Abstract
Siglecs are cell-surface proteins found primarily on hematopoietic cells. By definition, they are members of the immunoglobulin gene super-family and bind sialic acid. Most contain cytoplasmic tyrosine motifs implicated in cell signaling. This review will first summarize characteristics common and unique to Siglecs, followed by a discussion of each human Siglec in numerical order, mentioning in turn its closest murine ortholog or paralog. Each section will describe its pattern of cellular expression, latest known immune functions, ligands, and signaling pathways, with the focus being predominantly on CD33-related Siglecs. Potential clinical and therapeutic implications of each Siglec will also be covered.
Keywords: Siglec, human, mouse, leukocyte, ITIM, ITAM, sialic acid, lectin
Introduction
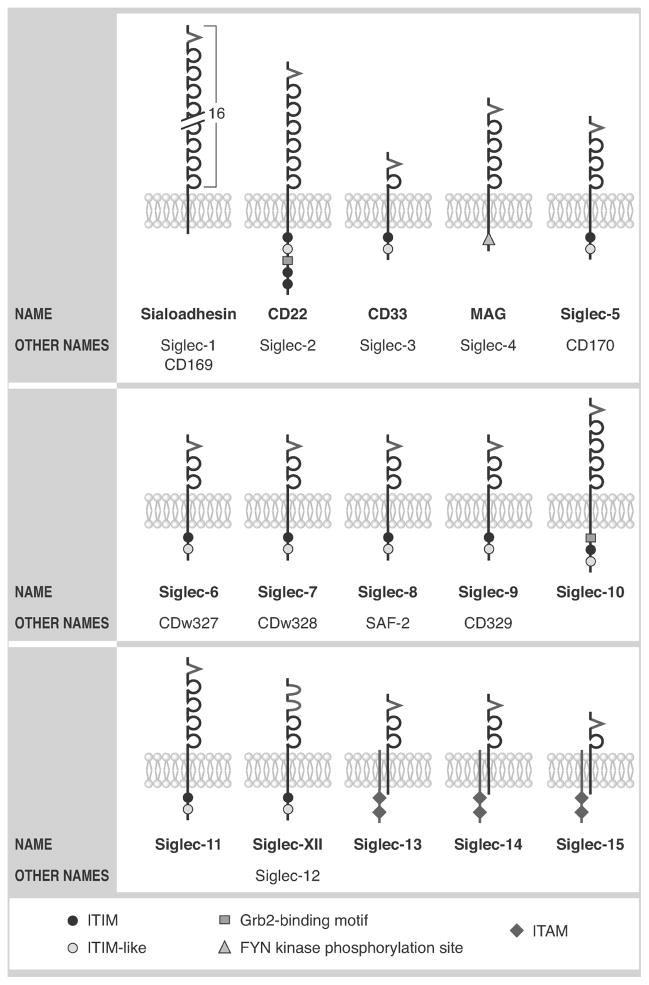
The term “Siglec,” or sialic acid–binding, immunoglobulin (Ig)-like lectin, was coined in 1998 to describe a subset of the Ig gene super-family that bound sialic acid.1 Siglecs are type I (single-pass) transmembrane surface proteins that can also be considered a subset of the I-type, or Ig-like lectins.2 Siglecs characteristically contain an N-terminal V-set (or variable set) domain for binding of sialic acid–containing glycan ligands, and within this domain is a conserved arginine residue for carbohydrate binding activity. Membrane proximal to the V-set domain is a series of repeats resembling C2 Ig domains that vary in number from one to sixteen, but commonly are two to four in number (Fig. 1). Most, but not all, Siglecs possess one or more cytoplasmic tyrosine residues located within characteristic signaling domain configurations, especially those involved in promoting inhibitory responses (so-called immunoreceptor tyrosine-based inhibitory motifs, or ITIMs). With respect to species homology, Siglec-1, Siglec-2, Siglec-3, and Siglec-4 (more commonly known as sialoadhesin, CD22, CD33, and myelin-associated glycoprotein, or MAG, respectively) along with Siglec-15 are highly conserved and therefore are given identical names in all mammals. In contrast, the remaining members of the CD33-related group, most of which are clustered together on chromosome 19q13.3–q13.4, are more diverse in their structural and evolutionary nature. This has resulted in a modification of nomenclature such that the remaining Siglecs in humans are numbered, but in mouse, they are lettered (Figs. 1 and 2).
Figure 1.
Nomenclature and key structural characteristics of human Siglecs. Although 15 are shown, Siglec-13 is present in nonhuman primates but not in man. V structures indicate the arginine-containing V-set domains with lectin activity; these are followed by C2-type Ig repeat domains. U-shaped structures for Siglec-XII indicate the mutated V-set domains missing arginine that have lost their lectin activity. Also shown is DAP12, illustrated as a shorter transmembrane structure co-associating with Siglec-13, Siglec-14, and Siglec-15. See key for symbols representing cytoplasmic signaling motifs.
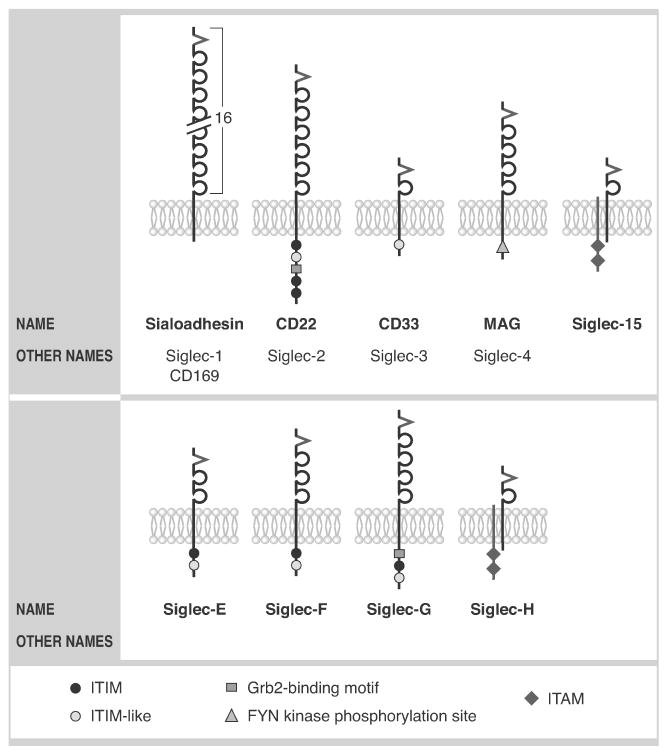
Figure 2.
Nomenclature and key structural characteristics of mouse Siglecs. V structures indicate the arginine-containing V-set domains with lectin activity; these are followed by C2-type Ig repeat domains. Also shown is DAP12, illustrated as a shorter transmembrane structure co-associating with Siglec-15 and Siglec-H. Whether Siglec-H, shown as having an arginine-containing V-set domains with lectin activity, can bind sialic acid ligands remains controversial (see text). Note that Siglecs-1–4 are conserved with humans, as is Siglec-15 (compare to Fig. 1). While not true orthologs, the closest functional paralog of Siglec-E is Siglec-9, while Siglec-F resembles Siglec-8 and Siglec-G resembles Siglec-10.
Each Siglec has a distinct pattern of expression on hematopoietic cells (Table 1), with important exceptions being Siglec-4, which is seen exclusively on cells of the nervous system; Siglec-6, on placental trophoblasts;3 and Siglec-12, found on epithelial and other cells. Siglec-12 deserves further discussion because, in humans, the critical arginine needed for lectin binding is absent; therefore, strictly speaking, it is not a Siglec since it cannot bind sialic acid and instead is referred as Siglec-XII.4 Siglec-13, which was initially identified in non-human primates, does not exist in humans.5 It can also be seen from Table 1 that some Siglecs are broadly expressed while others are much more restricted. For example, Siglec-1 expression is specific to macrophages, Siglec-2 expression is selective for B cells, and Siglec-8 expression is only found on eosinophils, mast cells, and weakly on basophils. Regarding expression patterns of CD33-related mouse Siglecs, Siglec-E is found on neutrophils, monocytes, and dendritic cells and is considered to most closely resemble Siglec-9, whereas Siglec-F (prominent on eosinophils)6–8 and Siglec-G (prominent on B cells)6,9 are considered the closest functional paralogs of Siglec-8 and Siglec-10, respectively. However, even though CD33-related Siglecs typically share roughly 50% identity, cellular expression patterns have not necessarily been conserved across species, as will be discussed in greater detail below. Also notable is the human-specific loss of Siglec expression on CD4+ T cells.10 Therefore, Siglecs are often thought of as cell-surface proteins contributing to innate immunity, even though some Siglecs are expressed on CD8+ T cells (Table 1), and under some inflammatory states in mice, low levels of Siglec-F can be detected on CD4+ T cells.11
TABLE 1.
