Abstract
In order to better understand how concepts might be represented in the brain, we used a cross-modal conceptual priming paradigm to examine how repetition-related activity changes in the brain are related to conceptual priming. During scanning, subjects made natural/manmade judgments on a continuous stream of spoken nouns, written nouns and pictures of objects. Each stimulus either repeated in the same or a different modality with 1-4 intervening trials between repetitions. Behaviorally, participants showed significant perceptual and conceptual priming effects. The fMRI data showed that the conditions associated with the greatest behavioral priming exhibited the largest decreases in BOLD activity in left perirhinal cortex (PRc), as well as a few other regions. Furthermore, the PRc was the only region to show this relationship for the cross-modal conditions alone, where the concept but not the percept repeated. Conversely, repetition-related increases in PRc activity predicted better subsequent memory as assessed by a post-scan recognition test. These results suggest that repetition-related activity changes in the PRc are related both to the speed of access to a repeated concept and to that concept’s later memorability.
1.0 INTRODUCTION
The human brain has a remarkable ability to support internal representations of concepts irrespective of the modality of the perceptual sensory input. For example, the presentation of the word “guitar” can generate the same internal representation whether it was presented visually or aurally. Furthermore, conceptual representations are themselves multi-modal in the sense that we know not only what a guitar looks like, but also what it sounds like and what it feels like to hold one. While we can simply intuit the existence of conceptual representations, behavioral evidence for the existence of such representations comes from conceptual priming. Specifically, exposure to a concept can facilitate the subsequent processing of that same conceptual information, even when the repeated concept is presented in a different modality than the cue (Bassili, Smith, & MacLeod, 1989; Graf, Shimamura, & Squire, 1985; McClelland & Pring, 1991). Thus, the facilitation in response times observed during conceptual priming is not dependent on the repetition of the perceptual aspects of the presented stimuli, but rather on the conceptual similarity between the two presentations. While conceptual priming has long been appreciated, the nature of the neural representation of conceptual information remains elusive.
There is emerging evidence that the perirhinal cortex (PRc), a brain region in the anterior portion of the medial temporal lobes, may contribute to the representation of conceptual information. Recent electrophysiological recordings in rodents demonstrate that the PRc has unique physiological properties that allow for the association of spatially disparate signals from multiple sensory cortices (Unal, Apergis-Schoute, & Pare, 2012). Furthermore, it is known that PRc receives auditory, visual and somatosensory unimodal inputs (Jones & Powell, 1970; Suzuki & Amaral, 1994), as well as polymodal cortical afferent projections from orbitofrontal cortex, dorsal superior temporal sulcus, cingulate cortex and posterior parahippocampal cortex (Suzuki & Amaral, 1994). Thus, existing anatomical and physiological data suggests that PRc can support the convergence and integration of featural representations that may contribute, at least in part, to a coherent conceptual representation.
To date, a few fMRI studies have reported results consistent with a role of the PRc in representing conceptual information. Taylor et al. (2006) found that PRc activation was sensitive to the congruency of two presented stimuli. Specifically, PRc activation was greater when a presented picture and sound were drawn from two different concepts (i.e. were incongruent) than when they were congruent (e.g. hearing a bark while seeing a picture of a dog). One interpretation of this finding is that in the incongruent condition, two concepts may be represented as compared to the congruent condition, where only one may be represented. Thus, PRc BOLD activity may track the number of concepts being considered. Similar results were reported by Haskins et al. (2008), where greater PRc activation was observed when a novel concept was being processed/learned. On a related note, a recent study by Wang et al. (2010) found increased PRc activation during the encoding of words that were later produced during an exemplar generation task. Finally, O’Kane et al. (2005) asked whether regions in the medial temporal lobes were sensitive to perceptual and conceptual repetitions. They found that repetition suppression in the left PRc was greater when the same task was performed with repeated words, relative to a new task. This suggests that the PRc representations were sensitive to the features of the items being accessed on each trial. These studies demonstrate that BOLD activation in PRc shows some relationship to conceptual processing, but is also sensitive to the specific features of a concept that are being accessed.
To expand on this literature, one complementary approach may be to leverage behavioral priming effects to identify brain regions that show sensitivity to conceptual repetition that is related to their behavioral facilitation. Voss and Paller (2009) recently utilized this approach in a perceptual priming paradigm. They demonstrated that repetition suppression in PRc during visual word presentation is correlated with the magnitude of behavioral priming across subjects. This study was the first to establish a relationship between measures of behavioral priming and repetition suppression in PRc. However, to our knowledge, this approach has not been applied to conceptual priming. Here, we adopted a similar approach to Voss and Paller and used a paradigm that allowed us to measure both perceptual and conceptual priming across different conditions. Sixteen subjects were presented with stimuli that repeated in either the same or a different modality within a span of 1-4 intervening trials. Each initial exposure could be a picture of an object, a written noun, or a spoken noun. Each repeated exposure could be any of these three modalities. To foreshadow the results, while other brain regions were sensitive to conceptual repetitions, the PRc emerged as the one region showing the most robust and consistent relationship with behavioral measures of priming. In addition, repetition-related enhancements in PRc activation also related to subsequent memory. Thus, we believe these data strengthen the evidence in favor of the PRc as being part of a network supporting conceptual representations.
2.0 METHODS
2.1 Subjects
Sixteen (9 female) healthy, native English-speaking volunteers from New York University (NYU) participated in the study. All subjects gave informed written consent for the experiment in accordance with protocols approved by the University Committee on Activities Involving Human Subjects at NYU.
2.2 Stimuli
We presented 285 items (234 objects, 51 scenes) twice each. Scenes were included to help in localizing object and scene specific regions of cortex. While scenes were always presented as full color photographs (IMSI MasterClips© and MasterPhotosTM Premium Image Collection, 1895 Francisco Blvd., East, San Rafael, CA 94901-5506, USA), objects could be presented in any of three modalities: spoken nouns, written nouns, and pictures of objects. Pictures were full color photographs collected from two online photography databases (www.photoobject.net, www.cepolina.com). In a separate phase, a different cohort of participants was presented the photographs and instructed to label each with the first word that came to mind. Only photographs that received the same label across 80% of participants were included in the present study. Furthermore, these labels were in turn the basis of the spoken and written noun stimuli. Spoken nouns were presented using .wav files collected from the LDC American English Spoken Lexicon (http://www.ldc.upenn.edu/cgi-bin/aesl/aesl). Object stimuli were grouped into 9 sets of 26 items each. Each set was presented in one of the 9 repetition conditions, and the exact mapping was counterbalanced across subjects.
