Abstract
The ectonucleotidases CD39 and CD73 degrade ATP to adenosine which inhibits immune responses via the A2A adenosine receptor (ADORA2A) on T and NK cells. The current study investigates the potential therapeutic use of the specific anti CD39- and anti CD73-antibodies A1 (CD39) and 7G2 (CD73) as these two ectonucleotidases are overexpressed in ovarian cancer (OvCA). As expected, NK cell cytotoxicity against the human ovarian cancer cell lines OAW-42 or SK-OV-3 was significantly increased in the presence of A1 or 7G2 antibody. While this might partly be due to antibody-dependent cell-mediated cytotoxicity, a luciferase-dependent assay for quantifying biologically active adenosine further showed that A1 and 7G2 can inhibit CD39 and CD73-dependent adenosine-generation. In turn, the reduction in adenosine levels achieved by addition of A1 and 7G2 to OAW-42 or SK-OV-3 cells was found to de-inhibit the proliferation of CD4+ T cells in coculture with OvCA cells. Likewise, blocking of CD39 and CD73 on OvCA cells via A1 and 7G2 led to an increased cytotoxicity of alloreactive primed T cells. Thus, antibodies like A1 and 7G2 could improve targeted therapy in ovarian cancer not only by specifically labeling overexpressed antigens but also by blocking adenosine-dependent immune evasion in this immunogenic malignancy.
Keywords: Ovarian cancer, immune escape, adenosine, CD39, CD73
Introduction
In spite of all efforts to develop new therapeutic strategies for ovarian cancer (OvCA), its bleak prognosis has remained largely unchanged since the introduction of the platinum-based chemotherapies about thirty years ago [1]. For the so-called “developed countries” where treatment options should be optimal, epidemiological data still report an annual number of more than 64.500 deaths from ovarian cancer which indicates that a majority of the annually 100.300 patients diagnosed with the disease will ultimately succumb to it [2]. Therefore, new approaches for improving the medical management of OvCA-patients urgently need to be identified.
There is growing evidence that interactions with the immune system are clinically most relevant for this type of cancer [3]. For example, increased recruitment of regulatory T cells (Treg) by OvCAs correlates with a significantly increased mortality, presumably by suppressing the host’s spontaneous immune responses to tumor antigens [4,5]. One key mechanism for immunomodulation by Treg seems to be the generation of extracellular adenosine. CD4+CD25+FoxP3+ Treg were shown to use CD39/ENTPD1 and CD73/ecto-5’-nucleotidase to hydrolyze adenosine tri- and diphosphate (ATP/ADP) to adenosine which in turn exerts immunosuppression on various immune cell populations [6-9].
Investigating CD39- and CD73-positive cells in ovarian cancer we found that OvCA cells themselves express both ectonucleotidases and produce about 30-60 times more biologically active adenosine than Treg. Furthermore, we could show that CD39- and CD73-mediated adenosine-production by OvCA cells suppresses NK and T cell cytotoxicity as well as CD4+ T cell proliferation. Also immunohistochemical ex situ stainings of OvCA tissue showed strongly increased ectonucleotidase expression compared to benign ovarian tissue (all: [10]).
This prompted us to investigate if CD39 and CD73 could be new targets for immunomodulatory therapies in ovarian cancer. Therefore, we tested if specific antibodies against CD39 and CD73, A1 and 7G2, could improve immune responses against ovarian cancer cells. A special focus was placed on the ability of the antibodies to inhibit adenosine generation by both ectonucleotidases.
Materials and methods
Cell culture
The human ovarian cancer cell lines SK-OV-3 (American Type Culture Collection (ATCC) HTB-77) and OAW-42 (European Cell Culture Collection 85073102) were cultured in RPMI 1640 medium with 10% FCS (Biochrom, Berlin, Germany), 0.02% sodium pyruvate, penicillin (100 IU/ml) and streptomycin (100 μg/ml) (all from PAA, Pasching, Austria). In order to detach the cells for further experimental use, Accutase (PAA) was used. Cell line identity was confirmed using the single tandem repeat fingerprint system as performed by the Deutsche Sammlung für Mikroorganismen und Zellkulturen (Braunschweig, Germany).
Flow cytometric analysis of specific CD39 and CD73 surface expression on OvCa cells using antibodies A1 and 7G2
Detached Ovarian cancer cells (106/sample) were blocked and stained with mouse anti-human CD39 (clone A1, #MCA1268XZ, AbD serotec, Oxford, UK) or mouse anti-human CD73-antibody (clone 7G2, #ab54217, Abcam, Cambridge, UK). FITC-conjugated goat anti mouse antibodies (22549913, Immunotools, Friesoythe, Germany) were used for visualization. 50,000 cells were assessed for expression of CD39 or CD73 using a FACScan flow cytometer (BD Biosciences, San Jose, USA). Specific fluorescence indices (SFI) were obtained by dividing mean fluorescence recorded with the specific antibodies by the fluorescence intensity obtained with the corresponding isotype controls (n=3).
