Abstract
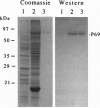
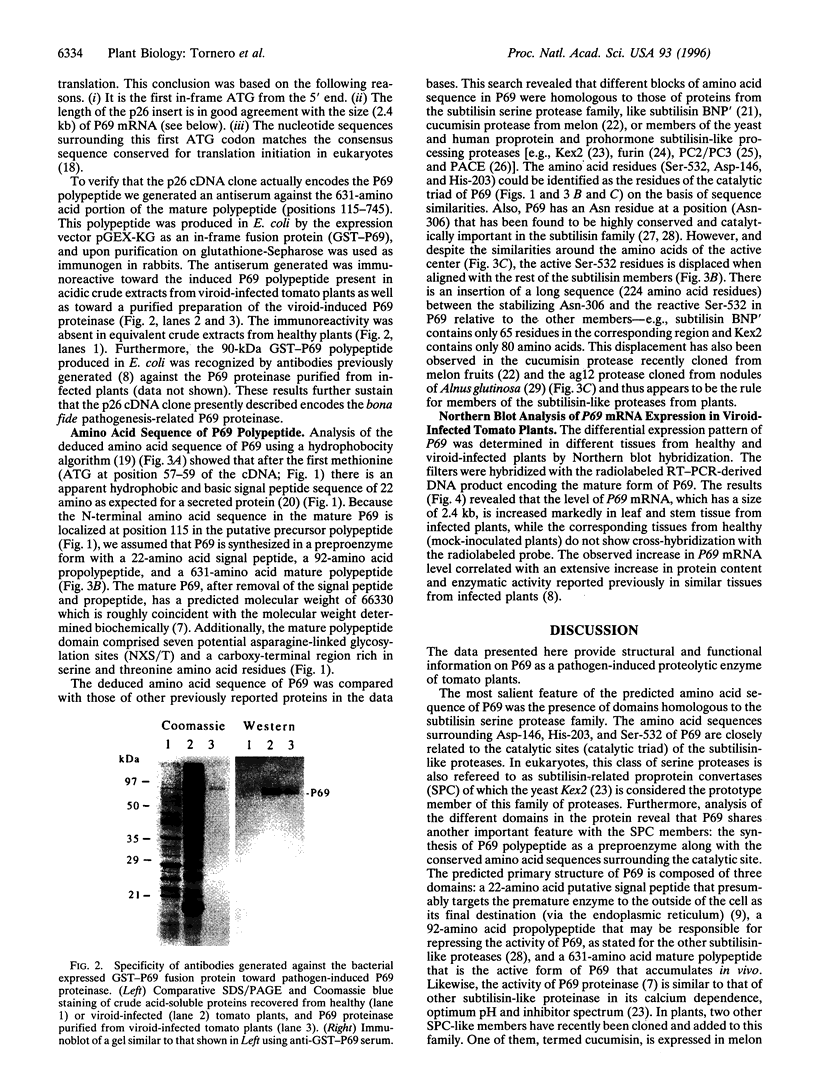
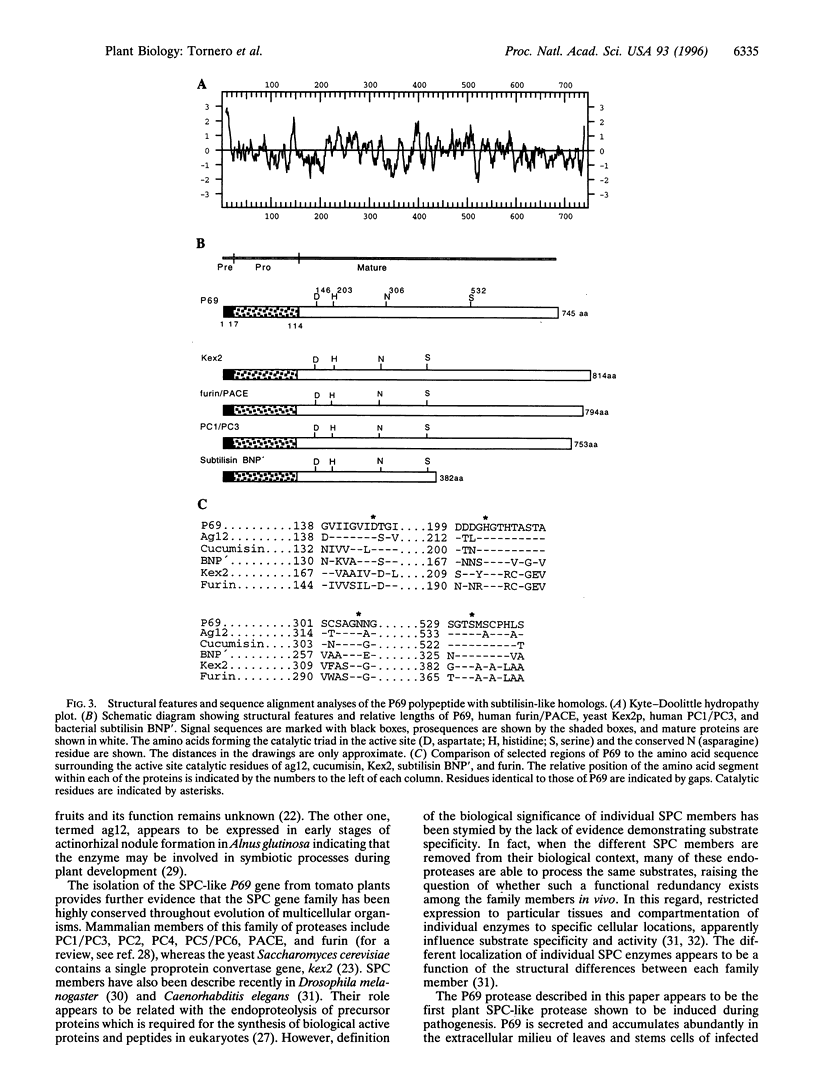
A 69-kDa proteinase (P69), a member of the pathogenesis-related proteins, is induced and accumulates in tomato (Lycopersicon esculentum) plants as a consequence of pathogen attack. We have used the polymerase chain reaction to identify and clone a cDNA from tomato plants that represent the pathogenesis-related P69 proteinase. The nucleotide sequence analysis revealed that P69 is synthesized in a preproenzyme form, a 745-amino acid polypeptide with a 22-amino acid signal peptide, a 92-amino acid propolypeptide, and a 631-amino acid mature polypeptide. Within the mature region the most salient feature was the presence of domains homologous to the subtilisin serine protease family. The amino acid sequences surrounding Asp-146, His-203, and Ser-532 of P69 are closely related to the catalytic sites (catalytic triad) of the subtilisin-like proteases. Northern blot analysis revealed that the 2.4-kb P69 mRNA accumulates abundantly in leaves and stem tissues from viroid-infected plants, whereas the mRNA levels in tissues from healthy plants were undetectable. Our results indicate that P69, a secreted calcium-activated endopeptidase, is a plant pathogenesis-related subtilisin-like proteinase that may collaborate with other defensive proteins in a general mechanism of active defense against attacking pathogens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr P. J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991 Jul 12;66(1):1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Bresnahan P. A., Leduc R., Thomas L., Thorner J., Gibson H. L., Brake A. J., Barr P. J., Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990 Dec;111(6 Pt 2):2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T. Membrane proteases: roles in tissue remodeling and tumour invasion. Curr Opin Cell Biol. 1992 Oct;4(5):802–809. doi: 10.1016/0955-0674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo C., Gómez M. D., Cañas L., Hernández-Yago J., Conejero V., Vera P. A novel extracellular matrix protein from tomato associated with lignified secondary cell walls. Plant Cell. 1994 Aug;6(8):1035–1047. doi: 10.1105/tpc.6.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Brake A., Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Pautot V., Holzer F. M., Reisch B., Walling L. L. Leucine aminopeptidase: an inducible component of the defense response in Lycopersicon esculentum (tomato). Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9906–9910. doi: 10.1073/pnas.90.21.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power S. D., Adams R. M., Wells J. A. Secretion and autoproteolytic maturation of subtilisin. Proc Natl Acad Sci U S A. 1986 May;83(10):3096–3100. doi: 10.1073/pnas.83.10.