Abstract
Multivitamin use is common in the United States. It is not known whether multivitamins with minerals supplements (MVM) used by women already diagnosed with invasive breast cancer would affect their breast cancer mortality risk. To determine prospectively the effects of MVM use on breast cancer mortality in postmenopausal women diagnosed with invasive breast cancer, a prospective cohort study was conducted of 7,728 women aged 50–79 at enrollment in the women's health initiative (WHI) in 40 clinical sites across the United States diagnosed with incident invasive breast cancer during WHI and followed for a mean of 7.1 years after breast cancer diagnosis. Use of MVM supplements was assessed at WHI baseline visit and at visit closest to breast cancer diagnosis, obtained from vitamin pill bottles brought to clinic visit. Outcome was breast cancer mortality. Hazard ratios and 95 % confidence intervals (CIs) for breast cancer mortality comparing MVM users to non-users were estimated using Cox proportional hazard regression models. Analyses using propensity to take MVM were done to adjust for potential differences in characteristics of MVM users versus non-users. At baseline, 37.8 % of women reported MVM use. After mean post-diagnosis follow-up of 7.1 ± 4.1 (SD) years, there were 518 (6.7 %) deaths from breast cancer. In adjusted analyses, breast cancer mortality was 30 % lower in MVM users as compared to non-users (HR = 0.70; 95 % CI 0.55, 0.91). This association was highly robust and persisted after multiple adjustments for potential confounding variables and in propensity score matched analysis (HR = 0.76; 95 % CI 0.60–0.96). Postmenopausal women with invasive breast cancer using MVM had lower breast cancer mortality than non-users. The results suggest a possible role for daily MVM use in attenuating breast cancer mortality in women with invasive breast cancer but the findings require confirmation.
Keywords: Breast cancer, Multivitamins, Vitamins, Women's health initiative, WHI, Breast cancer mortality
Introduction
Multivitamin use is common in the United States [1] in adults and in women with breast cancer [2], yet findings regarding multivitamins with minerals (MVM) influence on breast cancer incidence and outcome are mixed. With respect to breast cancer incidence, the women's health initiative (WHI) investigated multivitamin use in 161,608 women and reported no effect on incident breast cancer or on other common cancer, cardiovascular disease, or total mortality [3]. Park et al. [4] found no association between multivitamin use and breast cancer incidence or overall mortality in the Multiethnic Cohort. In addition, no association between multivitamin use and breast cancer incidence was seen in a recent meta-analysis of observational studies which included over 350,000 women [5]. A recent report from the Iowa Women's Health Study of 38,772 older women found that several commonly used vitamin and mineral supplements were associated with increased total mortality risk [6].
The use of multivitamins, especially regarding those with anti-oxidant activity, in women with diagnosed breast cancer has been controversial. A prospective cohort study of 4,877 women with invasive breast cancer in Shanghai, China found lower mortality and recurrence risk with vitamin supplement use regardless of concurrent or non-concurrent chemotherapy, but only among those who did not receive radiotherapy [7]. In contrast, in the life after cancer epidemiology study, multivitamin use was associated with a non-significant lower recurrence and mortality, but limited to women who had been treated with radiation only [8]. Observational analyses investigating the relationship between dietary supplement use, primarily vitamins, and breast cancer recurrence and/or survival have generally provided mixed results [8–11]. In a comparative study involving 90 women with non-metastatic breast cancer, women who were prescribed a mega dose vitamin and mineral regimen as compared to matched controls had somewhat higher, though not statistically significant breast cancer mortality [12]. Another study reported multivitamin use in patients with early-stage breast cancer may be associated with lower mortality [8]. Multivitamins contain antioxidants, including carotenoids. In the life after cancer epidemiology study of 2,264 breast cancer survivors, vitamin C and E supplementation was associated with a 27 % lower risk for recurrent breast cancer and carotenoid supplementation was associated with increased risk of death from breast cancer [11]. Given the mixed results, and the generally small number of breast cancer cases in these studies, whether the use of multivitamins affects survival in women diagnosed with breast cancer has not been established.
The WHI has the largest cohort of breast cancer cases among postmenopausal women and includes 8,166 incident invasive breast cancer cases of which 1,358 died during follow-up. Thus it provides an excellent opportunity to examine the associations of multivitamin use and breast cancer mortality in women diagnosed with incident breast cancer during participation in WHI.
Methods
Population and enrollment
The WHI enrolled 161,608 postmenopausal women aged 50–79 years in 40 clinical centers throughout the US during the years 1993–1998. The design and baseline characteristics of participants are described in detail elsewhere [13]. All participants signed an informed consent and Institutional Review Boards at all participating institutions approved the protocols and procedures.
Women could be enrolled into overlapping clinical trials (WHI-CT; N = 67,932) or a long-term follow-up observational study (WHI-OS; N = 93,676). There were two clinical trials evaluating postmenopausal hormones with cancer and coronary heart disease as primary endpoints: estrogen plus progestin versus placebo (N = 16,608) for women with an intact uterus and estrogen versus placebo (N = 10,739) for women who had a hysterectomy. A dietary modification trial (DM) was also performed to evaluate a low total fat diet on breast and colorectal cancer incidence (N = 48,836). Women who were either in the hormone or diet trials could also join a calcium/vitamin D supplementation trial (N = 36,282) whose primary interest was colorectal cancer and osteoporotic fractures. Excluded from the WHI were participants who had medical conditions predictive of survival of less than 3 years, had conditions (like alcoholism or dementia) making it unlikely they could participate. In addition for the clinical trials, they were excluded if they had breast cancer prior to enrollment or any cancer except non-melanoma skin cancer in the past 10 years. Follow-up in WHI was high (95 % overall); 5.2 % of women stopped follow-up or were otherwise lost to follow-up (5.8 % in CT and 4.8 % in OS).