2.3 Task
While scanning, participants were instructed to indicate, by making a button press as quickly as possible, whether the presented item was natural or manmade (Figure 1). Items were presented in a continuous stream with a jittered inter-trial interval. Pictures and written nouns were presented for a fixed duration of .25 seconds while spoken nouns varied from .5-1 seconds. Initial trial presentations could be pictures of objects, written nouns and spoken nouns. Item repetitions could be in any of these three modalities. Thus, 1/3 of the time, the trial would repeat identically and 2/3 of the time the concept would switch modalities. Repetitions were separated by 1- 4 (mean 2.5) intervening trials. During jittered inter-trial intervals (Dale, 1999), participants were instructed to fixate on a blue fixation cross which flashed green at the onset of each trial. There were a total of 3 within-modal conditions (SS – spoken nouns preceded by spoken nouns, PP – pictures preceded by pictures, WW – written nouns preceded by written nouns) and 6 cross-modal conditions (PS - spoken nouns preceded by pictures, WS – spoken nouns preceded by written nouns, SP – pictures preceded by spoken nouns, WP – pictures preceded by written nouns, PW – written nouns preceded by pictures, SW – written nouns preceded by spoken nouns). Throughout the paper, first presentations of items are referred to by a single letter (P, S or W) while repeated presentations are referred to by two letters where the first letter indicates the modality of the first presentation and the second letter indicates the modality of the repeated item presentation (e.g. PP or PW). After scanning, we administered a test assessing subsequent memory for all concepts. Subjects were presented with 234 old stimuli (26 trials from each condition) and 120 new stimuli, and asked to indicate their memory confidence on a scale from 1 (highly confident the item was studied) to 6 (highly confident the item was new). This test was self-paced. Importantly, all stimuli were presented as written words.
Figure 1.
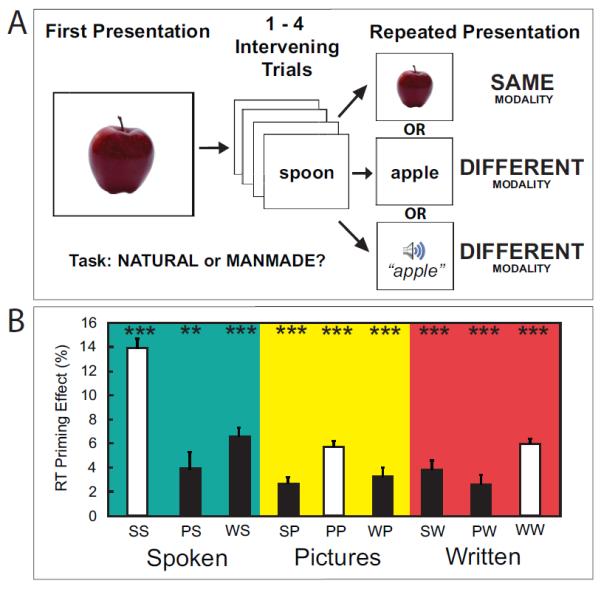
A) Schematic of the Paradigm. Participants made natural/manmade judgments on a continuous stream of items in one of three modalities. Each concept/item was presented twice, but offset by lag of 1-4 intervening items. Repeated items could be in the same modality (within-modal) or a different modality (cross-modal). B) Response Time Priming. A significant effect of repeated item presentation was observed for all within-modal and cross-modal conditions [all pairwise p<.05]. The y-axis represents the average percent decrease in response time from the first to second presentation of an item. The x-axis represents condition type where white bars are within-modal conditions and black bars are cross-modal conditions (***p<.005, **p<.01, *p<.05; condition labels: SS – spoken-spoken, PS – picture-spoken, WS – written-spoken, PP – picture-picture, WP – written-picture, SP – spoken-picture, WW – written-written, PW – picture-written, SW – spoken-written).
2.4 Imaging Parameters
Imaging data were collected with a 3 Tesla Siemens Allegra scanner. We acquired whole-brain functional data using an echoplanar imaging sequence across three scans each containing 297 volumes (TR = 2000 ms, TE = 30 ms, flip angle = 85, 35 slices, 3 × 3 × 3 mm voxels, 20% distance factor) using coronal slices angled perpendicular to the long axis of the hippocampus. Because of this slice orientation, data from the most posterior parts of visual cortex were not collected. The first four volumes, collected for stabilization purposes, were discarded. A high-resolution, T1-weighted, full brain, anatomical scan (magnetization-prepared rapid-acquisition gradient echo; MPRAGE) was collected for visualization and registration.
2.5 Imaging Preprocessing and Registration
Preprocessing and analysis of fMRI data was conducted using FSL (version 4.1.9, http://www.fmrib.ox.ac.uk/fsl/) as well as custom Matlab (version 2010A, http://www.mathworks.com/products/matlab/) scripts. First, the data were motion corrected using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002). Then, we performed slice-timing correction using fourier-space time-series phase-shifting. The data were spatially smoothed using a gaussian kernel of 8.0mm. To remove slow drift components of the BOLD signal, we low-pass filtered the data using a gaussian-weighted least-squares straight line fitting approach (with sigma=50.0s). The data were “pre-whitened” using FILM in order to assure statistical validity and efficiency (Woolrich, Ripley, Brady, & Smith, 2001). Finally, registration was carried out in two steps. First, all functional data were registered using a normal linear search with 6 degrees of freedom to an intermediate inplane image derived from a field map magnitude image collected at the beginning of the experiment. Then, the data were registered to a subject-specific MPRAGE structural image using a normal linear search with 6 degrees of freedom. Finally, the data were registered to a standard space template from the Montreal Neurological Institute (MNI) using a normal linear search and 12 degrees of freedom. All registration procedures were carried out using FLIRT (Jenkinson, et al., 2002; Jenkinson & Smith, 2001).
2.6 General Linear Model (GLM)
Based on behavioral performance data, trials were sorted into correct and incorrect trials for each of the 9 conditions. Only trials for which correct responses were made on both presentations of the experiment were included in subsequent analyses. To create regressors for each condition, we convolved a series of impulse functions representing trial onsets with a double gamma hemodynamic response function. Incorrect trials were modeled as covariates of no interest, with a separate regressor for each condition. We included a temporal derivative for each covariate of interest to account for temporal variability in the onset of the BOLD signal. We also included motion spikes as covariates of no interest, using a separate regressor for each spike. As with the fMRI data, the entire model was filtered using a 50 second low-pass filter. We created a separate GLM for each run for each subject. Then, we performed a second-level fixed effects analysis across runs within a subject. Finally, group-level effects were obtained by performing a mixed effects analysis across subjects using FLAME (Beckmann, Jenkinson, & Smith, 2003).
