Abstract
On a recognition test, stimuli originally encoded in the context of shock threat show an enhanced late parietal positivity during later recognition compared to stimuli encoded during safety, particularly for emotionally arousing stimuli. The present study investigated whether this ERP old/new effect is further influenced when a threat context is reinstated during the recognition test. ERPs were measured in a yes-no recognition test for words rated high or low in emotional arousal that were encoded and recognized in the context of cues that signaled threat of shock or safety. Correct recognition of words encoded under threat, irrespective of reinstatement, was associated with an enhanced old-new ERP difference (500–700 ms; centro-parietal), and this difference was only reliable for emotionally arousing words. Taken together, the data suggest that information processed in a stressful context are associated with better recollection on later recognition, an effect that was not modulated by reinstating the stressful context at retrieval.
Keywords: Emotion, Memory, Stress, Threat, ERPs, Context-dependent memory, Reinstatement
1. Introduction
Anticipating the presentation of an aversive event such as electric shock not only activates measurable defensive reactions, such as skin conductance elevation, startle reflex potentiation and cardiac deceleration (Bradley et al., 2005; Bradley et al., 2008; Grillon et al., 1991; Grillon and Davis, 1995; Melzig et al., 2008), but also impacts later memory for items encoded under threat. For instance, when participants are informed at encoding that they will be shocked if an item is later forgotten, items processed under threat of shock are better recognized than items not encoded under threat (Murty et al., 2012). Measuring ERPs at recognition, we found that participants who incidentally encoded words under threat of shock showed an enhanced late parietal ERP old/new difference during later recognition, compared to words encoded in the context of safety (Weymar et al., 2013), suggesting better recollection (Rugg & Curran, 2007; Voss & Paller, 2008). Interestingly, this ERP difference was most reliable for emotional words encoded under threat, suggesting that threat of shock specifically facilitates recollection of emotional stimuli.
Some evidence suggests that events are better remembered when the original learning environment is reinstated at test, compared to when testing occur in a different environmental context (Smith & Vela, 2001). For instance, rats show better memory in a maze if the room lights are the same during test and learning (Carr, 1917). In humans, evidence suggests that the external environment (Smith, Glenberg, & Bjork, 1978; Godden & Baddeley, 1975), pharmacological state (Eich, 1980), mood state (Bower, Monteiro & Gilligan, 1978), level of arousal (Clark et. al. 1984), and posture (Rand & Wapner, 1967) can be salient contextual cues. Effects of contextual cues at retrieval are assumed to reflect the fact that focal stimuli are associated with incidental background cues and the presence of these contextual cues at test facilitates episodic retrieval (Smith, 1994; Smith & Vela, 2001). The present study investigated the role of reinstating a threat context during later recognition. Specifically, we tested whether the enhanced old/new ERP difference previously found for words encoded during shock threat is further enhanced when the recognition test is also conducted under threat of shock.
Previous ERP memory studies have reported multiple old/new differences (larger positivity for correctly recognized items compared to correctly rejected new items) during recognition. An early (300–500 ms) frontal old/new difference has been linked to familiarity-based recognition (Rugg and Curran, 2007) and a later occuring (> 500 ms) centro-parietal old/new effect has been related to explicit recollection, as it is largest for correct source and remember judgments (e.g., Wilding & Rugg, 1996; Düzel, Yonelinas, Mangun, Heinze, & Tulving, 1997, Weymar, Löw, Schwabe, & Hamm, 2010) and those made with high confidence ratings (e.g., Weymar et al., 2009). The late parietal ERP old/new effect is also enhanced when recognizing emotional, compared to neutral stimuli (e.g., Ferrari et al, 2012; Inaba et al., 2005; Johansson et al., 2004; Newsome, Dulas, & Duarte, 2012; Weymar et al., 2009, 2010, 2011), suggesting that recognition of emotional events may be more often mediated by explicit recollection (see for review: LaBar & Cabeza, 2006).