The study population includes the 8,163 women in the OS and CT with invasive breast cancer diagnosed through September 2012. Excluded were 110 women with multiple breast cancer occurrences, 5 women with breast cancer diagnosed on their date of death, and 320 who took multivitamins without minerals, resulting in 7,728 for the primary analyses, of whom N = 3,266 were clinical trial participants and N = 4,462 were Observational Study participants.
Breast cancer screening and diagnosis
Mammogram and breast cancer screening frequency were protocol defined in the clinical trials and were performed at baseline and annually in the hormone trials and at baseline and at two-year intervals in the DM trial. Mammography and breast exam frequency were not protocol defined in the observational study but information on their usage was collected at baseline and annually. The details of identification and adjudication of incident breast cancer cases have been published previously [14]. In brief, medical records were obtained for self-reported breast cancers identified on annual (WHI-OS) or semi-annual (WHI-CT) questionnaires or by report of third parties to WHI staff. Medical records were reviewed and locally adjudicated by trained physicians. Final central adjudication and coding of histology, stage, and hormone receptor status (by local laboratory determination) were performed at the clinical coordinating center by adjudicators blind to study arm.
Multivitamin exposure
Participants completed multiple questionnaires about their physical and mental health and co-morbid conditions and had a baseline clinic visit with physical measurements, and a fasting blood draw. Participants brought in their medications and dietary supplements in original pill bottles to their baseline visit and annual visits (WHI-CT) or 3-year follow-up visit (WHI-OS). Information on multivitamin use, with or without minerals, was collected in a standardized manner for vitamins used at least once a week in the last 2 weeks. We do not have information on frequency of use, however, multivitamins with minerals usually have 20–30 vitamins and minerals, often at levels of 100 % US RDA or less and are usually recommended on the bottle to be taken daily.
Outcomes ascertainment
The primary outcome was breast cancer mortality among women previously diagnosed with incident invasive breast cancer. There were no deaths during follow-up among the 1,839 women who had in situ breast cancer only, and these are not included in any analyses in this report. Cause of death was ascertained by review of death certificates, medical records or autopsy reports at the Clinical Coordinating Center. The National Death Index was searched periodically to identify deaths of participants lost to follow-up. Ascertainment and adjudication methods are described in detail elsewhere [14].
Psychosocial covariates
Depression was assessed with the Burnam algorithm [15] which is a regression equation giving different weights to six items from the CES-D scale [16] and two items from the Diagnostic Interview Schedule [17]. Social functioning, social support, and emotional well-being are subscales from the SF-36 developed for the Medical Outcomes Study, with higher values indicated better function or well being [18].
Statistical methods
In the analytic cohort of 7,728, baseline characteristics of those using MVM were compared to non-users by Pearson Chi square (or Fishers exact Chi square) or t test (Mann–Whitney U test) as appropriate. Similarly, baseline characteristics were compared by mortality status (alive vs. breast cancer death). Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95 % confidence intervals (CIs) of death from breast cancer, for users of MVM as compared to non-users. All Cox models were stratified on study arms (HRT, DM and OS) allowing the baseline hazard function to vary by study arm.
Primary analyses considered MVM use reported at baseline. Median time between baseline and breast cancer diagnosis was 5.92 years [mean (SD) = 6.46 (4.06)]. Secondary analyses took into account MVM use at the visit closest to breast cancer diagnosis; 82.4 % of women had their closest MVM use recorded at a visit prior to their breast cancer diagnosis (median = 2.92 years prior to diagnosis) and 17.6 % had their closest visit after diagnosis (median = 0.75 years after diagnosis). Of women taking MVM at baseline 76.7 % also took MVM at the visit closest to breast cancer diagnosis, indicating fairly stable use of MVM; We additionally looked at these consistent users (N = 2,239), i.e., those using MVM both at baseline and at closest to diagnosis visit (excluding those who took MVM at baseline but not at closest visit to diagnosis or vice versa). The comparison group for these consistent users was the consistent non-users of MVM at both baseline and closest visit (N = 3,358).
Cox regression analyses were conducted with serial adjustment for potential confounders: Model 1 reports HRs adjusted for age at breast cancer diagnosis and race/ethnicity; Model 2 additionally adjusts for three key variables related to the outcome including stage of breast cancer, tumor estrogen receptor, and progestin receptor status; Model 3 further adjusts for variables significantly related to both MVM use and breast cancer mortality which included education, smoking status, BMI, alcohol use, physical activity, self-reported health, and diabetes. Follow-up time after breast cancer diagnosis was determined as the later of time to death or if no death occurred, time to end of follow-up as of September 2012. Mean follow-up time after breast cancer diagnosis was 7.1 years, SD = 4.13; median time was 6.7 years.
As an additional means of controlling for potential confounding which may be due to the different overall characteristics of users versus non-users, we calculated propensity to use MVM by running a logistic regression with MVM use as the dependent variable and 33 covariates in the model to predict MVM use from demographic, lifestyle, risk factor, and comorbidity variables measured at baseline. Thus these propensity scores are a weighted composite of the 33 individual covariates for each person (list of covariates in Appendix A). The C-statistic was 0.61 indicating only fair ability of the variables used in the propensity score analysis to discriminate use of multivitamins. We divided the propensity scores (or probability of MVM use) into deciles and then used the propensity decile for each participant as a covariate in the Cox regression (Model 4) which included the variables in Model 2 and the propensity decile. We also ran Model 4 further adjusting for grade of breast cancer. In addition to adjusting for propensity decile as described in Model 4 above, we matched those taking MVM with those not taking MVM by propensity probability which resulted in a diminished sample size of 4404. We then compared the matched groups on breast cancer mortality. The matched groups were balanced on age at diagnosis, race/ethnicity, education, income, smoking status, BMI, waist–hip ratio, alcohol use, physical activity, depression, self-reported health, social functioning, history of CVD, hypertension, and, history of diabetes. Women taking MVM were more likely to also take additional individual supplements of vitamins C, E, calcium or zinc, than those who were non-MVM users, so use of any single supplements was controlled for in the Cox regressions comparing the matched groups.
