Abstract
Background: Recently there has been an increased interest in the role of tumor-associated stroma in prostate tumorigenesis, but little is known about the respective roles of stomal ERα and ERβ in prostate cancer (PCa). This study characterizes the expression patterns of ERα and ERβ in tumor-associated stroma in association with various clinicopathological factors of importance in PCa prognosis and treatment. Design: Immunohistochemistry was performed using antibodies against ERα and ERβ to characterize their expression patterns in PCa tissue. Stromal ER levels (ERα and ERβ) on tissue sections (n=47), were compared between tumor associated stroma and adjacent benign associated stroma. Immunohistochemistry was also performed on a PCa tissue microarray (TMA) (n=177) to correlate stromal expression with various clinicopathological parameters. The levels of ER nuclear expression were scored semi-quantitatively. Results: The expression levels of both ERα and ERβ were significantly lower in tumor-associated stroma than stroma surrounding benign prostatic glands on the same tissue section (ERα: p<0.01; ERβ: p=0.01). When correlated with clinicopathological factors, the level of ERα expression in tumor-associated stroma showed a positive correlation with Gleason score (R2=0.8638). The expression of ERα was higher in PCa with advanced tumor stage (p=0.05) and not significantly different in extraprostatic extension (p>0.05). The level of ERβ expression in tumor-associated stroma was decreased in patients older than 60 years compared to younger patients (p=0.01). Conclusion: This study demonstrates significant down-regulation of ERα and ERβ expression in the tumor-associated stroma of PCa. However, the level of ERα expression in tumor-associated stroma shows a positive correlation with cancer differentiation and tumor stage.
Keywords: Estrogen receptors, prostate cancer, stromal
Introduction
Prostate cancer (PCa) is the most common cancer among men, as well as one of the leading causes of cancer related deaths [1]. Curative therapy is a challenge because late stage disease often evolves to a state that is refractory to therapy, developing the androgen-independent disease [2]. A better understanding of PCa progression is crucial to the discovery of reliable prognostic markers and novel therapies for the disease.
Recently, there has been an increased interest in role of tumor-associated stroma in neoplastic progression, including in PCa. Stromal cells in the surrounding matrix play an important role in cancer, communicating with nearby tumor cells via either direct or paracrine signaling [3-6]. The introduction of tumor-associated stroma to normal epithelial cells has been shown to cause alterations of the epithelium leading to hyperplasia and tumorigenesis [7]. Though non-cancerous themselves, these stromal cells often show different patterns of expression for specific factors as compared to stromal cells distant from cancer [8]. A recent study has been performed to identify key factors specific to PCa tumor-associated stroma, including CAV1 a predictor of early PCa tumor recurrence [9]. Importantly, some of these stromal factors may be potential predictive and prognostic markers of cancer.
Nuclear hormone receptors, including androgen receptor (AR), ER and progesterone receptor (PR), have previously been reported to be important modulators of prostate growth and differentiation. Once activated by their respective hormones, they regulate gene expression, modifying differentiation and proliferation pathways [10-13]. ER, activated by estrogen, has activity as both a DNA binding transcription factor regulating gene expression as well as non-genomic functions, including membrane signaling leading to post-translational modifications of many existing proteins [14]. ER is expressed in two separate forms, α and β, that are regulated separately and have different expression patterns in PCa [15-19]. ER plays a role in cell proliferation in the prostate, both as a stimulatory factor and a growth inhibitor, via activation of its two separate isoforms [20,21]. ERβ may have anti-proliferative effects to counter the proliferative effects of ERα [22,23]. One study found that the inability to activate ERβ by estrogen in tissue recombinant mice leads to prostate hyperplasia, which can be resolved by an ERβ specific agonist [24].
The roles of androgen and estrogen receptors in PCa have been primarily focused on epithelial cells [22,25], while their roles in stromal cells (i.e. effects on prostate tumorigenesis and cancer progression) have been far less studied. ER α and β are both expressed in PCa associated stromal cells; however, prior to this study, no correlation between stromal ER expression and clinicopathological factors of PCa have been reported. We previously reported an association between decreased stromal AR and PCa differentiation and androgen-independence [26]. Additionally, PR expression is reduced in tumor associated stroma when compared to benign associated stroma [27]. The purpose of this study was to evaluate the expression of both isoforms of ER in tumor-associated stroma compared to benign stroma to determine any link between stromal ER expression and clinicopathological factors of disease.