In the current study, a color was used to cue periods in which the participant could receive an electric shock, whereas another color signaled there was no possibility of receiving an electric shock; these threat and safety periods varied in length from 12 to 36 s. Words rated high or low in emotional arousal were encoded in the context of threat or safety cue without an intentional memory instruction. We expected to replicate our previous finding of a larger late old/new difference over parietal sensors for emotionally arousing words encoded under threat of shock, compared to safety, when there was no threat of shock during the recognition test (Weymar et al., 2013). If reinstating the threat context further facilitates recognition, a larger late parietal positivity should be found when stimuli are encoded and tested under threat of shock (e.g., threat-threat), compared to when items are encoded under threat but tested in a safe context (i.e. threat-safe).
In contrast to predictions that reinstating a threat context may facilitate episodic memory, some data indicate that stress during testing can impair memory performance (Schwabe & Wolf, 2013). For instance, when participants learn word lists and are then exposed to a psychosocial stressor (free speech in front of a committee) just prior to testing, memory performance was impaired compared to participants who did not experience stress (Kuhlmann et al., 2005). If stress generally impairs memory processing, effects exactly opposite to those predicted by the reinstatement account are expected: a larger late parietal potential should be found when recognizing words encoded during threat and tested during safety (i.e. threat-safe), compared to when encoded and tested under threat (threat-threat). Moreover if a threat context at test generally impairs recognition performance, items encoded during safety and tested under threat (i.e. safe-threat) should show smaller recognition ERPs compared to safe items tested in the context of safety (safe-safe).
2. Material and methods
2.1. Participants
Participants were 28 students (13 female, 15 male; mean age: 19.4 years; six left-handed) from a General Psychology course at the University of Florida who participated for course credit. All participants had normal or corrected-to normal vision, were native speakers of English, and provided informed written consent for a protocol approved by the UF Institutional Review Board.
2.2 Materials and Procedure
Overall, 240 nouns were selected from the Affective Norms for English Words (ANEW; Bradley and Lang, 1999), consisting of 48 unpleasant words (24 high arousal vs. 24 low arousal words), 48 pleasant words (24 high arousal vs. 24 low arousal words) and 24 neutral low arousal words. Two sets of 120 stimuli were matched on the basis of hedonic valence, arousal (see ANEW norms, Bradley and Lang, 1999) and word frequency (Kucera and Francis, 1967)1. During encoding, each of the two word sets was presented to approximately half of the participants.
Of the 120 words presented at encoding, half were printed in a font color that signaled threat of shock (blue or yellow; counterbalanced across participants), and half were printed in a font color that denoted safety (blue or yellow). Each word was presented for 6 sec with no inter-trial interval (ITI). Threat and safety periods varied in duration from 12 to 36 s (i.e., 2 to 6 words in the same font color). To encourage processing of the words, participants were told to press a button whenever the word "window" appeared (this word was only presented on the last trial).
During recognition, each participant viewed both sets of words (240 words), resulting in 120 old words and 120 new words. Of the old words, half had been encoded in the context of threat of shock (half of each hedonic content), and half had been encoded in safe context. Old and new words were presented in black font color and a colored background frame signaled threat of shock or safety (blue or yellow; same assignment as during encoding)2. Of the old words that had been encoded under threat or safety, half were presented in the same context (congruent) during recognition as during encoding (e.g., threat-threat or safe-safe), and half were presented in the incongruent context (e.g., threat-safe; safe-threat). Each word was presented for 2 sec followed by the question “old/new?” for 3 s. Threat and safety periods varied in duration from 10 to 30 s (i.e., 2 to 6 words and the question slide with the same frame color). An electric shock occurred only once in the experiment, at the end of the recognition phase, when the previously unexperienced mild shock (5 mA, 32 msec duration) was delivered through the shock sensor using a constant current electro stimulator (Grass Instruments Co., Model SIU7, Quincy, Mass, USA) during a 5 s threat period. This trial was not included in the analyses.
The experiment took place in a comfortable chair in a sound-attenuated dimly lit room. Participants were instructed that when a word was presented in one color, an electric shock could be delivered through a stimulating bar electrode attached to the inner surface of the right wrist, whereas no shock was possible if the word was presented in the other color. No shock work-up was conducted and no mention of a memory test was made (incidental encoding). Immediately after encoding, the recognition memory task occurred, in which old and new words were presented. Participants were instructed that a background frame of one color again indicated periods where the participant might receive an electric shock, whereas no shock was possible if the background frame was in the other color. Participants were instructed to decide whether each word had previously been seen in the experiment or not Following word offset3 the question “Old/ New?” appeared, and the participants pressed the “old” button if they remembered the word, or else the “new” button. The assignment of left and right button presses to old/new responses was counterbalanced across participants.
