Abstract
From microscopic organelles and sub-cellular domains to the level of whole tissues, organs, and body parts, living organisms must continuously maintain and renovate structural components. Matrix metalloproteinases (MMPs) comprise a family of over two dozen Zn-dependent endopeptidases thought to be primary effectors of extracellular tissue renewal and remodeling processes. Endogenous inhibitors, particularly the tissue inhibitors of MMPs (TIMPs), counteract MMP-2 proteolytic activity, but also participate in conversion of several pro-MMPs to proteolytically active forms. Numerous pathologies are characterized by imbalances in activities of MMPs relative to TIMPs. MMPs are synthesized and stored in cytoplasmic domains prior to secretion or expression in cell surface-associated form. Several proteases have been identified in cell nuclei, but their functions, regulation, and substrates remain largely unknown. Here we showed that the catalytically active gelatinase MMP-2 is expressed in nuclei of endothelial cells and neurons, but not in glial or Schwannoma cell lines, in a pattern resembling nuclear speckles, and colocalizes with TIMP-1.
Keywords: Nuclear localization, catalytically active MMP-2, endothelial cells, neurons
Introduction
A number of proteases have been found in the nucleus, including members of the cysteine protease [1-6] and cathepsin [7] families. The function of these is not well understood, but may include functions such as chromatin remodeling, maintenance of nuclear matrix structure, apoptosis, and regulation of cellular proliferation. Protease inhibitors such as calpastatin [4], the cathepsin inhibitor Myeloid and Erythroid Nuclear Termination stage-specific protein (MENT) [8] and serpins [9,10] have been detected in nuclei of several cell types, including endothelial cells. TIMPs localize to some cell nuclei as well, for example in proliferating but not in cell cycle-arrested fibroblasts [11-13]. Besides inhibition of MMPs proteolytic activity, TIMP-1 plays a role in apoptosis and potentiates growth of a number of human cell types [14-18]. We hypothesized that because at least some TIMPs localize to the nucleus, one or more of their MMP substrates may also be present in the nucleus.
To determine this, we performed immunostaining on vascular endothelial cells. We detected MMP-2 in both the cytoplasm and the nucleus of human vascular endothelial cells. Further immunostaining demonstrated the presence of MMP-9 and membrane type (MT)1-, MT2-, MT3-, MT4-, and MT5-MMPs in the cytoplasm and/or pericellular surface as expected, but no nuclear immunofluorescence for these antigens was ever observed. To ensure that these findings were not due to some unique feature of the particular endothelial cells (ECs), we also determined expression of all these MMPs in a number of other cell types.
Materials and methods
Cell culture
Human ECs (HSVEC) were harvested enzymatically from saphenous vein using type II collagenase as described [19]. Cells were grown in Medium 199 containing 20 mM HEPES, 50 μg/ml endothelial cell growth factor, 5 mM glutamine, 5% fetal calf serum, and an antibiotic mixture of penicillin (100 units/ml), streptomycin (100 μg/ml), and Fungizone (1.25 μg/ml). The isolation and characterization of human aortic EC were previously described [20]. EC were routinely characterized by phase contrast microscopy (Zeiss ICM 405, X40 objective) and expression of von Willebrand factor antigen [19]. EC within three passages were used throughout the experiments. Cells were studied at confluence in all treatment conditions. Cellular viability was assessed by Trypan blue exclusion. Treatment of EC with cytokines was performed essentially as described [21].