To assess variables which may be modifiers of the effects of MVM on breast cancer mortality, we examined potential interactions between MVM use and age at breast cancer diagnosis, race/ethnicity, BMI, stage of breast cancer, ER or PR assay positivity and use of other supplements both statistically by including the product terms in the regression models and qualitatively by comparing magnitudes of the stratified HR's. Sensitivity analyses were performed excluding those women in the calcium/Vitamin D trial, as well as women taking individual supplements of vitamins C, D, E, Calcium or Zinc.
All reported Cox models were assessed for violations of the proportional hazards assumptions using graphical approaches and goodness of fit tests with no substantial violations found. All analyses were done using STATA version 12 or SAS version 9.1.
Results
Of 8,048 women who had incident invasive breast cancer, did not have multiple occurrences of breast cancer and were diagnosed with breast cancer prior to their death, 40.4 % (N = 3,240) used any type of multivitamin at baseline, 36.3 % (N = 2,920) used MVM, and 4 % (N = 320) used multivitamins without minerals. Our primary analyses excluded the 320 women who used multivitamins without minerals, although we did a sensitivity analysis including them. Of the 7,728 women in our analytic cohort, 518 (6.7 %) died from breast cancer. Table 1 shows characteristics of women with invasive breast cancer overall and stratified by MVM use. Mean age at diagnosis was 69.8 years (SD = 7.6). Women taking MVM at baseline (N = 2,920, 38 %) as compared to non-users at baseline (N = 4,808, 62 %) were slightly older, more likely to be white, have higher education and income, less likely to be current smokers, more likely to have lower BMI and WHR, more active, reported higher levels of self-reported general health, and less likely to have a history of diabetes; all p < 0.05. They were also more likely than non-MVM users to be taking additional supplement of vitamins C (37.5 vs. 20.9 %), E (42.5 vs. 24.4 %), calcium (30.9 vs. 18.9 %), and zinc (4.4 vs. 3.0 %); all p ≤ 0.001.
Table 1. Characteristics overall and by multivitamin with mineral use at baseline.
| All women (N = 7,728) | Multivitamin with minerals | |||
|---|---|---|---|---|
|
| ||||
| No (N = 4,808) | Yes (N = 2,920) | p value | ||
| Age at breast cancer diagnosis (years) | ||||
| Mean ± SD | 69.8 ± 7.6 | 69.7 ± 7.5 | 70.1 ± 7.6 | <0.01 |
| N (%) | N (%) | N (%) | ||
| 50–59 | 724 (9.4) | 456 (9.5) | 268 (9.2) | 0.05 |
| 60–69 | 3,009 (38.9) | 1,918 (39.9) | 1,091 (37.4) | |
| 70+ | 3,995 (51.7) | 2,434 (50.6) | 1,561 (53.5) | |
| Race/Ethnicity | <0.001 | |||
| White | 6,758 (87.6) | 4,103 (85.6) | 2,655 (91.0) | |
| Black | 532 (6.9) | 410 (8.6) | 122 (4.2) | |
| Other | 423 (5.5) | 282 (5.9) | 141 (4.8) | |
| Education | <0.001 | |||
| <High school | 1,425 (18.6) | 941 (19.7) | 484 (16.7) | |
| Some college | 2,842 (37.0) | 1,801 (37.7) | 1,041 (35.9) | |
| College graduate | 3,409 (44.4) | 2,030 (42.5) | 1,379 (47.5) | |
| Income | <0.001 | |||
| <$20,000 | 946 (13.1) | 648 (14.4) | 298 (10.9) | |
| $20,000-$34,999 | 1,737 (24.0) | 1,087 (24.1) | 650 (23.7) | |
| $35,000-$49,999 | 1,560 (21.5) | 967 (21.5) | 593 (21.6) | |
| $50,000+ | 3,006 (41.5) | 1,805 (40.1) | 1,201 (43.8) | |
| Smoking | <0.001 | |||
| Current | 492 (6.5) | 347 (7.3) | 145 (5.0) | |
| Former | 3,443 (45.1) | 2,081 (43.8) | 1,362 (47.2) | |
| Never | 3,697 (48.4) | 2,321 (48.9) | 1,376 (47.7) | |
| Stage | 0.18 | |||
| Localized | 5,754 (75.3) | 3,551 (74.9) | 2,203 (76.0) | |
| Regional | 1,784 (23.4) | 1,118 (23.6) | 666 (23.0) | |
| Distant | 101 (1.3) | 71 (1.5) | 30 (1.0) | |
| ER assay | 0.28 | |||
| Positive | 6,063 (84.4) | 3,767 (84.1) | 2,296 (85.0) | |
| Negative | 1,117 (15.6) | 713 (15.9) | 404 (15.0) | |
| PR assay | 0.23 | |||
| Positive | 5,035 (71.1) | 3,115 (70.5) | 1,920 (71.9) | |
| Negative | 2,052 (29.0) | 1,301 (29.5) | 751 (28.1) | |
| Mortality status | 0.51 | |||
| Alive | 6,446 (83.4) | 4,000 (83.2) | 2,446 (83.8) | |
| Dead | 1,282 (16.6) | 808 (16.8) | 474 (16.2) | |
| Cause of death | <0.01 | |||
| Breast cancer | 518 (40.4) | 352 (43.6) | 166 (35.0) | |
| Other | 764 (59.6) | 456 (56.4) | 308 (65.0) | |
| Region | <0.001 | |||
| North East | 1,803 (23.3) | 1,122 (23.3) | 681 (23.3) | |
| South | 1,874 (24.3) | 1,256 (26.1) | 618 (21.2) | |
| Mid-west | 1,692 (21.9) | 1,066 (22.2) | 626 (21.4) | |
| West | 2,359 (30.5) | 1,364 (28.4) | 995 (34.1) | |
| BMI | ||||
| Mean ± SD | 28.3 ± 5.9 | 28.6 ± 6.0 | 27.7 ± 5.6 | <0.001 |
| Normal (<24.9) | 2,522 (32.9) | 1,472 (30.9) | 1,050 (36.2) | <0.001 |