Expression of Siglecs on Various Human Cells
| Siglecs expressed
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell type | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 14* | 15 |
| B cell | + | ± | ± | + | + | |||||||||
| Basophil | + | + | + | ± | + | |||||||||
| CD4+ T cell | n | o | n | e | e | x | p | r | e | s | s | e | d | |
| CD8+ T cell | + | + | ||||||||||||
| CD34+ cell¶ | + | + | + | + | + | |||||||||
| Dendritic cell | + | + | + | + | ||||||||||
| Eosinophil | + | ± | ||||||||||||
| Epithelial cell | + | |||||||||||||
| Macrophage | + | + | + | + | + | + | ||||||||
| Mast cell† | ± | + | + | + | + | + | ||||||||
| Microglial cell | + | |||||||||||||
| Monocyte | + | + | + | + | + | + | + | + | ||||||
| Neutrophil | + | + | + | + | ||||||||||
| NK cell | + | ± | + | |||||||||||
| Oligodendrocyte | + | |||||||||||||
| Placental trophoblast | + | |||||||||||||
| Schwann cell | + | |||||||||||||
Expression is likely similar to Siglec-5, but this has not yet been confirmed; also note that the Siglec-13 gene is deleted in humans
Expressed intracellularly or only weakly on the cell surface
Based on data using cells from peripheral blood
Based on data using human CD34+-derived mast cells, mast cell lines, and/or mature tissue mast cells
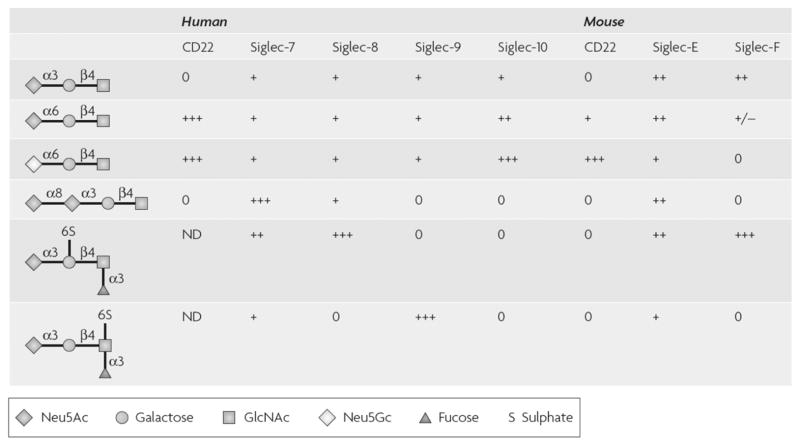
Another important general characteristic of Siglecs is their varied yet often overlapping glycan-binding characteristics.2 Indeed, the identity of specific ligands for Siglecs had been unknown until very recently. This had previously required investigators interested in studying downstream biology to resort to the use of artificial ligands, typically specific antibodies, to engage Siglecs. Therefore, much of our knowledge of Siglec biology was generated through surface crosslinking with antibodies. Fortunately, however, with the launch of several efforts including the Consortium for Functional Glycomics (www.functionalglycomics.org) by the National Institutes of General Medical Sciences in 2001, glycan arrays have been utilized to screen for Siglec binding partners, and this approach has been particularly fruitful in identifying similarities and differences among carbohydrate-binding activities for many Siglecs12 (Fig. 3). Along with this progress came the realization that in many cell systems, Siglecs often can bind to other endogenous cell surface sialic acids in a so-called “cis” configuration.13 Thus, in a variety of experiments, neuraminidase treatment of the cell surface is required to remove these cis sialic acids in order to facilitate Siglec binding activity for “trans” soluble glycans or glycans on other cells.
Figure 3.
Relative affinities of Siglecs with selected sialoside sequences. A comparison of the sialoside specificities, obtained from glycan array analysis and competitive binding experiments, for several sialic acid–binding immunoglobulin-like lectins (Siglecs) against selected sialoside sequences found in mammalian glycoproteins and glycolipids8,35,143,164 (see also the Functional Glycomics web site). Despite the general low affinity of Siglecs toward the common mammalian sialoside structures containing the N-acetylneuraminic acid (Neu5 Ac) α2–6 and α2–3 linkages, examination of the binding of each individual Siglec to a range of common sialoside structures shows that a unique sialoside specificity profile is characteristic of each Siglec. +++, strong binding; ++, moderate binding; +, low binding; ±, detectable binding; 0, no detectable binding; GlcNAc, N-acetylglucosamine; ND, not determined; Neu5 Gc, N-glycolylneuraminic acid. Legend and figure reproduced from Ref. 13 with permission.
Despite these numerous advances, the exact biological functions of most Siglecs in mouse and human systems are still poorly understood. The fact that Siglecs recognize sialylated glycans and that most have cytoplasmic signaling motifs (exceptions include sialoadhesin, mouse CD33, MAG, Siglec-14, Siglec-15, Siglec-H, and an isoform of Siglec-814 [Figs. 1 and 2]), either within their own cytoplasmic regions where they contain at least one ITIM domain, or via co-association in the cell membrane with adapter proteins such as DAP12 (DNAX-activating protein of molecular mass 12 kDa; also called KARAP or killer-cell activating receptor-associated protein) that instead contains immunoreceptor tyrosine-based activation motifs (ITAMs) (Siglec-13, Siglec-14, Siglec-15, and Siglec-H, for example), strongly suggests that Siglec crosslinking is likely to induce cell signaling.15
Given the nature of this review and its goal of identifying new developments in the field, each Siglec will be discussed with respect to its latest known immune functions, with the focus being predominantly on CD33-related Siglecs. The human Siglecs will be reviewed in numerical order, and where appropriate we will include a discussion of the murine ortholog or paralog when discussing its human counterpart. For additional discussion of other aspects of Siglec immunobiology, glycobiology, phylogeny, and therapeutic implications, the reader is referred to a number of additional outstanding reviews.2,12,13,16–27
Sialoadhesin (Siglec-1)
Sialoadhesin was initially identified as an erythrocyte ligand expressed by macrophages.13,28–30 It is by far the largest Siglec, with 16 C2-type repeats, and its surface expression is both positively and negatively regulated by cytokines, toll receptor activation, rhinovirus infection, and glucocorticoids.31–34 Among potential sialic acid ligands, sialoadhesin prefers α2–3-linkages for binding, a very commonly found terminal sequence on many mammalian sialosides.2,35 Sialoadhesin has been reported to bind a variety of ligands including sialylated structures in CD43,36 MUC1,37 certain porcine viruses,38,39 Neisseria meningitides,40 and Trypanisoma cruzi.41 In the latter situations, it has been suggested that sialoadhesin may play a role in pathogen internalization. Because it lacks any known cytoplasmic signaling domains, its role if any in cell signaling remains unknown.29 Levels of sialoadhesin on monocytes have reportedly been useful disease activity markers in patients with scleroderma34 or lupus.42 Its expression by monocytes is also markedly upregulated during HIV infection.43,44 However, an immunosuppressive role for sialoadhesin has also been proposed based on its ability to inhibit dendritic cell antigen presentation capabilities.33
In mice with selective sialoadhesin deficiency, beneficial effects are seen in various disease models with respect to myelin degeneration45,46 and uveoretinitis,47 even though these mice have a rather mild baseline phenotype consisting of slight shifts in B and T cell populations and diminished IgM levels.48
CD22 (Siglec-2)
CD22 is a B cell (not plasma cell) surface protein with known inhibitory cell function due an unusually extensive arrangement of tyrosine-based motifs in its cytoplasmic tail (Refs. 13 and 49 and see Fig. 1). Somewhat paradoxically, the cytoplasmic domain of CD22 contains both ITIM motifs and motifs that can recruit activating molecules such as Grb2 (growth factor receptor bound protein 2). CD22 is best known for its ability to regulate B cell function and survival, especially involving signaling via antigen, the IgM-containing B cell antigen receptor, or CD40+ B cell receptor activation,50 but CD22 may not influence signaling mediated through the IgG-containing B cell antigen receptor.51 CD22 has a strong predilection for binding α2–6-linked sialoside ligands (Ref. 52 and see Fig. 3).
Deficiencies of CD22 in mice result in an enhanced ability for B cells to proliferate and mature into plasma cells,50,53 presumably due to elimination of the inhibitory function of this molecule. The same biology occurs in mice in which CD22 has been altered to abrogate its ability to bind to its preferred α2–6-linked sialoside ligands54 but when the ability of mice to generate such ligands is prevented by deletion of the enzyme ST6Gal1, an unexpected phenotype appeared in which the mice were markedly immunodeficient in their B cell responses, including homing.55 This phenotype reverts to normal when CD22 is also deleted.56 CD22 is the target of the therapeutic monoclonal antibody Epratuzumab, which is in late-stage clinical trials for B cell malignancies, non-Hodgkin lymphoma, and autoimmune diseases such as lupus and Sjögren syndrome. 57,58
CD33 (Siglec-3)
In the early 1980s, CD33 was discovered as a myeloid cell–specific surface structure on progenitors and leukemic cells.59,60 In 1988, the mapping of the human CD33 gene to chromosome 19q13·3 was described.61 In 1995, the Crocker group reported CD33 as a novel member of the sialoadhesin receptor family.62
During hematopoietic differentiation, CD33 is expressed at an earlier stage than other Siglecs, which are more restricted to committed cells.63–65 Although CD33 is highly expressed on early multilineage hematopoietic progenitors and myelomonocytic precursors, it is absent from pluripotent hematopoietic stem cells.59,60,66,67 CD33, together with Siglec-5 and Siglec-10, is constitutively expressed on the surface of CD34+ precursor cells from peripheral blood.63 Upon differentiation, membrane display of CD33 is down-regulated on mature granulocytes but retained on macrophages, monocytes, and dendritic cells.59,60 Besides myelomonocytic cells CD33 has also been found to be expressed on human mast cells and blood basophils (Refs. 63, 68–70 and see Table 1).
Tyrosine phosphorylation of the two intra-cellular tyrosine-based motifs of CD33 by antibody cross-linking of the receptor or by treatment with the protein tyrosine phosphatase inhibitor pervanadate has been shown to result in the recruitment of several SH2 domain-containing proteins such as SHP-1 and SHP-2, Syk, CrkL, and PLC-γ1.71–74 Based on mutagenesis studies, the membrane-proximal ITIM motif appears to be dominant in CD33 interactions with the inhibitory tyrosine phosphatases SHP-1 and SHP-2.71 CD33 tyrosine phosphorylation appears to depend on Src family kinases,71 and the Src kinase Lck has been shown to be effective at phosphorylating the proximal, but not the distal, tyrosine residue of human CD33.73 Interestingly, protein tyrosine phosphatases associated with CD33 appear to catalyze CD33 dephosphorylation, suggesting a potential inhibitory feedback control of CD33 signaling.71 After coligation, CD33 has been shown to inhibit FcγRI-induced calcium mobilization, in the manner of a classical inhibitory receptor.13,15
Antibody crosslinking of CD33 results in inhibition of growth of normal CD34+ myeloid progenitor cells and leukemic cells isolated from the blood of patients suffering from chronic myeloid leukemia (CML),75 as well as inhibition of proliferation and induction of apoptosis in leukemic cells from acute myeloid leukemia (AML) patients and some AML cell lines.76 Subsequent proliferation studies revealed that cells from 56% of patients were responsive to the inhibitory effects of anti-CD33 antibody crosslinking, while 44% were unresponsive.77 The latter correlated with reduced Syk and/or zeta chain-associated protein kinase 70 (ZAP-70) expression levels or function, pointing to significant biochemical differences between responder and nonresponder AML populations.
