Abstract

A series of Cp*IrIII dimers have been synthesized to elucidate the mechanistic viability of radical oxo-coupling pathways in iridium-catalyzed O2 evolution. The oxidative stability of the precursors toward nanoparticle formation and their oxygen evolution activity have been investigated and compared to suitable monomeric analogues. We found that precursors bearing monodentate NHC ligands degraded to form nanoparticles (NPs), and accordingly their O2 evolution rates were not significantly influenced by their nuclearity or distance between the two metals in the dimeric precursors. A doubly chelating bis-pyridine–pyrazolide ligand provided an oxidation-resistant ligand framework that allowed a more meaningful comparison of catalytic performance of dimers with their corresponding monomers. With sodium periodate (NaIO4) as the oxidant, the dimers provided significantly lower O2 evolution rates per [Ir] than the monomer, suggesting a negative interaction instead of cooperativity in the catalytic cycle. Electrochemical analysis of the dimers further substantiates the notion that no radical oxyl-coupling pathways are accessible. We thus conclude that the alternative path, nucleophilic attack of water on high-valent Ir-oxo species, may be the preferred mechanistic pathway of water oxidation with these catalysts, and bimolecular oxo-coupling is not a valid mechanistic alternative as in the related ruthenium chemistry, at least in the present system.
INTRODUCTION
The attractiveness of water as an alternative fuel source is growing steadily with the growth of the worldwide population as well as increase in living standards, which demand increased exploration of solar power.1,2 One of the key steps in using water as a viable solar fuel is its oxidation to dioxygen and reducing equivalents as shown in eq 1.3,4
The water oxidation half-reaction
| (1) |
Plants, algae, and cyanobacteria oxidize water using the oxygen evolving complex (OEC), an oxo-bridged Mn4Ca cluster in the enzyme photosystem II.5 Despite a growing number of homogeneous water oxidation catalysts utilizing a variety of transition metals (Mn, Fe, Co, Ni, Cu, Ru, Ir),6–14 a synthetic system combining low overpotentials with high turnover rates and sufficient robustness for large-scale application has yet to be developed.
In 1982, Meyer et al. reported a ruthenium dimer as the first homogeneous water oxidation catalyst (WOC), and in 1999 Brudvig and Crabtree described a mangenese terpy dimer.15,16 One proposed mechanism for water oxidation is radical oxo-coupling (ROC) with the O–O bond formation being the rate-determining step (RDS). On the basis of the analogy with the tetrametallic oxo-cluster in the biological OEC, it was believed that polymetallic catalysts were generally beneficial for this type of mechanism.17
In 2005, however, a series of mononuclear Ru-based WOCs were reported, showing that a single metal center can be capable of mediating the water oxidation cycle through nucleophilic attack of water (WNA) on an electrophilic metal oxo.18 This finding was followed by Bernhard’s report in 2008 on mononuclear IrIII complexes as precatalysts for water oxidation with Ce(IV).19 Since then, IrIII half-sandwich complexes have been extensively studied as WOCs.11,12,20–22 Both Cp* (Cp* = pentamethylcyclopentadienyl) and Cp (Cp = cyclopentadienyl) half-sandwich IrIII complexes are viable precatalysts, not only for water oxidation but also selective CH oxidations.23,24 We have used NaIO4 as a primary oxidant in this chemistry because of its ease of handling, good solubility, and lack of absorptions in the visible and near-UV. The disadvantage is that we cannot be certain if the reaction catalyzed in this case is true water oxidation rather than periodate dismutation: 2 IO4− → 2 IO3− + O2. The analogue to the WNA mechanism would then become a periodate nucleophilic attack (PNA) on the metal oxo. Hetterscheid and Reek have proposed such a PNA mechanism for periodate-driven O2 evolution with [Cp*Ir(NHC)(OH)2] (NHC = N-dimethylimidazolin-2-ylidene) based on DFT calculations.25 Unfortunately, experimental distinction between these two cases of nucleophilic attack by oxygen isotope labeling is obscured by the fast oxygen exchange of periodate with water in aqueous solution.26 The fact that these precursors also evolve O2 from water using Ce(IV) or electrochemical potentials12 shows, however, that these catalysts are able to perform true water-oxidation.
In cases where both closed- and open-shell pathways are accessible, WNA or PNA may be expected to be slower than ROC because higher oxidation states need to be generated to obtain a sufficiently electrophilic oxo that can undergo WNA or PNA. Both WNA and ROC pathways are known to be viable, for instance, for molecular Co- and Ru-based WOCs,27,28 and in these systems the ligand framework dictates the mechanism by actively favoring or disfavoring bimolecular oxo-coupling. Recent reports on a series of Ru-WOCs demonstrate strongly accelerated catalysis through ligand-enhanced oxo-coupling.29–31
In order to correctly interpret mechanistic analyses, however, one has to ascertain the integrity of the precursor. Some WOC precursors have been shown to undergo rapid ligand degradation under reaction conditions to form polymeric species in situ, which in some cases are also active WOCs.32 It is thus crucial to investigate the fate of the ligands in the catalyst precursor in order to elucidate the mechanism by which a given system operates.