| Overweight (25.0–29.9) | 2,642 (34.5) | 1,619 (34.0) | 1,023 (35.3) | |
| Obesity I (30.0–34.9) | 1,532 (20.0) | 1,016 (21.3) | 516 (17.8) | |
| Obesity II–III (≥35.0) | 967 (12.6) | 659 (13.8) | 308 (10.6) | |
| Waist–hip ratio | 0.813 ± 0.084 | 0.814 ± 0.082 | 0.810 ± 0.087 | 0.04 |
| Alcohol | 0.01 | |||
| Non-drinker | 716 (9.3) | 480 (10.1) | 236 (8.1) | |
| Past drinker | 1,284 (16.8) | 817 (17.2) | 467 (16.1) | |
| <1 Drink per week | 2,512 (32.8) | 1,568 (32.9) | 944 (32.5) | |
| 1 ≤ 7 a week | 2,073 (27.0) | 1,249 (26.2) | 824 (28.4) | |
| 7+ a week | 1,081 (14.1) | 651 (13.7) | 430 (14.8) | |
| Physical activity | <0.001 | |||
| No activity | 1,119 (15.3) | 740 (16.4) | 379 (13.5) | |
| Limited duration activity | 2,929 (40.0) | 1,882 (41.8) | 1,047 (37.3) | |
| 2–4 episodes/week | 1,369 (18.7) | 846 (18.8) | 523 (18.6) | |
| 4 episodes/week | 1,901 (26.0) | 1,039 (23.1) | 862 (30.7) | |
| Psychosocial measures depression | 0.43 | |||
| Depressive symptoms below cut point (<0.06) | 6,804 (90.1) | 4,214 (89.9) | 2,590 (90.5) | |
| Depressive symptoms above cut point ≤ 0.06 | 746 (9.9) | 473 (10.1) | 273 (9.5) | |
| Social support | 0.83 | |||
| Less than maximum (score < 45) | 6,418 (84.8) | 3,989 (84.9) | 2,429 (84.7) | |
| Maximum (score = 45) | 1,150 (15.2) | 711 (15.1) | 439 (15.3) | |
| Social functioning | 0.95 | |||
| Some problems (score < 100) | 2,542 (33.2) | 1,580 (33.2) | 962 (33.1) | |
| No problems (score = 100) | 5,119 (66.8) | 3,178 (66.8) | 1,941 (66.9) | |
| Emotional well-being | 0.82 | |||
| Below mean (<84) | 3,523 (46.1) | 2,193 (46.3) | 1,330 (46.0) | |
| Above mean (≥84) | 4,112 (53.9) | 2,549 (53.8) | 1,563 (54.0) | |
| Self-reported health | 0.02 | |||
| Excellent/very good | 4,695 (61.1) | 2,865 (60.0) | 1,830 (62.9) | |
| Good | 2,403 (31.3) | 1,526 (31.9) | 877 (30.2) | |
| Fair/poor | 588 (7.7) | 387 (8.1) | 201 (6.9) | |
| Co-Morbidities hypertension | 0.21 | |||
| No | 4,799 (66.0) | 2,920 (65.3) | 1,879 (67.2) | |
| Untreated | 546 (7.5) | 337 (7.5) | 209 (7.5) | |
| Treated | 1,925 (26.5) | 1,216 (27.2) | 709 (25.4) | |
| History of diabetes | 0.03 | |||
| No | 7,329 (94.9) | 4,540 (94.5) | 2,789 (95.6) | |
| Yes | 397 (5.1) | 267 (5.6) | 130 (4.5) | |
| History of CVD | 0.48 | |||
| No | 7,084 (91.7) | 4,399 (91.5) | 2,685 (92.0) | |
| Yes | 644 (8.3) | 409 (8.5) | 235 (8.1) | |
| High cholesterol requiring pills | 0.87 | |||
| No | 6,252 (86.4) | 3,839 (86.4) | 2,413 (86.5) | |
| Yes | 984 (13.6) | 607 (13.1) | 377 (13.5) | |
| Vitamin supplementation use | ||||
| Vitamin c single supplement | <0.001 | |||
| No | 5,626 (72.8) | 3,802 (79.1) | 1,824 (62.5) | |
| Yes | 2,102 (27.2) | 1,006 (20.9) | 1,096 (37.5) | |
| Vitamin E single supplement | <0.001 | |||
| No | 5,316 (68.8) | 3,636 (75.6) | 1,680 (57.5) | |
| Yes | 2,412 (31.2) | 1,172 (24.4) | 1,240 (42.5) | |
| Vitamin D single supplement | 0.69 | |||
| No | 7,401 (95.8) | 4,608 (95.8) | 2,793 (95.6) | |
| Yes | 327 (4.2) | 200 (4.2) | 127 (4.4) | |
| Calcium single supplement | <0.001 | |||
| No | 5,917 (76.6) | 3,900 (81.1) | 2,017 (69.1) | |
| Yes | 1,811 (23.4) | 908 (18.9) | 903 (30.9) | |
| Zinc Single Supplement | 0.001 | |||
| No | 7,458 (96.5) | 4,666 (97.1) | 2,792 (95.6) | |
| Yes | 270 (3.5) | 142 (3.0) | 128 (4.4) | |
| Vitamin E, C, D, calcium or zinc single supplement use | <0.001 | |||
| No | 3,901 (50.5) | 2,875 (59.8) | 1,026 (35.1) | |
| Yes | 3,827 (49.5) | 1,933 (40.2) | 1,894 (64.9) | |
Table 2 contains the hazard ratios (HR) from Cox proportional hazards models. The minimally adjusted hazard ratio of MVM use for breast cancer mortality finds a protective association of MVM (HR = 0.77, 95 % CI 0.64–0.93). Neither additional adjustment for receptor status variables, and stage of disease (Model 2), nor full adjustment for multiple covariates (Model 3), changed this protective association. Although stage of the disease can be in the pathway between MVM use and breast cancer mortality, adjusting for it did not alter the HR of MVM use from the unadjusted model. A model adjusting for age at diagnosis, race, receptor status, other supplement use, and deciles of propensity to be taking MVM (Model 4) yielded similar results (HR = 0.70, 95 % CI 0.55–0.90). In addition adjusting for grade (well differentiated N = 1,957 [27.9 %], moderately differentiated N = 3,091 [44.1 % ] and poorly differentiated or anaplastic N = 1,957 [27.9 % ]) did not change the hazard ratio estimate (HR = 0.73, 95 % CI 0.57–0.95), nor was it changed after adjustment for hormone use at baseline (HR = 0.69, 95 % CI 0.54, 0.89), nor after excluding women assigned to the treatment arm of the hormone trials (HR = 0.70, 95 % CI 0.54–0.91), data not displayed.