3.0 RESULTS
3.1 Behavior
3.1.1 Task Accuracy
Across all conditions and trial presentations, overall mean accuracy during encoding was high [92.61%, Standard Deviation (SD) =.04]. Accuracy on the second presentation of an item [94.45%, SD=.03] was significantly higher than first the first [90.79%, SD=.04; Paired t-test, p<.001]. Accuracy also varied as a function of modality [One-way ANOVA, F(2,45)=21.44, p<.0001], where first presentation written noun (W) and picture (P) trials were significantly more accurate than first presentation spoken noun (S) trials [both p<.01], but not significantly different from each other [p>.10; all raw accuracy and response time data in Table 1].
Table 1.
Raw Encoding Accuracy, Response Times and Subsequent Memory. On the top, raw encoding task accuracy is listed for each condition. Conditions with one letter are first presentations. Conditions with two letters are repeated trial presentations. In the middle, response times are listed for each condition. On the bottom, high confidence subsequent memory hit rates are listed by condition (SD = standard deviation; condition labels: S – first spoken noun, P – first picture, W – first word, SS – spoken-spoken, PS – picture-spoken, WS – written-spoken, PP – picture-picture, WP – written-picture, SP – spoken-picture, WW – written-written, PW – picture-written, SW – spoken-written).
| Encoding Task Accuracy (Proportion Correct) | ||||
| S | SS | PS | WS | |
| Mean | 0.8438 | 0.9038 | 0.9111 | 0.887 |
| SD | 0.0963 | 0.0807 | 0.0608 | 0.1037 |
| P | PP | SP | WP | |
| Mean | 0.9399 | 0.9712 | 0.9591 | 0.9663 |
| SD | 0.054 | 0.0329 | 0.0454 | 0.0418 |
| W | WW | SW | PW | |
| Mean | 0.9127 | 0.9375 | 0.9591 | 0.9736 |
| SD | 0.0571 | 0.0441 | 0.0328 | 0.046 |
| S | SS | PS | WS | |
| Encoding Response Times (Milliseconds) | ||||
| Mean | 1243.74 | 1058.01 | 1191.84 | 1156.97 |
| SD | 91.81 | 108.27 | 121.43 | 102.83 |
| P | PP | SP | WP | |
| Mean | 777.79 | 700.92 | 741.61 | 734.74 |
| SD | 112.83 | 105.87 | 108.76 | 124.69 |
| W | WW | SW | PW | |
| Mean | 787.64 | 708.47 | 736.32 | 752.38 |
| SD | 1221.35 | 113.49 | 98.07 | 101.79 |
| Subsequent Memory (High Confidence Hits) | ||||
| S | SS | PS | WS | |
| Mean | - | 0.6057 | 0.8317 | .07329 |
| SD | - | 0.1907 | 0.1655 | 0.1881 |
| P | PP | SP | WP | |
| Mean | - | 0.8125 | 0.8101 | 0.8269 |
| SD | - | 0.2000 | 0.1449 | 0.1544 |
| W | WW | SW | PW | |
| Mean | - | 0.6850 | 0.6851 | 0.7541 |
| SD | - | 0.2208 | 0.2117 | 0.1890 |
3.1.2 Response Times and Priming
Overall, participants were significantly slower to respond to S trials, compared to P and W trials [One-way ANOVA, F(2,45)=517.37, p<.0001]. This was expected given that sounds trials take longer to present. P and W trials were not different from each other [Paired t-test, p>.10]. Response times (RTs) to second presentation of sounds were also longer than pictures and written words [One-way ANOVA, F(2,45)=337.69, p<.0001]. RTs for second presentation written noun (WW trials) and picture trials (PP trials) were not different from each other [Paired t-test, p>.10]. Importantly, perceptual and conceptual priming was evident in all conditions. Specifically, for all within- and cross-modal conditions, participants were significantly faster in making the study decision for the second item presentation relative to the first [All pairwise p<.05, see Figure 1]. To calculate cross-modal priming, we first calculated the mean RT for all first presentations of items in a given modality. Then, we subtracted the mean RT for second presentations of items that were presented in the same modality. For example, second presentation picture trials that were preceded by spoken nouns (SP condition) were subtracted from first presentations pictures (P). This was important since there were baseline differences in RTs between modalities. As expected, on average, priming was significantly greater for within-modal trials, which include both perceptual and conceptual repetitions, compared to cross-modal trials where the concept repeats but the perceptual representation is novel [Paired t-test, p<.0001].
3.1.3 Subsequent Memory
Overall memory was relatively high (collapsed across conditions: d’ mean: 2.31 and standard error of the mean: .06). Average high confidence hit rates for each condition are listed in Table 1. We tested whether high confidence hit rates as well as overall hit rates (sum of proportion of high, medium and low confidence hits) varied as a function of condition. There was significant variability in subsequent memory across conditions for both overall hit rates [One-way ANOVA, F(8,135)=5.88, p<.001] as well as high confidence hit rates alone [One-way ANOVA, F(8,135)=5.38,p<.001].
3.2 FMRI Analyses
We took two main approaches to identify brain regions whose activity may be sensitive to conceptual repetitions. The first approach was to ask whether there are regions that show significant repetition suppression in all of our conditions (Dehaene et al., 2001; Horner & Henson, 2008). In order to do this, we used a conjunction analysis to identify regions of interest (ROIs) that showed repetition suppression effects in the three within-modal conditions, and then queried those ROIs for cross-modal suppression.
Our second approach utilized the behavioral priming measures to ask which brain regions displayed a sensitivity to repetitions that correlated with the amount of priming evident on those conditions. In order to do this, repetition-related decreases in brain activity were correlated with behavioral priming across conditions. We also applied this approach to ask whether repetition-related decreases (and increases) correlated with subsequent memory across conditions. Those data are described below.
3.2.1 Within-Modality Repetition Suppression Effects
To identify regions showing suppression effects for the three within-modal conditions, we first identified regions showing significant suppression effects (a contrast of first presentation items > repeated presentation) in each condition separately (Figure 2 and Table 2). For pictures (P>PP), we saw reduced BOLD activation on PP trials in left PRc, bilateral fusiform gyrus, left premotor cortex and bilateral dorsal cingulate cortex (p<.001, voxel cluster k>10; see Table 2 for complete list of all within-modal repetition suppression effects for all contrasts). The written noun repetition suppression contrast (W>WW) revealed suppression in left lateral inferotemporal cortex and bilateral cerebellum (p<.001, k>10). Finally, the spoken noun repetition suppression contrast (S>SS) failed to reveal significant voxels with a threshold of p<.001. However, using a more liberal threshold of p<.005 repetition suppression effects were evident in left fusiform gyrus, lateral inferotemporal cortex, superior temporal gyrus, thalamus, lingual gyrus, posterior inferior frontal gyrus and right inferior frontal gyrus.