NK cell preparation and cytotoxicity assays
Polyclonal NK cell populations were obtained by co-culturing peripheral blood leucocytes (PBL) from healthy volunteers with irradiated (30 Gy) RPMI 8866 feeder cells [11]. PKH26 (Sigma-Aldrich St. Louis, MO, USA) was used to label the NK cells according to the manufacturer’s instructions. Their lytic activity against 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFDA-SE, LifeTechnologies, Darmstadt, Germany) target cells (50.000 target cells/well) [12] was determined in modified 4h FATAL assays. For triggering antibody-dependent cellular cytotoxicity (ADCC), anti-human CD39 (A1, AbD serotec) or anti-human CD73-antibody (7G2, Abcam) or, respectively, an isotype control antibody were added at 1 μg/ml. For further control purposes, the A2A adenosine receptor inhibitor SCH58261 (100 nM, Tocris, Bristol, UK) or a suitable solvent control was applied. Using a FACScan flow cytometer, tumor cell lysis was measured at different effector/target cell ratios. CFDA-SEdim cells within the PKH-26 negative cell population were counted as lysed cells. Spontaneous leakage of CFDA-SE was controlled by culture with solvent only.
Adenosine production via CD39 and CD73
Biologically active adenosine within the cellular microenvironment was determined as described in [13] and [10]. 104 freshly detached OAW-42 cells were co-incubated with equal numbers of RIP1-CRE.luc- and pRL-CMV-transfected HEK-293 ADORA2A+/- cells in 96-well plates. During this incubation, A1 (anti-human CD39) or 7G2 (anti-human CD73) were added at 10 μg/ml to block CD39 or CD73 function. After 4h, the cells were lysed in passive lysis buffer (Promega). Using a non-commercial dual luciferase assay [14], the biophotonic signals were quantified in an Orion II Microplate Luminometer (Berthold Detection Systems, Pforzheim, Germany). All values were measured in triplicates.
Proliferation of CD4+ T cells in co-culture with OvCA cells
The “CD4+ T cell isolation kit II” was used to isolate CD4+ T cells from PBL; CD4+CD25+ and CD4+CD25- T cells were obtained using the “CD4+CD25+ regulatory T cell isolation kit” (both from Miltenyi Biotec, Bergisch Gladbach, Germany). Directly after isolation the T cells were labeled with 2.5 μM CFDA-SE (Invitrogen). To induce proliferation, anti-human CD3 (clone UCHT-1, Immunotools) at 1 μg/ml was immobilized on 96 well Maxisorp-plates (Nunc, Roskilde, Denmark) by overnight-incubation in PBS. In each pre-coated well, 2×106 T cells were then co-incubated with anti-human CD28 (clone 15E8, ImmunoTools, at 1 μg/ml) and with 5×105 SK-OV-3 or OAW-42 cells. CD39 and CD73 were blocked by addition of anti-human CD39 (A1, AbD serotec) or anti-human CD73 antibody (7G2, Abcam). Specificity was ensured by use of an isotype control (MG1-45, BioLegend, San Diego, USA) or the ADORA2A antagonist SCH58261 (100 nM, Tocris). All antibodies were used at 10 μg/ml. Where indicated, adenosine (Sigma-Aldrich, St. Louis, USA) was added at 100 μM. A FACScan flow cytometer (BD Biosciences) was used to measure proliferation on day 7 [15]. Calculation of proliferation rates in FACS analyses was performed as outlined in [10]: the absolute number of counts in each peak-region of the corresponding CFDA-SE histogram was divided by 2n (n standing for the number of the peak, being counted from right to left and beginning with 0) to calculate the number of cells “x” which originally divided into the cells of the peak. Equation 1 is equal to the total number of cell division per peak n.
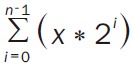 |
T cell response to recall antigens
Co-culturing PBMC with IL-2 (30 U/ml) and irradiated (10 Gy) ovarian cancer cell lines SK-OV-3 or OAW-42 at a ratio of 15:1 provided antigen-specific, alloreactive CD8+ T cells [12,16,17]. To specifically block CD39 or CD73 during priming of the PBMC, anti-human CD39 (A1), anti-human CD73 (7G2) or an isotype control were added at 1 μg/ml. SCH58261 (100 nM) was used to inhibit ADORA2A. After an incubation period of 14 days, the PBMC were labeled with PKH-26. The lytic activity against fresh CFDA-SE-labeled SK-OV-3 or OAW-42 cells at different effector to target cell ratios was determined after 4h, using modified FATAL assays as described above.
Statistics
Flow cytometric data was evaluated using Summit v4.1 and v4.3 (DakoCytomation, Fort Collins, USA). Further calculations including statistical computations were done using Microsoft Office Excel 2007 and 2013 (Microsoft, Redmond, USA). Significance levels were determined by Student’s t-test. p-values < 0.05 were considered as significant (*), p < 0.01 as highly significant (**). In flow cytometric assays, two samples were considered to be significantly different (*) when they were separated by at least twice the sum of the standard deviations for the respective regions. A difference exceeding four times the sum of the respective standard deviations was considered as highly significant (**).
Results
Specific labeling of SK-OV-3 and OAW-42 by the anti-human CD39- and anti-human CD73-antibodies A1 and 7G2
To confirm the binding of A1 (anti CD39) and 7G2 (anti CD73) to the human ovarian cancer cell lines SK-OV-3 and OAW-42, the cells were incubated with either antibody or an isotype control. Binding was visualized with FITC-conjugated secondary antibodies and analysed by flow cytometry. For A1, the Specific Fluorescence Indices (SFIs) of 3.3 (SK-OV-3) resp. 20.0 (OAW-42) were determined (Figure 1, upper panels). The CD73-antibody 7G2 yielded SFI values of 48.1 for SK-OV-3 and 105.6 for OAW-42 (Figure 1, lower panels).
Figure 1.