2.3. EEG Recording
EEG signals were recorded continuously from 128 electrodes using an Electrical Geodesic system and digitized at a rate of 250 Hz, using the vertex sensor (Cz) as recording reference. Scalp impedance for each sensor was kept below 50 kΩ, as recommended by the manufacturer guidelines. All channels were bandpass filtered online from 0.1 to 48 Hz. Off-line reduction was performed using EMEGS (Peyk, De Cesarei, & Junghöfer, 2011) and included low-pass filtering at 40 Hz, artifact detection, sensor interpolation, baseline correction, and conversion to an average reference (Junghöfer et al., 2000). Stimulus-synchronized epochs were extracted from 100 msec before to 1200 msec after picture onset and baseline corrected (100 msec prior to stimulus onset).
2.4. Data analysis
ERPs were computed for each sensor and participant. For recognition, only trials with correct responses were included in ERP averages. In consideration of previous research and based on inspection of the waveforms (Rugg & Curran, 2007; Weymar et al., 2013), mean ERP amplitudes were analyzed in a window 500 and 700 msec over centro-parietal and occipital brain regions, where the difference between old and new conditions was maximal. The ERP old/new difference was analyzed in an ANOVA including the factors context during encoding (threat vs. safe), context during during recognition (2: threat vs safe), and word emotionality (2: high arousal, low arousal).
For behavioral performance hit rate (H), false alarm rate (FA), recognition accuracy (Pr = H - FA), and response bias (Br = p(FA) / p(1-Pr)) were analyzed using an ANOVA involving the factors context (2: threat, safe), congruency (2: congruent, incongruent) and arousal (2: arousal high, arousal low).
For effects involving repeated measures, the Greenhouse-Geisser procedure was used to correct violations of sphericity.
3. Results
3.1. Ratings
Post-experimental ratings of aversiveness (7-point scale: 1 aversive – 7 pleasant) confirmed that cues signaling threat of shock were rated as more unpleasant than cues signaling safe periods, both during encoding (threat: Mean = 3.7, SD = 1.3; safe: Mean = 5.8, SD = 1.3, F(1,27)= 63.72, p< .0001) and during recognition (threat: Mean = 4.1, SD 1.4; safe: Mean = 5.6, SD = 1.4, F(1,27)= 28.46, p< .0001).
3.2. Recognition
Table 1 lists memory performance for old and new words encoded in the context of threat and safe cues and tested either in a threat (threat-threat, safe-threat) or safe context (threat-safe, safe-safe). For hits, a main effect of word emotionality (F(1,27) = 5.84, p< .05), indicated significantly overall higher accuracy when recognizing emotionally arousing words, compared to words low in emotional arousal. False alarms, F(1,27) = 1.06, p= .31 on the other hand, neither threat of shock at encoding (context: for hit rate, (F(1,27) = 2.70, p= .11 ; for Pr, (F(1,27) = 1.04, p= .32) or recognition (both F(1,27) <1) affected performance.
Table 1.
Hits, false alarms (FA) and discrimination performance (Pr) for emotionally arousing words and low arousal words that were encoded under threat of shock or safety, and tested under threat of shock or safety.
| Encoding Context | ||||||
|---|---|---|---|---|---|---|
| Recognition Context | Threat | Safe | ||||
| Hits | FA | Pr | Hits | FA | Pr | |
| Threat | ||||||
| Emotionally arousing words | .75 (.17) | .24 (.17) | .51 (.25) | .75 (.16) | .25 (.16) | .51 (.23) |
| Low arousal words | .73 (.14) | .23 (.16) | .50 (.22) | .73 (.15) | .23 (.13) | .50 (.19) |
| Safe | ||||||
| Emotionally arousing words | .77 (.14) | .27 (.16) | .50 (.24) | .80 (.12) | .22 (.17) | .58 (.22) |
| Low arousal words | .73 (.14) | .22 (.17) | .50 (.21) | .74 (.15) | .23 (.14) | .51 (.18) |
Numbers in parentheses indicate standard deviation. Higher Pr values (Hits minus False Alarms) indicate better discrimination ability between old and new items.