For our Cp*IrIII precursors, we succeeded in distinguishing homogeneous from heterogeneous water oxidation catalysts by monitoring the in situ formation of IrOx material using an electrochemical quartz nanobalance (EQCN)20 and time-resolved dynamic light scattering (DLS).33 On the basis of kinetic analyses and previous DFT calculations, we proposed a mononuclear WNA pathway as the preferred mechanism in the homogeneous iridium systems (Figure 1).11,12
Figure 1.
Postulated mechanisms for Ir-catalyzed water oxidation. Left: Water nucleophilic attack (WNA) on a singlet oxo. Right: Radical oxo-coupling (ROC) of two triplet oxo moieties (charges and ligands omitted for clarity).
In the mononuclear WNA mechanism, a sequential proton-coupled one-electron oxidation of the IrIII precursor to an IrIV and finally a high-valent IrV-oxo species is proposed. Recent experimental results support these consecutive one-electron oxidation steps through the observation of transient IrIV species.34–40 The closed-shell IrV-oxene intermediate subsequently undergoes nucleophilic attack by water, leading to the formation of an IrIII-(hydro)peroxide intermediate, which upon further oxidation would dissociate dioxygen and close the catalytic cycle. Computations confirmed the highly electrophilic character of an oxo-unit in an octahedral IrV, and the experimental observation of first-order rate dependences on [Ir] suggested a mononuclear transformation (plausibly the IV→V oxidation) to be the RDS in these systems.12,40
An alternative pathway for the formation of dioxygen might be energetically favorable when two metals are held close together. In this scenario (ROC, Figure 1), two open-shell Ir-oxyl species could undergo radical coupling leading to the formation of the O–O bond. The reverse of this reaction, the direct oxygenation of two IrIII with O2 to yield two IrVO, is known for Wilkinson’s trimesityl-iridium (http://pubs.acs.org/doi/abs/10.1021/ic025700e). For our Cp*IrIII precursors, WNA barriers were found to be significantly lower on the singlet energy surface by DFT,12 but ROC barriers can be expected to be lower on the triplet energy surface. Since facile S–T interconversion was found for the formal (V) oxidation state (<5 kcal/mol), both pathways might be accessible through ligand control as in the related ruthenium chemistry.29 In Figure 1 only coupling pathways from the formal (V) oxidation state are shown for the sake of simplicity, but other, potentially lower-energy, ROC pathways might be accessible from lower formal oxidation states (i.e., the doublet Ir(IV)).42 In order to experimentally probe the viability of ROC mechanisms in Ir-catalyzed WO, we prepared some dimeric Cp*IrIII precursors and investigated their oxidative stabilities, kinetics in WO catalysis, and electrochemical behaviors, all compared to their respective monomers.
RESULTS AND DISCUSSION
Since Cp*IrIII precursors with NHC ligands are easily accessible and have shown good performance in WOC,43 we first synthesized a series of flexibly linked dimers, 2a–d. Varying the number of methylene groups between the two NHC moieties in the bridging ligands 2a–d allowed us to vary the distance between the two metals without affecting the electronics.44 Figure 2 shows the synthesis of the half-sandwich iridium dimers 2a–d via transmetalation45–47 from the corresponding AgI complexes 1a–d derived from known bis-imidazolium salts.44,48
Figure 2.

Synthesis of the NHC-linked half-sandwich iridium dimers 2a–d.
Transmetalation of the silver-NHC complexes 1a–d onto [Cp*IrCl2]2 proceeded in nearly quantitative yields in dichloro-methane (DCM) at room temperature. Using isolated Ag-NHC complexes with a coordinating halide anion ensured binding of only one NHC moiety per silver to afford stoichiometric NHC donor reagents. This strategy prevents halide abstraction from the iridium precursor and thus suppresses the formation of monomeric byproducts with chelating bis-NHC ligands.49 Due to the presence of different conformers with distinct symmetry in solution, the dimers 2a–d show two sets of peaks for the bridging NHCs in the NMR spectra. This phenomenon has been observed previously with a variety of bridging ligands with flexible linkers.44,50–52 The exemplary crystal structure of 2c shown in Figure 3 confirms the expected connectivity.
Figure 3.
Crystal structure of dimer 2c (thermal ellipsoids shown at the 50% probability level, hydrogen atoms and CH2Cl2 solvent molecules omitted for clarity).
From a simple space model it becomes clear that dimer 2c in particular would provide an ideal distance between the two iridium centers for oxo-coupling, providing the integrity of the precatalyst is retained.
Catalytic activity of oxygen evolution was assessed for complexes 2a–d using NaIO4 as the oxidant. NaIO4 has been demonstrated to be a superior alternative to CAN (cerium ammonium nitrate, [NH4]2[CeIV(NO3)6])53 because of its mild pH and the absence of potentially seeding precipitates.54 Activity was measured with a Clarke-type electrode in the liquid phase, and rates were determined from the initial linear regime of O2 evolution.
As shown in Table 1, the dimers 2a–d exhibited rather similar turnover frequencies ranging from 14 min−1 to 18 min−1, whereas the monomer 3 (shown in Figure 4) reached slightly higher rates of ~25 min−1.55
Table 1.