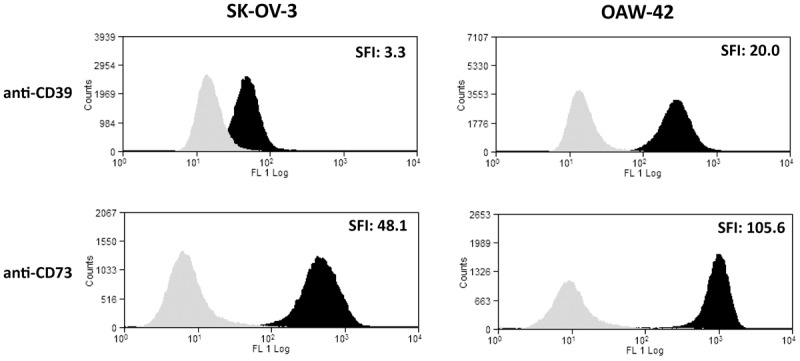
Specific binding of antibodies A1 and 7G2 to SK-OV-3 and OAW-42 cells. Freshly detached ovarian cancer cell lines SK-OV-3 (left) or OAW-42 (right) were stained either with the mouse anti human CD39-antibody A1 or the mouse anti human CD73-antibody 7G2 (dark), both used at 10 μg/ml. After application of a secondary FITC-conjugated anti mouse-antibody, the specific fluorescence indices (“SFI”, indexed in the upper right corner of the histograms) for either CD39 or CD73 were determined in comparison to isotype-matched control antibodies of irrelevant specificity (light).
Lytic activity of polyclonal NK cells against SK-OV-3 or OAW-42 is increased by antibodies A1 and 7G2
To assess the effect of the antibodies A1 and 7G2 on NK cell-mediated killing of OvCA targets, polyclonal NK cell cultures were obtained from healthy volunteers [11] and stained with PKH-26. Freshly detached SK-OV-3 and OAW-42 cells were stained with CFDA-SE. The PKH-26-labeled polyclonal NK cells were co-incubated with CFDA-SE-loaded OvCA cells at different effector to target ratios in modified 4h FATAL assays [12]. During this incubation A1, 7G2 or an isotype control antibody were present. Flow cytometry was used to determine the percentage of PKH-26- CFDA-SEdim OvCa cells after 4h. Applying A1 and 7G2 caused significantly improved lytic activity of polyclonal NK cells against SK-OV-3 (Figure 2, left) and OAW-42 (Figure 2, right) cells as compared to isotype controls. Both antibody-dependent cellular cytotoxicity (ADCC) mediated by NK cells recognizing IgG-decorated OvCA targets and the relief of adenosine-dependent NK cell inhibition may contribute to this effect. In line with our previous findings [10], we also observed a significant increase in target cell lysis when the specific Adenosine Receptor 2a (ADORA2A)-Inhibitor SCH58261 was applied (data not shown) which suggests that the effect was not only due to ADCC. Due to the lack of suitable reagents, antibody-mediated blockade of ADORA2A could not be tested.
Figure 2.
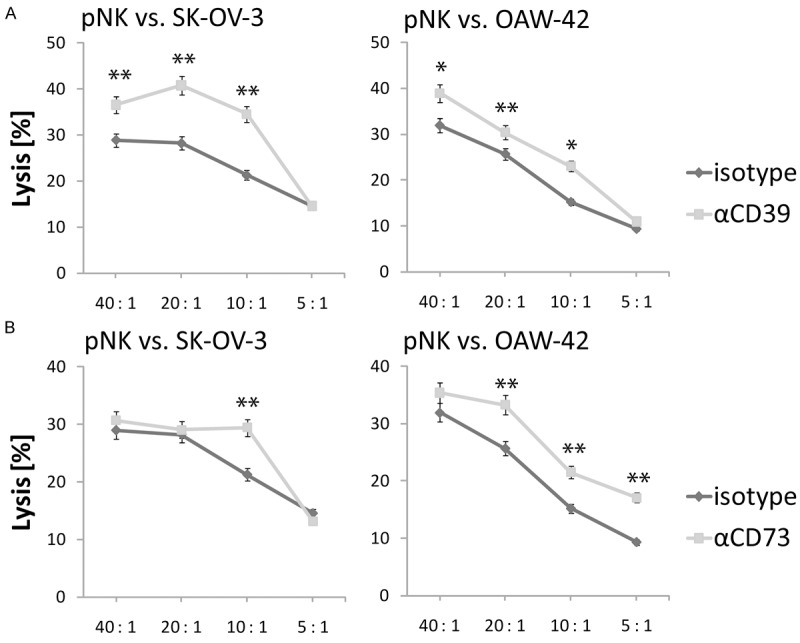
Anti-CD39 and anti-CD73 antibodies A1 and 7G2 enhance cytolytic activity of polyclonal NK cells against SK-OV-3 and OAW-42. PKH26 stained polyclonal NK cells (pNK) were co-incubated for 4h with CFDA-SE labeled SK-OV-3 (left) or OAW-42 (right) OvCA cells at effector to target cell (E:T) ratios from 40:1 to 5:1 in the presence or absence of the antibodies A1 (αCD39, grey, (A)), 7G2 (αCD73, grey, (B)) or an isotype control of irrelevant specificity (shaded). As dying OvCA cells release CFDA-SE, modified FATAL-assays were used to determine the percentage of lysed cells (“Lysis [%]”) [10] (n=3).