CD33 also has been shown to have inhibitory functions on mature myeloid cells. For instance, incubation with CD33 antibody dramatically reduced the survival not only of human CD34+ myeloid progenitor cells, but also of dendritic cells derived from monocytes in vitro, even though mature dendritic cells were resistant to these effects.78 CD33 appears to inhibit human monocyte production of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-8 via phosphatidylinositol 3-kinase (PI3K) and p38 mitogen-activated protein kinase (MAPK).79 Furthermore, suppressor of cytokine signaling 3 (SOCS3) has recently been shown to associate with the phosphorylated ITIM motif of CD33 resulting in its proteosomal degradation and inhibition of the effect of CD33 engagement on cytokine-induced proliferation.80 Finally, it has been demonstrated that when ITIMs, such as those found in CD33, are phosphorylated by tyrosine kinases, they can bind to the ubiquitin ligase Cbl and become ubiquitylated, leading to CD33 internalization.81 Additional studies are required to explain the role of CD33 in inflammatory diseases.
CD33, like other Siglecs, has been shown to prefer α2–6-linked sialic acid–containing glycans. Pretreatment of transfected COS-cells with neuraminidase is required for CD33-dependent binding to red blood cells, which are heavily sialylated on their surface, with subsequent rosette formation, suggesting that the binding activity of CD33 can be masked by endogenous carbohydrates on the surface of the same cell in cis.62 Mutation of the proximal tyrosine motif of CD3371 or inhibition of CD33 phosphorylation with pharmacological agents82 resulted in an increase of sialic acid–dependent rosette formation, suggesting that CD33 signaling through selective recruitment of SHP-1/SHP-2, may modulate its ligand binding activity.
Clinically, CD33 has received particular attention as the molecular target of antibody-based treatment strategies in the therapy of AML.83–87 Its use takes advantage of the fact that approximately 90% of AML patients have myeloblasts expressing CD33.88 Directing specific cytotoxic therapy to AML cells is facilitated by the particular surface expression pattern of CD33, which theoretically allows sparing of normal tissues or hematopoietic stem cells. Studies testing unconjugated, radio-iodinated, or toxin-conjugated monoclonal antibodies culminated in the approval of gemtuzumab ozogamicin (GO; Mylotarg™, Wyeth, Philadelphia, PA, USA) for the treatment of AML in the year 2000.89 GO consists of a recombinant humanized anti-CD33 antibody conjugated to a derivative of the cytotoxic antitumor agent calicheamicin isolated from Micromonospora echinospora subsp calichensis.87 The antibody portion of GO (hP67.7) binds specifically to the CD33 antigen on the surface of myeloid leukemic cells followed by drug internalization and induction of site-specific, double-stranded breaks in the DNA by the calicheamicin toxin.
Mouse CD33 is expressed mainly by granulocytes, and unlike human CD33 it lacks a membrane-proximal ITIM domain and fails to bind to either α2–3 linked or α2–6 linked sialic acid–containing ligands, instead preferring mucins.90,91 A mouse deficient in CD33 has been generated, but its phenotype was essentially normal.91 Mouse CD33 also contains a charged amino acid in its transmembrane domain, suggesting that it might associate with another molecule, perhaps DAP12, as is seen with Siglec-14, Siglec-15, and Siglec-H.
Siglec-4 (Myelin-associated Glycoprotein, or MAG)
MAG is one of many membrane components found on the periaxonal Schwann cell and oligodendroglial membranes of myelin, where it makes up less than 1% of all such proteins.92–94 MAG is believed to function to stabilize myelin–axon interactions by binding to α2–3 linked sialic acid–containing gangliosides GD1a and GT1b on axons, as well as glycosylphosphatidylinositol-anchored Nogo receptors, influencing axonal growth and survival under normal conditions as well as following nerve injury.95 Mice missing the Galgt1 gene cannot express complex brain gangliosides, including GD1a and GT1b, and exhibit axon degeneration and dysmyelination with motor deficits along with selective loss of MAG from the brain.96 It was recently reported that MAG can also bind to mucin-1 (MUC1) and that this interaction can contribute to tumor spread.97 MAG-deficient mice develop central and peripheral axon degeneration.98 Thus, MAG binding to its axon-based ligands is felt to contribute to long-term axon–myelin stability and to inhibit neurite outgrowth. With respect to its role in immune-mediated diseases, an epitope on MAG shared with other molecules may become antigenic, leading to conditions such as the oligodendrogliopathy associated with multiple sclerosis and autoimmune peripheral neuropathy associated with IgM gammopathy.93
Siglec-5
In 1998 Cornish and colleagues cloned a novel ITIM-containing member of the Ig superfamily with a high degree of sequence similarity to CD33, extending to both extracellular domains and the cytoplasmic tail and called this molecule Siglec-5.99 Several studies have subsequently shown that Siglec-5 is expressed in a myeloid-restricted manner on the surface of neutrophils, monocytes, basophils, mast cells, and macrophages.99–103 Four isoforms of Siglec-5, with three or four extracellular domains linked to long or short cytoplasmic tails including a soluble isoform, have been described.100 However, nothing is currently known about the functional role of these isoforms. Siglec-5 is absent from early CD34+ hematopoietic progenitor cells and is upregulated later than CD33 during in vitro myeloid differentiation of CD34+ progenitor cells.10 An analysis of blasts from patients with AML revealed aberrant expression of Siglec-5 in 50–60% of AML cases.102 In this same study, 100% of acute lymphoblastic leukemic (ALL) cells tested, including CD33+ ALL, were Siglec-5 negative. Based on these findings, it has been suggested that Siglec-5 might be a useful marker for both normal myelopoiesis and AML.
Interestingly, Siglec-5 and sialoadhesin have been shown to recognize sialylated meningococcal lipopolysaccharide and to enhance bacterial uptake.40 Sialylated, but not nonsialylated, strains of Neisseria meningitidis were specifically bound and phagocytosed in a Siglec- and sialic acid–dependent manner by Siglec-5-transfected cell lines and macrophages from wild-type and sialoadhesin-deficient mice. Increased Siglec-5 expression has been appreciated on alveolar macrophages and macrophages within reactive lymph nodes activated by bacterial or viral pathogens, suggesting that macrophages, upon activation during normal clearance activity in the lung and upon immune responses in lymph nodes, respectively, upregulate Siglec-5 surface expression.100
Respiratory burst activity is a key component of antimicrobial activity of phagocytes.104,105 Using luminol-dependent chemiluminescence assays, one study showed that priming of neutrophils with antibodies to Siglec-5 enhanced the respiratory burst activity of neutrophils induced by N-formyl-methionyl-leucyl-phenylalanine (fMLP), whereas antibodies alone had no significant effect.103 The endocytic and respiratory burst priming capacity of Siglec-5 might act in concert in the defense against sialylated pathogens. On the other hand, it is possible that pathogens might exploit internalization by Siglec-5 as a route to gain access to the intracellular compartment and eventually to penetrate beyond epithelial and endothelial barriers in a “Trojan horse” manner.40 However, Siglec-5 might also play a role in the clearance of endogenous sialoconjugate-bearing molecules. For instance, the phagocytosis of apoptotic bodies by macrophages was inhibited with sialo-oligosaccharide ligands of Siglec-5 and antibodies to Siglec-5, suggesting that this receptor could participate in the engulfment and clearance of such particles.106 Taken together, these findings imply that Siglec-5 might play an important role in both the phagocytosis of pathogens and the clearance of endogenous substrates, and based on the characteristics of this receptor, as described, the efficacy of these processes might be significantly enhanced in an inflammatory microenvironment.
Like other family members, Siglec-5 also appears to act as an inhibitory receptor. For example, primary human T cells, which do not express Siglec-5, show reduced T cell receptor responsiveness after transfection with Siglec-5.10 In a transfected rat basophilic leukemia (RBL) model system, Siglec-5 could efficiently recruit SHP-1 and SHP-2 after tyrosine phosphorylation and inhibit calcium flux and serotonin release after co-ligation with the ITAM-containing high-affinity IgE receptor (FcεRI).107 Surprisingly, however, mutagenesis studies showed that inhibition of serotonin release could still occur efficiently after a double tyrosine-to-alanine substitution in the membrane proximal ITIM and the membrane distal ITIM-like motif. A potential mechanism for tyrosine phosphorylation-independent inhibitory signaling was provided by results of in vitro phosphorylation assays, which demonstrated low levels of activation of SHP-1 by the Siglec-5 cytoplasmic tail in the absence of tyrosine phosphorylation. Contrasting results were found in another study showing that the acute-phase protein α1-acid glycoprotein, by binding to and interacting with Siglec-5, increased calcium levels in neutrophils.108 A possible explanation for the discrepancy of these findings is provided by the recent identification of Siglec-14 (see Siglec-14 section below), a molecule that is almost identical to Siglec-5 with regard to sequence and glycan-binding properties, that instead potentially acts as an activating receptor since it associates with the ITAM-containing adaptor protein DAP12.109 These authors proposed that Siglec-5 and Siglec-14 are the first glycan binding “paired receptors” with antagonizing signaling properties. Furthermore, they showed that because of the extreme sequence identity of Siglec-5 and Siglec-14 at the amino-terminal region, some Siglec-5 antibodies cross-react with Siglec-14, so some prior reports of the expression pattern of Siglec-5 in humans might represent expression of Siglec-5, Siglec-14, or both. Obviously, for future research purposes antibodies need to be selected that can clearly discriminate between these two receptors. Future studies are required to investigate the expression and the functional role of these paired receptors in the immune system.
Siglec-6
Siglec-6 was originally identified both as a leptin-binding protein (obesity binding protein-1 or OB-BP1) and as a placental protein.110,111 Based on mRNA screening, highest levels were found in placenta, and antibody-based analyses confirmed expression on cyto- and syncytiotrophoblasts along with one of its preferred ligands, α2–6-linked sialosides, on placenta and uterine epithelium.3,111 Lower levels of mRNA expression were also detected in spleen, leukocytes, and small intestine. The leukocyte and splenic sources of Siglec-6 were traced to B cells,111 but subsequent studies found high levels of Siglec-6 on CD34+-derived human mast cells and the HMC-1 mast cell line, but not basophils or lung mast cells.63,112 Its function, including its involvement in gestation, remains unknown.
