FLU, AmB and CAS in combination with FFA was also examined using light microscopy. Proliferation of cells within the biofilm was substantially reduced for prevention of biofilm formation, and the multiple layers of a typical biofilm were not apparent (Fig. 2d). For treatment of mature biofilms, only AmB and CAS caused a visible reduction in biofilm mass (Fig. 3d); and a gross increase in the visual biofilm density was observed, consistent with the paradoxical effect noted by XTT assay for the combination of FFA with CAS.
4. Discussion
In this study, FFA was tested at serially increasing concentrations alone or in combination with standard antifungal agents against C. albicans biofilms. The results demonstrated that high doses of FFA exhibited excellent activity for the prevention and treatment of biofilms from six C. albicans isolates, including two echinocandin-resistant strains. In previous studies, it has been observed that the NSAIDs ibuprofen and naproxen cause an alteration in the fluidity and permeability of mitochondrial cell membranes [9]; a similar mechanism might be present when FFA is used for the prevention and treatment of C. albicans biofilms, however further studies of the effects of FFA on fungal cell biology are needed to support this hypothesis.
The activity of combinations of FFA with commonly used antifungals varied according to the specific antifungal type and the concentration of FFA used. When used in combination with FLU (both for prevention and treatment of biofilms), a dose-dependent enhanced effect was observed compared with the effect of either agent alone. Notably, for the treatment of mature biofilms, a substantial reduction in biofilm metabolic activity was observed when FLU (32 mg/L) was combined with low concentrations of FFA (≥8 mg/L). Previous findings have indicated little or no effect for treatment of mature biofilms when FLU is used in combination with other antifungals, including AmB and CAS [10], whereas synergistic effects (similar to the enhanced effect reported in this study) have been observed when FLU is combined with calcium channel inhibitors [11] and doxycycline [12], among others.
Prevention of biofilm formation with AmB alone caused a 75% decrease in biofilm metabolic activity, and the combination with low concentrations of FFA (≥8 mg/L) caused a maximum inhibitory effect (99% reduction in metabolic activity); similarly, treatment of biofilms with AmB alone caused a 50% decrease in biofilm metabolic activity and the combination with FFA (≥32 mg/mL) caused a 75–90% reduction in biofilm metabolic activity. Previous studies have suggested that AmB may facilitate the entry of tetracycline derivatives into the fungal cell [13]; however, the molecular mechanisms associated with AmB combined with NSAIDs against C. albicans planktonic cells or biofilms remain undefined.
An enhanced effect of the combination of FFA with CAS was observed for prevention of biofilm formation; surprisingly, for treatment of mature biofilms a paradoxical effect (defined as a reduced fungicidal effect at high concentrations) [14] was observed when increased concentrations of FFA (64–256 mg/L) were combined with low-dose CAS (0.125 mg/L). Previous studies have linked the paradoxical effect with cell wall stress responses that permit growth in the presence of high concentration of CAS, such as upregulation of chitin biosynthesis, high-osmolarity glycerol responses and calcium/calcineurin signalling pathways [14]. The surprising observation that low-dose CAS resulted in a paradoxical effect in combination with FFA may provide additional insights towards further elucidating the molecular pathway(s) responsible for the paradoxical effect.
The concentration of FFA in plasma varies from 6 mg/L to 20 mg/L and the terminal elimination is long, remaining in plasma for ca. 13.3 h and up to 22 h [15]. According to these pharmacokinetic parameters, FFA might be an effective adjunctive antifungal agent when combined with FLU, AmB and CAS if administered at standard doses (normal dosage varies from 200 mg to 600 mg per day) [6]. Although systemic administration of FFA alone would not achieve levels that would be effective against Candida biofilms, high-dose FFA could also serve as a potential antifungal lock strategy for both prevention and treatment of catheter-related Candida biofilm infections. These findings demonstrate that FFA may be useful in combination with AmB, FLU or CAS as an adjunctive systemic agent, or in high doses as an antifungal lock therapy.
Acknowledgments
Funding: SAL was supported by funding from the Department of Veterans Affairs (Merit Award) and the Biomedical Research Institute of New Mexico; AACD was supported by the National Institute of General Medical Sciences (NIGMS) through the Institutional Research and Career Development Award and the Academic Science Education and Research Training Program (IRACDA-ASERT) [grant no. K12GM088021].
Footnotes
Competing interests: None declared.
Ethical approval: Not required.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramage G, Saville SP, Thomas DP, López-Ribot JL. Candida biofilms: an update. Eukaryot Cell. 2005;4:633–8. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mermel LA, Farr BM, Sherertz RJ, Raad II, O’Grady N, Harris JS, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249–72. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 3.Walraven CJ, Lee SA. Antifungal lock therapy. Antimicrob Agents Chemother. 2013;57:1–8. doi: 10.1128/AAC.01351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alem MAS, Douglas LJ. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob Agents Chemother. 2004;48:41–7. doi: 10.1128/AAC.48.1.41-47.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alem MAS, Douglas LJ. Prostaglandin production during growth of Candida albicans biofilms. J Med Microbiol. 2005;54:1001–5. doi: 10.1099/jmm.0.46172-0. [DOI] [PubMed] [Google Scholar]
- 6.Rajan KT, Hill AGS, Barr A, Whitwell E. Flufenamic acid in rheumatoid arthritis. Ann Rheum Dis. 1967;26:43–6. doi: 10.1136/ard.26.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micelli MH, Bernardo SM, Lee SA. In vitro analyses of the combination of high-dose doxycycline and antifungal agents against Candida albicans biofilms. Int J Antimicrob Agents. 2009;34:326–32. doi: 10.1016/j.ijantimicag.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Ramage G, López-Ribot JL. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol Med. 2005;118:71–9. doi: 10.1385/1-59259-943-5:071. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenberger LM, Zhou Y, Jayaraman V, Doyen JR, O’Neil RG, Dial EJ, et al. Insight into NSAID-induced membrane alterations, pathogenesis and therapeutics: characterization of interaction of NSAIDs with phosphatidylcholine. Biochim Biophys Acta. 2012;1821:994–1002. doi: 10.1016/j.bbalip.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmann SP, Ramage G, VandeWalle K, Patterson TF, Wickes BL, López-Ribot JL. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrob Agents Chemother. 2003;47:3657–9. doi: 10.1128/AAC.47.11.3657-3659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uppuluri P, Nett J, Heitman J, Andes D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother. 2008;52:1127–32. doi: 10.1128/AAC.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur R, Castano I, Cormack BP. Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: roles of calcium signaling and mitochondria. Antimicrob Agents Chemother. 2004;48:1600–13. doi: 10.1128/AAC.48.5.1600-1613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lew MA, Beckett KM, Levin MJ. Antifungal activity of four tetracycline analogues against Candida albicans in vitro: potentiation by amphotericin B. J Infect Dis. 1977;136:263–70. doi: 10.1093/infdis/136.2.263. [DOI] [PubMed] [Google Scholar]
- 14.Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for β-1,6-glucan synthesis inhibition by caspofungin. Antimicrob Agents Chemother. 2006;50:3160–1. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentjes EG, van Ginneken CA. Pharmacokinetics of flufenamic acid in man. Int J Clin Pharmacol Ther Toxicol. 1987;25:185–7. [PubMed] [Google Scholar]