Figure 2.
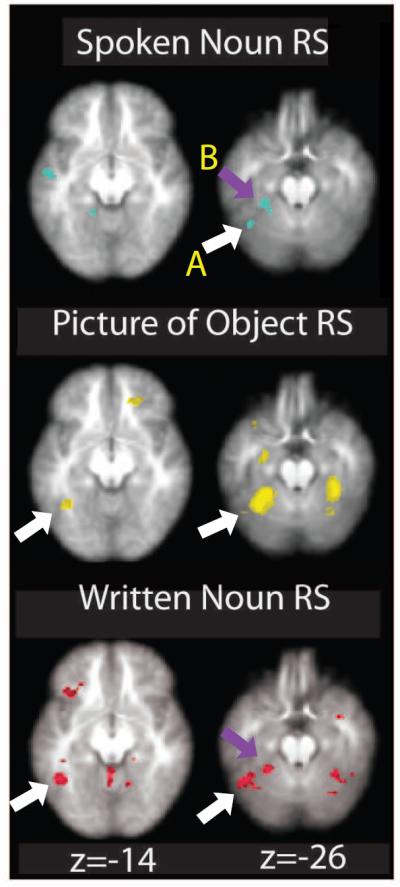
Within-modal Repetition Suppression Effects. Contrast of initial>repeated item exposures for each modality separately (p<.005, uncorrected for display purposes). Arrows indicate overlapping clusters that overlap between modalities. Each contrast elicited repetition suppression (RS) effects in modality-specific cortical regions as well as overlapping activity in the left inferior temporal cortex (A; white arrows) and left fusiform gyrus (B; purple arrows). Numbers at the bottom indicate slice number in the Z plane.
Table 2.
List of Regions from Repetition Suppression Analyses. This table lists all regions from the repetition suppression analyses. Coordinates are in MNI space. All analyses are thresholded at p<.001, k>10 except for the within-modal spoken noun repetition suppression (RS) contrast where no voxels reached significance at p<.001. This analysis was thresholded at p<.005, k>10.
|
Within-Modality Repetition
Suppression | |||||
| Analysis and Brain Region | # of Voxels |
Peak t-
statistic |
Peak MNI Coordinates | ||
| x | y | z | |||
| Spoken Words | |||||
| L Inferior Frontal Gyrus, pars triangularis |
30 | 3.93 | −44 | 30 | 14 |
| L Thalamus | 27 | 3.75 | −6 | −16 | 4 |
| R Insula | 23 | 3.93 | 34 | 26 | 0 |
| L Lingual Gyrus | 17 | 3.63 | −16 | −46 | −8 |
| L Fusiform Gyrus | 13 | 3.58 | −30 | −36 | −26 |
| Pictures | |||||
| L Fusiform Gyrus | 279 | 6.44 | −30 | −48 | −26 |
| R Fusiform Gyrus | 81 | 4.84 | 34 | −38 | −26 |
| L Cingulate Gyrus | 63 | 4.44 | −2 | 18 | 50 |
| L Middle Frontal Gyrus | 35 | 4.49 | −46 | 10 | 32 |
| L Perirhinal Cortex | 18 | 4.39 | −28 | −10 | −28 |
| Written Words | |||||
| L Inferior Temporal Cortex | 67 | 4.99 | −44 | −54 | −20 |
| R Cerebellum, VIIIb | 34 | 4.64 | 24 | −46 | −58 |
| R Cerebellum, VIIIa | 33 | 4.86 | 14 | −70 | −56 |
| L Superior Frontal Gyrus | 18 | 4.35 | −12 | 14 | 72 |
| Within-Modality Repetition Suppression Conjunction Analysis | |||||
| L Fusiform Gyrus | 79 | - | −26 | −46 | −28 |
| L Inferior Temporal Gyrus | 51 | - | −46 | −60 | −30 |
3.2.2 Within-modal Repetition Suppression Conjunction Analysis
To identify brain regions that displayed repetition suppression effects in all three modalities, we performed a conjunction analysis using the three within-modal contrasts described above. Specifically, we created group-level binary masks for P>PP, W>WW, and S>SS contrasts and then used these to identify voxels that overlapped in each within-modal contrast. Using a threshold of p<.05 for each contrast (joint probability of p<.000125), a conjunction between these three contrasts revealed activation clusters in left fusiform gyrus and in a region spanning the left inferior temporal cortex and the left anterior cerebellum, near the anterior portion of a region described as the lateral occipital complex (Figure 3; Kanwisher, Chun, McDermott & Ledden, 2001; Spiridon, Fischl & Kanwisher, 2006; Malach et al., 1995). While the center of this region falls in the inferior temporal cortex, the region also extends slightly into the left cerebellum. However, given the vast literature suggesting of a role for the inferior temporal lobe in representing visual objects (for review, see Dicarlo, Zoccolan & Rust, 2012) and the fact that we observed within- and cross-modal repetition suppression in this region for pictures of objects and written words, we think it is more likely that this activity is coming from the inferior temporal cortex. Thus, we will refer to it as such for the remainder of the manuscript.
Figure 3.
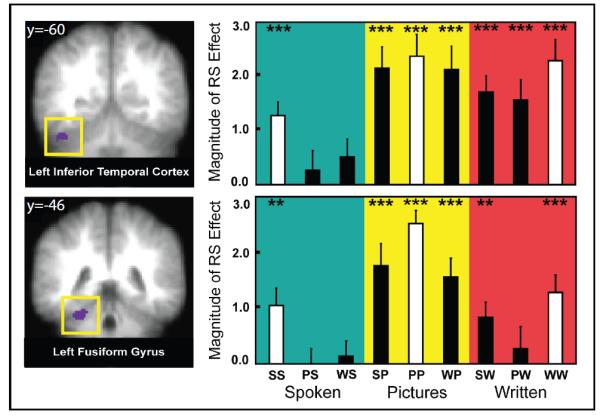
Within-modal Repetition Suppression Conjunction Analysis. Left: Regions of interest identified from a conjunction analysis showing overlapping repetition suppression effects across the three within-modal conditions. Left lateral inferior temporal cortex (A; on top left) and left fusiform gyrus (B; bottom left) showed repetition suppression (RS) effects to all three within-modal trial types (written nouns, spoken nouns and pictures; conjunction analysis joint probability of p<.000125). Coordinates listed at the top of the images represent MNI coordinates in the Y plane. Right: Bar graph of repetition suppression effects in left inferior temporal cortex (top right) and left fusiform gyrus (bottom right) for each condition. The y-axis represents repetition suppression (RS) and the x-axis represents different experimental conditions. RS was calculated as the difference in z-transformed signal change between first and repeated item presentations where a more positive effect indicates greater repetition suppression Within-modal repetition suppression (white bars) was robustly significant for all conditions (by definition), while cross-modal repetition suppression (black bars) varied as a function of condition, but was significant for most trial types (***p<.005, **p<.01, *p<.05; condition labels: SS – spoken-spoken, PS – picture-spoken, WS – written-spoken, PP – picture-picture, WP – written-picture, SP – spoken-picture, WW – written-written, PW – picture-written, SW – spoken-written).