Immunofluorescence staining
Human ECs (106/well) were seeded on poly-DL-lysine coated glass cover slips in serum-containing media for 16 h. Cells were washed with PBS, fixed in 4% paraformaldehyde, permeabilized with 0.05% Triton X-100, blocked with 5% normal goat serum, incubated with anti-MMP2 mAb (1:250 dilution) together with MMP-2 pAb (1:500 dilution), washed with PBS, and incubated in PE-conjugated goat anti-mouse (1:500; Caltag Laboratories, Burlingame, CA), Alexa 568-conjugated goat anti-rabbit (1:500; Molecular Probes, Eugene, OR) and nuclear stain TOTO-3 (1:15,000; Molecular Probes, Eugene, OR). After wash, the cover slips were mounted in Immuno Fluore (ICN, Costa Mesa, CA), and visualized using a Leica (Heidelberg, Germany) TCS SP laser scanning confocal microscope (inverted) equipped with Argon (Ar, 488 nm) and Krypton (Kr, 568 nm) lasers. Alexa568 signal for MMP-2 and PE-stained MT3-MMP were obtained by sequential scans. To establish possible colocalization of MT3-MMP (green channel) and MMP-2 (red channel), corresponding 2D sections from both channels were merged, using Leica software, and viewed as maximum intensity projections, as well as xz and yz projections of selected cellular regions.
Isolation of EC nuclei
Cells were washed with PBS and incubated with trypsin-EDTA, then centrifuged (5 min at 300 xg), homogenized, centrifuged (10 min at 1000 xg), resuspended in homogenization medium with an equal volume of OptiPrep (Sigma), and added onto 30% and 35% iodixanol gradient solutions. The homogenate and gradient solutions were centrifuged for 20 min at 10,000 xg, nuclei were extracted from the interface of the gradient and stained with 0.5% Azure C in a 0.25% sucrose solution.
Nuclear protein extraction
Isolated nuclei were centrifuged (10 min at 10,000 xg) in PBS, resuspended in KCl buffer with proteinase inhibitors at pH 7.3. Tubes were then agitated gently for 30 min and were centrifuged for 15 min at 9,000 xg. Supernatant was collected for further analysis.
Immunoprecipitation
Protein supernatants were incubated with polyclonal anti-MMP-2 antibodies (Chemicon) and 2x IP buffer (2% Triton X-100, 300 mM NaCl, 20 mM Tris-HCl, 2 mM EDTA, 2 mM EGTA, 0.4 mM PMSF, 1% NP-40) and agitated overnight. Immunoprecipitation was performed using Seize Classic Immunoprecipitation Kits (Pierce) according to the manufacturer’s instructions.
Immunoblotting
Endothelial cell lysates (50 μg) and known molecular weight markers were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinyl difluoride membranes, and incubated for one hour at room temperature with blocking solution (5% skimmed milk in phosphate buffered saline). Membranes were washed and incubated overnight at 4°C with primary monoclonal anti-MMP-2 antibodies (Oncogene; 1:1000) followed by incubation with goat anti-mouse, IgG-HRP secondary antibodies (Santa Cruz; 1:5,000) for one hour at room temperature. Immunodetection was accomplished with an enhanced chemiluminescence.
Results and discussion
We found that all ECs and primary neurons examined consistently exhibited nuclear MMP-2 immunofluorescence, but other MMPs were never observed in the nuclei of these cells. However, MMP-2 was not detected in the nuclei of primary astrocytes, primary oligodendrocytes (both from neonatal rat brain), or a rat schwannoma cell line (Figure 2). As previously reported in fibroblasts and other cell types [11-13], TIMP-1 was also detected in nuclei isolated from ECs (Figure 3). TIMP-1 immunofluorescence colocalized with MMP-2 immunofluorescence, but fluorescence resonance energy transfer (FRET) experiments failed to demonstrate direct association of MMP-2 with TIMP-1. No colocalization or positive FRET was seen between MMP-2 and any other of the previously tested MMPs listed above. These observations indicate that TIMP-1 and MMP-2 are localized nearby one another in the nucleus, but do not directly interact.
Figure 2.