Material and methods
Case selection and TMA construction
Two sets of PCa cases were used in this study. In the first study set, the tissue sections from forty-seven PCa cases were studied to compare stromal ER (ERα and ERβ) levels in PCa and adjacent benign prostate on the same slide. Gleason score ranges from 5 to 10, stages from T2 to T3b and PSA ranges from 0.05 to 50 (including both recurrent and non-recurrent tumor) were used. In the second study set, ERα and ERβ expression patterns were studied in 177 samples of PCa on a tissue microarray (TMA) (177 out of 200 with IHC scores available). The samples on the TMA were stratified by various clinicopathological factors including age at diagnosis, preoperative PSA, grade, and stage (Table 1).
Table 1.
Clinical characteristics of the study population on TMA
| Characteristic | No. of Patients | % |
|---|---|---|
| PSA Recurrence Status | ||
| PSA Recurrence | 43 | 21.5 |
| Post-Prostatectomy Residual Tumor | 48 | 24 |
| None | 60 | 30 |
| Unknown | 49 | 24.5 |
| Vital Status | ||
| Alive | 157 | 78.5 |
| Dead | 36 | 18 |
| Lost to Follow-Up | 7 | 3.5 |
| Extraprostatic Extension | ||
| Focal | 47 | 23.5 |
| Established | 20 | 10 |
| Multifocal | 23 | 11.5 |
| None | 109 | 54.5 |
| Unknown | 1 | 0.5 |
| pT Stage | ||
| pT1b | 1 | 0.5 |
| pT2 | 1 | 0.5 |
| pT2+ | 4 | 2 |
| pT2a | 23 | 11.5 |
| pT2b | 77 | 38.5 |
| pT3 | 1 | 0.5 |
| pT3a | 67 | 33.5 |
| pT3b | 26 | 13 |
| pN Stage | ||
| pN0 | 166 | 83 |
| pN1 | 2 | 1 |
| pNX | 32 | 16 |
| pM Stage | ||
| pM0 | 178 | 89 |
| pMX | 22 | 11 |
Immunohistochemistry (IHC)
Archival formalin-fixed, paraffin-embedded tissue sections and TMAs of PCa cases were used in this study with NYU IRB approval. The IHC was performed using single label immunohistochemistry by the NexES automated immunostainer and detection system (Ventana Medical Systems, Tucson, AZ, USA). 4 micron sections were deparaffinized in xylene, rehydrated through graded alcohols, and rinsed in distilled water. All incubations were carried out at 37°C unless otherwise noted. After deparaffinization, heat induced epitope retrieval was performed by microwaving sections with 0.01 M, pH 6.0 citrate buffer for 20 min in a 1200 watt microwave oven. Endogenous peroxidase was blocked by application of hydrogen peroxide for 4 minutes. ERα and ERβ specific antibodies (Cell Signaling) were detected by the application of a biotinylated goat anti-rabbit for 8 minutes, followed by the application of streptavidin-horseradish peroxidase for 8 minutes. The chromogen, 3,3’-diaminobenzidine/hydrogen peroxide mix was applied for 8 minutes and then enhanced with copper sulfate for 4 minutes. Slides were then counterstained with hematoxylin, dehydrated, and mounted in permanent media. The levels of ER nuclear expression were scored semi-quantitatively: 0 as negative, 1 as weak, 2 as moderate, and 3 as strong expression. Breast cancer cases were used as positive control for ERα and ERβ expression. No primary antibody controls were used to serve as a negative control.
Statistical analysis
Statistical analyses of the above results were performed by Student’s t-test and linear regression and correlation analysis. Differences were considered statistically significant if p≤0.05.