We then wanted to assess whether the regions that show repetition suppression in the three within-modal conditions also showed repetition suppression when only concepts repeated. To that end, we examined BOLD activation in these regions of interest for the cross-modal conditions. While these regions did indeed show suppression effects in some cross-modal condition, it was not consistently the case. Specifically, the left lateral inferior temporal ROI showed significant cross-modal repetition suppression in all (all p’s < .05, see figure 2) conditions except two: S>PS (p=.47) and S>WS trials (p=.13). The left fusiform gyrus ROI showed significant cross-modal repetition suppression effects in three cross-modal conditions: SP, WP and SW conditions. However, suppression effects were not seen for S>PS (p=.97), S>WS (p=.66) and W>PW (p=.57) conditions. Thus, taken together, these results indicate that while left inferotemporal cortex and left fusiform gyrus show repetition suppression effects for all the within-modal conditions, they only show significant suppression for some cross-modal conditions.
3.2.3 Cross-modal Repetition Suppression Conjunction Analyses
To more directly identify whether a single cluster (or multiple clusters) would show evidence of cross-modal repetition suppression effects in all conditions, we conducted a conjunction analysis across all cross-modal contrasts (S>WS, S>PS, P>SP, P>WP, W>SW, W>PW). No regions emerged this analysis, even when using a liberal threshold of p<.05 for each contrast.
3.2.4 Condition-level Priming Correlation Analyses
Next, we aimed to utilize the variance in behavioral priming across conditions as a proxy for the extent to which a concept was accessed upon repeated exposure. We did this in order to help identify brain regions whose activation was sensitive to conceptual repetition. To that end, we performed a whole-brain condition-level correlation analysis between repetition-related decreases in BOLD activation and behavioral priming across conditions (Figure 4 and Table 3). First, we computed group averaged contrast maps for each condition (note: subjects were not treated separately in this analysis) separately for first and repeated items (vs. baseline). Then, we computed a difference map between first and repeated items for each condition. Finally, we calculated a whole-brain correlation between the group-averaged difference maps and group-averaged behavioral priming scores across the nine conditions. This analysis revealed a set of brain regions where increases in the behavioral priming effect linearly predicted repetition-related BOLD decreases across all conditions.
Figure 4.

Condition-level Priming Correlation Analysis. Regions showing a significant correlation between repetition suppression and behavioral priming across conditions are displayed on the right (displayed at p<.005, k>10). Brain areas that show this correlation were (A) left inferior frontal gyrus (IFG), (B) left perirhinal cortex (PRc), (C) left lingual gyrus, (D) left fusiform gyrus and (E) right cerebellum. To the left of the coronal brain slices are two plots (IFG and PRc) of the correlation between repetition suppression and behavioral priming across condition for illustrative purposes. Repetition suppression was calculated as the difference in z-transformed parameter estimates for the first and repeated presentations of an item, where a more positive effect indicates greater repetition suppression. Behavioral priming was calculated as the percent decrease in reaction time from first to second item presentation, where a greater value indicates a larger priming effect. Coordinates listed at the top of the images represent MNI coordinates in the Y plane (condition labels: SS – spoken-spoken, PS – picture-spoken, WS – written-spoken, PP – picture-picture, WP – written-picture, SP – spoken-picture, WW – written-written, PW – picture-written, SW – spoken-written).
Table 3.
List of Regions from Correlation Analyses. This table lists correlation values as well as MNI coordinates for each region defined by the condition-level correlation analyses (p<.001, k=10).
|
Condition-level Repetition Suppression - Priming
Correlation Analysis | |||||
| Analysis and Brain Region |
# of
Voxels |
Peak
Correlation |
Peak MNI Coordinates | ||
| L Inferior Frontal Gyrus | 35 | 0.949 | −40 | 10 | −18 |
| L Fusiform Gyrus | 35 | 0.939 | −34 | −34 | −22 |
| L Lingual Gyrus | 21 | 0.932 | −20 | −48 | −8 |
| L Perirhinal Cortex | 15 | 0.946 | −32 | −6 | −48 |
| R Cerebellum | 13 | 0.933 | 40 | −60 | −38 |
|
Condition-level Repetition Suppression -
Subsequent Memory Correlation Analysis | |||||
| R Dorsolateral Prefrontal Cortex |
54 | 0.966 | 42 | 36 | 34 |
| R Frontopolar Cortex | 40 | 0.97 | 44 | 46 | 0 |
| L Frontopolar Cortex | 37 | 0.96 | −36 | 56 | 0 |
|
Condition-level Repetition Enhancement-
Subsequent Memory Correlation Analysis | |||||
| L Orbital Frontal Sulcus | 26 | 0.972 | −34 | 34 | −16 |
| R Cerebellum | 24 | 0.965 | 46 | −64 | −36 |
| L Perirhinal Cortex | 16 | 0.932 | −36 | −4 | −44 |
|
Condition-level Overall BOLD - Subsequent Memory
Correlation Analysis | |||||
| L Cerebellum | 187 | 0.967 | −36 | −42 | −30 |
| L Perirhinal Cortex | 58 | 0.981 | −32 | −8 | −40 |
| R Cerebellum | 39 | 0.975 | 46 | −64 | −34 |
| R Hippocampus | 32 | 0.973 | 24 | −8 | −28 |
Regions that emerged from this analysis were the left PRc, left fusiform gyrus, left lingual gyrus, left posterior inferior frontal gyrus (a region near the insula), and right cerebellum (all r’s>.89; all p’s <.001, k>10). For visualization purposes, we plot the brain-behavior correlation in left PRc and left inferior frontal gyrus in Figure 4. Perhaps not surprisingly, in all regions, the within-modal conditions were associated with the greatest behavioral priming and BOLD repetition suppression effects compared to the cross-modal conditions. Importantly, however, visual inspection of the correlations suggests that the significant brain-behavioral correlations are not solely driven by the within-modal conditions. For example, all cross-modal data points lie very close to the repetition suppression-priming regression line, particularly in the PRc ROI (Figure 4). To assure that these effects were not driven by overall response time variability between the stimulus modalities, we also correlated overall response times (as opposed to priming) and BOLD activity to repeated concepts across conditions. None of the regions reported above emerged in this analysis. Therefore, the repetition-related changes in BOLD activity we report here are related to variability in RT priming rather than gross RT differences between stimulus modalities.