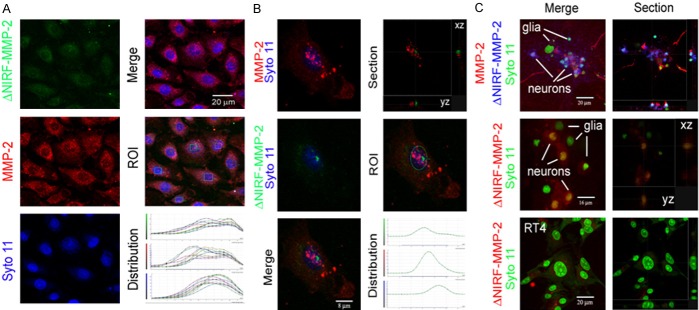
Fluorescence-based, microscopic assay for MMP-2 catalytic activity in nuclei of human ECs and rat neurons and glia. Cells were co-incubated for 60 min with the nuclear stain Syto 11 and a synthetic NIRF probe that is quenched when intact but fluoresces when specifically cleaved by MMP-2 and excited at 633 nm. After incubation, cells were fixed and stained with anti-MMP-2 polyclonal antibody followed by Alexa 568-conjugated secondary antibody. A: Left column: maximum intensity projections of confocal images of endothelial cells showing MMP-2 activity specific fluorescent product from NIRF probe (green), MMP-2 immunolocalization (red) and nuclear signal with Syto 11 (blue); right column: merged image (top), regions of interest within nuclei (middle), and the corresponding distribution of fluorescent signals for NIRF probe (green channel), MMP-2 (red channel) and syto 11 (blue channel) through the stacks of images. B: Left column: high magnification merged confocal images of an endothelial cell showing nuclear localization of MMP-2 (top, red), fluorescent product from NIRF probe (middle, green), and merged view (bottom); right column: section through the nuclear volume (top), nuclear region of interest (middle), and corresponding distribution of signals (bottom) for MMP-2 catalytic activity (green channel), MMP-2 immunolocalization (red channel), and nuclear staining with syto 11 (green channel). C: MMP-2 catalytic activity in the nuclei of neurons but not glia. Primary cultures of rat hippocampal neurons with a few glial cells, or RT4 schwannoma cells were incubated for 60 min with Syto 11 and MMP-2 specific NIRF probe, fixed, and stained with anti-MMP-2 antibody. Left column: maximum intensity projections of merged confocal images; Right column: sections though the stack of images. Neuronal nuclei (top, middle) but neither contaminating glia (top, middle) nor RT4 cells (bottom) show the fluorescent product from MMP-2 specific catalytic cleavage of the NIRF probe.
Figure 3.
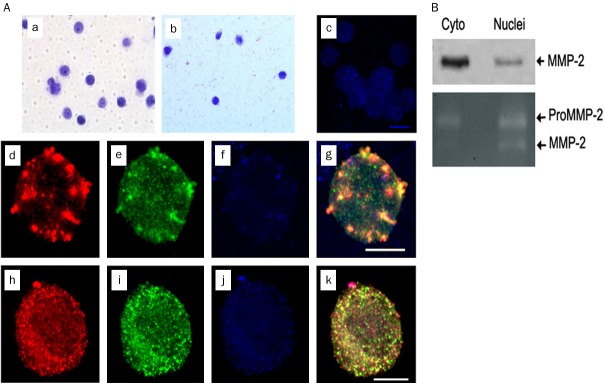
MMP-2 and TIMP-1 colocalize in isolated nuclei of endothelial cells. Nuclei from cultured ECs were isolated by OptiPrep centrifugation. (A) crude nuclei (a) and nuclei isolated following OptiPrep centrifugation (b) stained with Azure C or TOPRO-3 (c). Isolated nuclei were fixed and stained with anti-MMP-2 polyclonal and anti-TIMP1 monoclonal antibodies followed by Alexa 488 and Alexa 568 conjugated secondary antibodies, respectively. MMP-2 (red, d, h) and TIMP1 (green, e, i) colocalize within isolated, TOPRO-3 stained (blue, f, j) nuclei; merged images are shown as maximum intensity projections (g, k). Scale bar, 8 mm. (B) Western blot (top) and zymograph (bottom) of MMP-2 immunoprecipitated from OptiPrep isolated nuclei of ECs using anti-MMP-2 polyclonal antibody. MMP-2 immunoreactive band of relative MW 69 kDa identified with an anti-MMP-2 monoclonal antibody on Western blot and both pro-MMP2 and catalytically active MMP-2 bands in zymograph from isolated nuclei are shown.