Table 2. Hazard Ratio (95 % CI) for breast cancer mortality.
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Baseline multivitamin with mineral (MVM) use | N = 7,713 | N = 7,010 | N = 6,415 | N = 5,178 |
| 2918 MVM users | 2651 MVM users | 2470 MVM users | 2039 MVM users | |
| 516 breast cancer deaths | 427 breast cancer deaths | 387 breast cancer deaths | 303 breast cancer deaths | |
| 0.77 (0.64, 0.93) | 0.76 (0.62, 0.94) | 0.76 (0.61, 0.95) | 0.70 (0.55, 0.90) | |
| Closest multivitamin with mineral (MVM) use | N = 7,713 | N = 7,010 | N = 6,415 | N = 5,178 |
| 3736 MVM users | 3411 MVM users | 3131 MVM users | 2560 MVM users | |
| 516 breast cancer deaths | 427 breast cancer deaths | 387 breast cancer deaths | 303 breast cancer deaths | |
| 0.70 (0.59, 0.84) | 0.69 (0.57, 0.84) | 0.70 (0.57, 0.86) | 0.64 (0.50, 0.81) | |
| Consistent multivitamin with mineral (MVM) use | N = 5,585 | N = 5,060 | N = 4,654 | N = 3,739 |
| 2234 MVM users | 2030 MVM users | 1896 MVM users | 1562 MVM users | |
| 408 breast cancer deaths | 333 breast cancer deaths | 305 breast cancer deaths | 240 breast cancer deaths | |
| 0.69 (0.56, 0.85) | 0.67 (0.53, 0.85) | 0.66 (0.52, 0.85) | 0.59 (0.44, 0.78) |
All models stratified on study arms (HRT and DM)
Model 1: Adjusted for age at breast cancer diagnosis and race/ethnicity
Model 2: Adjusted for age at breast cancer diagnosis, race/ethnicity, estrogen receptor status, progestin receptor status and stage of cancer at diagnosis (regional/distant vs. localized)
Model 3: Adjusted for variables in Model 2 plus education, smoking status, body mass index, alcohol use, physical activity, self-reported health and diabetes
Model 4: Adjusted for variables in Model 2 plus propensity deciles
We also assessed the association between MVM use and breast cancer mortality taking MVM use reported at the visit closest to the woman's breast cancer diagnosis and similar associations were found (Table 2—Model 4 adjustment HR = 0.64, 95 % CI 0.50–0.81). Analyses of the consistent users (both at baseline and at closest visit to diagnosis) also showed robust finding of a protective association Model 4 adjustment: HR = 0.59, 95 % CI 0.44, 0.78.
We did a number of sensitivity analyses (Table 3). Excluding from analyses those in the calcium and vitamin D arm, we found no substantial differences in any of the regression models for breast cancer mortality (Model 4 HR = 0.68, 95 % CI 0.52–0.89). Excluding women taking single supplements of vitamins C, D, E, calcium or zinc also did not substantially affect the magnitude of association for breast cancer mortality (Model 4 adjustment HR = 0.68, 95 % CI 0.47–0.99). Excluding women who were in the OS who had reported a history of breast cancer at baseline (N = 323) but who were included in our analytic cohort because they had a subsequent breast cancer diagnosis (i.e., a new or recurring cancer) the Model 4 adjustment HR was 0.69, 95 % CI 0.54–0.90). Lastly, excluding women who were in the clinical trials, and were enrolled in the observational study with no history of breast cancer at baseline, the Model 4 adjustment hazard ratio was 0.59, 95 % CI 0.42–0.82. Additional adjustment for baseline hormone use did not change this estimate (HR = 0.61, 95 % CI 0.44–0.85). We also did an analysis of all multivitamin users, including the 320 women who used multivitamins without minerals and found similar results (HR = 0.77, 95 % CI 0.63–0.94).
Table 3. Sensitivity analyses of baseline MVM use for breast cancer mortality.