Siglec-7
In 1999 Falco and colleagues reported the cloning of p75/AIRM1 (adhesion inhibitory receptor molecule 1) as a novel NK cell inhibitory receptor and a member of the sialoadhesin family.113 Two other groups cloned and identified the identical molecule as a member of the CD33-related-Siglecs and called it Siglec-7.114,115 Siglec-7 is highly expressed on human NK cells and weakly on monocytes and a minor subpopulation of T cells expressing the αβ T cell receptor (TCR) subunits.113,114,116 Based on the observation that Siglec-7 is expressed on a subpopulation of T cells, Ikehara and colleagues used stably transfected Jurkat cells as a model to study the effects of this receptor on TCR signaling.116 Following either pervanadate treatment or TCR stimulation by CD3 antibodies or phytohaemagglutinin (PHA), Siglec-7 exhibited increased tyrosine phosphorylation and recruitment of SHP-1. The expression of Siglec-7 on Jurkat cells alone was sufficient to result in reduced TCR downstream signaling events, including reduced phosphorylation of ZAP-70 and nuclear factor of activated T cells (NFAT)-mediated transactivation of gene transcription. This receptor was also found in myeloid leukemias, notably AML and CML.75,76 Siglec-7 has been shown to preferentially recognize α2–8-linked sialylated glycans (Ref. 117 and see Fig. 3).
Multiple studies have contributed evidence for the inhibitory function of Siglec-7. In an RBL cell model, co-crosslinking of Siglec-7 and FcεRI inhibited serotonin release, and site-directed mutagenesis experiments revealed that the membrane-proximal ITIM motif was essential for both the inhibitory function and the recruitment of the inhibitory phosphatases SHP-1 and SHP-2.118 The capacity of Siglec-7 to recruit SHP-1 after tyrosine phosphorylation has previously been observed in polyclonal NK cell populations isolated from human peripheral blood leukocytes.113 These studies showed that Siglec-7, in a manner consistent with other classical inhibitory receptors of the immune system, has the capacity to antagonize the effector function of an activating receptor by recruitment of inhibitory phosphatases to counteract tyrosine phosphorylation of an ITIM motif.
Using standard killing assays, the inhibitory effects of Siglec-7 on NK-mediated cytotoxicity as initially observed in cell lines113 were subsequently shown to occur in primary human cells.114 Nicoll and colleagues showed that Siglec-7 binds to the ganglioside GD3, which displays α2–8-linked disialic acids. Surprisingly, NK cells more effectively killed GD3 synthase-transfected P815 cells, and this occurred via Siglec-7-independent mechanisms. However, Siglec-7-dependent inhibition of killing was observed following sialidase treatment of NK cells. Sialidase treatment presumably leads to the liberation of masked Siglec-7 binding sites bound in cis, allowing the interaction of the inhibitory receptor in trans with GD3 ligands on the target cell resulting in downregulation of NK cell activity. This hypothesis was supported by data showing that the surface of NK cells is decorated with α2–8-linked sialosides with high affinity for Siglec-7.119 Although Siglec-7 function might be regulated via masking of binding sites by endogenous ligands on the cell surface in cis, another alternative mechanism was suggested. The molecule SOCS3 (suppressor of cytokine signaling 3), which is thought to compete with inhibitory phosphatases for binding to ITIM-like motifs, bound to the phosphorylated ITIM of Siglec-7 and targeted it for proteasomal degradation.120 In this study, SOCS3 efficiently blocked the inhibitory effects of Siglec-7 on cytokine-induced proliferation of a transfected Ba/F3 cell line. Since SOCS3 is upregulated during inflammation, the authors suggested that this might be one mechanism by which an inflammatory response might be potentiated.
Despite its high expression on NK cells, the inhibitory activity of Siglec-7 is not restricted to these cells, but has also been found in other cell types. For example, crosslinking of Siglec-7 inhibited the proliferation of normal CD34+ myeloid precursor cells or malignant cells isolated from blood of CML and AML patients.75,76,113 However, in contrast to CD33 engagement, Siglec-7 crosslinking did not result in apoptosis of AML cells.121 Based on its expression pattern and antiproliferative capacity, Siglec-7, like some other Siglecs, might be exploited for new therapeutic approaches in myeloid leukemias as a target for antibody-mediated treatment strategies. Indeed, one such reported approach exploited Siglec-7 binding and internalization to deliver nanoparticles.122
Siglec-8 (and Siglec-F)
Siglec-8 was co-discovered by two laboratories from the same eosinophil cDNA library generated from a patient with hyper-eosinophilic syndrome.123,124 Though highly and selectively expressed by eosinophils, it soon became clear that Siglec-8 also was expressed by human mast cells and weakly, but consistently, by human basophils.63,123,125,126 Siglec-8 was initially felt to encode for a molecule with an extremely short cytoplasmic domain that did not possess any known signaling motifs, but subsequent work determined that this gene could be alternatively spliced.127,128 Indeed, the predominant form expressed by cells is identical in its extracellular and transmembrane regions, but instead contains a longer cytoplasmic tail with a membrane-proximal ITIM and a membrane-distal ITIM-like domain similar to that found in CD150 or SLAM (signaling lymphocyte activation molecule).125 The presence of both isoforms of Siglec-8 has been detected in normal cells, but the relative importance and function of each remain unknown.14,129
Siglec-8 ligation with monoclonal antibodies, as well as autoantibodies contained in commercial gamma globulin (IVIg) preparations, has been used to explore biological function. On eosinophils, this results in caspase-, mitochondrial-, and reactive oxygen species–dependent apoptosis.130–132 Somewhat paradoxically, cytokines that would normally prolong eosinophil survival actually enhance this death process, but do so in a caspase-independent manner.126,130,132,133 Eosinophils isolated from the lungs of allergen-challenged allergic subjects, which are primed by cytokines in vivo, also display enhanced susceptibility to apoptosis when exposed to Siglec-8 antibodies in vitro, suggested that activated eosinophils might be especially sensitive to Siglec-8-induced death.126,133 The presence of Siglec-8 autoantibodies in IVIg mentioned above may help to explain the somewhat controversial and inconsistent nature of the efficacy of IVIg in disorders associated with eosinophil-driven diseases such as severe asthma,134 chronic urticaria,135 and Churg-Strauss syndrome.136
Although little is known about Siglec-8 function on basophils, it is now known that Siglec-8 engagement on mast cells does not affect their survival but instead counteracts stimulatory signals delivered via FcεRI crosslinking, resulting in 50% or greater inhibition of release of histamine and prostaglandin D2 but not release of cytokines such as IL-8.137 Mast cell–dependent contraction of human airway rings in vitro was inhibited by pre-incubation with Siglec-8 antibody. Siglec-8 engagement was also shown to inhibit mast cell calcium flux associated with FcεRI activation, and this inhibitory effect was lost in Siglec-8-transfected RBL cells when a point mutation was introduced into the membrane-proximal ITIM domain of Siglec-8.
There is no exact Siglec-8 ortholog in the mouse. Siglec-F appears to be the most closely related functional paralog, but it is expressed on many other cells, and poorly if at all on mouse mast cells (Refs. 6–8, 11, 138–140 and see Table 2). Siglec-F, like Siglec-8, has a cytoplasmic tail containing an ITIM-like motif as well as a second membrane distal tyrosine motif. Recent studies in which Siglec-F-deficient mice were generated revealed no baseline eosinophilia, but exaggerated eosinophilic allergic inflammatory responses in the bone marrow, blood, and lungs.11 This was felt to be due to reduced rates of apoptosis, given the absence of the Siglec-F eosinophil death pathway. Systemic administration of monoclonal antibodies to Siglec-F reduced eosinophil numbers in models of allergic asthma141 and reduced circulating and gastrointestinal eosinophils in models of hypereosinophilia, namely IL-5 transgenic mice and a mouse model of hypereosinophilic syndrome.142 In addition, administration of Siglec-F antibodies to normal mice resulted in reduced eosinophil numbers, and, as with Siglec-8, these effects appear to be due to increased rates of eosinophil apoptosis.142
TABLE 2.
Comparison of Surface Expression Patterns of Siglec-8 and Siglec-F
| Siglec-8 | Siglec-F | |
|---|---|---|
| Eosinophils | +++ | +++ |
| Mast cells | ++ | ± |
| Basophils | + | ? |
| Alveolar macrophages | − | ++ |
| T cells | − | ± |
| Neutrophils | − | ± |
| Monocytes | − | − |
Expressed only weakly or under inflammatory conditions; ? unknown
Both Siglec-8 and Siglec-F have evolved to recognize the same unique glycan ligand, namely 6′-sulfated sialyl Lewis X or sialyl Lewis X in which the penultimate galactose is sulfated in the 6 position (NeuAcα2-3Galβ1-4(Fucα1-3)(6-O-sulfo)GlcNAc).8,126,143,144 Using mouse eosinophils, Siglec antibodies, and a polymer decorated with this sulfated glycan, it was shown that Siglec-F engagement results in its endocytosis.145 This process required the cytoplasmic tyrosine motifs, ADP ribosylation factor 6, and lysosomal trafficking but was clathrin and dynamin independent. Given the endogenous expression of Siglec-F ligands on normal mouse airway epithelium and enhanced diffuse expression in allergic inflamed lungs,11,146 eosinophils entering the airway may encounter such Siglec-F ligands and have their tissue lifespan reduced. Further studies are needed to fully characterize these endogenous ligands.
Siglec-9 (and Siglec-E)
Siglec-9 is highly expressed on neutrophils and monocytes, and at lower levels on subpopulations of T and B lymphocytes and NK cells.65,116,147,148 Siglec-9 protein expression is absent from eosinophils,65 and little or no mRNA is produced in normal hematopoietic progenitor cells or human mast cells derived by culture of CD34+ peripheral blood precursors.63,64 Immature neutrophils gain Siglec-9 surface expression late in differentiation, appearing after the myelocyte stage but before CD16 is expressed.65 Siglec-9 is highly expressed on AML cells at levels similar to CD33, particularly on a subset with myelomonoblastic features.64 Like CD33, Siglec-9 mediates rapid endocytosis of bound specific antibody. Based on these features it was suggested that Siglec-9 might provide a novel marker for myelomonoblastic leukemias that could be exploited as a potential target of antibody-based treatment strategies, either alone or in conjunction with other treatments.
Similar to other members of the CD33 subfamily, Siglec-9 contains two cytoplasmic tyrosine motifs: a membrane-proximal ITIM and a distal ITIM-like domain. Cell activation or exposure to cytokines within minutes leads to rapid tyrosine phosphorylation of Siglec-9 by tyrosine kinases.65,116 Upon tyrosine phosphorylation, Siglec-9 recruits the inhibitory phosphatases SHP-1 and SHP-2.116,118,149 Functional evidence that Siglec-9 acts as an inhibitory receptor comes from RBL cell transfectants, where cocrosslinking with FcεRI inhibited serotonin release.118 Site-directed mutagenesis experiments revealed that the classical membrane-proximal ITIM motif is essential for this inhibitory function. Siglec-9 was also shown to be capable of negative regulation of T cell receptor signaling using Jurkat cells transfected with these receptors.116 The finding that cell activation by TCR engagement increased tyrosine phosphorylation and recruitment of SHP-1 is suggestive for a negative feedback role.