It is noteworthy that the within-modal conditions all displayed repetition suppression effects, but the cross-modal conditions displayed both repetition suppression and enhancement (that is, the second presentation of an item was associated with greater activation than the first presentation). In the left PRc and left lingual gyrus, all cross-modal conditions displayed numerical repetition enhancement effects, while cross-modal conditions in the left fusiform gyrus, the left inferior frontal gyrus and right cerebellum displayed either repetition suppression, repetition enhancement or no repetition-related difference. Critically, however, in those regions that showed enhancements for the cross-modal repetitions, greater priming is still related to less activation. In other words, greater priming was associated with a reduction in the repetition enhancement effect. Thus, in these regions, lower BOLD activation during concept repetition correlated with greater behavioral priming.
Next, and most importantly, we tested which of these regions would show a brain-behavior correlation for the cross-modal conditions alone. Since the within-modal conditions showed more priming and more suppression, we wanted to eliminate the possibility that any significant correlations were being carried by the within-modal conditions anchoring the data. Thus, to verify that a significant correlation in the prior analyses is seen for the cross-modal conditions, we removed the three within-modal conditions from the condition-level across-condition correlation analysis and recomputed the correlation values for each ROI, now only including the six remaining cross-modal conditions. Remarkably, the only region that still displayed a significant correlation for the cross-modal conditions alone (p<.001, all other p’s>.05 except the right cerebellum which was p=.045) was the PRc.
3.2.5 Condition-level Subsequent Memory Correlation Analyses
Our final set of analyses assessed whether the repetition-related activity changes we observed in the previous analysis might relate to subsequent memory. To answer this question, we first examined whether repetition-related activity decreases correlated with high confidence subsequent memory across all nine conditions. This whole-brain correlation revealed three frontal regions (bilateral frontopolar cortex and right dorsolateral prefrontal cortex; see Table 3 for coordinates) where repetition-related decreases predicted better subsequent memory. These regions did not overlap with any from the previous priming analyses. Next, we examined if repetition-related activity increases correlated with subsequent memory across conditions. This analysis revealed a set of three brain regions, including the left PRc (as well as the right cerebellum and left orbital frontal sulcus; p<.001, k=10; see Table 3). Thus, while repetition-related reductions in activity were associated with greater priming, enhanced repetition-related activity in the PRc was associated with better subsequent memory.
One explanation for this finding could be that BOLD activation on the second presentation itself (rather than the change in activation from first to second presentation) may predict later memory, which would be consistent with the finding that PRc BOLD activation during item presentations predicts later memory (for review, see Davachi, 2006). Thus, our final analysis focused on identifying brain regions whose magnitude of activity during the repeated presentation of a concept predicted subsequent memory. To that end, we correlated group averaged high confidence “remember” rates with group averaged BOLD activity during repeated concepts, across all nine conditions. Interestingly, four regions (left PRc, right hippocampus and bilateral cerebellum) displayed a significant relationship, where greater BOLD activation was associated with better memory performance (p<.001, k=10; Table 3).
Notably, the PRc region from this analysis was highly overlapping with the region found in the correlation between repetition-related activity increases and subsequent memory, as well as the region found in the correlation between repetition-related activity decreases and behavioral priming (Figure 5). Thus, taken together, we see that 1) PRc decreases in repetition-related activity predicted facilitated behavioral priming, 2) PRc increases in repetition-related activity predicted better subsequent memory and finally 3) the magnitude of PRc activity during the encoding of a repeated concept also predicted better subsequent memory.
Figure 5.
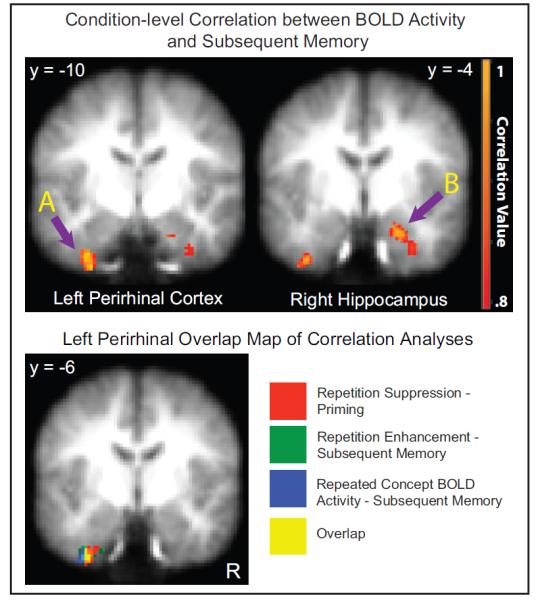
Subsequent Memory Correlation Analysis and Perirhinal Overlap Map. The top two images display the condition-level correlation between BOLD activity and subsequent memory in the left PRc (A) and right hippocampus (B). The Y coordinates at the top of the images are in MNI space. For illustrative purposes, the correlation maps are each thresholded at p<.005, k=10. On the bottom, an overlap map of the three condition-level correlation analyses is displayed. Each color represents a different analysis, and the overlap between them is displayed in yellow. These clusters of activity are displayed at p<.005.
4.0 DISCUSSION
These results reveal a network of brain regions that display repetition-related BOLD activation changes associated with conceptual priming and subsequent memory. Significant behavioral measures of priming were obtained in all nine experimental conditions: three within-modal and six cross-modal repetition conditions (Figure 1). These data are consistent with findings from previous cross-modal priming studies (Bassili et al., 1989; Graf et al., 1985; McClelland & Pring, 1991) demonstrating faster processing upon the repeated exposure of conceptual information. While we identified two regions (left fusiform gyrus and left lateral inferotemporal cortex) that displayed repetition suppression effects for all three within-modal conditions (Figure 3), activity in these regions did not consistently display cross-modal repetition suppression. Thus, in order to gain leverage on the brain regions supporting the representation of conceptual information in all conditions, we examined whether brain activation changes with repetition were related to across-condition variability in the behavioral priming measures. Using this approach, we found that repetition-related activity changes in a number of brain regions, including PRc, were highly correlated with behavioral priming (Figure 4). Importantly, when considering the cross-modal conditions alone, the left PRc was the only region to display repetition-related activation still significantly correlated with conceptual priming.