| Adjusteda HR (95 % CI) | |
|---|---|
| Excluding women in calcium and vitamin D arm | N = 4,086 |
| MVM users = 1,639 | |
| Breast cancer deaths = 246 | |
| 0.68 (0.52, 0.89) | |
| Excluding women taking single supplements of vitamins C, D, E, calcium or zinc | N = 2,585 |
| MVM users = 712 | |
| Breast cancer deaths = 171 | |
| 0.68 (0.47, 0.99) | |
| Excluding women with history of breast cancer at baseline | N = 4,949 |
| MVM users = 1,946 | |
| Breast cancer deaths = 289 | |
| 0.69 (0.54–0.90) | |
| Excluding women in any clinical trial (i.e. among OS women only and free of breast cancer at baseline) | N = 2,900 |
| MVM users = 1,233 | |
| Breast cancer deaths = 174 | |
| 0.59 (0.44, 0.85) | |
| Any MV use (MV with minerals and/or without minerals) at baseline | N = 7,308 |
| Any MV users: 2,949 | |
| Breast cancer deaths = 445 | |
| 0.77 (0.63, 0.94) |
Adjusted for age at breast cancer diagnosis, race/ethnicity, estrogen receptor status, progestin receptor status and stage of cancer at diagnosis (regional/distant vs. localized) and propensity deciles
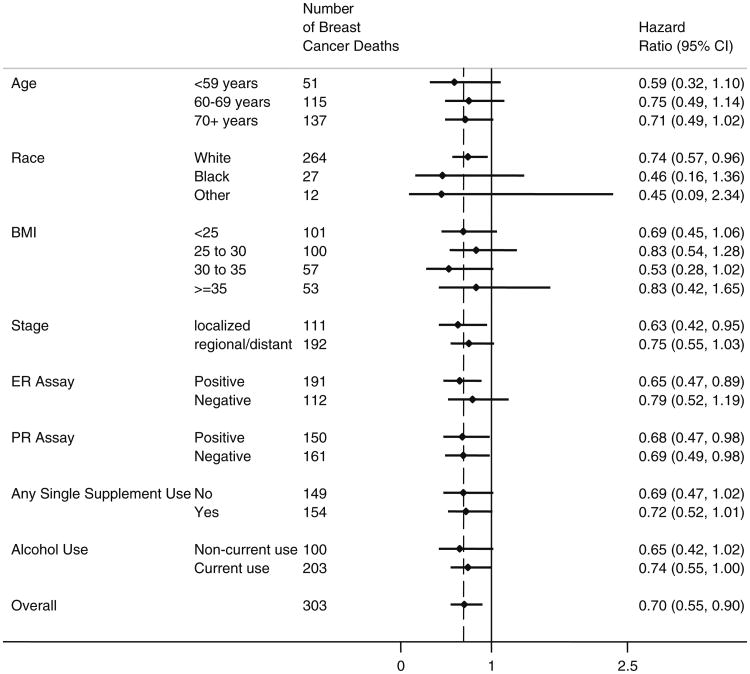
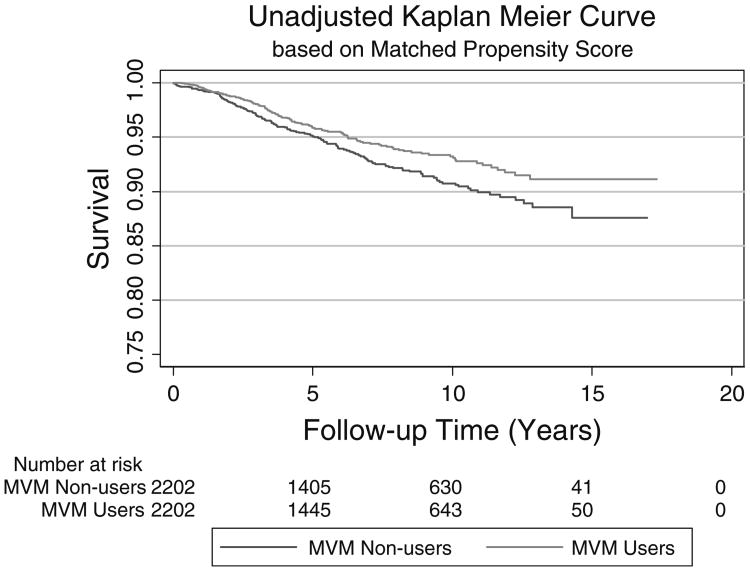
Several potential effect modifiers were evaluated. None of the interaction terms tested indicated significant statistical interaction (all p-interaction > 0.20). Figure 1 shows that the MVM association was similar in most subgroups and the HR's did not differ from the overall HR of 0.70. Analysis of the two groups matched on propensity for taking MVM resulted in an unadjusted HR of 0.76 (95 % CI 0.60–0.96). Adjusting for age at breast cancer diagnosis, race, estrogen, and progestin receptor status, stage of breast cancer and use of any individual supplements did not substantially change this estimate (HR = 0.75, 95 % CI 0.57,1.00). Figure 2 shows the Kaplan–Meier survival curves of the matched groups indicating lower breast cancer mortality for MVM users as compared to non-users.
Fig. 1.
Stratified adjusted* hazard ratios (95 % CI) for baseline multivitamin with mineral (MVM) Use with breast cancer mortality by selected characteristics *adjusted for age at breast cancer diagnosis and race/ethnicity, estrogen receptor status, progestin receptor status, stage of cancer at diagnosis and propensity deciles (Model 4)
Fig. 2.
Unadjusted Kaplan–Meier curve comparing MVM users versus non-users at baseline among those in matched propensity score analysis (N = 4,404)
We were not able to obtain hazard ratios for the effects on breast cancer mortality of taking MVM after diagnosis of breast cancer because we do not have sufficient data to answer this question. In the observational study, vitamin and medications use was assessed only at baseline and at year 3 so that anyone who had a diagnosis after year 3 would not have had another assessment of MVM use. In the clinical trials, although assessment was made more frequently, 51 % of the women with breast cancer in the clinical trials have no information on MVM use after diagnosis, leaving only N = 1,588 who have such information. Among those 1,588, there were 312 women who had not used MVM before diagnosis but did use MVM after diagnosis; 7.7 % (N = 24) of them died of breast cancer as compared to 34 of the 632 (5.4 %) women who used MVM both before and after diagnosis.
Discussion
In the WHI's study of 7,728 postmenopausal women with incident invasive breast cancer followed for an average of 7.1 years (SD = 4.1) after diagnosis, use of multivitamins with minerals was associated with a 30 % significantly lower breast cancer mortality as compared to non-use. This protective association was highly robust and persisted after multiple adjustments and for all subgroups examined.