In a story that parallels data from Siglec-8, ligation of Siglec-9 by antibodies induced apoptosis in human neutrophils.65 Siglec-9-mediated cell death was enhanced by cytokines such as granulocyte/macrophage colony-stimulating factor (GM-CSF), interferon-α (IFN-α), and IFN-γ, and “primed” neutrophils from patients with sepsis or rheumatoid arthritis were more susceptible to Siglec-9-mediated death than normal cells. Incubation with GM-CSF resulted in rapid tyrosine phosphorylation of Siglec-9 and significantly increased the potency and efficacy of Siglec-9-dependent death upon antibody crosslinking. The increased Siglec-9-mediated death was caspase independent, but dependent on reactive oxygen species and was characterized by a non-apoptotic, autophagy-like morphology. The observation that cytokine priming might recruit alternative death pathways is suggestive of a complex interplay between cytokine receptor and Siglec signaling pathways.24
As was reported for Siglec-8, there is evidence that humans produce functional autoantibodies against Siglec-9.150 Siglec-9 autoantibodies present in IVIg triggered neutrophil death in vitro in a cytokine-dependent fashion. The authors suggested that the increased efficacy and potency of IVIg in a cytokine-rich environment might explain the local anti-inflammatory effects of systemically administered IVIg. On the other hand, Siglec-9-dependent neutrophil death may provide a potential mechanism for unwanted IVIg-induced neutropenia observed in some patients.151
The most likely paralog of Siglec-9 in the mouse is Siglec-E, which is broadly expressed on leukocytes and in tissues rich in leukocytes, but with little or no overlap in expression pattern with Siglec-F.6,7,152 Equally broad is its sialic acid binding activity, showing affinity for α2–3-, α2–6-, and α2–8-linked sialosides but a preference for the latter.2 Its function on murine leukocytes remains unknown.
Siglec-10 (and Siglec-G)
Siglec-10 (also referred to as Siglec-like gene (SLG2)) was discovered in 2001 by four independent groups of investigators.128,153–155 Siglec-10 is expressed on monocytes, dendritic cells, a CD16+/CD56− subpopulation of cells, and weakly on eosinophils and B cells.128,153,154 Four splice variants of Siglec-10 have been identified, but their biological significance remains unclear.155,156 Like other family members, Siglec-10 mediates sialic acid–dependent binding of transfected COS cells to human erythrocytes and binding is masked by endogenous ligands in cis on these cells.128,153 Sialoconjugates in α2–3 and α2–6 linkage are recognized by Siglec-10, with a preference for the latter (see Fig. 3). In kinase assays Siglec-10 was phosphorylated in decreasing order by Lck, Jak3, and Emt, but not ZAP-70.157 SHP-1 and SHP-2 have been shown to associate with Siglec-10, whereas no interaction with the SH2-protein SAP (SLAM-associated protein) was observed.154,156 Functional studies on Siglec-10 are lacking.
Siglec-G is considered an ortholog of Siglec-10. Siglec-10 and Siglec-G both have five extracellular domains and three similarly positioned intracellular tyrosine-based motifs, including a membrane-proximal Grb2-binding motif, an ITIM domain, and a membrane-distal ITIM-like domain (Figs. 1 and 2). Siglec-G is expressed throughout the B cell lineage, being especially high in pre-B cells.9 Its tissue expression is also increased in IL-5 transgenic mice, and this was initially interpreted as being eosinophil-associated.6 A more likely explanation is that B cells are also increased in these tissues because IL-5 is also a B cell growth and differentiation factor for which it was originally named.158 Particularly striking was the observation that Siglec-G-deficient mice spontaneously developed a substantial increase in B1 cells and higher titers of IgM but not IgG.9 These B cells displayed enhanced anti-IgM-induced calcium flux as well. In contrast, when Siglec-G was overexpressed in cell lines and engaged by antibody, there was inhibition of B cell receptor-mediated calcium signaling, supporting the notion that Siglec-G is a negative regulator of B1 cells.
Siglec-11
The cloning of Siglec-11 was reported in 2002.159 Its extracellular domain has high sequence similarity with Siglec-10, but its cytoplasmic tail is more closely related to that of Siglec-5. A genomic analysis suggested that Siglec-11 is a chimeric molecule that arose from relatively recent gene duplication and recombination events.159 Although tyrosine-phosphorylation of Siglec-11 following pervanadate treatment leads to recruitment of SHP-1 and SHP-2 as is typical for CD33-related Siglecs, Siglec-11 has several novel features.159 Siglec-11 was not found on peripheral blood leukocytes, and instead its expression was observed on cells in tissues such as Kupffer cells, intestinal lamina propria macrophages, perifollicular cells in the spleen, and brain microglia. Siglec-11 transfected COS or fixed cells failed to show the typical sialic acid–dependent binding to red blood cells, but did bind α2–8-linked sialoconjugates.159 No functional studies on Siglec-11 have been reported.
Siglec-12 (Siglec-XII, Siglec-L1) and Siglec-13
The first of these two Siglecs was identified in humans in 2001 and was called Siglec-L1 (Siglec-like molecule-1).4 This molecule has two unique characteristics among Siglecs: first, it contains two similar V-set domains rather than one, and secondly, both have lost the arginine required for sialic acid binding, even though its ortholog, designated as chimpanzee Siglec-12, retains this arginine and thus its ability to bind carbohydrates, especially 5-N -glycolylneuraminic acid.4 The change of name from Siglec-12 to Siglec-XII for the human molecule was done to acknowledge that it represents the arginine-mutated ortholog of chimpanzee Siglec-12.5 Siglec-13 was discovered by sequencing the chimpanzee and baboon Siglec gene cluster regions; this gene is deleted in humans.5 Nothing yet is known about Siglec-12 or Siglec-13 function.
Siglec-14
The existence of Siglec-14 was reported in 2006.109 Based on the high mutual sequence similarity and the potentially antagonizing signaling properties of Siglec-14 with Siglec-5, the authors suggested that these molecules represent paired receptors (see Siglec-5 section above). Initially Siglec-14 was located as a genomic DNA segment upstream of Siglec-5 and was called SIGLEC5*, because it showed >99% identity with the 5′ end of several SIGLEC5 exons.109
Siglec-14 contains three Ig-like domains, whereas Siglec-5 contains four, of which the first two Ig-like domains share almost complete sequence identity.109 Not surprisingly, both receptors showed similar glycan binding preferences, but Siglec-14 showed more robust glycoconjugate and erythrocyte binding. RT-PCR analysis of human tissues revealed a similar expression pattern for both Siglec-5 and Siglec-14, suggesting that these receptors are expressed simultaneously in the same tissues, possibly on the same cell. Future carefully conducted expression studies are required, taking into account the fact that many of the currently available anti-Siglec-5 antibodies cross-react with Siglec-14. The cytoplasmic tail of Siglec-14 lacks the ITIM found in Siglec-5 and other CD33-related Siglecs and via an arginine residue in the transmembrane domain it associates with the activating adapter protein DAP12, suggesting that Siglec-14 functions as an activating receptor.109 Studies are needed to explore Siglec-14 function and to compare its biology to that of Siglec-5.
Siglec-15 (and Siglec-H)
There is limited information on Siglec-15, the smallest Siglec, possessing an unusually short cytoplasmic region. It is expressed on macrophages and dendritic cells.160 Its extracellular domain preferentially binds to α2–3-linked sialic acid–containing ligands, and the molecule associates via a charged transmembrane lysine residue (like Siglec-14) with the activating adaptor protein DAP12. This suggests that unlike most other Siglecs, Siglec-15 may function as an activating molecule. Unlike Siglec-14, Siglec-15 does not have another counterpart with inhibitory activity. Siglec-15 appears to be the only Siglec expressed in the immune system that has been conserved throughout vertebrate evolution. Thus, Siglec-15 has been proposed to play a critical regulatory role in immunity of a wide range of vertebrates, but its exact function is not yet known.160
Siglec-H was identified by one group of investigators as a marker of mouse plasmacytoid dendritic cells that produce type I IFNs.161,162 Like Siglec-15, Siglec-H is extremely small, lacks its own cytoplasmic signaling domains, and associates with DAP12. Engagement of either Siglec-H or DAP12 altered type I IFN responses of these cells to a TLR9 agonist. Zhang and coworkers used a partial sequence of Siglec-H obtained from mouse brain cDNA that they had previous used to search for novel murine Siglec-like genes7 to isolate a full-length sequence from a murine spleen cDNA library. They found that Siglec-H was expressed not only on plasmacytoid dendritic cells but also on subsets of macrophages in spleen and lymph nodes.163 These investigators were unable to detect carbohydrate binding ability (raising a concern that this molecule may lose its status as a true Siglec) or Siglec-H-modulated cytokine production, even in the presence of TLR9 agonists. Instead, they proposed that Siglec-H might function as an endocytic receptor. Experiments utilizing ovalbumin-conjugated anti-Siglec-H antibody to immunize mice in the presence of TLR9 agonists resulted in the elicitation of antigen-specific CD8+ T cells. These data suggest that Siglec-H may be a useful molecule for delivering antigens to plasmacytoid dendritic cells. Siglec-16, found in many different cells and tissues, was until recently felt to represent a pseudogene (SIGLEC16P). However, a polymorphism was just identified containing a four-base pair deletion. This latest version of Siglec-16 was indeed found to be expressed in the presence of DAP12 (like other late-numbered Siglecs) on tissue macrophages and brain, as well as lung and esophageal cancer.165
Conclusions
The last decade has witnessed an explosion of discoveries regarding the Siglec family of surface proteins. While much has been learned regarding their molecular, immunologic, and cellular biology as well as carbohydrate binding specificities, our knowledge of their in vivo biology and the true identity of their natural glycan ligands lags in comparison. Several Siglecs have already been exploited for diagnostic and therapeutic benefits, and others are likely being considered. Future studies will hopefully shed additional light on the roles these receptors play in innate and other immune responses and in disease pathogenesis.