Antibodies A1 and 7G2 specifically block enzymatic adenosine generation by CD39 and CD73 expressed on SK-OV-3 and OAW-42 cells
As blocking of the adenosine signal by SCH58261 led to a similar increase in lytic activity of NK cells against OvCA cells as the application of the anti CD39-/anti CD73-antibodies, we wondered if A1 and 7G2 could interfere with adenosine generation by ectonucleotidases. Therefore, we used the luciferase-based reporter assay for the measurement of biologically active adenosine described in [13]. In this assay, ADORA2A-overexpressing HEK-293-“sensor cells” containing a cAMP reporter plasmid are placed in the cellular microenvironment of potentially adenosine-producing cells. To normalize for transfection efficiency, the firefly luciferase-based cAMP reporter plasmid is co-transfected with a constitutively expressed renilla reniformis luciferase (pRL-CMV). These sensor cells then express a firefly luciferase in response to adenosine receptor stimulation which can be quantified and normalized by conventional dual luciferase assays. When OAW-42 were co-incubated with these sensor cells, addition of A1 anti-CD39 antibody resulted in a > 60%-decrease of the measured adenosine concentration compared to an unspecific isotype control (Figure 3). Application of 7G2 anti-CD73 mAb in the same setting reduced adenosine levels by 62% (Figure 3), the combination of A1 and 7G2 by 64% (Figure 3) confirming that these anti CD39 and anti CD73 antibodies inhibit enzymatic adenosine generation by ectonucleotidases.
Figure 3.
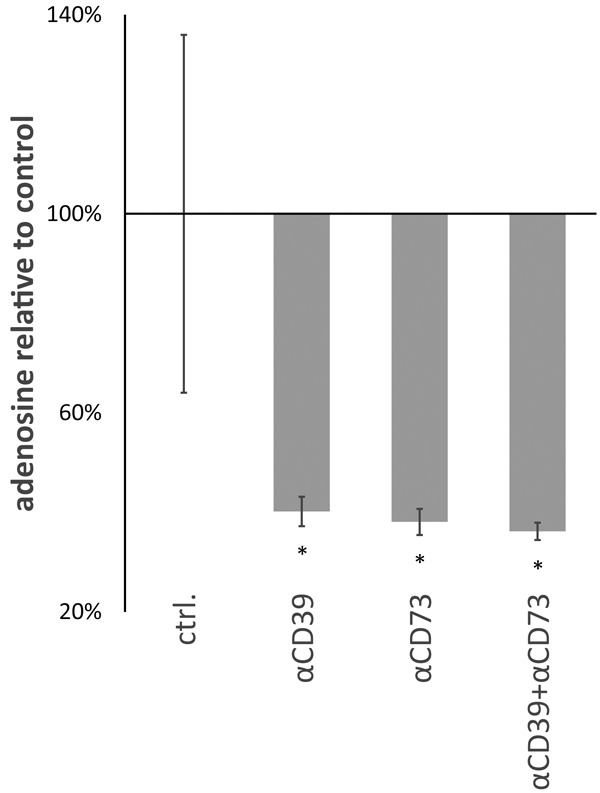
Antibodies A1 and 7G2 block adenosine production by CD39 and CD73 expressed on SK-OV-3 and OAW-42. OAW-42 cells were coincubated with RIP1-CRE-luc+ pRL-CMV+ ADORA2A+ HEK-293 reporter cells at a ratio of 1:1 (104 cells of each type/well) for 4h. Adenosine produced during the co-culture induces expression of firefly luciferase in the HEK-293 reporter cells. Quantification of biologically active adenosine is based on a standard curve [13]. Samples containing an isotype control antibody (“ctrl.”) were compared with samples in which A1 (“αCD39”) or 7G2 (“αCD73”) or both antibodies (“αCD39 + αCD73”) were present. Adenosine levels are depicted relative to the controls (n=3).
Blockade of CD39 and CD73 via A1 and 7G2 antibodies increases proliferation of CD4+ T cells in co-culture with SK-OV-3 and OAW-42 ovarian cancer cells
To investigate if the anti-CD39 and anti-CD73 antibodies A1 and 7G2 de-inhibit adenosine-mediated suppression of CD4+ T cell proliferation, activated CFDA-SE-labeled CD4+ T cells were incubated with irradiated SK-OV-3 or OAW-42 OvCA cells and both antibodies. An example for the effect of A1 on co-cultures of CFDA-SE-labeled CD4+ T and SK-OV-3 cells is shown in Figure 4A. Compared to an isotype antibody of irrelevant specificity, CD4+ T cell proliferation measured by flow cytometry almost doubled (Figure 4A). Likewise, the combination of A1 and 7G2 antibodies with either OAW-42 or SK-OV-3 cells also yielded highly significant increases in CD4+ T cell proliferation, again compared to isotype control antibodies directed against an irrelevant control antigen (Figure 4B). When instead of A1 or 7G2 antibodies the specific ADORA2A-inhibitor SCH-58261 was present in the OvCA/T cell co-cultures, comparable increases in CD4+ T cell proliferation were achieved (Figure 4B). Additionally, we wanted to confirm that the observed de-inhibition of CD4+ T cell proliferation in the presence of A1 and 7G2 antibodies could be reverted by exogenous addition of adenosine. Therefore, the aforementioned co-culture with OAW-42 and CD4+ T cells was repeated either in the absence or presence of 100 μM exogenously added adenosine. As shown in Figure 4C, the pro-proliferative effect of blocking CD39 (left panel) and CD73 (middle panel) with A1 and 7G2 in these co-culture settings could be more than abolished by exogenous adenosine. SCH58261, in contrast, still increased the T cell proliferation when applied in presence of exogenous adenosine confirming the rescue achieved via blockade of ADORA2A (Figure 4C, right panel).