Rates of Catalytic Oxygen Evolution Using Cp*Ir(NHC) Precursorsa
| compound | initial rate (μmol O2 L−1 min−1)a | TOF (min−1) |
|---|---|---|
| 2a, dimer n = 1 | 145.6 ± 9.8 | 15.7 ± 1.1 |
| 2b, dimer n = 2 | 128.4 ± 22.4 | 14.3 ± 1.9 |
| 2c, dimer n = 3 | 164.1 ± 16.8 | 18.2 ± 2.1 |
| 2d, dimer n = 4 | 119.2 ± 18.6 | 13.2 ± 2.1 |
| 3, monomer | 224.5 ± 25.0 | 25.0 ± 5.4 |
Conditions: 10 μM [Ir], 100 mM NaIO4, 25 °C (for details see Experimental Section and the Supporting Information).
Figure 4.

Monomeric analogue 3.
Provided that the NHC ligands were retained throughout the WO cycle, these results would indicate that WNA was largely favored over ROC, and tying two metals together would rather obstruct turnover. In order to test the validity of this data set, we investigated the oxidative stability of the NHC precursors by time-resolved in operando DLS. As can be seen in Figure 5, both the dimer 2c and the monomer 3 degraded to form nanoparticles upon oxidation with excess NaIO4 in water. We have previously investigated this behavior in detail and shown that heterogeneous nanoparticles, formed under various conditions, originate from the iridium precursors in an oxidative degradation process.33
Figure 5.
Light-scattering intensities and mean particle sizes of diffusional mixtures of complex 3 (orange) or 2c (green) at 2.5 mM [Ir] with 250 mM NaIO4 in water at room temperature.
Although no heterogeneous material formed during the initial stage of the reaction used for the O2 evolution kinetics and higher iridium concentrations were required for the DLS measurements, the observation that the active Ir component ultimately polymerized to oxide NPs strongly suggests complete loss of the organic ligands in the precursors. This observation, coupled with the very similar rates for the different dimers 2a–d, casts doubt on the stability and utility of monodentate NHC ligands for homogeneous oxidation catalysis. Oxy-functionalization of metal-coordinated NHCs has been observed earlier.56,57
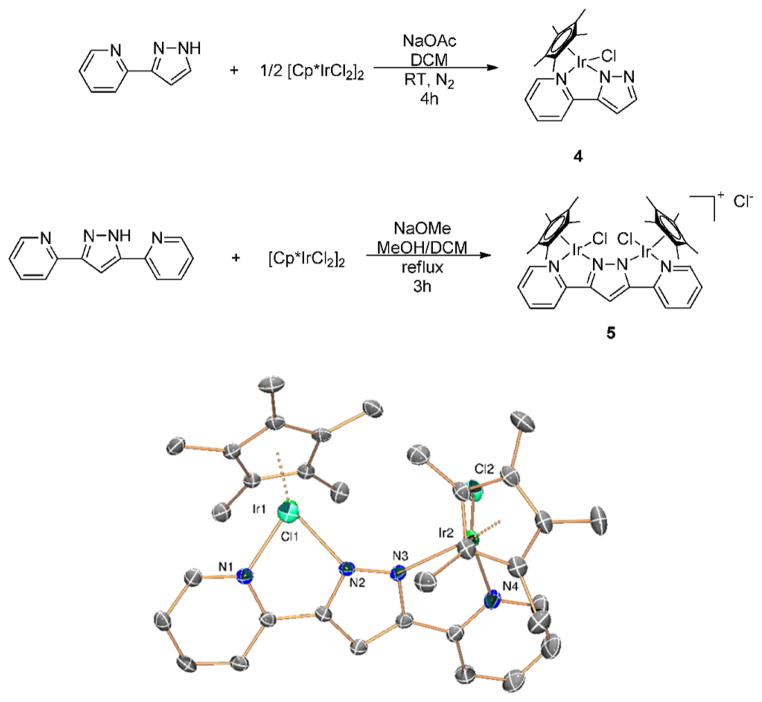
We thus turned to a more robust chelating ligand that would be more stable under reaction conditions to allow studying the effects of nuclearity on WO rates. The doubly chelating bis-pyridine–pyrazolide ligand shown in Figure 6 is known to be an effective framework in ROC water oxidation with binuclear Ru and Co complexes,58,59 and synthesis of the Cp*Ir compounds 4 and 5 was straightforward. Assessment of the oxidative stability of the monomer 4 and dimer 5 by time-resolved DLS showed that no heterogeneous material formed under catalytic conditions in this case (Figure 7 and Supporting Information), suggesting retention of the chelate ligand.
Figure 6.
Syntheses of complexes 4 and 5 and crystal structure of 5 (thermal ellipsoids shown at 50% probability, hydrogen atoms and counterion omitted for clarity).
Figure 7.

Light-scattering intensities and mean particle sizes of diffusional mixtures of complex 4 (red) or 5 (blue) at 2.5 mM [Ir] with 250 mM NaIO4 in water at room temperature.
With a more reliable ligand framework in hand, we compared O2 evolution rates of monomer 4 and dimer 5 across different iridium concentrations (Figure 8). Both exhibited a linear rate dependence on [Ir], indicating the RDS to be first-order in [Ir] even in the dimer.