Acknowledgments
This work was supported by grant #PBBEB-113394 from the Swiss National Foundation (to Dr. von Gunten), by grants AI41472 and AI72265 from the National Institutes of Health (to Dr. Bochner), and a grant from the Dana Foundation (to Dr. Bochner). Dr. Bochner also received support as a Cosner Scholar in Translational Research from Johns Hopkins University. The authors thank medical illustrator Jacqueline Schaffer for Figures 1 and 2.
Footnotes
Conflicts of Interest
Dr. Bochner is a co-author on existing and pending Siglec-8-related patents. If Siglec-8-related products are developed in the future, under a licensing agreement between Glaxo-SmithKline and the Johns Hopkins University, Dr. Bochner may be entitled to a share of royalties received by the University on the potential sales of such products. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Crocker PR, et al. Siglecs: a family sialic-acid binding lectins. Glycobiology. 1998;8:v–vi. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- 2.Varki A, Angata T. Siglecs – the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 3.Brinkman-Van der Linden EC, et al. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17:922–931. doi: 10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- 4.Angata T, Varki NM, Varki A. A second uniquely human mutation affecting sialic acid biology. J Biol Chem. 2001;276:40282–40287. doi: 10.1074/jbc.M105926200. [DOI] [PubMed] [Google Scholar]
- 5.Angata T, et al. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 2004;101:13251–13256. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aizawa H, et al. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G) Genomics. 2003;82:521–530. doi: 10.1016/s0888-7543(03)00171-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JQ, et al. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 8.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6′-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann A, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen DH, et al. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci USA. 2006;103:7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, et al. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulson JC, Blixt O, Collins BE. Sweet spots in functional glycomics. Nat Chem Biol. 2006;2:238–248. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]
- 13.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 14.Aizawa H, Plitt J, Bochner BS. Human eosinophils express two siglec-8 splice variants. J Allergy Clin Immunol. 2002;109:176. doi: 10.1067/mai.2002.120550. [DOI] [PubMed] [Google Scholar]
- 15.Avril T, et al. Negative regulation of leucocyte functions by CD33-related siglecs. Biochem Soc Trans. 2006;34:1024–1027. doi: 10.1042/BST0341024. [DOI] [PubMed] [Google Scholar]
- 16.Crocker PR, et al. Siglecs: a family of sialic-acid binding lectins. Glycobiology. 1998;8:v–vi. doi: 10.1093/oxfordjournals.glycob.a018832. [DOI] [PubMed] [Google Scholar]
- 17.Crocker PR, Varki A. Siglecs in the immune system. Immunology. 2001;103:137–145. doi: 10.1046/j.0019-2805.2001.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crocker PR, Varki A. Siglecs, sialic acids and innate immunity. Trends Immunol. 2001;22:337–342. doi: 10.1016/s1471-4906(01)01930-5. [DOI] [PubMed] [Google Scholar]
- 19.Crocker PR. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr Opin Struct Biol. 2002;12:609–615. doi: 10.1016/s0959-440x(02)00375-5. [DOI] [PubMed] [Google Scholar]
- 20.Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol. 2005;5:431–437. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Collins BE, Paulson JC. Cell surface biology mediated by low affinity multivalent protein-glycan interactions. Curr Opin Chem Biol. 2004;8:617–625. doi: 10.1016/j.cbpa.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Altheide TK, et al. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: Evidence for two modes of rapid evolution. J Biol Chem. 2006;281:25689–25702. doi: 10.1074/jbc.M604221200. [DOI] [PubMed] [Google Scholar]
- 23.Bock N, Kelm S. Binding and inhibition assays for Siglecs. Methods Mol Biol. 2006;347:359–375. doi: 10.1385/1-59745-167-3:359. [DOI] [PubMed] [Google Scholar]
- 24.von Gunten S, Simon HU. Sialic acid binding immunoglobulin-like lectins may regulate innate immune responses by modulating the life span of granulocytes. Faseb J. 2006;20:601–605. doi: 10.1096/fj.05-5401hyp. [DOI] [PubMed] [Google Scholar]
- 25.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 26.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 2007;87:851–857. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillan SJ, Crocker PR. CD33-related sialic-acid-binding immunoglobulin-like lectins in health and disease. Carbohydr Res. 2008;343:2050–2056. doi: 10.1016/j.carres.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Crocker PR, et al. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. Embo J. 1991;10:1661–1669. doi: 10.1002/j.1460-2075.1991.tb07689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munday J, Floyd H, Crocker PR. Sialic acid binding receptors (siglecs) expressed by macrophages. J Leukoc Biol. 1999;66:705–711. doi: 10.1002/jlb.66.5.705. [DOI] [PubMed] [Google Scholar]
- 30.Hartnell A, et al. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97:288–296. doi: 10.1182/blood.v97.1.288. [DOI] [PubMed] [Google Scholar]
- 31.McWilliam AS, Tree P, Gordon S. Interleukin 4 regulates induction of sialoadhesin, the macrophage sialic acid-specific receptor. Proc Natl Acad Sci USA. 1992;89:10522–10526. doi: 10.1073/pnas.89.21.10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Den Berg TK, et al. Regulation of sialoadhesin expression on rat macrophages. Induction by glucocorticoids and enhancement by IFN-beta, IFN-gamma, IL-4, and lipopolysaccharide. J Immunol. 1996;157:3130–3138. [PubMed] [Google Scholar]
- 33.Kirchberger S, et al. Human rhinoviruses inhibit the accessory function of dendritic cells by inducing sialoadhesin and B7-H1 expression. J Immunol. 2005;175:1145–1152. doi: 10.4049/jimmunol.175.2.1145. [DOI] [PubMed] [Google Scholar]
- 34.York MR, et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56:1010–1020. doi: 10.1002/art.22382. [DOI] [PubMed] [Google Scholar]
- 35.Blixt O, et al. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem. 2003;278:31007–31019. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 36.Van Den Berg TK, et al. Cutting edge: CD43 functions as a T cell counterreceptor for the macrophage adhesion receptor sialoadhesin (Siglec-1) J Immunol. 2001;166:3637–3640. doi: 10.4049/jimmunol.166.6.3637. [DOI] [PubMed] [Google Scholar]
- 37.Nath D, et al. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology. 1999;98:213–219. doi: 10.1046/j.1365-2567.1999.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanderheijden N, et al. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J Virol. 2003;77:8207–8215. doi: 10.1128/JVI.77.15.8207-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delputte PL, et al. Porcine arterivirus attachment to the macrophage-specific receptor sialoadhesin is dependent on the sialic acid-binding activity of the N-terminal immunoglobulin domain of sialoadhesin. J Virol. 2007;81:9546–9550. doi: 10.1128/JVI.00569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones C, Virji M, Crocker PR. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol Microbiol. 2003;49:1213–1225. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- 41.Monteiro VG, et al. Increased association of Trypanosoma cruzi with sialoadhesin positive mice macrophages. Parasitol Res. 2005;97:380–385. doi: 10.1007/s00436-005-1460-1. [DOI] [PubMed] [Google Scholar]
- 42.Biesen R, et al. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1136–1145. doi: 10.1002/art.23404. [DOI] [PubMed] [Google Scholar]
- 43.Pulliam L, Sun B, Rempel H. Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J Neuroimmunol. 2004;157:93–98. doi: 10.1016/j.jneuroim.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 44.Van Der Kuyl AC, et al. Sialoadhesin (CD169) expression in CD14+ cells is upregulated early after HIV-1 infection and increases during disease progression. PLoS ONE. 2007;2:e257. doi: 10.1371/journal.pone.0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ip CW, et al. Sialoadhesin deficiency ameliorates myelin degeneration and axonopathic changes in the CNS of PLP overexpressing mice. Neurobiol Dis. 2007;25:105–111. doi: 10.1016/j.nbd.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 46.Kobsar I, et al. Attenuated demyelination in the absence of the macrophage-restricted adhesion molecule sialoadhesin (Siglec-1) in mice heterozygously deficient in P0. Mol Cell Neurosci. 2006;31:685–691. doi: 10.1016/j.mcn.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Jiang HR, et al. Sialoadhesin promotes the inflammatory response in experimental autoimmune uveoretinitis. J Immunol. 2006;177:2258–2264. doi: 10.4049/jimmunol.177.4.2258. [DOI] [PubMed] [Google Scholar]
- 48.Oetke C, et al. Sialoadhesin-deficient mice exhibit subtle changes in B- and T-cell populations and reduced immunoglobulin M levels. Mol Cell Biol. 2006;26:1549–1557. doi: 10.1128/MCB.26.4.1549-1557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tedder TF, Poe JC, Haas KM. CD22: A Multifunctional Receptor That Regulates B Lymphocyte Survival and Signal Transduction. Adv Immunol. 2005;88:1–50. doi: 10.1016/S0065-2776(05)88001-0. [DOI] [PubMed] [Google Scholar]
- 50.Nitschke L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr Opin Immunol. 2005;17:290–297. doi: 10.1016/j.coi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Wakabayashi C, et al. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science. 2002;298:2392–2395. doi: 10.1126/science.1076963. [DOI] [PubMed] [Google Scholar]
- 52.Kimura N, et al. Human B-lymphocytes express alpha2–6-sialylated 6-sulfo-N-acetyllacto-samine serving as a preferred ligand for CD22/Siglec-2. J Biol Chem. 2007;282:32200–32207. doi: 10.1074/jbc.M702341200. [DOI] [PubMed] [Google Scholar]
- 53.Onodera T, et al. CD22 regulates time course of both B cell division and antibody response. J Immunol. 2008;180:907–913. doi: 10.4049/jimmunol.180.2.907. [DOI] [PubMed] [Google Scholar]
- 54.Poe JC, et al. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat Immunol. 2004;5:1078–1087. doi: 10.1038/ni1121. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh S, Bandulet C, Nitschke L. Regulation of B cell development and B cell signalling by CD22 and its ligands alpha2,6-linked sialic acids. Int Immunol. 2006;18:603–611. doi: 10.1093/intimm/dxh402. [DOI] [PubMed] [Google Scholar]