Figure 4.
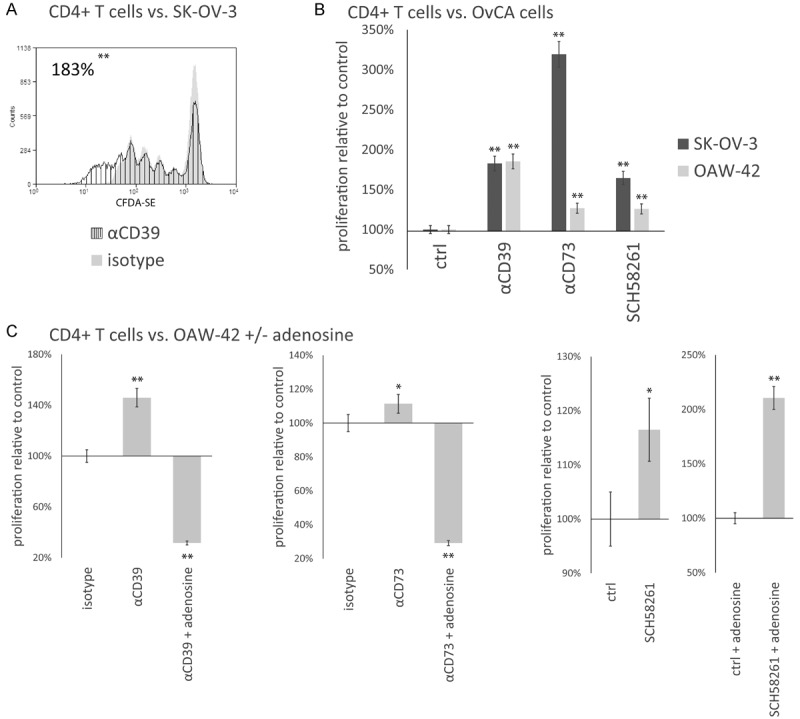
Antibodies A1 and 7G2 de-inhibit CD4+ T cell proliferation in co-culture with OAW-42 and SK-OV-3 OvCA cells. CFDA-SE-labeled CD4+ T cells were stimulated with plate-bound anti CD3- and soluble anti CD28-antibodies (1 μg/ml each) and co-incubated with SK-OV-3 or OAW-42 OvCA cells. Antibodies A1 (“αCD39”) or 7G2 (“αCD73”) or the small molecule ADORA2A inhibitor SCH58261 (“SCH58261”) were added to specifically block the function of CD39, CD73 or the Adenosine Receptor 2a (ADORA2A). Control settings (“ctrl.”) contained an isotype-matched antibody of irrelevant specificity as control (“ctrl.”) for A1 and 7G2, for SCH58261 a control with solvent only was performed. Upon each cell division CFDA-SE gets equally distributed between the resulting daughter cells. After seven days, the T cell proliferation was determined by flow cytometry. In order to calculate the number of T cell divisions per sample, the counts of each peak in its corresponding CFDA-SE-histogram were divided by 2n with n being the number of the peak (counting from right to left and beginning with 0) yielding “x” as the number of the cells from which the cells found in peak n arose by cell division. Equation 1 is equal to all cell divisions in the sample [10]. The “proliferation relative to control” is calculated by dividing the number of cell divisions of the specific setting with the number of the suitable control. A: A representative sample CFDA-SE histogram is shown. In this example, SK-OV-3 cells were co-incubated with the CFDA-SE-labeled CD4+ T cells in presence of A1 antibody (“αCD39”, dark) or, respectively, a non-binding isotype control (“ctrl”, shaded). B: The proliferation of CD4+ T cells in co-culture with SK-OV-3 (dark) or OAW-42 (shaded) cells in the presence or absence of the antibodies A1 (“αCD39”), 7G2 (“αCD73”) or SCH58261 was quantified relative to isotype/solvent controls. A representative experiment is shown. C: CFDA-SE-labeled CD4+ T cells were co-incubated with OAW-42 OvCA cells and antibodies A1 (“αCD39”, left), or 7G2 (“αCD73”, middle), or the ADORA2A inhibitor SCH58261 (“SCH58261”, right) or isotype/solvent controls (“isotype”/“ctrl.”) in the presence or absence of an excess of exogenous adenosine (“adenosine”). n=3 for all experiments.
T cell responses to recall antigens from SK-OV-3 or OAW-42 cells are increased when antibodies A1 or 7G2 block the function of CD39 or, respectively, CD73
Adenosine generated by OvCA cells via CD39 and CD73 was already shown to interfere with the priming of T cell responses against recall antigens [10]. To investigate whether blockade of CD39 and CD73 by antibodies could relieve this suppression, PBMC were isolated from healthy volunteers and co-cultured with irradiated human ovarian cancer cell lines SK-OV-3 and OAW-42 [16,17] in the absence or presence of A1 or 7G2 antibody. As additional control, ADORA2A signaling was blocked with SCH58261. In line with our previous findings, inhibition of adenosine generation or signaling during the 14 days of priming resulted in a significantly increased cytotoxic activity of primed PBMC against fresh SK-OV-3 OvCA targets (Figure 5).
Figure 5.