Figure 8.
Initial O2 evolution rates of oxidatively stable monomer 4 (red) and dimer 5 (blue) at various iridium concentrations with 10 mM NaIO4 in water at 25 °C.
The fact that dimer 5 afforded a first-order rate constant of kobs = 0.83 ± 0.06 min −1, whereas the corresponding monomer 4 was almost 6 times faster on a per metal basis (kobs = 4.77 ± 0.12 min−1) suggests that no cooperative effects prevailed under the conditions applied and that the two metals rather hinder each other in their individual turnover. To test whether this was due to the formation of stable peroxo species, as reported for a related cobalt dimer,59 we attempted substitution of the chlorides in 5 with a μ-[O2] ligand by reaction with Na2O2 in an ionizing solvent (Figure 9). Under the conditions utilized, however, only unreacted starting material could be recovered from these mixtures. This result shows the difficulty of obtaining a stable peroxo IrIII–IrIII species, although the active catalytic species is likely to be different electronically and structurally.60
Figure 9.

Attempted reaction of 5 with excess sodium peroxide.
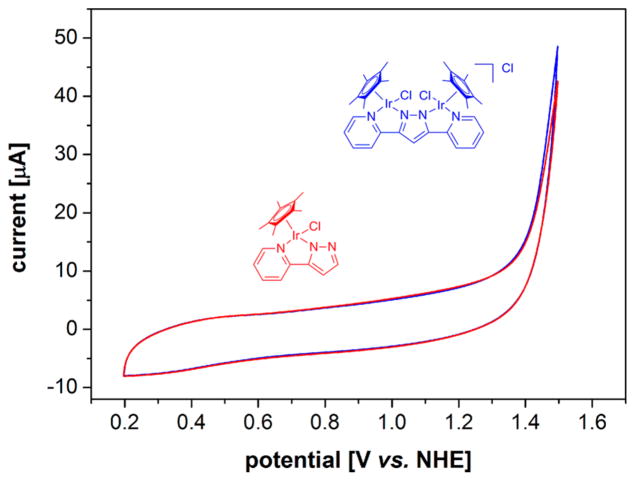
To investigate whether the observed detrimental effect of the second metal in dimers of the type of 5 was due to electronic communication, the aqueous electrochemistry of 4 and 5 was assessed by cyclic voltammetry (CV). As can be seen from Figure 10, no significant differences between monomer and dimer were observed, suggesting that lower oxidation-state ROC pathways are not directly accessible from these precursors.
Figure 10.
Cyclic voltammograms of 4 (red) and 5 (blue) at 1 mM [Ir] with 100 mV/s in 0.1 M aqueous KNO3 electrolyte (pH 4) at room temperature (for details see the Experimental Section).
Previous DFT studies confirmed an octahedral triplet “IrV” to have significant spin density on the oxyl ligand,12 whereas the SOMO of a related doublet IrIV species was found to be predominantly metal-centered.35 This might explain why ROC pathways, requiring radical spin density on the oxyl, are not accessible for iridium-based WOCs in the IV oxidation state and suggests that once in the formal V oxidation state these systems preferably operate via WNA mechanisms on the singlet energy surface (Figure 1). These results align well with the observation of stereo-retention in tertiary C-H hydroxylations with the same catalyst systems, ruling out radical rebound pathways.41
CONCLUSION
In summary, we report a series of novel Cp*IrIII dimers linked via monodentate NHCs that degrade to nanoparticles when exposed to NaIO4 in aqueous solution. Accordingly, these dimers show no significant difference in O2 evolution rates with NaIO4 when compared to an analogous monomer. A novel Cp*IrIII dimer with an electronically communicating chelate ligand and a suitable monomeric analogue were synthesized, showing resistance toward oxidative degradation to NPs under the same conditions. Both follow kinetics that are first-order in [Ir], but the dimer exhibits significantly lower rates for O2 evolution with NaIO4 as compared to the monomer. The aqueous electrochemistry of both monomer and dimer was found to be virtually identical, in concert indicating that no ROC pathways are accessible for the investigated Ir-based WOCs as opposed to Ru- and Co-based systems. Future design of iridium WOCs should therefore focus on WNA mechanisms.
EXPERIMENTAL SECTION
General Procedures
All organic solvents were dried using a Grubbs-type purification system. Deionized water was supplied through a centralized purification system (Dept. of Chemistry, Yale University). All chemicals were purchased from major commercial suppliers and used without further purification. [Cp*IrCl2]2,61 Cp*Ir(Me2Im)Cl2 (3),55 the bis(imidazolium) ligand precursor salts,44,48 and the silver NHC complexes 1a–d62 were synthesized following literature procedures. NMR spectra were recorded at room temperature on a 400 MHz Bruker or 500 MHz Varian spectrometer and referenced to the residual solvent peak (δ in ppm, J in Hz). Elemental analyses were performed by Robertson Microlit Laboratories (Ledgewood, NJ, USA).