Figure 1 illustrates grand average posterior ERPs for emotionally arousing (top panel) and low arousal (bottom panel) words that were encoded in threat or safety and tested in threat (left) or safety (right), as well as new words. Correct recognition of old words, compared to new words, was accompanied by enhanced positivity that was most pronounced over posterior scalp regions4, F(1,27)= 7.14, p< .01 (see Table 2). A larger ERP old/new difference was found for words encoded in the context of threat, compared to safety, F(1,27)= 5.40, p< .05 and an interaction between emotional arousal and encoding context (threat, safe), F(1,27)= 4.47, p< .05, indicated that emotionally arousing words encoded under threat, relative to safety, evoked larger ERP positivity than new words. This enhanced old-new difference was not found for words rated low in emotional arousal.
Figure 1.
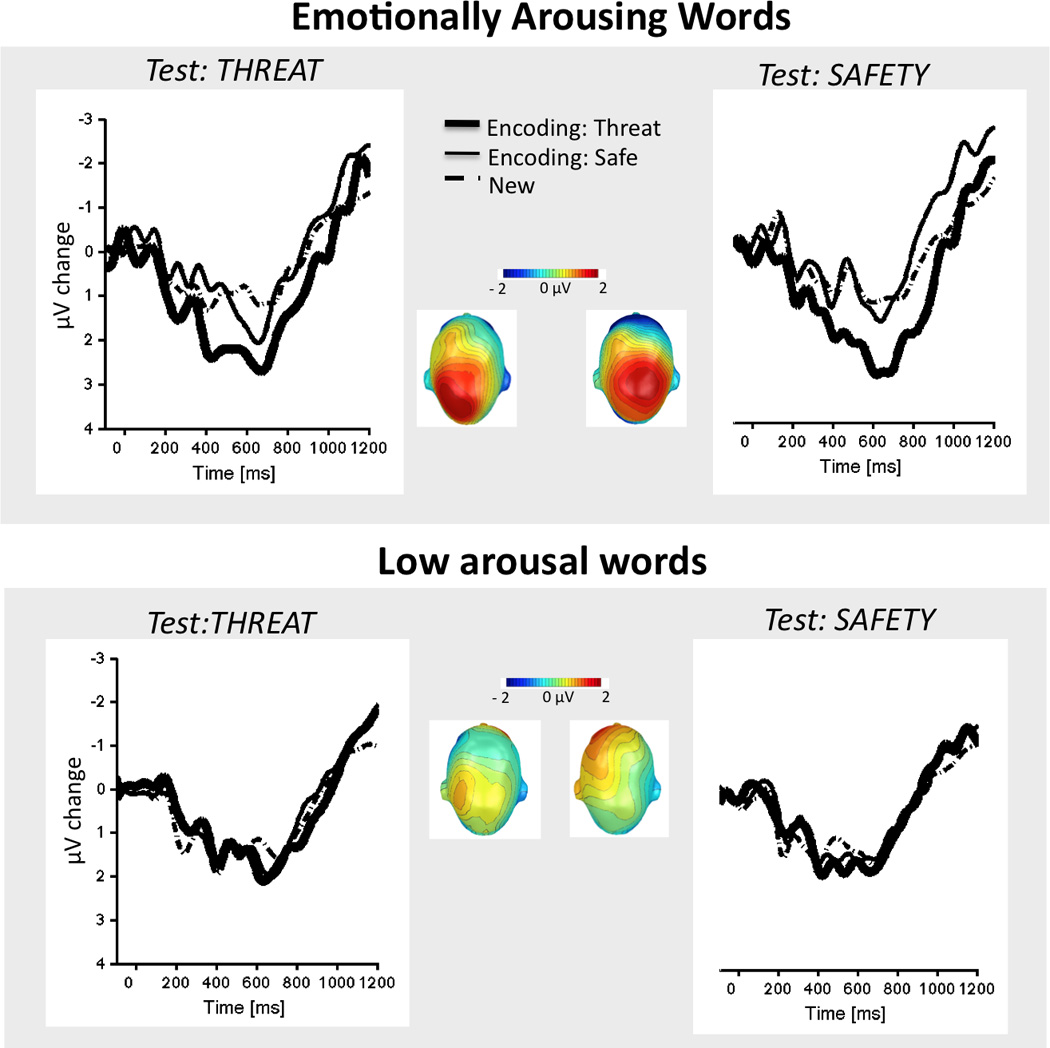
Grand average ERP waveforms over posterior sensors for correctly recognized words that were emotionally arousing (top panel) or low in emotional arousal (bottom panel) and tested in a context of threat (left panel) or safety (right panel) that had been encoded in a font color that signaled threat of shock (thick line) or safety (thin line) and new words (dotted line). Scalp topographies of the ERP old-new difference (500–700 msec) are illustrated for words encoded under threat and tested under threat of shock or safety.