Inflammatory cytokines such as TNF alpha caused markedly increased expression of a number of MMPs. However, no change in nuclear MMP-2 immunofluorescence was observed following TNF alpha treatment (data not shown). Treatment of ECs for 1 hour with agents that disrupt Golgi endosomal structure (Brefeldin A), inhibit protein synthesis (Actinomycin D), or disrupt microtubules (Colchicine) had no effect on the gross pattern or amount of nuclear MMP-2 immunofluorescence (Figure 1B), consistent with the conclusion that MMP-2 is relatively stable in the nucleus, and that nucleocytoplasmic flux of MMP-2 is minimal.
Figure 1.
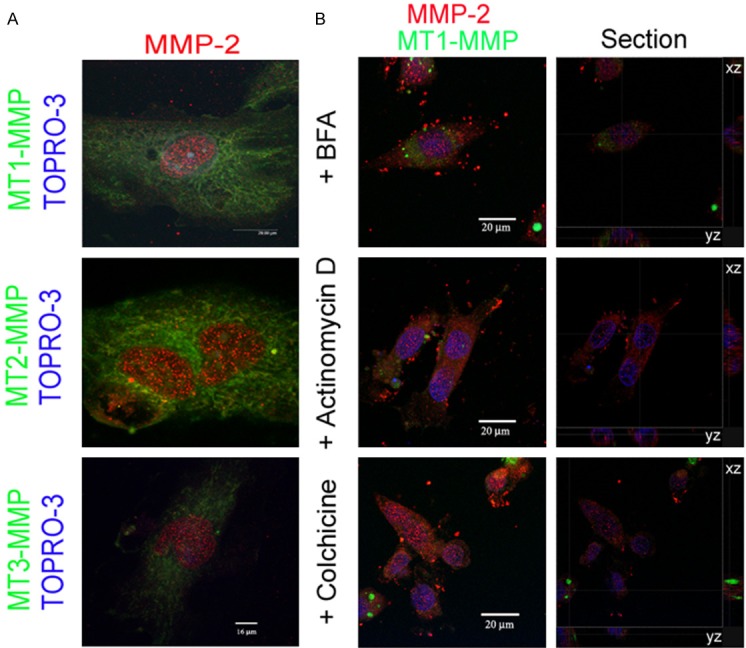
MMP-2 localization in subnuclear structures in human coronary artery endothelial cells. A: Merged, maximum intensity projections from confocal images of cultured cells stained with anti-MMP-2 (hinge region specific) polyclonal antibody together with monoclonal antibodies to MT1-MMP (left), MT2-MMP (middle) or MT3-MMP (right), and the nuclear stain TOPRO-3 (blue), show localization of MMP-2 (red) but not MT1-, MT2- or MT3-MMPs (green) in subnuclear structures. B: Subnuclear MMP-2 (red) localization persists but MT1-MMP (green) localization is lost in endothelial cells following 30 min treatment with BFA, actinomycin D and colchicine. Maximum intensity projections of merged confocal images acquired as sequential z-series of 0.3 mm thick optical sections (left column) and sections through the nuclear volume along xz and yz axes (right column) are shown.
It is possible that our observations were due to some type of immunologic artifact. To exclude this possibility, we performed Western blotting and immunoprecipitation experiments on cytoplasmic and nuclear extracts prepared from ECs. Both Western blotting and immunoprecipitation confirmed the immunofluorescence results, showing the presence of MMP-2 in both cytoplasmic as well as nuclear fractions (Figure 3B).
Separation of nuclear and cytoplasmic fractions may not be entirely complete, and contaminants may have inadvertently influenced our results. We therefore isolated intact nuclei from ECs, using a particularly rigorous isolation protocol designed to more completely remove cytoplasmic contaminants [22]. Visual inspection of isolated nuclei stained with Azure C revealed intact nuclei with one or more nucleoli and an apparently preserved nuclear membrane and internal structure. Sorting of nuclei by FACS sorting using anti-MMP-2 antibodies after permeabilizing the nuclei indicated that a major proportion of nuclei contained MMP-2 (data not shown). Confocal microscopic analysis in these isolated nuclei again revealed MMP-2 and TIMP-1 immunofluorescence in a punctate pattern that appeared grossly similar or identical to that observed in nuclei in whole cells (Figure 3A). However, in some isolated nuclei we also observed a more homogeneous distribution of both MMP-2 and TIMP-1 (Figure 3A). To further exclude the possibility of immunologic artifact, we repeated immunoprecipitation experiments on the isolated nuclei, and again found evidence of the presence of MMP-2 in the nuclei of these ECs (Figure 3B).