Cp*IrIII-NHC Complexes 2a–d
A 25 mL round-bottomed flask equipped with a stir bar was charged with the appropriate Ag-NHC complex (0.9157 mmol) and [Cp*IrCl2]2 (0.869 mmol, 692.2 mg), and the mixture was evacuated and backfilled with nitrogen. Dry CH2Cl2 (10 mL) was added via syringe, and the mixture stirred for one hour at room temperature. The resulting mixture was filtered through Celite, the solution was reduced to ~5 mL under reduced pressure, and then pentane (50 mL) was added. The resulting yellow-orange precipitate was collected by filtration, washed with pentane (3 × 10 mL), and dried in vacuo.
2a
Yield: 803 mg (95%). 1H NMR (400 MHz, CD2Cl2): δ 7.42 (s, 2H), 7.08–7.04 (s, 2H), 6.84 (s, 2H), 3.92 (s, 6H), 1.54 (s, 30H). 13C NMR (126 MHz, CD2Cl2): δ 154.3, 124.7, 124.4, 122.6, 89.4, 38.7, 9.2. Anal. Calcd for C29H42Cl4Ir2N4: C, 35.80; H, 4.35; N, 5.76. Found: C, 35.61; H, 4.13; N, 5.79.
2b
Yield: 814 mg (95%). 1H NMR (500 MHz, CD2Cl2): δ 8.06 (d, J = 2.0 Hz, 2H), 7.01 (d, J = 2.0 Hz, 2H), 5.13 (d, J = 6.0 Hz, 2H), 4.38 (d, J = 6.0 Hz, 2H), 3.95 (d, J = 1.9 Hz, 6H), 1.56 (s, 30H). 13C NMR (126 MHz, CD2Cl2): δ 157.2, 124.1, 123.2, 89.3, 51.6, 38.7, 9.2. Anal. Calcd for C29H42Cl4Ir2N4: C, 37.12; H, 4.82; N, 5.59. Found: C, 37.81; H, 4.71; N, 5.33.
2c
Yield: 844 mg (97%). 1H NMR (400 MHz, CD2Cl2): δ 7.14 (d, J = 2.2 Hz, 2H), 6.97 (d, J = 2.1 Hz, 2H), 4.77–4.66 (m, 2H), 3.91 (d, J = 2.3 Hz, 6H), 3.90–3.81 (m, 2H), 2.44 (s, 2H), 1.57 (d, J = 1.3 Hz, 30H). 13C NMR (126 MHz, CD2Cl2): δ 156.1, 124.1, 122.1, 89.0, 47.9, 38.6, 35.9, 9.2. Anal. Calcd for C31H46Cl4Ir2N4: C, 37.20; H, 4.63; N, 5.60. Found: C, 36.91; H, 4.30; N, 5.11.
2d
Yield: 846 mg (96%). 1H NMR (400 MHz, CD2Cl2): δ 7.16 (d, J = 2.4 Hz, 2H), 7.00 (d, J = 1.8 Hz, 2H), 4.73 (m, 2H), 3.92 (s, 6H), 3.73 (m, 2H), 1.99 (m, 4H), 1.55 (s, 30H). 13C NMR (101 MHz, CD2Cl2): δ 156.6, 123.9, 121.7, 88.9, 50.7, 38.7, 29.5, 9.2. Anal. Calcd for C32H48Cl4Ir2N4: C, 37.60; H, 4.77; N, 5.52. Found: C, 37.60; H, 4.53; N, 5.33.
Synthesis of Cp*Ir(pp)Cl (4)
[Cp*IrCl2]2 (0.5 equiv, 0.125 mmol, 100 mg), 2-pyrazolylpyridine (1 equiv, 0.251 mmol, 36.4 mg), and NaOAc (6 equiv, 0.753 mmo, 62 mg) are added to a 100 mL Schlenk flask and degassed with nitrogen. Fifteen mL of dry dichloromethane is added and the reaction is stirred for 3 h at room temperature. The reaction mixture is then filtered through Celite and the solvent reduced to 5 mL. Excess pentane is added and the resulting precipitate is filtered and washed with pentane to yield a yellow solid. Yield: 121 mg (96%). 1H NMR (400 MHz, CD2Cl2): δ 8.55 (ddd, J = 5.8, 1.5, 0.8 Hz, 1H), 7.76 (ddd, J = 8.1, 7.4, 1.5 Hz, 1H), 7.66 (ddd, J = 8.1, 1.5, 0.9 Hz, 1H), 7.60 (d, J = 2.0 Hz, 1H), 7.13 (ddd, J = 7.3, 5.8, 1.5 Hz, 1H), 6.61 (d, J = 2.0 Hz, 1H), 1.72 (s, 15H). 13C NMR (126 MHz, CD2Cl2): δ 155.5, 150.5, 148.2, 141.3, 138.6, 122.2, 119.3, 103.0, 87.3, 8.8. Anal. Calcd for C31H46-Cl4Ir2N4: C, 42.64; H, 4.17; N, 8.29. Found: C, 42.99; H, 3.79; N, 8.12.