Use of dietary supplements is estimated at greater than 50 % in US adults and use of multivitamin/multimineral supplements at 33 % [2,11]. MVM remain the most commonly consumed dietary supplement among US adults, including cancer survivors. Evidence also suggests an increase in supplement use, including MVM, after breast cancer therapy [19]. While a comprehensive review of publications suggested that between 45 and 80 % of breast cancer patients in the US use antioxidant supplements, including during cancer therapy [11, 20], a recent report during a multi-center breast cancer clinical trial (S0221) suggested substantially lower usage of vitamins C, D, E, B6, B12, folic acid, and calcium, with usage even lower during chemotherapy [21]. The use of multivitamins with minerals in breast cancer survivors was not examined in that trial setting. In our cohort of 7,728 breast cancer cases, 38 % (N = 2,920) used MVM at baseline which was before their breast cancer diagnosis, and an additional 9.5 % (N = 731) used MVM at any visit after diagnosis.
Yet, there remains limited evidence of the relationship between MVM use and breast cancer mortality. Relatively few studies have examined the influence of multivitamin use on breast cancer outcome and these have had a much smaller number of breast cancer cases than the current report. None have examined this issue exclusively in postmenopausal women diagnosed with invasive breast cancer. Cross study comparison of results from these studies has proven difficult, given differences in study design and endpoints. Many of the studies pertain to use of antioxidants such as vitamins C and E, rather than multivitamins with minerals which contain antioxidants but also generally more than 20 other vitamins and minerals. The Iowa Women's Health Study, whose general findings were that several commonly used vitamin and mineral supplements may increase total mortality risk is not comparable to our study since it pertained to overall mortality in generally healthy women rather than among invasive breast cancer survivors who were the subjects of our study. In regard to overall cancer mortality, the Iowa study found that there was no effect of multivitamin use (HR = 1.00; 95 % CI 0.94–1.07).
The current findings of lower breast cancer mortality with MVM use in the largest number to date of breast cancer cases in postmenopausal women, suggest possible benefit for multivitamin use in women with diagnosed invasive breast cancer. However, cautious interpretation is needed, especially for concurrent use during chemotherapy and radiation therapy. We do not have data on treatment of breast cancer in the cases and cannot comment on the potential interactions between MVM use and breast cancer therapy in this study.
Several identified mechanisms could mediate an effect of multivitamins and minerals, perhaps especially antioxidants, on breast cancer mortality. Mitochondrial oxidative stress is a driver of tumor progression and metastases, and in preclinical models, oxidative stress is reduced by anti-oxidants [22]. In addition, metastatic breast cancer is dependent on angiogenesis and antioxidants have anti-angiogenic activity in preclinical models. In the clinic, in a study of 84 breast cancer patients receiving tamoxifen, the anti-oxidants CoQ (10), riboflavin, and niacin reduced proangiogenic marker levels [23]. It is also possible that MVM use is a modifier of some other currently unknown risk factor effects leading to breast cancer mortality. Since MVM contain antioxidant vitamins as well as minerals, we cannot determine if the observed beneficial association is largely due to some of the individual components of the MVM preparations. A sizable percentage of women who used MVM in our study also used vitamin C (37.5 %) and/or vitamin E (42.5 %) as a single supplement. Breast cancer mortality did not differ between those who used vitamin C as a single supplement and those who did not, but those who took vitamin E had a slightly lower breast cancer unadjusted mortality rate (5.9 %) as compared to those who did not (7.1 %), p < 05. A sensitivity analysis excluding those women who took any single supplement did not affect our results of a protective association of MVM use.
The strengths of our study are the detailed characterization of the WHI participants, serial assessment of mammography, breast cancer verification by central medical record and pathology report review, and the large number of breast cancer cases with long-term follow-up for breast cancer mortality determination. Another strength is that the exposure, MVM use, was assessed through an inventory system where participants were asked to bring supplement bottles to the clinic visit where staff transcribed the contents using a standardized protocol. This led to a uniform definition of MVM across the entire WHI and participants did not need to rely on memory for their current supplement use. In addition we took great care to attempt to control for confounding due to the different characteristics of women who use MVM versus those who do not, by controlling for those variables related to both to exposure and outcome, by calculating propensity to take MVM and controlling for the propensity score and by comparing two groups matched on propensity score. Nevertheless, study limitations include possible residual confounding due to baseline differences between MVM users and non-users despite the analytic model adjustments. Another possible limitation is the absence of breast cancer treatment or recurrence information. However, since treatment is usually dependent on the stage and receptor status, we controlled for these in our multivariate analyses. The observational study design limits causal interpretation; however, it is a prospective study of women diagnosed with invasive breast cancer and is superior to cross-sectional or case–control studies. The findings cannot be generalized to premenopausal women diagnosed with breast cancer or to other populations of women.
In conclusion, this large prospective study among US older women showed a consistent association between multivitamin and mineral use and lower breast cancer mortality. Such suggestive evidence of benefit of MVM in the breast cancer setting is intriguing but should be followed up in future investigations.
Acknowledgments
The authors thank the WHI participants, Investigators and staff for their important contributions. The Women's Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The funding organization (NIH) had no role in the design and conduct of the study; analysis or interpretation of the data or preparation, review, or approval of the manuscript or the decision to submit the manuscript for publication. The funding organization was involved in designing the WHI protocol and in management of WHI.
Appendix. Variables use in logistic regression for propensity analysis
Age,
Race/ethnicity
Education
Income
Region of the country
Having a health care provider
Having last visit to health care provider within past year
Alcohol use
High cholesterol requiring pills
History of diabetes
Being on treatment for diabetes
Smoking
Hormone use
Body mass index
Waist/Hip ratio
Calories from diet
Hypertension treatment
Systolic blood pressure
Diastolic blood pressure
Hysterectomy
Physical activity
Activities of daily living
Life events score
General health
Social support
Social functioning
Emotional well-being
Depression score
History of atrial fibrillation
History of pulmonary embolism
History of cardiovascular disease
History of heart failure
History of any cancer (except non-melanoma skin cancer)
Footnotes
Conflict of interest: All authors state they have no conflicts of interest.