MMP-2 is synthesized in zymogen form, and the only known function of MMP-2 is proteolytic degradation of substrate. Nevertheless, expression of MMP-2 in the nucleus may or may not be accompanied by proteolytic activity. To determine whether MMP-2 in the nucleus was catalytically active, we performed gel zymography on lysates prepared from isolated nuclei. Gelatinase activity from a protease with a molecular weight identical to both active forms of MMP-2, but not from MMP-9 (also a gelatinase) was observed (Figure 3B). However, it is possible that preparation of the nuclear lysate somehow activated pro-MMP-2 into its catalytically active forms resulting in gelatinase activity noted on the zymograms.
To address this possibility and to corroborate zymographic results, we utilized a peptide substrate probe that incorporates a Cy5 moiety which emits fluorescence only upon proteolytic degradation by MMP-2 [23]. This probe is readily taken up by cells and has been successfully utilized to visualize proteolytic activity in vivo [23-26]. When ECs were incubated for 1 hour at 37°C with the synthetic peptide substrate, fluorescence typical of the Cy5 emission wavelength appeared in EC nuclei and cytoplasm when the cells were excited at 633 nm. Thus, both zymography and results from experiments with the synthetic peptide substrate fluorescent reporter indicate that nuclear MMP-2 is catalytically active.
MMP-2 and other MMPs contain nuclear localization signals that allow trafficking into the nucleus from the cytoplasm and may specify subnuclear destination. The function of nuclear MMP-2 is unknown, but could involve nuclear structural remodeling, degradation of nuclear proteins such as transcription factors and RNA processing proteins and ribonucleoproteins, or other undetermined functions.
We conclude that human ECs and primary neonatal rat neurons express catalytically active MMP-2 as well as TIMP-1 that both localize to the nucleus and colocalize within nearby nuclear subdomains. Nuclear proteases may activate or deactivate transcription factors [27-29] and regulate mitotic events [30]. Other possible roles of nuclear proteases include chromatin remodeling [31], apoptosis [5], alteration of nuclear matrix structural elements [32], and participation in molecular events leading to cellular proliferation and carcinogenesis [33]. The functional role of nuclear MMP-2 could include one or more of these intriguing possibilities, and is therefore the focus of ongoing investigation.
Acknowledgements
Supported by grants from the National Institutes of Health, Bethesda MD, HL51980 and HL58555 (to TBR). VM was supported by a fellowship from the Council of Scientific and Industrial Research (CSIR), India. A research associate funding from the Indian Council of Medical Research (ICMR) supported SN. TBR was supported by a Ramalingaswami Fellowship from the Department of Biotechnology (DBT), Government of India and the Philip Morris External Research Program (to TBR). SKS was supported by NIH grants (U54MD007598 and S21MD000103) at Charles R Drew University.
Disclosure of conflict of interest
None.