- 56.Collins BE, et al. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat Immunol. 2006;7:199–206. doi: 10.1038/ni1283. [DOI] [PubMed] [Google Scholar]
- 57.Steinfeld SD, Youinou P. Epratuzumab (humanised anti-CD22 antibody) in autoimmune diseases. Expert Opin Biol Ther. 2006;6:943–949. doi: 10.1517/14712598.6.9.943. [DOI] [PubMed] [Google Scholar]
- 58.Leonard JP, Goldenberg DM. Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies. Oncogene. 2007;26:3704–3713. doi: 10.1038/sj.onc.1210370. [DOI] [PubMed] [Google Scholar]
- 59.Andrews RG, Torok-Storb B, Bernstein ID. Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood. 1983;62:124–132. [PubMed] [Google Scholar]
- 60.Griffin JD, et al. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984;8:521–534. doi: 10.1016/0145-2126(84)90001-8. [DOI] [PubMed] [Google Scholar]
- 61.Peiper SC, Ashmun RA, Look AT. Molecular cloning, expression, and chromosomal localization of a human gene encoding the CD33 myeloid differentiation antigen. Blood. 1988;72:314–321. [PubMed] [Google Scholar]
- 62.Freeman SD, et al. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85:2005–2012. [PubMed] [Google Scholar]
- 63.Yokoi H, et al. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy. 2006;61:769–776. doi: 10.1111/j.1398-9995.2006.01133.x. [DOI] [PubMed] [Google Scholar]
- 64.Biedermann B, et al. Analysis of the CD33-related siglec family reveals that Siglec-9 is an endocytic receptor expressed on subsets of acute myeloid leukemia cells and absent from normal hematopoietic progenitors. Leuk Res. 2007;31:211–220. doi: 10.1016/j.leukres.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 65.von Gunten S, et al. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 2005;106:1423–1431. doi: 10.1182/blood-2004-10-4112. [DOI] [PubMed] [Google Scholar]
- 66.Andrews RG, et al. The L4F3 antigen is expressed by unipotent and multipotent colony-forming cells but not by their precursors. Blood. 1986;68:1030–1035. [PubMed] [Google Scholar]
- 67.Robertson MJ, et al. Human bone marrow depleted of CD33-positive cells mediates delayed but durable reconstitution of hematopoiesis: clinical trial of MY9 monoclonal antibody-purged autografts for the treatment of acute myeloid leukemia. Blood. 1992;79:2229–2236. [PubMed] [Google Scholar]
- 68.Valent P, et al. Mast cell typing: demonstration of a distinct hematopoietic cell type and evidence for immunophenotypic relationship to mononuclear phagocytes. Blood. 1989;73:1778–1785. [PubMed] [Google Scholar]
- 69.Valent P, et al. Further characterization of surface membrane structures expressed on human basophils and mast cells. Int Arch Allergy Appl Immunol. 1990;91:198–203. doi: 10.1159/000235115. [DOI] [PubMed] [Google Scholar]
- 70.Valent P, Bettelheim P. Cell surface structures on human basophils and mast cells. Biochemical and functional characterization. Adv Immunol. 1992;52:333–423. doi: 10.1016/s0065-2776(08)60879-2. [DOI] [PubMed] [Google Scholar]
- 71.Taylor VC, et al. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. J Biol Chem. 1999;274:11505–11512. doi: 10.1074/jbc.274.17.11505. [DOI] [PubMed] [Google Scholar]
- 72.Ulyanova T, et al. The sialoadhesin CD33 is a myeloid-specific inhibitory receptor. Eur J Immunol. 1999;29:3440–3449. doi: 10.1002/(SICI)1521-4141(199911)29:11<3440::AID-IMMU3440>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 73.Paul SP, et al. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood. 2000;96:483–490. [PubMed] [Google Scholar]
- 74.Walter RB, et al. ITIM-dependent endocytosis of CD33-related Siglecs: role of intracellular domain, tyrosine phosphorylation, and the tyrosine phosphatases, Shp1 and Shp2. J Leukoc Biol. 2008;83:200–211. doi: 10.1189/jlb.0607388. [DOI] [PubMed] [Google Scholar]
- 75.Vitale C, et al. Engagement of p75/AIRM1 or CD33 inhibits the proliferation of normal or leukemic myeloid cells. Proc Natl Acad Sci USA. 1999;96:15091–15096. doi: 10.1073/pnas.96.26.15091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vitale C, et al. Surface expression and function of p75/AIRM-1 or CD33 in acute myeloid leukemias: engagement of CD33 induces apoptosis of leukemic cells. Proc Natl Acad Sci USA. 2001;98:5764–5769. doi: 10.1073/pnas.091097198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balaian L, Zhong RK, Ball ED. The inhibitory effect of anti-CD33 monoclonal antibodies on AML cell growth correlates with Syk and/or ZAP-70 expression. Exp Hematol. 2003;31:363–371. doi: 10.1016/s0301-472x(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 78.Ferlazzo G, et al. Engagement of CD33 surface molecules prevents the generation of dendritic cells from both monocytes and CD34+ myeloid precursors. Eur J Immunol. 2000;30:827–833. doi: 10.1002/1521-4141(200003)30:3<827::AID-IMMU827>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 79.Lajaunias F, Dayer JM, Chizzolini C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur J Immunol. 2005;35:243–251. doi: 10.1002/eji.200425273. [DOI] [PubMed] [Google Scholar]
- 80.Orr SJ, et al. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood. 2007;109:1061–1068. doi: 10.1182/blood-2006-05-023556. [DOI] [PubMed] [Google Scholar]
- 81.Walter RB, et al. Phosphorylated ITIMs enable ubiquitylation of an inhibitory cell surface receptor. Traffic. 2008;9:267–279. doi: 10.1111/j.1600-0854.2007.00682.x. [DOI] [PubMed] [Google Scholar]
- 82.Grobe K, Powell LD. Role of protein kinase C in the phosphorylation of CD33 (Siglec-3) and its effect on lectin activity. Blood. 2002;99:3188–3196. doi: 10.1182/blood.v99.9.3188. [DOI] [PubMed] [Google Scholar]
- 83.Linenberger ML. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia. 2005;19:176–182. doi: 10.1038/sj.leu.2403598. [DOI] [PubMed] [Google Scholar]
- 84.Pagano L, et al. The role of Gemtuzumab Ozogamicin in the treatment of acute myeloid leukemia patients. Oncogene. 2007;26:3679–3690. doi: 10.1038/sj.onc.1210364. [DOI] [PubMed] [Google Scholar]
- 85.Tsimberidou AM, et al. The role of gemtuzumab ozogamicin in acute leukaemia therapy. Br J Haematol. 2006;132:398–409. doi: 10.1111/j.1365-2141.2005.05872.x. [DOI] [PubMed] [Google Scholar]
- 86.Sievers EL. Clinical studies of new “biologic” approaches to therapy of acute myeloid leukemia with monoclonal antibodies and immunoconjugates. Curr Opin Oncol. 2000;12:30–35. doi: 10.1097/00001622-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 87.Sievers EL. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukaemia in first relapse. Expert Opin Biol Ther. 2001;1:893–901. doi: 10.1517/14712598.1.5.893. [DOI] [PubMed] [Google Scholar]
- 88.Legrand O, et al. The immunophenotype of 177 adults with acute myeloid leukemia: proposal of a prognostic score. Blood. 2000;96:870–877. [PubMed] [Google Scholar]
- 89.Bross PF, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid. Leukemia Clin Cancer Res. 2001;7:1490–1496. [PubMed] [Google Scholar]
- 90.Tchilian EZ, et al. Molecular cloning of two isoforms of the murine homolog of the myeloid CD33 antigen. Blood. 1994;83:3188–3198. [PubMed] [Google Scholar]
- 91.Brinkman-Van der Linden EC, et al. CD33/Siglec-3 binding specificity, expression pattern, and consequences of gene deletion in mice. Mol Cell Biol. 2003;23:4199–4206. doi: 10.1128/MCB.23.12.4199-4206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vyas AA, et al. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci USA. 2002;99:8412–8417. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100:1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 94.Kursula P. Structural properties of proteins specific to the myelin sheath. Amino Acids. 2008;34:175–185. doi: 10.1007/s00726-006-0479-7. [DOI] [PubMed] [Google Scholar]
- 95.Mehta NR, et al. Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells. J Biol Chem. 2007;282:27875–27886. doi: 10.1074/jbc.M704055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun J, et al. Myelin-associated glycoprotein (Siglec-4) expression is progressively and selectively decreased in the brains of mice lacking complex gangliosides. Glycobiology. 2004;14:851–857. doi: 10.1093/glycob/cwh107. [DOI] [PubMed] [Google Scholar]
- 97.Swanson BJ, et al. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4 a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67:10222–10229. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 98.Pan B, et al. Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: neuropathology and behavioral deficits in single- and double-null mice. Exp Neurol. 2005;195:208–217. doi: 10.1016/j.expneurol.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cornish AL, et al. Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood. 1998;92:2123–2132. [PubMed] [Google Scholar]
- 100.Connolly NP, Jones M, Watt SM. Human Siglec-5: tissue distribution, novel isoforms and domain specificities for sialic acid-dependent ligand interactions. Br J Haematol. 2002;119:221–238. doi: 10.1046/j.1365-2141.2002.03808.x. [DOI] [PubMed] [Google Scholar]
- 101.Ghannadan M, et al. Detection of novel CD antigens on the surface of human mast cells and basophils. Int Arch Allergy Immunol. 2002;127:299–307. doi: 10.1159/000057747. [DOI] [PubMed] [Google Scholar]
- 102.Virgo P, et al. Identification of the CD33-related Siglec receptor, Siglec-5 (CD170), as a useful marker in both normal myelopoiesis and acute myeloid leukaemias. Br J Haematol. 2003;123:420–430. doi: 10.1046/j.1365-2141.2003.04625.x. [DOI] [PubMed] [Google Scholar]
- 103.Erickson-Miller CL, et al. Characterization of Siglec-5 (CD170) expression and functional activity of anti-Siglec-5 antibodies on human phagocytes. Exp Hematol. 2003;31:382–388. doi: 10.1016/s0301-472x(03)00046-8. [DOI] [PubMed] [Google Scholar]
- 104.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 105.Winterbourn CC, et al. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 106.Rapoport EM, et al. Sialoside-binding macrophage lectins in phagocytosis of apoptotic bodies. Biochemistry (Mosc) 2005;70:330–338. doi: 10.1007/s10541-005-0119-y. [DOI] [PubMed] [Google Scholar]
- 107.Avril T, et al. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J Biol Chem. 2005;280:19843–198451. doi: 10.1074/jbc.M502041200. [DOI] [PubMed] [Google Scholar]
- 108.Gunnarsson P, et al. The acute-phase protein alpha 1-acid glycoprotein (AGP) induces rises in cytosolic Ca2+ in neutrophil granulocytes via sialic acid binding immunoglobulin-like lectins (siglecs) Faseb J. 2007;21:4059–4069. doi: 10.1096/fj.07-8534com. [DOI] [PubMed] [Google Scholar]