Next, we investigated the relationship between BOLD activity and subsequent memory. Across conditions, we found that increased repetition-related activity in the left PRc, left orbital frontal sulcus and right cerebellum predicted better subsequent recognition memory. Given the prior literature establishing a strong relationship between PRc activity during encoding and subsequent memory (Davachi et al., 2003; Ranganath, Yonelinas, Cohen, Sy, Tom & D’Esposito, 2004; for review Davachi, 2006), we hypothesized that this correlation might not be solely driven by repetition-related enhancements per se, but rather could be attributed to the overall BOLD magnitude during the second presentation of repeated concepts. Consistent with this idea, we found that greater activity in the left PRc (as well as the right hippocampus and bilateral cerebellum) during the repeated presentation of a concept predicted better subsequent memory performance (Figure 5, top). Remarkably, the voxels within the left PRc that emerged from all three correlation analyses described above were highly overlapping (Figure 5, bottom). Taken together, these data suggest that PRc activity predicts both conceptual priming benefits as well as enhanced encoding of those concepts.
It is worth mentioning that the priming effects reported here may be partially driven by decision priming. Previous work has suggested that varying the task decision on a repeated stimulus can diminish behavioral as well as neural priming effects (Dobbins, Schnyer, Verfaellie, & Schacter, 2004). However, if the priming effects reported here were solely driven by decision priming, one might expect equivalent amounts of priming for each condition. Thus, the variability across conditions likely reflects ease and speed of access to the meaning of each item in the service of producing a decision. Nonetheless, the contribution of decision priming to the response time priming effects observed in our study cannot be ruled out entirely; thus, the extent to which these are ‘pure’ estimates of conceptual access cannot be addressed.
4.1 The role of the PRc in conceptual priming and long-term memory
Previous fMRI and lesion data have implicated the PRc using different paradigms all focused on the processing of concepts. Specifically, using implicit conceptual priming (Wang, Lazzara, Ranganath, Knight, & Yonelinas, 2010), task-related conceptual priming (O'Kane, Insler, & Wagner, 2005), and cross-modal object feature integration (Taylor, Moss, Stamatakis, & Tyler, 2006), the PRc has been shown to play a broad role in representing conceptual information. Consistent with this notion, the PRc receives input from both unimodal sensory cortices and polymodal association cortices (Jones & Powell, 1970; Suzuki & Amaral, 1994). Given that multimodal cortical regions project to the PRc and that properties of the PRc allow for the integration of multiple cortical signals (Unal et al., 2012), the PRc appears to be one region of the brain that is anatomically and functionally well situated to play a role in the integration of multimodal information that might support conceptual processing.
While the precise role of the PRc in conceptual representation is still under debate, an influential model of visual object representation suggests that PRc resides at the apex of a system that binds conjunctions of complex visual features (Bussey, Saksida, & Murray, 2005; Murray & Bussey, 1999; Saksida & Bussey, 2010). In this framework, posterior regions in the ventral visual stream represent simple object features while more anterior regions represent conjunctions between multiple stimulus features. The current data, as well as evidence suggesting that the PRc is sensitive to the integration of conceptually-related cross-modal information (Taylor et al., 2006), are consistent with this integration account while extending it to include auditory representations. Specifically, in the current study, we found that repetition-related activity in the PRc was related to the magnitude of priming evident across those conditions. Importantly, this effect was seen when considering the cross-modal conditions alone.
It is noteworthy that while all within-modal repetitions were associated with a mean suppression of the BOLD signal compared to the initial presentation, cross-modal repetitions elicited, on average, repetition-related activity enhancements. Importantly, however, even though the second presentation of a concept led to greater overall BOLD activation compared to the first presentation in the cross-modal conditions, a smaller enhancement was correlated with more priming. In other words, smaller repetition enhancements in PRc activity predicted faster response times. In the current study, the repeated ‘stimulus’ in cross-modal conditions was always perceptually novel. We speculate that the PRc response reflects a combination, or integration, of perceptual and conceptual information. Specifically, it is likely that the novel perceptual features of a repeated concept would lead to an enhanced PRc response while, at the same time, the repeated concept may be associated with a reduced PRc response. Thus, the two signals, which may be carried in different inputs, may become integrated in PRc, where overall BOLD activation reflects a complex interplay between perceptual and semantic (or conceptual) features of a stimulus. Further work will be necessary to test these hypotheses more directly.
Interestingly, while we found that repetition-related reductions in PRc activity during a repeated concept were related to greater priming, enhanced PRc activity (as well as right hippocampal activity) during concept repetition predicted successful subsequent memory. Prior work has demonstrated a mnemonic cost for items associated with greater behavioral and neural priming (Wagner, Maril & Schacter, 2000) in a perceptual priming paradigm. The current results are consistent with those findings and extend them to the cross-modal domain. Functional imaging in humans has consistently provided a direct link between BOLD activity in the PRc during encoding and subsequent item recognition or item-feature memory, while activity in the hippocampus has been related to the successful binding of an item to it’s spatiotemporal context (Davachi, 2006; Davachi, Mitchell & Wagner, 2003; Diana, Yonelinas & Ranganath, 2007; Staresina & Davachi, 2006; Staresina & Davachi, 2010; Haskins, Yonelinas, Quamme & Ranganath, 2008). In the data we report here, enhancements in left PRc activity during concept repetition predict better memory performance across conditions. We also see that when considering overall activity on the concept repetition itself (irrespective of activity on the first presentation), greater activity in the left PRc and right hippocampus predicts later memorability. One potential interpretation of these findings is that the repetition of a concept when the percept changes may be associated with the integration of novel item features and that this feature integration process can support later long-term memory retrieval. Future work should be designed test this interpretation directly.