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro A., Akkermans A. D., van Kammen A., Bisseling T., Pawlowski K. A nodule-specific gene encoding a subtilisin-like protease is expressed in early stages of actinorhizal nodule development. Plant Cell. 1995 Jun;7(6):785–794. doi: 10.1105/tpc.7.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller A., Ryan C. A. Identification of a 50-kDa systemin-binding protein in tomato plasma membranes having Kex2p-like properties. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11802–11806. doi: 10.1073/pnas.91.25.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Day R., Hamelin J., Gaspar A., Collard M. W., Chrétien M. Testicular expression of PC4 in the rat: molecular diversity of a novel germ cell-specific Kex2/subtilisin-like proprotein convertase. Mol Endocrinol. 1992 Oct;6(10):1559–1570. doi: 10.1210/mend.6.10.1448111. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Thacker C., Peters K., Srayko M., Rose A. M. The bli-4 locus of Caenorhabditis elegans encodes structurally distinct kex2/subtilisin-like endoproteases essential for early development and adult morphology. Genes Dev. 1995 Apr 15;9(8):956–971. doi: 10.1101/gad.9.8.956. [DOI] [PubMed] [Google Scholar]
- Tornero P., Conejero V., Vera P. A gene encoding a novel isoform of the PR-1 protein family from tomato is induced upon viroid infection. Mol Gen Genet. 1994 Apr;243(1):47–53. doi: 10.1007/BF00283875. [DOI] [PubMed] [Google Scholar]
- Vera P., Conejero V. Effect of Ethephon on Protein Degradation and the Accumulation of ;Pathogenesis-Related' (PR) Proteins in Tomato Leaf Discs. Plant Physiol. 1990 Jan;92(1):227–233. doi: 10.1104/pp.92.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera P., Conejero V. Pathogenesis-related proteins of tomato : p-69 as an alkaline endoproteinase. Plant Physiol. 1988 May;87(1):58–63. doi: 10.1104/pp.87.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera P., Tornero P., Conejero V. Cloning and expression analysis of a viroid-induced peroxidase from tomato plants. Mol Plant Microbe Interact. 1993 Nov-Dec;6(6):790–794. [PubMed] [Google Scholar]
- Vera P., Yago J. H., Conejero V. Immunogold localization of the citrus exocortis viroid-induced pathogenesis-related proteinase p69 in tomato leaves. Plant Physiol. 1989 Sep;91(1):119–123. doi: 10.1104/pp.91.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K., Wada Y., Ishibashi F., Gojobori T., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1992 May 11;20 (Suppl):2111–2118. doi: 10.1093/nar/20.suppl.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. J., Barr P. J., Wong P. A., Kiefer M. C., Brake A. J., Kaufman R. J. Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9378–9382. doi: 10.1073/pnas.87.23.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Masuzawa T., Nagaoka Y., Ohnishi T., Iwasaki T. Cucumisin, a serine protease from melon fruits, shares structural homology with subtilisin and is generated from a large precursor. J Biol Chem. 1994 Dec 30;269(52):32725–32731. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]