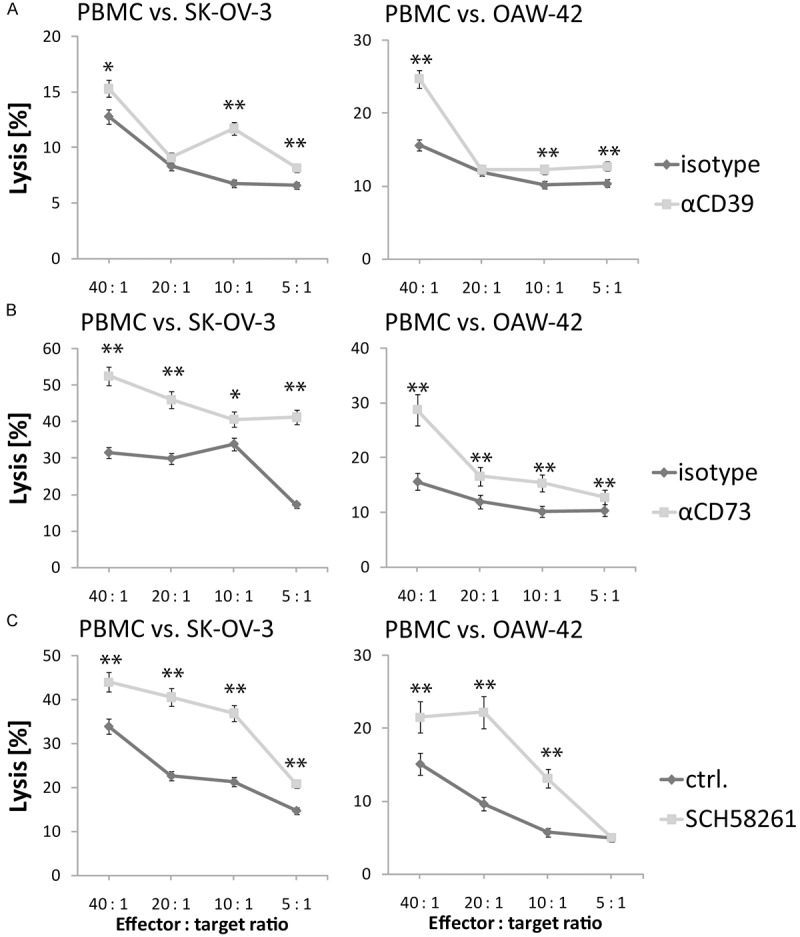
Antibodies A1 and 7G2 enhance the cytotoxic response of alloantigen-primed T cells against OAW-42 and SK-OV-3 OvCA targets. PBMC from healthy volunteers were co-incubated with SK-OV-3 (left) or OAW-42 cells (right) in the presence or absence of antibodies A1 (“αCD39”, shaded, (A)) or 7G2 (“αCD73”, shaded, (B)), or the ADORA2A inhibitor SCH58261 (“SCH58261”, shaded, (C)). After 14 days, the PBMC were harvested and their cytotoxicity against freshly detached CFDA-SE-labeled OAW-42 or SK-OV-3 cells was assessed via flow cytometry. Lysis rates (=CFDA-SEdim cells divided by total cell numbers, “Lysis [%]”) measured for cells primed in the presence of anti-CD39 or anti-CD73 antibody or SCH58261 (shaded lines) were compared to the lytic activity of cells to which only an irrelevant isotype control antibody or, respectively, solvent had been added during the priming phase (dark lines). A representative out of 3 experiments is shown. In all assays, several effector to target ratios were used.
Discussion
Epidemiological data on the impact of CD39 and CD73 expression on the prognosis of cancer patients are discordant which suggests that the effect may depend on the respective malignancy [18-21]. Human ovarian cancer cells, however, are known to over-express the two ectonucleotidases CD39 and CD73 which convert free ATP into immune-regulatory adenosine (compare Figure 1 and [10]). Knockdown of CD73 was already shown to increase the survival of tumor-bearing mice in the immune-competent ID-8 ovarian carcinoma model, especially when combined with adoptive immunotherapy [22]. In vitro, human T and NK cells were shown to mount improved responses to ovarian cancer targets when either CD39 or CD73 or the ADORA2A receptor was blocked by small molecule inhibitors [10]. The data summarized here suggest that specific antibodies against CD39 (here shown for clone A1) or CD73 (clone 7G2) could be valid alternatives to small molecule inhibitors for the targeting of ectonucleotidases in cancer. Apart from the generally good biosafety profile of therapeutic antibodies, this would enable further effector mechanisms like antibody dependent cellular cytotoxicity (ADCC) [23] which likely contributed to the significant enhancement of target cell killing observed when A1 or 7G2 were applied together with polyclonal NK cells (Figure 2). In vivo, this might be a very significant benefit as evidenced by the pivotal role of ADCC for the treatment of HER2-positive breast cancer with Trastuzumab [24-26]. Moreover, antibodies against CD73 were not only shown to activate immune responses against breast cancer cells [27]. A most recent study in immune deficient mice also found that an antibody which induced clustering and internalization of CD73 on the surface of cancer cells could inhibit metastasis formation in a breast cancer model, independent of any effect on CD73 catalytic activity [28]. As this effect involved internalization of the antibody, antibody-drug conjugates might also be attractive therapeutic tools for the treatment of CD73 overexpressing cancers. Likewise, antibody engineering techniques enable additional functionalities to be built in, e.g. to enhance ADCC or to improve anti-cancer specificity via bispecific targeting. Drawbacks are, of course, the high cost of biologicals and possibly a lesser tissue penetration as compared to small molecule inhibitors. In addition, the antibody clones available for our study may not have been the ones that would be optimal for further clinical development.