Synthesis of [Cp*2Ir2(bpp)Cl2]Cl (5)
[Cp*IrCl2]2 (1 equiv, 0.44 mmol, 360 mg) is dissolved in 10 mL of degassed dichloromethane and added to a solution of Hbpp (1 equiv, 0.44 mmol, 100 mg) in 10 mL of degassed MeOH under N2. The mixture is heated to reflux for 3 h. The crude is purified on a neutral silica column. The column is first eluted with acetone to remove a yellow impurity. The product is then eluted using 3% MeOH/DCM. The fractions are collected, and the solvent is reduced to 5 mL. Excess pentane is added, and the resulting precipitate is filtered and washed with pentane to yield a yellow-orange solid. Yield: 411 mg (95%). 1H NMR (500 MHz, CD2Cl2): δ 8.57 (dt, J = 5.7, 1.1 Hz, 2H), 8.55 (s, 1H), 8.41 (dt, J = 8.0, 1.2 Hz, 2H), 8.03 (td, J = 7.8, 1.4 Hz, 2H), 7.49 (ddd, J = 7.4, 5.7, 1.4 Hz, 2H), 1.56 (s, 30H). 13C NMR (126 MHz, CD2Cl2): δ 155.0, 150.6, 138.8, 122.7, 121.5, 119.6, 87.5, 8.8. Anal. Calcd for C31H46Cl4Ir2N4: C, 47.29; H, 4.14; N, 9.59. Found: C, 46.61; H, 3.98; N, 9.01.
Oxygen Evolution Assays
Oxygen evolution assays were performed as described previously.12,35 Measurements of O2 evolution were made with a YSI standard oxygen electrode inserted into a bubble-free, water-cooled jacket at 25 °C. In a typical experiment, 5 mL of a freshly prepared solution of NaIO4 (100 mM) was placed in the electrode chamber and left to equilibrate for several minutes. Subsequently, the appropriate volume of aqueous catalyst solution was injected via syringe to start the reaction. The procedure was repeated at least three times to ensure reproducibility.
Dynamic Light Scattering
Light-scattering experiments and data analyses were performed as described previously.33 NaIO4 (1 mmol, 214 mg) was dissolved in water (3 mL) just prior to analysis, and the clear solution passed through a hydrophobic syringe filter (Teflon, 0.2 μm pore size) into the sample vial in the temperature-controlled scattering chamber at 23 °C. New cylindrical screw-cap glass vials (15 × 45 mm) were used for each experiment. The automated measurement was started, and after collection of a few data points, 1 mL of a filtered aqueous catalyst solution (pH = 2.5 with H2SO4 in the case of 4) (10 mM in [Ir] = 2.5 mM final [Ir]) was added via syringe to start the reaction. The diffusional mixture was left for the analysis in a dark, undisturbed room.
Electrochemical Studies
Electrochemical measurements were made on a CH Instruments CHI1200B potentiostat using a standard three-electrode configuration. A glassy carbon electrode (surface area: 0.09 cm2) was used as the working electrode to minimize background oxidative current. A platinum wire was used as the counter electrode, and a Ag/AgCl reference was used. The measurements were carried out in 0.1 M KNO3 supporting electrolyte in Milli-Q water, and the pH was between 4.3 and 4.5 for all measurements.
Supplementary Material
Acknowledgments
This material is based in part upon work supported as part of the Argonne-Northwestern Solar Energy Research (ANSER) Center, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Award Number DE-SC0001059, a catalysis grant from the Division of Chem. Sci.s, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy, through Grant DE-FG02-84ER13297, and the National Science Foundation under Research Grant CBET-0828795. U.H. thanks the Alexander von Humboldt Foundation for a Feodor Lynen Research Fellowship, supplemented by a grant from the Yale Institute for Nanoscience and Quantum Engineering, and the Centre for Sustainable Chemical Technologies at the University of Bath for a Whorrod Research Fellowship.
Footnotes
The authors declare no competing financial interest.
NMR spectra, catalysis data, and crystallographic details. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lewis NS. Science. 2007;315:798. doi: 10.1126/science.1137014. [DOI] [PubMed] [Google Scholar]
- 2.Wild M, Gilgen H, Roesch A, Ohmura A, Long CN, Dutton EG, Forgan B, Kallis A, Russak V, Tsvetkov A. Science. 2005;308:847. doi: 10.1126/science.1103215. [DOI] [PubMed] [Google Scholar]