Table 2.
Mean change (in εv) in a 500–700 ms window over posterior sensors for correctly recognized emotionally arousing and low arousal words that were encoded under threat of shock or safety, and recognized under threat of shock or safety, and new words tested under threat of shock or safety.(standard error of the mean).
| Recognition Context | |||
|---|---|---|---|
| Old words | New words | ||
| Threat during encoding | Safe during encoding | ||
| Threat | |||
| Emotionally arousing words | 2.46 (.49) | 1.65 (.62) | 1.02 (.62) |
| Low arousal words | 1.75 (.44) | 1.65 (.35) | 1.33 (.38) |
| Safe | |||
| Emotionally arousing words | 2.65 (.51) | 1.33 (.55) | 1.10 (.39) |
| Low arousal words | 1.65 (.37) | 1.42 (.37) | 1.30 (.31) |
However, context during recognition did not significantly enhance or attenuate these late parietal potentials, F(1,27)<1.
4. Discussion
ERPs were measured to assess whether memory for items encoded under threat of shock is affected by whether a threat context at test is reinstated or not. The results indicate that threat reinstatement did not influence behavioral or electrophysiological measures of recognition memory for words that were encoded during threat or safety. Rather, replicating previous data, correct recognition of words encoded in a threatening context evoked enhanced ERP positivity over posterior electrodes (500–700 ms), compared to words encoded under safety (Weymar et al., 2013). Because the late parietal old/new difference is often interpreted as an electrophysiological correlate of successful episodic recollection (Rugg and Curran, 2007), the data suggest that recognition of emotional items encoded in the context of threat is more likely to be mediated by explicit recollection.
Reinstating the encoding context did not modulate either memory performance or ERPs, which could result from a number of different mechanisms, including encoding processes such as overshadowing or retrieval processes such as outshining, which reduce the effectiveness of reinstated context cues (Smith, 1988; Smith & Manzano, 2010). Overshadowing occurs when contextual cues are less effective because multiple targets (e.g., words) are encoded in the same context during encoding (overloaded cue). In retrieval, outshining occurs when a non-contextual cue, such as the word itself, is a more effective retrieval cue than the background context. Thus, on an explicit recognition test, presenting the item itself can attenuate effects of context on retrieval, which may have occurred here.
Another possibility, of course, is that the threat manipulation was not successful during the recognition test. Threat of shock, however, particularly in the absence of any shock experience (as in the current study) has proven to be a robust manipulation for eliciting defensive reactions, as reflected in sustained skin conductance elevation, startle reflex potentiation and cardiac deceleration (Bradley et al., 2005; Grillon et al., 1991; Costa, Bradley, & Lang, 2009). Nonetheless, although the post-experimental ratings indicated that threat periods during recognition were rated as more aversive than safe periods, collecting additional physiological measures of aversive anticipation during recognition would provide a more definitive manipulation check.