Still, the enhancement in NK cell cytotoxicity achieved with antibodies against either CD39 or CD73 was similar to the effect observed upon blockade of adenosine-triggered, immunomodulatory signaling by SCH58261, a highly potent ADORA2A inhibitor [29] (Figure 2). Considering that ADCC was likely present with the antibodies, but not with SCH58261, this raises the question if the antibodies could efficiently suppress adenosine generation by OvCA cells. In fact, we found adenosine production to be significantly (60-70%) reduced by the antibodies (compare Figure 3) though not fully abrogated. With regard to the level of ADCC, limitations arise from the fact that both antibodies were generated in mice which could result in suboptimal recognition of surface-bound A1 or 7G2 by human effector cells, even though recognition as such should still be possible (e.g. [23]). Apart from increased NK cell killing which can be ascribed to both ADCC and reduced adenosine levels, the antibodies A1 and 7G2 could also de-inhibit the proliferation of CD4+ T cells in co-culture with OvCA cells, an effect that could be reverted by exogenous addition of adenosine (Figure 4). Finally, the cytotoxic capacity of alloreactive, OvCA-antigen-primed CD8+ T cells was significantly enhanced when the antibodies were present during the priming phase (Figure 5). This is fully compatible with studies performed by Stagg et al. and Jin et al. who have shown impressive effects upon inhibition of CD73 in animal models for breast and ovarian cancer [22,27]. Our data are also in line with findings from Ohta and colleagues who described that mice lacking the most potent immunomodulatory adenosine receptor, ADORA2A, could generate improved antitumoral CD8+ T cell responses leading to reduced growth of experimental tumors [8].
Even though the targets are not strictly tumor-specific, studies which explored in vivo targeting of ectonucleotidases (or ADORA2A) for cancer treatment did not report any critical side effects. Moreover, the manifold immune regulatory effects of adenosine (e.g. [30]) were described to be involved in the suppressive action of regulatory T cells expressing CD39 and CD73 [6,7]. Considering that Treg are recruited by human ovarian cancers and that infiltration of ovarian tumor tissue by Treg is strongly associated with poor prognosis [4], an additional targeting of Treg via antibodies directed at CD39 and CD73 could have further beneficial effects. In fact, CD39-based strategies aiming at the selective functional inhibition of Treg without depletion are already being developed [31]. Moreover, the increasing repertoire of targeted anti-cancer therapies is also beginning to offer manifold possibilities for intelligent combinations. In this context, reports that CD39 and CD73 promote the survival of cells in hypoxic areas are of interest: necrotic cells tend to release high levels of ATP which is not only an immunological danger signal, but which can also have direct cytotoxic effects on tumor cells [32,33]. Prevention of ATP hydrolysis via targeting of CD39 and CD73 could thus increase hypoxic stress on cancer cells. Ovarian cancer cells are known to counteract hypoxia by secreting the pro-angiogenic cytokine vascular endothelial growth factor (VEGF) [34]. The four phase III trials ICON7 [35], GOG-0128 [36], OCEANS [37] and AURELIA [38] have recently shown that the blockade of VEGF with the humanized antibody Bevacizumab is beneficial both for the adjuvant as well as for the palliative treatment of ovarian cancer. Accordingly, a combination of Bevacizumab with antibodies directed at CD39 or CD73 could yield synergistic effects by decreasing tumor vascularization and by reducing the ability of cancer cells to survive within hypoxic areas.
Taken together, the results obtained in our human in vitro model system support the murine studies which have shown that CD39 and CD73 are expressed on OvCA cells and that their inhibition can relieve adenosine-dependent immunosuppression. Using antibodies instead of small molecule inhibitors offers additional effects like ADCC or antibody-induced internalization of the receptor. Antibody-based therapeutics like bispecific constructs could be generated to further enhance tumor specificity or actively induce the recruitment of immune effector cells. Consequently, antibodies to CD39 and CD73 should be further investigated in vivo, as they may become valuable tools for the treatment of otherwise incurable cases of ovarian cancer.
Disclosure of conflict of interest
None.