- 3.Dau H, Limberg C, Reier T, Risch M, Roggan S, Strasser P. ChemCatChem. 2010;2:724. [Google Scholar]
- 4.Brudvig GW. Philos Trans R Soc B. 2008;363:1211. doi: 10.1098/rstb.2007.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEvoy JP, Brudvig GW. Chem Rev. 2006;106:4455. doi: 10.1021/cr0204294. [DOI] [PubMed] [Google Scholar]
- 6.Concepcion JJ, Jurss JW, Templeton JL, Meyer TJ. J Am Chem Soc. 2008;130:16462. doi: 10.1021/ja8059649. [DOI] [PubMed] [Google Scholar]
- 7.Concepcion JJ, Tsai MK, Muckerman JT, Meyer TJ. J Am Chem Soc. 2010;132:1545. doi: 10.1021/ja904906v. [DOI] [PubMed] [Google Scholar]
- 8.Fillol JL, Codolà Z, Garcia-Bosch I, Gómez L, Pla JJ, Costas M. Nat Chem. 2011;3:807. doi: 10.1038/nchem.1140. [DOI] [PubMed] [Google Scholar]
- 9.Barnett SM, Goldberg KI, Mayer JM. Nat Chem. 2012;4:498. doi: 10.1038/nchem.1350. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Wang F. Coord Chem Rev. 2012;256:1115. [Google Scholar]
- 11.Hull JF, Balcells D, Blakemore JD, Incarvito CD, Eisenstein O, Brudvig GW, Crabtree RH. J Am Chem Soc. 2009;131:8730. doi: 10.1021/ja901270f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blakemore JD, Schley ND, Balcells D, Hull JF, Olack GW, Incarvito CD, Eisenstein O, Brudvig GW, Crabtree RH. J Am Chem Soc. 2010;132:16017. doi: 10.1021/ja104775j. [DOI] [PubMed] [Google Scholar]
- 13.Savini A, Bellachioma G, Bolaño S, Rocchigiani L, Zuccaccia C, Zuccaccia D, Macchioni A. ChemSusChem. 2012;5:1415. doi: 10.1002/cssc.201200067. [DOI] [PubMed] [Google Scholar]
- 14.McEvoy JP, Brudvig GW. Phys Chem Chem Phys. 2004;6:4754. [Google Scholar]
- 15.Gersten SW, Samuels GJ, Meyer TJ. J Am Chem Soc. 1982;104:4029. [Google Scholar]
- 16.Limburg J, Vrettos JS, Liable-Sands LM, Rheingold AL, Crabtree RH, Brudvig GW. Science. 1999;283:1524. doi: 10.1126/science.283.5407.1524. [DOI] [PubMed] [Google Scholar]
- 17.Romain S, Vigara L, Llobet A. Acc Chem Res. 2009;42:1944. doi: 10.1021/ar900240w. [DOI] [PubMed] [Google Scholar]
- 18.Zong R, Thummel RP. J Am Chem Soc. 2005;127:12802. doi: 10.1021/ja054791m. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel ND, Coughlin FJ, Tinker LL, Bernhard S. J Am Chem Soc. 2008;130:210. doi: 10.1021/ja074478f. [DOI] [PubMed] [Google Scholar]
- 20.Schley ND, Blakemore JD, Subbaiyan NK, Incarvito CD, D’Souza F, Crabtree RH, Brudvig GW. J Am Chem Soc. 2011;133:10473. doi: 10.1021/ja2004522. [DOI] [PubMed] [Google Scholar]
- 21.Lalrempuia R, McDaniel ND, Müller-Bunz H, Bernhard S, Albrecht M. Angew Chem, Int Ed. 2010;49:9765. doi: 10.1002/anie.201005260. [DOI] [PubMed] [Google Scholar]
- 22.Savini A, Bellachioma G, Ciancaleoni G, Zuccaccia C, Zuccaccia D, Macchioni A. Chem Commun. 2010;46:9218. doi: 10.1039/c0cc03801f. [DOI] [PubMed] [Google Scholar]
- 23.Zhou M, Hintermair U, Hashiguchi BG, Parent AR, Hashmi SM, Elimelech M, Periana RA, Brudvig GW, Crabtree RH. Organometallics. 2013;32:957. doi: 10.1021/om400658a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou M, Schley ND, Crabtree RH. J Am Chem Soc. 2010;132:12550. doi: 10.1021/ja1058247. [DOI] [PubMed] [Google Scholar]
- 25.Hetterscheid DGH, Reek JNH. Eur J Inorg Chem. 2013 n/a. [Google Scholar]
- 26.Pecht I, Luz Z. J Am Chem Soc. 1965;87:4068. [Google Scholar]
- 27.Wasylenko DJ, Palmer RD, Berlinguette CP. Chem Commun. 2013;49:218. doi: 10.1039/c2cc35632e. [DOI] [PubMed] [Google Scholar]
- 28.Hetterscheid DGH, Reek JNH. Angew Chem, Int Ed. 2012;51:9740. doi: 10.1002/anie.201202948. [DOI] [PubMed] [Google Scholar]
- 29.Maji S, Vigara L, Cottone F, Bozoglian F, Benet-Buchholz J, Llobet A. Angew Chem, Int Ed. 2012;51:5967. doi: 10.1002/anie.201201356. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Duan L, Stewart B, Pu M, Liu J, Privalov T, Sun L. J Am Chem Soc. 2012;134:18868. doi: 10.1021/ja309805m. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, Li F, Zhang B, Li X, Wang X, Huang F, Sun L. Angew Chem, Int Ed. 2013;52:3398. doi: 10.1002/anie.201209045. [DOI] [PubMed] [Google Scholar]