Results
Characteristics of study population
Analyses of the correlation of stromal ERα and ERβ expression with clinicopathological factors were performed on 177 samples of PCa and compared with race, age at diagnosis, Gleason score, grade and stage, extraprostatic extension, preoperative PSA, and PSA recurrence. The demographic and clinicopathological data from the 177 patients on the TMA are summarized in Table 1.
The expression levels of both ERα and ERβ are significantly lower in tumor-associated stroma
First, we compared the expression levels of ERα and ERβ between tumor-associated stroma and the stroma surrounding benign prostatic glands. The expression levels of ER in the stroma were scored based on the intensity of immunostaining and the percentage of stromal fibroblasts and myofibroblasts adjacent to either the benign or malignant prostatic glands. The staining patterns of the infiltrating lymphocytes, macrophages, and other cells in the stromal compartment were not taken into account. On the whole tissue section of 47 PCa cases, ERα and ERβ are expressed in 40% of stromal cells in benign prostate tissue. The expression levels of both ERα and ERβ were significantly lower in tumor-associated stroma (ERα: p<0.01; ERβ: p=0.01) (Figures 1 and 2).
Figure 1.
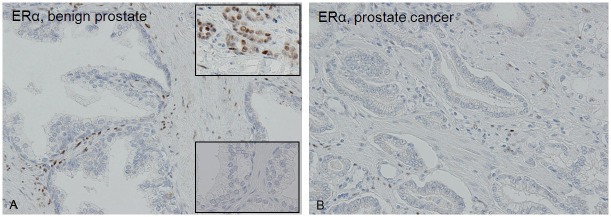
The expression of ERα is significantly lower in tumor-associated stroma (B) compared with stroma around benign glands (A) (p<0.01). Upper inset: ERα in breast cancer as positive control. Lower inset: Negative control for ERα in prostate.
Figure 2.
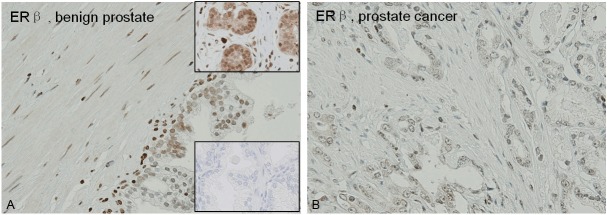
The expression of ERβ is significantly lower in tumor-associated stroma (B) compared with stroma around benign glands (A) (p=0.01). Upper inset: ERβ in breast cancer as positive control. Lower inset: Negative control for ERβ in prostate.
Increased expression of stromal ERα is associated with advanced disease
Since the expression of stromal ERα and ERβ was decreased in PCa in general, we performed a correlation analysis between stromal ER expression and various clinicopathological factors of PCa (including race, age at diagnosis, Gleason score, grade and stage, extraprostatic extension, preoperative PSA, and status of PSA recurrence). The expression of ERα in tumor-associated stroma showed a positive correlation with the Gleason score (r=0.93, p=0.05) (Figure 3) and tumor stage (p=0.05) (Figure 4). In addition, the cases with extraprostatic extension showed a trend of higher stromal expression of ERα, but the difference was not statistically significant (p>0.05). No significant correlation was detected between the expression levels of ERα and the other clinicopathological factors. The level of ERβ expression in tumor-associated stroma was significantly decreased in patients older than 60 years compared to younger patients (p=0.01) (Figure 5). No significant correlation was detected between the expression level of ERβ in tumor-associated stroma and any of the other clinicopathological factors.
Figure 3.
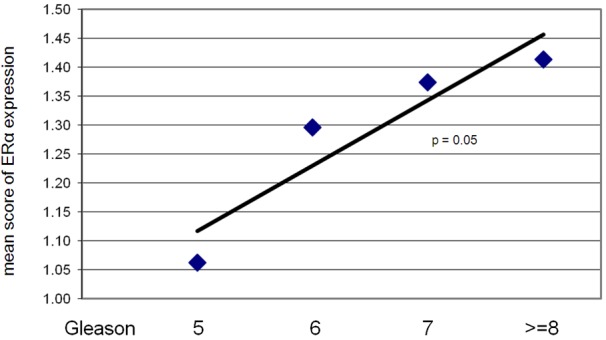
The level of ERα expression in tumor-associated stroma shows a positive correlation with Gleason score (r=0.93, p=0.05). No significant correlation was detected between the expression of ERβ in tumor-associated stroma and Gleason score.