4.2 Frontal retrieval of conceptual information
Consistent with previous research, we found that left inferior frontal activity was related to conceptual priming (Buckner & Koutstaal, 1998; Demb, Desmond, Wagner, Vaidya, Glover & Gabrieli, 1995; Dobbins et al., 2004; Orfanidou, Marslen-Wilson, & Davis, 2006; Raichle et al., 1994; Wagner, Koutstaal, Maril, Schacter, & Buckner, 2000). Repetition-related activity changes in the left posterior inferior frontal gyrus were correlated with behavioral priming across conditions. The left inferior frontal gyrus has been previously associated with the selection or retrieval of semantic information (Badre & Wagner, 2002; Danker, Gunn, & Anderson, 2008; Thompson-Schill et al., 1998; Wagner, Pare-Blagoev, Clark, & Poldrack, 2001). Specifically, anterior portions of the lateral inferior frontal gyrus are hypothesized to be involved in the retrieval of semantic information whereas more posterior and lateral inferior frontal gyrus areas are thought to be involved in the general selection of task relevant information (Badre, Poldrack, Pare-Blagoev, Insler, & Wagner, 2005). Here we found that activity in a posterior and lateral region of the inferior frontal gyrus was correlated with behavioral priming, but this relationship appeared to be primarily driven by the within-modal conditions. This could suggest that left posterior inferior frontal gyrus retrieval mechanisms are closely linked with the perceptual aspects of the to-be-retrieved stimulus rather than the underlying semantic or conceptual features. It is possible that a more anterior region may be important in conceptual priming but we did not see evidence for that in the current results. Interestingly, we observed increased repetition-related activity in the left orbital frontal sulcus [-36, 34, -14] that was predictive of subsequent memory. This finding is consistent with data from Wagner et al. (2000), who showed that greater repetition suppression across subjects in the anterior lateral inferior prefrontal cortex (as well as in this frontal region) is related to poorer subsequent memory. However, we also observed repetition-related decreases in three other frontal regions (~BA area 10 bilaterally and right dorsolateral prefrontal cortex) that were correlated to better later memory (see Table 3). Thus, future work must address how these repetition-related changes in the frontal cortex activity support or hinder subsequent memory.
4.4 Conclusions
Our findings link repetition-related activity changes in the PRc to cross-modal conceptual priming. This is consistent with existing data demonstrating (1) that PRc is a site of multimodal sensory input convergence, (2) has physiological properties that support associative binding of multimodal information and (3) PRc involvement in conceptual priming, cross-modal integration and long-term subsequent memory. However, it remains unknown how PRc mechanisms might support conceptual knowledge. One possibility is that PRc serves as a working memory space that allows for integration of stored inputs from distant cortical regions and that the integration happens ‘on-line’. However, another possibility is that over time, PRc neurons come to represent the higher-level amodal conceptual information through learning. Much future work will be needed to understand the precise mechanisms supported by PRc. Nonetheless, the current work suggests that whatever the nature of the PRc representation, it’s output and processing are intimately related to the speed of access to and the memorability of conceptual information.
Acknowledgments
This research was supported by NIMH RO1 –MH074692 to Lila Davachi.
References
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Semantic retrieval, mnemonic control, and prefrontal cortex. Behavioral and Cognitive Neuroscience Reviews. 2002;1(3):206–218. doi: 10.1177/1534582302001003002. [DOI] [PubMed] [Google Scholar]
- Bassili JN, Smith MC, MacLeod CM. Auditory and visual word-stem completion: separating data-driven and conceptually driven processing. The Quarterly Journal of Experimental Psychology. 1989;41A(3):439–453. [Google Scholar]
- Beckmann C, Jenkinson M, Smith SM. General multi-level linear modelling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. The Quarterly Journal of Experimental Psychology. 2005;58(3-4):269–282. doi: 10.1080/02724990544000004. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8(2-3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker JF, Gunn P, Anderson JR. A rational account of memory predicts left prefrontal activation during controlled retrieval. Cerebral Cortex. 2008;18(11):2674–2685. doi: 10.1093/cercor/bhn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memory. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinions in Neurobiology. 2006;6(16):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nature Neuroscience. 2001;4(7):752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15(9):5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobes: a three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dicarlo JJ, Zoccolan D, Rust NC. How does the brain solve visual object recognition? Neuron. 2012;73(3):415–434. doi: 10.1016/j.neuron.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Graf P, Shimamura AP, Squire LR. Priming across modalities and priming across category levels: extending the domain of preserved function in amnesia. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1985;11(2):386–396. doi: 10.1037//0278-7393.11.2.386. [DOI] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59(4):554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46(7):1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimization method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Chun MM, McDermott J, Ledden PJ. Functional imaging of human visual recognition. Cognitive Brain Research. 2001;5(1-2):55–67. doi: 10.1016/s0926-6410(96)00041-9. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proceeding of the National Academy of Sciences of the United States of America. 1995;92(18):8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland AGR, Pring L. An investigation of cross-modality effects in implicit and explicit memory. The Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology. 1991;43(1):19–33. [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends in Cognitive Science. 1999;3(4):142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- O'Kane G, Insler RZ, Wagner AD. Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus. 2005;15:326–332. doi: 10.1002/hipo.20053. [DOI] [PubMed] [Google Scholar]
- Orfanidou E, Marslen-Wilson WD, Davis MH. Neural response suppression predicts repetition priming of spoken words and pseudowords. Journal of Cognitive Neuroscience. 2006;18(8):1237–1252. doi: 10.1162/jocn.2006.18.8.1237. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42(1):2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod A-MK, Pardo JV, Fox PT, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cerebral Cortex. 1994;8(26):1047–3211. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ. The representational-hierarchical view of amnesia: translation from animal to human. Neuropsychologia. 2010;48(8):2370–2384. doi: 10.1016/j.neuropsychologia.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Spiridon M, Fischl B, Kanwisher N. Location and spatial profile of category-specific regions in human extrastriate cortex. Human Brain Mapping. 2006;27(1):77–89. doi: 10.1002/hbm.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience. 2006;26(36):9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Object unitization and associative memory formation are supported by distinct brain regions. Journal of Neuroscience. 2010;30(29):9890–9897. doi: 10.1523/JNEUROSCI.0826-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. The Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(21):8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal G, Apergis-Schoute J, Pare D. Associative properties of the perirhinal cortex. Cerebral Cortex. 2012;22(6):1318–1332. doi: 10.1093/cercor/bhr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Hauner KKY, Paller KA. Establishing a relationship between activity reduction in human perirhinal cortex and priming. Hippocampus. 2009;19:773–778. doi: 10.1002/hipo.20608. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DH. Interactions between forms of memory: when priming hinders new episodic learning. Journal of Cognitive Neuroscience. 2000;12:52–60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31(2):329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wang WC, Lazzara MM, Ranganath C, Knight RT, Yonelinas AP. The medial temporal lobe supports conceptual implicit memory. Neuron. 2010;68(5):835–842. doi: 10.1016/j.neuron.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]