- 32.Artero V, Fontecave M. Chem Soc Rev. 2013;42:2338. doi: 10.1039/c2cs35334b. [DOI] [PubMed] [Google Scholar]
- 33.Hintermair U, Hashmi SM, Elimelech M, Crabtree RH. J Am Chem Soc. 2012;134:9785. doi: 10.1021/ja3033026. [DOI] [PubMed] [Google Scholar]
- 34.Graeupner J, Brewster TP, Blakemore JD, Schley ND, Thomsen JM, Brudvig GW, Hazari N, Crabtree RH. Organometallics. 2012;31:7158. doi: 10.1021/om300696t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brewster TP, Blakemore JD, Schley ND, Incarvito CD, Hazari N, Brudvig GW, Crabtree RH. Organometallics. 2011;30:965. doi: 10.1021/om300696t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Bruin B, Hetterscheid DGH, Koekkoek AJJ, Grützmacher H. Progress in Inorganic Chemistry. John Wiley & Sons, Inc; New York: 2008. p. 247. [Google Scholar]
- 37.Rohde JU, Lee WT. J Am Chem Soc. 2009;131:9162. doi: 10.1021/ja9033445. [DOI] [PubMed] [Google Scholar]
- 38.Ip HF, So YM, Lee HK, Williams ID, Leung WH. Eur J Inorg Chem. 2012;2012:3289. [Google Scholar]
- 39.Takaoka A, Peters JC. Inorg Chem. 2011;51:16. doi: 10.1021/ic202079r. [DOI] [PubMed] [Google Scholar]
- 40.Savini A, Bucci A, Bellachioma G, Rocchigiani L, Zuccaccia C, Llobet A, Macchioni A. Eur J Inorg Chem. 2013 doi: 10.1002/ejic.201300530. [DOI] [Google Scholar]
- 41.Zhou M, Balcells D, Parent AR, Crabtree RH, Eisenstein O. ACS Catal. 2011;2:208. [Google Scholar]
- 42.Polyansky DE, Muckerman JT, Rochford J, Zong R, Thummel RP, Fujita E. J Am Chem Soc. 2011;133:14649. doi: 10.1021/ja203249e. [DOI] [PubMed] [Google Scholar]
- 43.Codolà Z, Cardoso JMS, Royo B, Costas M, Lloret-Fillol J. Chem—Eur J. 2013;19:7203. doi: 10.1002/chem.201204568. [DOI] [PubMed] [Google Scholar]
- 44.Mata JA, Chianese AR, Miecznikowski JR, Poyatos M, Peris E, Faller JW, Crabtree RH. Organometallics. 2004;23:1253. [Google Scholar]
- 45.Lee KM, Wang HMJ, Lin IJB. Dalton Trans. 2002;0:2852. [Google Scholar]
- 46.Chiu PL, Chen CY, Zeng JY, Lu CY, Lee HM. J Organomet Chem. 2005;690:1682. [Google Scholar]
- 47.Wang HMJ, Lin IJB. Organometallics. 1998;17:972. [Google Scholar]
- 48.Poyatos M, Sanaú M, Peris E. Inorg Chem. 2003;42:2572. doi: 10.1021/ic026212+. [DOI] [PubMed] [Google Scholar]
- 49.Hintermair U, Englert U, Leitner W. Organometallics. 2011;30:3726. [Google Scholar]
- 50.Herrmann WA, Elison M, Fischer J, Köcher C, Artus GR. J Chem—Eur J. 1996;2:772. [Google Scholar]
- 51.Enders D, Gielen H. J Organomet Chem. 2001;617–618:70. [Google Scholar]
- 52.Enders D, Gielen H, Runsink J, Breuer K, Brode S, Boehn K. Eur J Inorg Chem. 1998;1998:913. [Google Scholar]
- 53.Parent AR, Brewster TP, De Wolf W, Crabtree RH, Brudvig GW. Inorg Chem. 2012;51:6147. doi: 10.1021/ic300154x. [DOI] [PubMed] [Google Scholar]
- 54.Parent AR, Crabtree RH, Brudvig GW. Chem Soc Rev. 2013;42:2247. doi: 10.1039/c2cs35225g. [DOI] [PubMed] [Google Scholar]
- 55.Hetterscheid DGH, Reek JNH. Chem Commun. 2011;47:2712. doi: 10.1039/c0cc05108j. [DOI] [PubMed] [Google Scholar]
- 56.Herrmann WA, Roesky PW, Elison M, Artus G, Oefele K. Organometallics. 1995;14:1085. [Google Scholar]
- 57.Hong D, Mandal S, Yamada Y, Lee YM, Nam W, Llobet A, Fukuzumi S. Inorg Chem. 2013;52:9522. doi: 10.1021/ic401180r. [DOI] [PubMed] [Google Scholar]
- 58.Sens C, Romero I, Rodríguez M, Llobet A, Parella T, Benet-Buchholz J. J Am Chem Soc. 2004;126:7798. doi: 10.1021/ja0486824. [DOI] [PubMed] [Google Scholar]
- 59.Rigsby ML, Mandal S, Nam W, Spencer LC, Llobet A, Stahl SS. Chem Sci. 2012;3:3058. [Google Scholar]
- 60.Hintermair U, Sheehan SW, Parent AR, Ess DH, Richens DT, Vaccaro PH, Brudvig GW, Crabtree RH. J Am Chem Soc. 2013;135:10837. doi: 10.1021/ja4048762. [DOI] [PubMed] [Google Scholar]
- 61.Ball RG, Graham WAG, Heinekey DM, Hoyano JK, McMaster AD, Mattson BM, Michel ST. Inorg Chem. 1990;29:2023. [Google Scholar]
- 62.Gil-Rubio J, Cámara V, Bautista D, Vicente J. Organometallics. 2012;31:5414. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.