Figure 4.
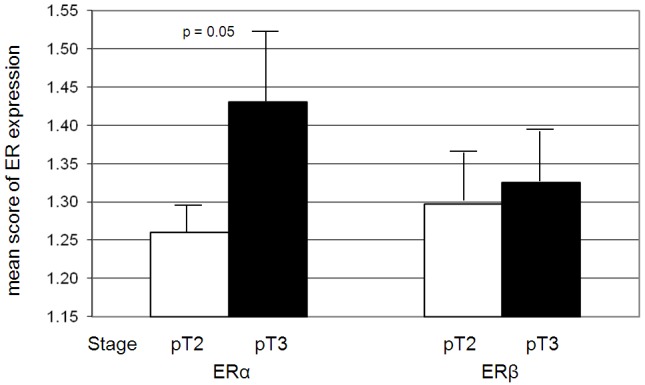
The expression level of ERα is higher in PCa with advanced tumor stage (n (pT2): 108, n (pT3): 86, p=0.05). No significant correlation was detected between the expression of ERβ in tumor-associated stroma and tumor stage.
Figure 5.
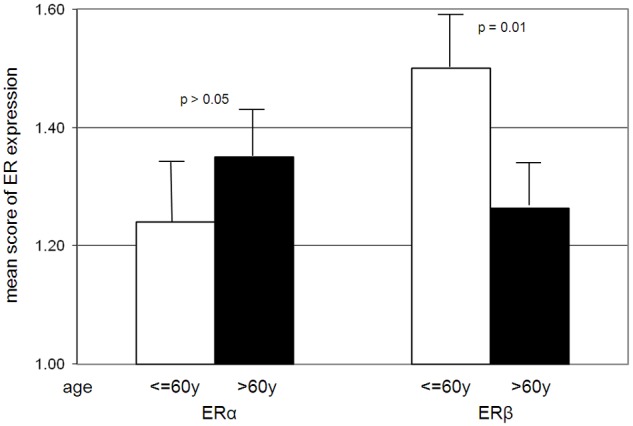
The level of ERβ expression in tumor-associated stroma is decreased in older patients (p=0.01). No significant correlation was detected between the expression of ERα in tumor-associated stroma and age.
Discussion
The stromal environment in the prostate is heterogeneous in nature, consisting of macrophages, lymphocytes, smooth muscle, and fibroblasts in benign tissue, while myofibroblasts predominate in the tumor-associated stroma of cancer [28]. The prostate stroma is also heterogeneous in its expression of steroid hormone receptors (including AR, ER and PR). Low levels of stromal AR have been correlated with more aggressive cancer and loss of stromal AR facilitates growth in vitro and in vivo and invasion [26,29]. Decreased PR expression has also been observed in PCa associated stromal cells [27].
Estrogen and ER have also been shown to have carcinogenic effects on the prostate [30,31]. Both ERα and ERβ show proliferative and anti proliferative properties in the prostate in mouse models [24,32]. These models shed light on ERα and β-specific effects that might otherwise be masked, such as by the indirect effects of estrogen. However, little is known about the expression and function of stromal ER and how it affects the neighboring epithelium. In benign prostate, ERβ is the primary isoform expressed in the epithelium, whereas ERα is primarily found in the stromal and basal cells. However, ERα levels increase in epithelial cells in cancer [17]. More recently, it has been reported that TMPRSS2-ERG, a commonly identified fusion protein under the regulation by androgen in PCa, is regulated by estrogen signaling in a subclass of PCa [22]. ERα is also significantly lower in the stroma of Caucasian and African American men, who are at higher risk for PCa, compared to Hispanic and Asian men [33]. In this study, we showed that the expression of stromal ER is distinct from that found in epithelial cells. There is a significant reduction in both ERα and ERβ in tumor-associated stroma compared with adjacent benign prostate. Thus, down-regulation of stromal ER may be a critical step in the early stages of tumorigenesis. Although both isoforms are down-regulated in cancer compared to benign prostate, higher levels of ERα was observed in high grade and advanced stage tumors in tumor-associated stroma compared to low grade and stage cancer. This data was consistent with a recent report showing a trend of higher stromal ERα expression with high grade PCa [34]. This data is in direct contrast to stromal AR expression patterns where the AR levels are decreased in relation with grade [26]. Thus, an eventual rise of ERα levels may actually be critical in the transition into more advanced disease, or conversely, this could represent a passive change in advanced cancers that no longer require down-regulation of stromal ERα for tumor growth. It would be of great interest to determine whether the same relationship is found in metastatic and androgen-independent PCa.