References
- 1.Sugiyama T, Konishi I. Emerging drugs for ovarian cancer. Expert Opin Emerg Drugs. 2008;13:523–536. doi: 10.1517/14728214.13.3.523. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Dietl J, Engel JB, Wischhusen J. The role of regulatory T cells in ovarian cancer. Int J Gynecol Cancer. 2007;17:764–770. doi: 10.1111/j.1525-1438.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 6.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 7.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 10.Hausler SF, Montalban del Barrio I, Strohschein J, Anoop Chandran P, Engel JB, Honig A, Ossadnik M, Horn E, Fischer B, Krockenberger M, Heuer S, Seida AA, Junker M, Kneitz H, Kloor D, Klotz KN, Dietl J, Wischhusen J. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother. 2011;60:1405–1418. doi: 10.1007/s00262-011-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valiante NM, Rengaraju M, Trinchieri G. Role of the production of natural killer cell stimulatory factor (NKSF/IL-12) in the ability of B cell lines to stimulate T and NK cell proliferation. Cell Immunol. 1992;145:187–198. doi: 10.1016/0008-8749(92)90322-g. [DOI] [PubMed] [Google Scholar]
- 12.Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J Immunol Methods. 2001;249:99–110. doi: 10.1016/s0022-1759(00)00329-x. [DOI] [PubMed] [Google Scholar]
- 13.Hausler SF, Ossadnik M, Horn E, Heuer S, Dietl J, Wischhusen J. A cell-based luciferase-dependent assay for the quantitative determination of free extracellular adenosine with paracrine signaling activity. J Immunol Methods. 2010 Sep 30;361:51–6. doi: 10.1016/j.jim.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Dyer BW, Ferrer FA, Klinedinst DK, Rodriguez R. A noncommercial dual luciferase enzyme assay system for reporter gene analysis. Anal Biochem. 2000;282:158–161. doi: 10.1006/abio.2000.4605. [DOI] [PubMed] [Google Scholar]
- 15.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 16.Blazevic V, Sahgal N, Kessler HA, Landay AL, Shearer GM. T cell responses to recall antigens, alloantigen, and mitogen of HIV-infected patients receiving long-term combined antiretroviral therapy. AIDS Res Hum Retroviruses. 2000;16:1887–1893. doi: 10.1089/08892220050195847. [DOI] [PubMed] [Google Scholar]
- 17.Wischhusen J, Jung G, Radovanovic I, Beier C, Steinbach JP, Rimner A, Huang H, Schulz JB, Ohgaki H, Aguzzi A, Rammensee HG, Weller M. Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res. 2002;62:2592–2599. [PubMed] [Google Scholar]
- 18.Dzhandzhugazyan KN, Kirkin AF, thor Straten P, Zeuthen J. Ecto-ATP diphosphohydrolase/CD39 is overexpressed in differentiated human melanomas. FEBS Lett. 1998;430:227–230. doi: 10.1016/s0014-5793(98)00603-6. [DOI] [PubMed] [Google Scholar]
- 19.Oh HK, Sin JI, Choi J, Park SH, Lee TS, Choi YS. Overexpression of CD73 in epithelial ovarian carcinoma is associated with better prognosis, lower stage, better differentiation and lower regulatory T cell infiltration. J Gynecol Oncol. 2012;23:274–281. doi: 10.3802/jgo.2012.23.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulte D, Olson KE, Broekman MJ, Islam N, Ballard HS, Furman RR, Olson AE, Marcus AJ. CD39 activity correlates with stage and inhibits platelet reactivity in chronic lymphocytic leukemia. J Transl Med. 2007;5:23. doi: 10.1186/1479-5876-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu XR, He XS, Chen YF, Yuan RX, Zeng Y, Lian L, Zou YF, Lan N, Wu XJ, Lan P. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol. 2012;106:130–137. doi: 10.1002/jso.23056. [DOI] [PubMed] [Google Scholar]
- 22.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellstrom I, Garrigues U, Lavie E, Hellstrom KE. Antibody-mediated killing of human tumor cells by attached effector cells. Cancer Res. 1988;48:624–627. [PubMed] [Google Scholar]
- 24.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 25.Gampenrieder SP, Rinnerthaler G, Greil R. Neoadjuvant Chemotherapy and Targeted Therapy in Breast Cancer: Past, Present, and Future. J Oncol. 2013;2013:732047. doi: 10.1155/2013/732047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reim F, Dombrowski Y, Ritter C, Buttmann M, Hausler S, Ossadnik M, Krockenberger M, Beier D, Beier CP, Dietl J, Becker JC, Honig A, Wischhusen J. Immunoselection of breast and ovarian cancer cells with trastuzumab and natural killer cells: selective escape of CD44high/CD24low/HER2low breast cancer stem cells. Cancer Res. 2009;69:8058–8066. doi: 10.1158/0008-5472.CAN-09-0834. [DOI] [PubMed] [Google Scholar]
- 27.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terp MG, Olesen KA, Arnspang EC, Lund RR, Lagerholm BC, Ditzel HJ, Leth-Larsen R. Anti-Human CD73 Monoclonal Antibody Inhibits Metastasis Formation in Human Breast Cancer by Inducing Clustering and Internalization of CD73 Expressed on the Surface of Cancer Cells. J Immunol. 2013;191:4165–4173. doi: 10.4049/jimmunol.1301274. [DOI] [PubMed] [Google Scholar]
- 29.Cacciari B, Pastorin G, Spalluto G. Medicinal chemistry of A2A adenosine receptor antagonists. Curr Top Med Chem. 2003;3:403–411. doi: 10.2174/1568026033392183. [DOI] [PubMed] [Google Scholar]
- 30.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J Biol Chem. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 31.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2013;32:1743–1751. doi: 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay D, Datta K. Multiple regulatory pathways of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in tumors. Semin Cancer Biol. 2004;14:123–130. doi: 10.1016/j.semcancer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Shi J, Wan Y, Di W. Effect of hypoxia and re-oxygenation on cell invasion and adhesion in human ovarian carcinoma cells. Oncol Rep. 2008;20:803–807. [PubMed] [Google Scholar]
- 34.Olson TA, Mohanraj D, Carson LF, Ramakrishnan S. Vascular permeability factor gene expression in normal and neoplastic human ovaries. Cancer Res. 1994;54:276–280. [PubMed] [Google Scholar]
- 35.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stahle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 36.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE, Boente M, Birrer MJ, Liang SX. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 37.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, Yi J, Nycum LR. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote IB, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag DT, Ray-Coquard I. AURELIA: A randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC) 2012 ASCO Annual Meeting. Oral Abstract Session, Gynecologic Cancer. J. Clin. Oncol. 2012;30 abstr LBA5002. [Google Scholar]