References
- 1.Doherty TM, Pei D, Uzui H, Wilkin DJ, Shah PK, Rajavashisth TB. Therapeutic developments in matrix metalloproteinase inhibition. Expert Opinion on Therapeutic Patents. 2002;12:655–697. [Google Scholar]
- 2.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 3.Shah PK, Galis ZS. Matrix metalloproteinase hypothesis of plaque rupture - players keep piling up but questions remain. Circulation. 2001;104:1878–1880. [PubMed] [Google Scholar]
- 4.Gil-Parrado S, Popp O, Knoch TA, Zahler S, Bestvater F, Felgentrager M, Holloschi A, Fernandez-Montalvan A, Auerswald EA, Fritz H, Fuentes-Prior P. Subcellular localization and in vivo subunit interactions of ubiquitous mu-calpain. J Biol Chem. 2003;278:16336–16346. doi: 10.1074/jbc.M208657200. [DOI] [PubMed] [Google Scholar]
- 5.Paroni G, Henderson C, Schneider C, Brancolini C. Caspase-2 can trigger cytochrome C release and apoptosis from the nucleus. J Biol Chem. 2002;277:15147–15161. doi: 10.1074/jbc.M112338200. [DOI] [PubMed] [Google Scholar]
- 6.Scholtz B, Lamb K, Rosfjord E, Kingsley M, Rizzino A. Appearance of nuclear protease activity after embryonal carcinoma cells undergo differentiation. Dev Biol. 1996;173:420–427. doi: 10.1006/dbio.1996.0037. [DOI] [PubMed] [Google Scholar]
- 7.Nishinaka T, Fu YH, Chen LI, Yokoyama K, Chiu RA. Unique cathepsin-like protease isolated from CV-1 cells is involved in rapid degradation of retinoblastoma susceptibility gene product, RB, and transcription factor SP1. Biochim Biophys Acta. 1997;1351:274–286. doi: 10.1016/s0167-4781(96)00210-2. [DOI] [PubMed] [Google Scholar]
- 8.Irving JA, Shushanov SS, Pike RN, Popova EY, Brömme D, Coetzer TH, Bottomley SP, Boulynko IA, Grigoryev SA, Whisstock JC. Inhibitory activity of a heterochromatin-associated serpin (MENT) against papain-like cysteine proteinases affects chromatin structure and blocks cell proliferation. J Biol Chem. 2002;277:13192–13201. doi: 10.1074/jbc.M108460200. [DOI] [PubMed] [Google Scholar]
- 9.Strik MC, Bladergroen BA, Wouters D, Kisiel W, Hooijberg JH, Verlaan AR, Hordijk PL, Schneider P, Hack CE, Kummer JA. Distribution of the human intracellular serpin protease inhibitor 8 in human tissues. J Histochem Cytochem. 2002;50:1443–1454. doi: 10.1177/002215540205001103. [DOI] [PubMed] [Google Scholar]
- 10.Bird CH, Blink EJ, Hirst CE, Buzza MS, Steele PM, Sun J, Jans DA, Bird PI. Nucleocytoplasmic distribution of the ovalbumin serpin PI-9 requires a nonconventional nuclear import pathway and the export factor Crm1. Mol Cell Biol. 2001;21:5396–5407. doi: 10.1128/MCB.21.16.5396-5407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritter LM, Garfield SH, Thorgeirsson UP. Tissue inhibitor of metalloproteinases-1 (TIMP-1) binds to the cell surface and translocates to the nucleus of human MCF-7 breast carcinoma cells. Biochem Biophys Res Commun. 1999;257:494–499. doi: 10.1006/bbrc.1999.0408. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WQ, Li H, Yamashita K, Guo XK, Hoshino T, Yoshida S, Shinya T, Hayakawa T. Cell cycle-associated accumulation of tissue inhibitor of metalloproteinases-1 (TIMP-1) in the nuclei of human gingival fibroblasts. J Cell Sci. 1998;111:1147–1153. doi: 10.1242/jcs.111.9.1147. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Nishio K, Yamashita K, Hayakawa T, Hoshino T. Cell cycle-dependent localization of tissue inhibitor of metalloproteinases-1 immunoreactivity in cultured human gingival fibroblasts. Nagoya J Med Sci. 1995;58:133–142. [PubMed] [Google Scholar]