Although an enhanced parietal ERP old/new difference was found for emotional words encoded under shock threat, these effects were not reflected in differential recognition performance. The late ERP old/new effect is often modulated by a variety of factors that are assumed to be related to recollection, such as depth of processing, correct source memory, high confidence ratings, "remember", compared to “know”, judgments, and the amount of information recollected (Vilberg et al., 2006). Consistent with this, Dunsmoor et al. (2012) found facilitation in memory performance for pictures of neutral objects (animals and tools) that had been reinforced with electric shock on a delayed (24 hour) recognition test for items reported as remembered with high confidence. And, Murty etal (2012) found enhanced recognition memory for neutral pictures of indoor/outdoor scences that had been preceded by a cue indicating the participant could avoid an electric shock if the picture was successfully remembered the next day (Murty et al., 2012). Based on these studies, effects of threat on memory performance may be more apparent when an aversive event is actually experienced (Dunsmoor et al., 2012), when memory encoding is intentional (Murty et al., 2012), when behavioral measures of recollection are included (e.g., confidence; Dunsmoor et al., 2012) or, perhaps most importantly, when recognition is tested after a delay (Dunsmoor et al., 2012; Murty et al., 2012).
Taken together, however, the present data suggest that larger ERP old-new differences for items encoded in the context of shock threat are likely to be mediated by processes occurring at encoding (e.g., depth, etc.) or storage, rather than at retrieval. Moreover, the facilitatory effects of encountering items under threat of shock on old-new ERPs was most pronounced for emotionally arousing words, which is consistent with both animal and human studies reporting that memory is particularly enhanced for emotionally arousing stimuli that are experienced in the context of stress (e.g., Joels et al., 2006; Diamond et al., 2007; Schwabe & Wolf, 2013). One hypothesis is that stress hormones (rapid nongenomic effects of catecholamines and glucocorticoids) that enhance vigilance and arousal in response to stress facilitate memory storage (see McGaugh, 2004) via interactions between the amygdalae and medial temporal lobe, with emotionally arousing stimuli and the stressful context both contributing to this modulation. The enhanced effects of threat stress on recognition found by Dunsmoor et al. (2012) and Murty et al. (2012) on delayed (24 hour) tests would support effects that occur during storage.
On the other hand, because a storage mechanism is more likely when memory is tested following a delay, the enhanced recollection for items encoded during threat on the immediate recognition test used here may instead reflect more elaborative processing at encoding. Previous studies have found that cues signaling threat of shock, compared to cues signaling safety, enhance electrophysiological components associated with heightened perceptual processing and increased selective attention (Baas et al., 2002; Böcker et al., 2004, Bublatzky & Schupp, 2012; Weymar et al., 2013), as well as measurable changes in skin conductance, startle reflex magnitude, heart rate, and other physiological measures associated with increased attention and arousal (Bradley, Moulder, & Lang, 2005). And, these measures of affective engagement are typically enhanced when encoding emotionally arousing stimuli (Bradley, 2009), consistent with an encoding interpretation of the current data.
Anticipating (dreading) upcoming aversive events is a critical factor in disorders that involve catastrophizing, worry, rumination and intrusive recollections (e.g., panic disorder, generalized anxiety disorder, depression or post-traumatic stress disorder). The present data suggest that information encountered during aversive anticipation may be more deeply encoded and therefore more easily recollected. To the extent that emotionally arousing events encoded during stress are more prone to enhanced recollection, a vicious cycle of heightened recollection of negative events may sustain and amplify aversive anticipation, mediating dysfunctional processes of worry and catastrophizing (e.g., Borkovec, 1985). If so, the current data suggest that ERPs may assist in indexing the influence of stress and anxiety on memory in clinical populations.
Highlights.
Episodic recollection, measured as the old-new difference in the ERP, was enhanced for items encoded under threat of shock
This effect was most reliable for emotionally arousing items
Enhanced recollection for emotional stimuli encoded under threat was not modulated by threat reinstatement at retrieval
Acknowledgments
This research was supported in part by NIMH grants R01-MH094386 and R01-MH098078 to Peter J. Lang, and by a post-doctoral stipend from the German Research Society (Deutsche Forschungsgemeinschaft, DFG) to Mathias Weymar (Forschungsstipendium, WE 4801/1-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Mean pleasure and arousal ratings from the ANEW (Bradley & Lang, 1999) were matched for words in the two sets: Pleasure ratings: Unpleasant words 3.4, 3.5; Neutral words: 5.2, 5.1; Pleasant words: 6.8, 6.5. Arousal ratings: Unpleasant 5.2, 5.1; Neutral 3.5, 3.5; Pleasant 5.2, 5.2. In addition word frequency did not differ for emotional and neutral words in each of the two sets (set x content, F(1,9)< 1).