ERβ levels were found to be markedly reduced in benign prostate stroma in older patients (>60), though ERβ levels did not show the same correlation with Gleason score and advanced tumor stage in this study. This observation may be of significance for the development and progression of PCa. It is reported that low stromal AR levels in normal and tumor prostate tissue are related to poor outcome in PCa patients [29]. ERβ may have similar effects. ERβ-specific agonists have shown promising results in PCa treatment [23]. Taken together, these observations suggest that stromal ERβ may have anti proliferative effects in the prostate as well as an inhibitory role in the progression to aggressive cancers.
We previously showed that stromal AR inhibits proliferation and invasion of malignant prostate epithelial cells. On the other hand, loss of AR in the stroma facilitated growth and invasion both in vitro and in vivo [26,29]. In light of a similar stromal ER expression pattern, ER may also have inhibitory effects in the prostate. It would be of great interest to assess the direct effects of stromal ER (ERα and ERβ) on prostate growth in future studies.
Acknowledgements
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development). This study is funded by NIH (1U01CA149556-01), DOD PCRP (PC080010 and PC111624) and VA Merit (1I01BX001505-01) grants to PL, NYU Molecular Oncology and Immunology Postdoctoral Training grant (T32 CA009161) to GD.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, Presti JC Jr, Kane CJ. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J. Clin. Oncol. 2005;23:7546–7554. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 3.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 4.Joesting MS, Cheever TR, Volzing KG, Yamaguchi TP, Wolf V, Naf D, Rubin JS, Marker PC. Secreted frizzled related protein 1 is a paracrine modulator of epithelial branching morphogenesis, proliferation, and secretory gene expression in the prostate. Dev Biol. 2008;317:161–173. doi: 10.1016/j.ydbio.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 7.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dakhova O, Ozen M, Creighton CJ, Li R, Ayala G, Rowley D, Ittmann M. Global gene expression analysis of reactive stroma in prostate cancer. Clin Cancer Res. 2009;15:3979–3989. doi: 10.1158/1078-0432.CCR-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orr B, Riddick AC, Stewart GD, Anderson RA, Franco OE, Hayward SW, Thomson AA. Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate. Oncogene. 2012;31:1130–1142. doi: 10.1038/onc.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linja MJ, Visakorpi T. Alterations of androgen receptor in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:255–264. doi: 10.1016/j.jsbmb.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 12.Ho SM. Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem. 2004;91:491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- 13.Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Progesterone receptor expression in human prostate cancer: correlation with tumor progression. Prostate. 2001;48:285–291. doi: 10.1002/pros.1108. [DOI] [PubMed] [Google Scholar]
- 14.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fixemer T, Remberger K, Bonkhoff H. Differential expression of the estrogen receptor beta (ERbeta) in human prostate tissue, premalignant changes, and in primary, metastatic, and recurrent prostatic adenocarcinoma. Prostate. 2003;54:79–87. doi: 10.1002/pros.10171. [DOI] [PubMed] [Google Scholar]