Contributor Information
S. Wassertheil-Smoller, Email: sylvia.smoller@einstein.yu.edu, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USA.
A. P. McGinn, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USA
N. Budrys, Department of Obstetrics and Gynecology, University of Oklahoma Health Science Center, Oklahoma City, OK, USA
R. Chlebowski, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA, USA
G. Y. Ho, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USA
K. C. Johnson, Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, TN, USA
D. S. Lane, Department of Preventive Medicine, Stony Brook University School of Medicine, Stony Brook, NY, USA
W. Li, Division of Preventive and Behavioral Medicine, University of Massachusetts Medical School, Worcester, MA, USA
M. L. Neuhouser, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA 98109-1024, USA
J. Saquib, Stanford Prevention Research Center, Stanford University, Stanford, CA, USA
J. M. Shikany, Division of Preventive Medicine, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA
Y. Song, Division of Preventive Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02215, USA
C. Thomson, Zuckerman College of Public Health, University of Arizona Cancer Center, Tucson, AZ, USA
References
- 1.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 2.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol. 2008;26(4):665–673. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- 3.Neuhouser ML, Wassertheil-Smoller S, Thomson C, Aragaki A, Anderson GL, Manson JE, Patterson RE, Rohan TE, van Horn L, Shikany JM, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women's Health Initiative cohorts. Arch Intern Med. 2009;169(3):294–304. doi: 10.1001/archinternmed.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Multivitamin use and the risk of mortality and cancer incidence: the multiethnic cohort study. Am J Epidemiol. 2011;173(8):906–914. doi: 10.1093/aje/kwq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan AL, Leung HW, Wang SF. Multivitamin supplement use and risk of breast cancer: a meta-analysis. Ann Pharmacother. 2011;45(4):476–484. doi: 10.1345/aph.1P445. [DOI] [PubMed] [Google Scholar]
- 6.Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR., Jr Dietary supplements and mortality rate in older women: the Iowa Women's Health Study. Arch Intern Med. 2011;171(18):1625–1633. doi: 10.1001/archinternmed.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nechuta S, Lu W, Chen Z, Zheng Y, Gu K, Cai H, Zheng W, Shu XO. Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20(2):262–271. doi: 10.1158/1055-9965.EPI-10-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan ML, Greenlee H, Lee VS, Castillo A, Gunderson EP, Habel LA, Kushi LH, Sweeney C, Tam EK, Caan BJ. Multivitamin use and breast cancer outcomes in women with early-stage breast cancer: the life after cancer epidemiology study. Breast Cancer Res Treat. 2011;130(1):195–205. doi: 10.1007/s10549-011-1557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleischauer AT, Simonsen N, Arab L. Antioxidant supplements and risk of breast cancer recurrence and breast cancer-related mortality among postmenopausal women. Nutr Cancer. 2003;46(1):15–22. doi: 10.1207/S15327914NC4601_02. [DOI] [PubMed] [Google Scholar]
- 10.Saquib J, Rock CL, Natarajan L, Saquib N, Newman VA, Patterson RE, Thomson CA, Al-Delaimy WK, Pierce JP. Dietary intake, supplement use, and survival among women diagnosed with early-stage breast cancer. Nutr Cancer. 2011;63(3):327–333. doi: 10.1080/01635581.2011.535957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenlee H, Kwan ML, Kushi LH, Song J, Castillo A, Weltzien E, Quesenberry CP, Jr, Caan BJ. Antioxidant supplement use after breast cancer diagnosis and mortality in the life after cancer epidemiology (LACE) cohort. Cancer. 2012;118(8):2048–2058. doi: 10.1002/cncr.26526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesperance ML, Olivotto IA, Forde N, Zhao Y, Speers C, Foster H, Tsao M, MacPherson N, Hoffer A. Mega-dose vitamins and minerals in the treatment of non-metastatic breast cancer: an historical cohort study. Breast Cancer Res Treat. 2002;76(2):137–143. doi: 10.1023/a:1020552501345. [DOI] [PubMed] [Google Scholar]
- 13.Group TWS. Design of the Women's Health Initiative clinical Trial and Observational Study. Control Clin Trials. 1988;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 14.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 15.Burnam M, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988;26:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 17.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National institute of mental health diagnostic interview schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 19.Velentzis LS, Keshtgar MR, Woodside JV, Leathem AJ, Titcomb A, Perkins KA, Mazurowska M, Anderson V, Wardell K, Cant-well MM. Significant changes in dietary intake and supplement use after breast cancer diagnosis in a UK multicentre study. Breast Cancer Res Treat. 2011;128(2):473–482. doi: 10.1007/s10549-010-1238-8. [DOI] [PubMed] [Google Scholar]
- 20.Greenlee H, Hershman DL, Jacobson JS. Use of antioxidant supplements during breast cancer treatment: a comprehensive review. Breast Cancer Res Treat. 2009;115(3):437–452. doi: 10.1007/s10549-008-0193-0. [DOI] [PubMed] [Google Scholar]
- 21.Zirpoli GR, Brennan PM, Hong CC, McCann SE, Ciupak G, Davis W, Unger JM, Budd GT, Hershman DL, Moore HC, et al. Supplement use during an intergroup clinical trial for breast cancer (S0221) Breast Cancer Res Treat. 2013;137(3):903–913. doi: 10.1007/s10549-012-2400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sotgia F, Martinez-Outschoorn UE, Lisanti MP. Mitochondrial oxidative stress drives tumor progression and metastasis: should we use antioxidants as a key component of cancer treatment and prevention? BMC Med. 2011;9:62. doi: 10.1186/1741-7015-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Premkumar VG, Yuvaraj S, Sathish S, Shanthi P, Sachdanandam P. Anti-angiogenic potential of Coenzyme Q10, riboflavin and niacin in breast cancer patients undergoing tamoxifen therapy. Vascul Pharmacol. 2008;48(4–6):191–201. doi: 10.1016/j.vph.2008.02.003. [DOI] [PubMed] [Google Scholar]