The threat cue was changed from font color during encoding to frame color during recognition in order to specifically reinstate the threat context rather than the physical appearance of the word itself.
Recognition button responses were delayed until the offset of the 2 sec picture presentation in order to avoid contamination by motor potentials. Reaction times are therefore not informative and were not analyzed.
Based on previous studies reporting early differences between old and new over frontal electrodes (Rugg & Curran, 2007), we tested the ERP old/new difference in an earlier time window (300–500 ms). However, this analysis did not reveal any significant memory effects (old vs. new) or interactions.
Contributor Information
Mathias Weymar, Email: mweymar@ufl.edu.
Margaret M. Bradley, Email: bradley@ufl.edu.
Alfons O. Hamm, Email: hamm@uni-greifswald.de.
Peter J. Lang, Email: langp@phhp.ufl.edu.
References
- Baas JM, Kenemans JL, Böcker KB, Verbaten MN. Threat-induced cortical processing and startle potentiation. NeuroReport. 2002;13(1):133–137. doi: 10.1097/00001756-200201210-00031. [DOI] [PubMed] [Google Scholar]
- Böcker KB, Baas JM, Kenemans JL, Verbaten MN. Differences in startle modulation during instructed threat and selective attention. Biological Psychology. 2004;67(3):343–358. doi: 10.1016/j.biopsycho.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Borkovec TD. Worry: A potentially valuable concept. Behaviour Research and Therapy. 1985;23:481–482. doi: 10.1016/0005-7967(85)90178-0. [DOI] [PubMed] [Google Scholar]
- Bower GH, Monteiro KP, Gilligan SG. Emotional mood as context for learning and recall. Journal of Verbal Learning and Verbal Behavior. 1978;22:650–666. [Google Scholar]
- Bradley MM, Lang PJ. Technical report C-1. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. Affective norms for English words (ANEW): Stimuli, instruction manual and affective ratings. [Google Scholar]
- Bradley MM, Moulder B, Lang PJ. When good things go bad: the reflex physiology of defense. Psychological Science. 2005;16:468–473. doi: 10.1111/j.0956-7976.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Silakowski T, Lang PJ. Fear of pain and defensive activation. Pain. 2008;137:156–163. doi: 10.1016/j.pain.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublatzky F, Schupp HT. Pictures Cueing Threat: Brain Dynamics in Viewing Explicitly Instructed Danger Cues. Social Cognitive and Affective Neuroscience. 2012;7:611–622. doi: 10.1093/scan/nsr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr H. Maze studies with the white rat. Journal of Animal Behavior. 1917;7:259–275. [Google Scholar]
- Clark MS, Milberg S, Ross J. Arousal cues arousal-related material in memory: Implications for understanding effects of mood on memory. Journal of Verbal Learning and Verbal Behavior. 1984;22:633–649. [Google Scholar]
- Costa VD, Bradley MM, Lang PJ. Fear less! Neural correlates of fear reversal. Psychophysiology. 2009;46:S63. [Google Scholar]
- Diamond D, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity: Article ID 60803. 2007:1–33. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düzel E, Yonelinas AP, Mangun GR, Heinze HJ, Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proceedings of the National Academy of Sciences of the USA. 1997;94:5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Martin A, LaBar KS. The role of conceptual knowledge in learning and retention of conditioned fear. Biological Psychology. 2012;89:300–305. doi: 10.1016/j.biopsycho.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eich JM. The cue-dependent nature of state-dependent retrieval. Memory & Cognition. 1980:157–173. doi: 10.3758/bf03213419. [DOI] [PubMed] [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Karlsson M, Lang PJ. Repetition and brain potentials when recognizing natural scenes: Task and emotion differences. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden DR, Baddeley AD. Context-dependent memory in two natural environments: Land and underwater. British Journal of Psychology. 1975;66:325–331. [Google Scholar]