- 16.Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Estrogen receptor gene expression and its relation to the estrogen-inducible HSP27 heat shock protein in hormone refractory prostate cancer. Prostate. 2000;45:36–41. doi: 10.1002/1097-0045(20000915)45:1<36::aid-pros4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol. 1999;155:641–647. doi: 10.1016/S0002-9440(10)65160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73:233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricke WA, Wang Y, Cunha GR. Steroid hormones and carcinogenesis of the prostate: the role of estrogens. Differentiation. 2007;75:871–882. doi: 10.1111/j.1432-0436.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 20.Cunha GR, Wang YZ, Hayward SW, Risbridger GP. Estrogenic effects on prostatic differentiation and carcinogenesis. Reprod Fertil Dev. 2001;13:285–296. doi: 10.1071/rd01010. [DOI] [PubMed] [Google Scholar]
- 21.Bonkhoff H, Berges R. The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol. 2009;55:533–542. doi: 10.1016/j.eururo.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Setlur SR, Mertz KD, Hoshida Y, Demichelis F, Lupien M, Perner S, Sboner A, Pawitan Y, Andren O, Johnson LA, Tang J, Adami HO, Calza S, Chinnaiyan AM, Rhodes D, Tomlins S, Fall K, Mucci LA, Kantoff PW, Stampfer MJ, Andersson SO, Varenhorst E, Johansson JE, Brown M, Golub TR, Rubin MA. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst. 2008;100:815–825. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci. 2009;1155:174–186. doi: 10.1111/j.1749-6632.2009.04360.x. [DOI] [PubMed] [Google Scholar]
- 24.McPherson SJ, Ellem SJ, Simpson ER, Patchev V, Fritzemeier KH, Risbridger GP. Essential role for estrogen receptor beta in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology. 2007;148:566–574. doi: 10.1210/en.2006-0906. [DOI] [PubMed] [Google Scholar]
- 25.Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O’Neill G, Kooner R, Stricker PD, Grygiel JJ, Gustafsson JA, Sutherland RL. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61:5331–5335. [PubMed] [Google Scholar]
- 26.Li Y, Li CX, Ye H, Chen F, Melamed J, Peng Y, Liu J, Wang Z, Tsou HC, Wei J, Walden P, Garabedian MJ, Lee P. Decrease in stromal androgen receptor associates with androgen-independent disease and promotes prostate cancer cell proliferation and invasion. J Cell Mol Med. 2008;12:2790–2798. doi: 10.1111/j.1582-4934.2008.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y, Liu L, Xie N, Xue H, Fazli L, Buttyan R, Wang Y, Gleave M, Dong X. Expression and function of the progesterone receptor in human prostate stroma provide novel insights to cell proliferation control. J Clin Endocrinol Metab. 2013;98:2887–2896. doi: 10.1210/jc.2012-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–2483. [PubMed] [Google Scholar]
- 29.Wikstrom P, Marusic J, Stattin P, Bergh A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate. 2009;69:799–809. doi: 10.1002/pros.20927. [DOI] [PubMed] [Google Scholar]
- 30.Santti R, Newbold RR, Makela S, Pylkkanen L, McLachlan JA. Developmental estrogenization and prostatic neoplasia. Prostate. 1994;24:67–78. doi: 10.1002/pros.2990240204. [DOI] [PubMed] [Google Scholar]
- 31.Singh PB, Matanhelia SS, Martin FL. A potential paradox in prostate adenocarcinoma progression: oestrogen as the initiating driver. Eur J Cancer. 2008;44:928–936. doi: 10.1016/j.ejca.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 32.Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- 33.Haqq C, Li R, Khodabakhsh D, Frolov A, Ginzinger D, Thompson T, Wheeler T, Carroll P, Ayala G. Ethnic and racial differences in prostate stromal estrogen receptor alpha. Prostate. 2005;65:101–109. doi: 10.1002/pros.20272. [DOI] [PubMed] [Google Scholar]
- 34.Hetzl AC, Montico F, Lorencini RM, Kido L, Candido E, Billis A, Ferreira U, Cagnon VH. Fibroblast growth factor, estrogen, and prolactin receptor features in different grades of prostatic adenocarcinoma in elderly men. Microsc Res Tech. 2013;76:321–330. doi: 10.1002/jemt.22170. [DOI] [PubMed] [Google Scholar]