- 109.Angata T, et al. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. Faseb J. 2006;20:1964–1973. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 110.Takei Y, et al. Molecular cloning of a novel gene similar to myeloid antigen CD33 and its specific expression in placenta. Cytogenet Cell Genet. 1997;78:295–300. doi: 10.1159/000134676. [DOI] [PubMed] [Google Scholar]
- 111.Patel N, et al. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J Biol Chem. 1999;274:22729–22738. doi: 10.1074/jbc.274.32.22729. [DOI] [PubMed] [Google Scholar]
- 112.Florian S, et al. Detection of novel leukocyte differentiation antigens on basophils and mast cells by HLDA8 antibodies. Allergy. 2006;61:1054–1062. doi: 10.1111/j.1398-9995.2006.01171.x. [DOI] [PubMed] [Google Scholar]
- 113.Falco M, et al. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J Exp Med. 1999;190:793–802. doi: 10.1084/jem.190.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nicoll G, et al. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J Biol Chem. 1999;274:34089–34095. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 115.Angata T, Varki A. Siglec-7: a sialic acid-binding lectin of the immunoglobulin superfamily. Glycobiology. 2000;10:431–438. doi: 10.1093/glycob/10.4.431. [DOI] [PubMed] [Google Scholar]
- 116.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004;279:43117–43125. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- 117.Yamaji T, et al. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J Biol Chem. 2002;277:6324–6332. doi: 10.1074/jbc.M110146200. [DOI] [PubMed] [Google Scholar]
- 118.Avril T, et al. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841–6849. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- 119.Avril T, et al. Probing the cis interactions of the inhibitory receptor Siglec-7 with alpha2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of alpha2,8-sialyltransferase gene expression. J Leukoc Biol. 2006;80:787–796. doi: 10.1189/jlb.1005559. [DOI] [PubMed] [Google Scholar]
- 120.Orr SJ, et al. SOCS3 Targets Siglec 7 for Proteasomal Degradation and Blocks Siglec 7-mediated Responses. J Biol Chem. 2007;282:3418–3422. doi: 10.1074/jbc.C600216200. [DOI] [PubMed] [Google Scholar]
- 121.Vitale C, et al. Surface expression and function of p75/AIRM-1 or CD33 in acute myeloid leukemias: Engagement of CD33 induces apoptosis of leukemic cells. Proc Natl Acad Sci USA. 2001;98:5764–5769. doi: 10.1073/pnas.091097198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scott CJ, et al. Immunocolloidal targeting of the endocytotic siglec-7 receptor using peripheral attachment of siglec-7 antibodies to poly(lactide-co-glycolide) nanoparticles. Pharm Res. 2008;25:135–146. doi: 10.1007/s11095-007-9400-7. [DOI] [PubMed] [Google Scholar]
- 123.Kikly KK, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J Allergy Clin Immunol. 2000;105:1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 124.Floyd H, et al. Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 125.Guo JP, et al. Siglec-8 and Siglec-F: inhibitory receptors on eosinophils, basophils and mast cells. Allergy Clin Immunol Inter J World Allergy Org. 2007;19:54–59. [Google Scholar]
- 126.Von Gunten S, Bochner BS. Expression and function of Siglec-8 in human eosinophils, basophils and mast cells. In: Pawankar R, Holgate S, Rosenwasser LJ, editors. Allergy Frontiers: From Epigenetics to Future Perspectives. Springer-Verlag; Tokyo: 2008. (in press) [Google Scholar]
- 127.Foussias G, Yousef GM, Diamandis EP. Molecular characterization of a Siglec-8 variant containing cytoplasmic tyrosine-based motifs, and mapping of the Siglec-8 gene. Biochem Biophys Res Commun. 2000;278:775–781. doi: 10.1006/bbrc.2000.3866. [DOI] [PubMed] [Google Scholar]
- 128.Munday J, et al. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem J. 2001;355:489–497. doi: 10.1042/0264-6021:3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nutku E, et al. Expression and function of Siglec-8 isoforms in human eosinophils, basophils and mast cells. In: Bienenstock J, Ring J, Togias AG, editors. Allergy Frontiers and Futures, Proceedings of the 24th Symposium of the Collegium Internationale Allergologicum. Hogrefe and Huber; Cambridge, Massachusetts: 2004. pp. 130–132. [Google Scholar]
- 130.Nutku E, et al. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 131.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–924. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 132.von Gunten S, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119:1005–1011. doi: 10.1016/j.jaci.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 133.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–124. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Landwehr LP, et al. Benefits of high-dose i.v. immunoglobulin in patients with severe steroid-dependent asthma. Chest. 1998;114:1349–1356. doi: 10.1378/chest.114.5.1349. [DOI] [PubMed] [Google Scholar]
- 135.O’Donnell BF, et al. Intravenous immunoglobulin in autoimmune chronic urticaria. Br J Dermatol. 1998;138:101–106. doi: 10.1046/j.1365-2133.1998.02033.x. [DOI] [PubMed] [Google Scholar]
- 136.Tsurikisawa N, et al. Treatment of Churg-Strauss syndrome with high-dose intravenous immunoglobulin. Ann Allergy Asthma Immunol. 2004;92:80–87. doi: 10.1016/S1081-1206(10)61714-0. [DOI] [PubMed] [Google Scholar]
- 137.Yokoi H, et al. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by Siglec-8 engagement. J Allergy Clin Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 138.Stevens WW, et al. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ohnmacht C, et al. Analysis of eosinophil turnover in vivo reveals their active recruitment to and prolonged survival in the peritoneal cavity. J Immunol. 2007;179:4766–4774. doi: 10.4049/jimmunol.179.7.4766. [DOI] [PubMed] [Google Scholar]
- 140.Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007;81:1434–1444. doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- 141.Kearley J, et al. Anti-Siglec-F antibody treatment during allergen-induced airway inflammation reduces eosinophil numbers but has no effect on airway hyperreactivity in vivo. Am J Respir Crit Care Med. 2007;175:A690. (abstr.) [Google Scholar]
- 142.Zimmermann N, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bochner BS, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- 144.Rapoport EM, et al. Probing sialic acid binding Ig-like lectins (siglecs) with sulfated oligosaccharides. Biochemistry (Mosc) 2006;71:496–504. doi: 10.1134/s0006297906050051. [DOI] [PubMed] [Google Scholar]
- 145.Tateno H, et al. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol. 2007;27:5699–5710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo J, et al. Ligands for Siglec-8 and Siglec-F: binding characteristics and tissue distribution. J Allergy Clin Immunol. 2007;119:S299. (abstr.) [Google Scholar]
- 147.Angata T, Varki A. Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. J Biol Chem. 2000;275:22127–22135. doi: 10.1074/jbc.M002775200. [DOI] [PubMed] [Google Scholar]
- 148.Zhang JQ, et al. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J Biol Chem. 2000;275:22121–22126. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- 149.Yamaji T, et al. Characterization of inhibitory signaling motifs of the natural killer cell receptor Siglec-7: attenuated recruitment of phosphatases by the receptor is attributed to two amino acids in the motifs. Glycobiology. 2005;15:667–676. doi: 10.1093/glycob/cwi048. [DOI] [PubMed] [Google Scholar]
- 150.von Gunten S, et al. Immunologic and functional evidence for anti-Siglec-9 autoantibodies in intravenous immunoglobulin preparations. Blood. 2006;108:4255–4259. doi: 10.1182/blood-2006-05-021568. [DOI] [PubMed] [Google Scholar]
- 151.Buenz EJ, Howe CL. Appropriate use of intravenous immunoglobulin in neonatal neutropenia. J Perinatol. 2007;27:196–197. doi: 10.1038/sj.jp.7211660. author reply 197. [DOI] [PubMed] [Google Scholar]
- 152.Yu Z, et al. mSiglec-E, a novel mouse CD33-related siglec (sialic acid-binding immunoglobulin-like lectin) that recruits Src homology 2 (SH2)-domain-containing protein tyrosine phosphatases SHP-1 and SHP-2. Biochem J. 2001;353:483–492. doi: 10.1042/0264-6021:3530483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li N, et al. Cloning and characterization of Siglec-10, a novel sialic acid binding member of the Ig superfamily, from human dendritic cells. J Biol Chem. 2001;276:28106–28112. doi: 10.1074/jbc.M100467200. [DOI] [PubMed] [Google Scholar]
- 154.Whitney G, et al. A new siglec family member, siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur J Biochem. 2001;268:6083–6096. doi: 10.1046/j.0014-2956.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 155.Yousef GM, et al. Molecular characterization, tissue expression, and mapping of a novel Siglec-like gene (SLG2) with three splice variants. Biochem Biophys Res Commun. 2001;284:900–910. doi: 10.1006/bbrc.2001.5053. [DOI] [PubMed] [Google Scholar]
- 156.Kitzig F, et al. Cloning of two new splice variants of Siglec-10 and mapping of the interaction between Siglec-10 and SHP-1. Biochem Biophys Res Commun. 2002;296:355–362. doi: 10.1016/s0006-291x(02)00885-9. [DOI] [PubMed] [Google Scholar]
- 157.Whitney G, et al. A new siglec family member, siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur J Biochem. 2001;268:6083–6096. doi: 10.1046/j.0014-2956.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 158.Azuma C, et al. Cloning of cDNA for human T-cell replacing factor (interleukin-5) and comparison with the murine homologue. Nucleic Acids Res. 1986;14:9149–9158. doi: 10.1093/nar/14.22.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Angata T, et al. Cloning and characterization of human Siglec-11. A recently evolved signaling that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem. 2002;277:24466–24474. doi: 10.1074/jbc.M202833200. [DOI] [PubMed] [Google Scholar]
- 160.Angata T, et al. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838–846. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- 161.Blasius AL, Colonna M. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends Immunol. 2006;27:255–260. doi: 10.1016/j.it.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 162.Blasius AL, et al. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang J, et al. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 164.Campanero-Rhodes MA, et al. Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem Biophys Res Commun. 2006;344:1141–1146. doi: 10.1016/j.bbrc.2006.03.223. [DOI] [PubMed] [Google Scholar]
- 165.Cao H, et al. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol. 2008;38:2303–2315. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]