- 14.Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 15.Lambert E, Boudot C, Kadri Z, Soula-Rothhut M, Sowa ML, Mayeux P, Hornebeck W, Haye B, Petitfrere E. Tissue inhibitor of metalloproteinases-1 signalling pathway leading to erythroid cell survival. Biochem J. 2003;372:767–747. doi: 10.1042/BJ20030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guedez L, Courtemanch L, Stetler-Stevenson M. Tissue inhibitor of metalloproteinase (TIMP)-1 induces differentiation and an antiapoptotic phenotype in germinal center B cells. Blood. 1998;92:1342–1349. [PubMed] [Google Scholar]
- 17.Wang T, Yamashita K, Iwata K, Hayakawa T. Both tissue inhibitors of metalloproteinases-1 (TIMP-1) and TIMP-2 activate Ras but through different pathways. Biochem Biophys Res Commun. 2002;296:201–205. doi: 10.1016/s0006-291x(02)00741-6. [DOI] [PubMed] [Google Scholar]
- 18.Lin H, Chen X, Wang J, Yu Z. Inhibition of apoptosis in rat mesangial cells by tissue inhibitor of metalloproteinase-1. Kidney Int. 2002;62:60–69. doi: 10.1046/j.1523-1755.2002.00403.x. [DOI] [PubMed] [Google Scholar]
- 19.Libby P, Ordovas JM, Auger KR, Robbins AH, Birinyi LK, Dinarello CA. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986;124:179–185. [PMC free article] [PubMed] [Google Scholar]
- 20.Navab M, Hough GP, Stevenson LW, Drinkwater DC, Laks H, Fogelman AM. Monocyte migration into the subendothelial space of a coculture of adult human aortic endothelial and smooth muscle cells. J Clin Invest. 1988;82:1853–1863. doi: 10.1172/JCI113802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng HB, Rajavashisth TB, Libby P, Liao JK. Nitric oxide inhibits macrophage-colony stimulating factor gene transcription in vascular endothelial cells. J Biol Chem. 1995;270:17050–17055. doi: 10.1074/jbc.270.28.17050. [DOI] [PubMed] [Google Scholar]
- 22.Nathanson L, Xia T, Deutscher MP. Nuclear protein synthesis: a re-evaluation. RNA. 2003;9:9–13. doi: 10.1261/rna.2990203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 24.Bremer C, Bredow S, Mahmood U, Weissleder R, Tung CH. Optical imaging of matrix metalloproteinase-2 activity in tumors: feasibility study in a mouse model. Radiology. 2001;221:523–529. doi: 10.1148/radiol.2212010368. [DOI] [PubMed] [Google Scholar]
- 25.Tung CH, Mahmood U, Bredow S, Weissleder R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000;60:4953–4958. [PubMed] [Google Scholar]
- 26.Weissleder R, Tung CH, Mahmood U, Bogdanov A Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77:53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 28.Moon NS, Premdas P, Truscott M, Leduy L, Bérubé G, Nepveu A. S phase-specific proteolytic cleavage is required to activate stable DNA binding by the CDP/Cut homeodomain protein. Mol Cell Biol. 2001;21:6332–6345. doi: 10.1128/MCB.21.18.6332-6345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palombella VJ, Maniatis T. Inducible processing of interferon regulatory factor-2. Mol Cell Biol. 1992;12:3325–3336. doi: 10.1128/mcb.12.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasmyth K. Segregating sister genomes: the molecular biology of chromosome separation. Science. 2002;297:559–565. doi: 10.1126/science.1074757. [DOI] [PubMed] [Google Scholar]
- 31.Gourdet C, Iribarren C, Morin V, Bustos P, Puchi M, Imschenetzky M. Nuclear cystein-protease involved in male chromatin remodeling after fertilization is ubiquitously distributed during sea urchin development. J Cell Biochem. 2007;101:1–8. doi: 10.1002/jcb.21056. [DOI] [PubMed] [Google Scholar]
- 32.Stein GS, van Wijnen AJ, Stein JL, Lian JB, Pockwinse SM, McNeil S. Linkages of nuclear architecture to biological and pathological control of gene expression. J Cell Biochem Suppl. 1998;30-31:220–231. [PubMed] [Google Scholar]
- 33.Blagosklonny MV, An WG, Melillo G, Nguyen P, Trepel JB, Neckers LM. Regulation of BRCA1 by protein degradation. Oncogene. 1999;18:6460–6468. doi: 10.1038/sj.onc.1203068. [DOI] [PubMed] [Google Scholar]