- Grillon C, Davis M. Acoustic startle and anticipatory anxiety in humans: effects of monaural right and left ear stimulation. Psychophysiology. 1995;32(2):155–161. doi: 10.1111/j.1469-8986.1995.tb03307.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fearpotentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28(5):588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Inaba M, Nomura M, Ohira H. Neural evidence of effects of emotional valence on word recognition. International Journal of Psychophysiology. 2005;57:165–173. doi: 10.1016/j.ijpsycho.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends in Cognitive Sciences. 2006;10(4):152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Johansson M, Mecklinger A, Treese AC. Recognition memory for emotional and neutral faces: An event-related potential study. Journal of Cognitive Neuroscience. 2004;16:1840–1853. doi: 10.1162/0898929042947883. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker D, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37(4):523–532. [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational Analysis of Present-Day American English. Providence: Brown University Press; 1967. [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Neuroscience Reviews. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Melzig CA, Michalowski JM, Holtz K, Hamm AO. The anticipation of interoceptive threat in highly anxiety sensitive persons. Behaviour Research and Therapy. 2008;46(10):1126–1134. doi: 10.1016/j.brat.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Murty VP, LaBar KS, Adcock RA. Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. Journal of Neuroscience. 2012;32:8969–8976. doi: 10.1523/JNEUROSCI.0094-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome RN, Dulas MR, Duarte A. The effects of aging on emotion-induced modulations of source retrieval ERPs: Evidence for valence biases. Neuropsychologia. 2012;50:3370–3384. doi: 10.1016/j.neuropsychologia.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Neuroscience Reviews. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Peyk P, De Cesarei A, Junghöfer M. ElectroMagnetoEncephalography software: overview and integration with other EEG/MEG toolboxes. Computational Intelligence and Neuroscience. 2011;2011:861705. doi: 10.1155/2011/861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand G, Wapner S. Postural status as a factor in memory. Journal of Verbal Learning and Verbal Behavior. 1967;6:268–271. [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress and multiple memory systems: from 'thinking' to 'doing'. Trends in Cognitive Sciences. 2013;17:51–98. doi: 10.1016/j.tics.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Smith SM. Environmental context-dependent memory. In: Davies G, Thomson D, editors. Memory in context: Context in memory. New York: Wiley; 1988. pp. 13–33. [Google Scholar]
- Smith SM. Theoretical principles of context-dependent memory. In: Morris P, Gruneberg M, editors. Aspects of memory (2nd edition): Theoretical aspects. Routledge Press; 1994. pp. 168–195. [Google Scholar]
- Smith SM, Glenberg AM, Bjork RA. Environmental context and human memory. Memory & Cognition. 1978;6:342–353. [Google Scholar]
- Smith SM, Vela E. Environmental context-dependent memory: A review and meta-analysis. Psychonomic Bulletin and Review. 2001;8:203–220. doi: 10.3758/bf03196157. [DOI] [PubMed] [Google Scholar]
- Smith SM, Manzano I. Video context-dependent memory. Behavior Research Methods. 2010;42(1):292–301. doi: 10.3758/BRM.42.1.292. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Moosavi RF, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Research. 2006;1122:161–170. doi: 10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Neural substrates of remembering: Electroencephalographic studies. In: Eichenbaum H, editor. Memory Systems. Oxford: Elsevier Press; 2008. pp. 79–98. Vol. 3 of Learning and Memory: A Comprehensive Reference, 4 vols. (J. Byrne, Ed.). [Google Scholar]
- Weymar M, Löw A, Melzig CA, Hamm AO. Enhanced long-term recollection for emotional pictures: Evidence from high-density ERPs. Psychophysiology. 2009;46(6):1200–1207. doi: 10.1111/j.1469-8986.2009.00869.x. [DOI] [PubMed] [Google Scholar]
- Weymar M, Löw A, Schwabe L, Hamm AO. Brain dynamics associated with recollective experiences of emotional events. Neuroreport. 2010;21:827–831. doi: 10.1097/WNR.0b013e32833d180a. [DOI] [PubMed] [Google Scholar]
- Weymar M, Löw A, Hamm AO. Emotional memories are resilient to time: Evidence from the parietal ERP old/new effect. Human Brain Mapping. 2011;32:632–640. doi: 10.1002/hbm.21051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymar M, Bradley MM, Hamm AO, Lang PJ. When fear forms memories: Threat of shock and brain potentials during encoding and retrieval. Cortex. 2013;49:819–826. doi: 10.1016/j.cortex.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]


