Abstract
The main determinant of glioblastoma (GBM) resistance to temozolomide (TMZ) is thought to be O6-methylguanine-DNA methyltransferase (MGMT), which is a DNA-repair enzyme that removes alkyl groups from the O6-position of guanine. Previously, we reported that a MGMT-siRNA/cationic liposome complex exerted a clear synergistic antitumor effect in combination with TMZ. Translation to a clinical setting might be desirable for reinforcing the efficacy of TMZ therapy for GBM. In this study, we aim to evaluate the safety of MGMT-siRNA/cationic liposome complexes and determine whether the convection-enhanced delivery of these complexes is suitable for clinical use by undertaking preclinical testing in laboratory animals. No significant adverse events were observed in rats receiving infusions of MGMT-siRNA/cationic liposome complex directly into the brain with or without TMZ administration. A pig which received the complex administered by CED also showed no evidence of neurological dysfunction or histological abnormalities. However, the complex did not appear to achieve effective distribution by CED in either the rat or the porcine brain tissue. Considering these results together, we concluded that insufficient distribution of cationic liposomes was achieved for tumor treatment by CED.
Keywords: Glioblastoma, MGMT, si-RNA, cationic liposome, convection-enhaced delivery
Introduction
Glioblastoma multiforme (GBM) is one of the most formidable solid tumors. In recent years, neuro-oncology has become one of the most rapidly developing fields in cancer research. A small-molecule alkylating agent, temozolomide (TMZ), has shown improvements in prognosis in a Phase III clinical study. However, the efficacy of TMZ for GBM is often very limited because of the inherent or acquired resistance of the tumor to TMZ. Thus, the median survival time was reported to be 14.6 months [1].
The main determinant of GBM resistance to alkylating agents (including TMZ) is thought to be O6-methylguanine-DNA methyltransferase (MGMT), which is a DNA-repair enzyme that removes alkyl groups from the O6-position of guanine, an important target site for DNA alkylation [2,3]. Thus, high MGMT expression in tumor cells diminishes the therapeutic effects of alkylating agents. Indeed, an increasing MGMT expression level correlates well with in vitro and in vivo glioma resistance to TMZ [4-7]. In a sub-analysis of the afore mentioned TMZ clinical trial [1], patients whose tumors did not exhibit methylation in the MGMT gene promoter derived almost no benefit from TMZ chemotherapy [8]. Thus, MGMT can be considered a suitable and important target for any adjuvant therapy aimed at improving the efficacy of concurrent chemotherapy for GBM.
Previously, we reported that a MGMT-siRNA/liposome complex (LipoTrust EX Oligo) delivered by convection-enhanced delivery (CED) exerted a clear synergistic antitumor effect in combination with TMZ [9]. Translation to a clinical setting might be desirable for reinforcing the efficacy of TMZ therapy for GBM. In this study, we aim to evaluate the safety of MGMT-siRNA/LipoTrust complexes and determine whether the CED of these complexes is suitable for clinical use by undertaking preclinical testing in laboratory animals.
Methods
TMZ and LipoTrust EX Oligo were supplied by Merck (Whitehouse Station, NJ, USA) and Hokkaido System Science (Sapporo, Japan), respectively. siRNA oligomers directed against both rat and human MGMT were procured from Hokkaido System Science (catalog no, 283SKSV_284). The sense and antisense strands were as follows: sense, 5’-CCAGACAGGUGUUAUGGAATT-3’ and antisense, 5’-UUCCAUAACACCUGUCUGGTT-3’. The negative control siRNA was also obtained from Hokkaido System Science (catalog no S5C-0600).
Preparation of a transfection complex
siRNAs were transduced by using LipoTrust EX Oligo. A vial of LipoTrust (1 µmol lipid) was reconstituted using 1 mL of nuclease-free water. For animal experiments, 100 µL of Opti-MEM (Life Technologies, Carlsbad, CA, USA) containing 80 pmol of siRNA was gently mixed with 8 µL of reconstituted LipoTrust solution.
Gadolinium encapsulation by LipoTrust
A vial of LipoTrust (1 µmol lipid) was reconstituted using 1 mL of nuclease-free water. Reconstituted LipoTrust (50 µL) was added to Omniscan (350 µL; Daiichi-Sankyo, Tokyo, Japan) and water (550 µL) while being vortexed at 55°C. Vortex mixing was further continued for 20 min at room temperature. Gadolinium encapsulation by LipoTrust was enriched by ultracentrifugation.
Direct injection of MGMT-siRNA/LipoTrust into rat brains
The safety of MGMT-siRNA/LipoTrust in rodent animals was tested in accordance with Good Laboratory Practice (GLP) guidelines. Five-week-old male specific pathogen-free sprague dawley (SPF SD) rats were purchased from Japan SLC (Shizuoka, Japan). They were housed in stainless steel cages and kept on a 12 h light/dark cycle. Water and food were given ad libitum. Following acclimatization for 1 week, the rats were divided into five groups containing five animals each: the control group, LipoTrust group, MGMT-siRNA/LipoTrust complex group, MGMT-siRNA/LipoTrust complex plus TMZ group, and TMZ group. For baseline data, blood samples were collected from a tail vein of all rats. In rats assigned to three of the groups (LipoTrust group, MGMT-siRNA/LipoTrust complex group, and MGMT-siRNA/LipoTrust complex plus TMZ group), burr holes were made on the skull 5 mm caudal to and 3 mm right from the bregma under general anesthesia (pentobarbital [50 mg/kg]), and 10 µL of LipoTrust (0.8 nmol) with or without MGMT-siRNA (8 pmol) was injected 5 mm inside the brain surface using a Hamilton syringe at Day 0. In rats of the MGMT-siRNA/LipoTrust complex plus TMZ group and the TMZ group, 160 µL TMZ was injected intraperitoneally once every day from Day 1 to Day 5. All rats were weighed once in 3 days, and blood samples were collected from the tail vein every 7 days during the observation period. After a 30-day observation period, blood samples for serum chemistry were collected from the abdominal vena cava under anesthesia. Then, the rats were euthanized and the bones, bone marrow, thymus, lungs and bronchi, heart, liver, pancreas, spleen, kidneys, adrenal gland, and brain were isolated. Each organ was fixed in 10% formalin, embedded in paraffin, and routinely stained with hematoxylin and eosin (H&E). For the hematological tests, red and white blood cell counts, differential leukocyte count, platelet count, hemoglobin, hematocrit, and reticulocyte counts were evaluated. The levels of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and 2’-5’-oligoadenylate synthetase 1 (2-5-OAS1) were measured during serum chemical analysis in addition to other general measurements of blood chemical analysis (i.e., total protein, albumin, globulin, albumin/globulin ratio, glucose, total cholesterol, triglyceride, bilirubin, urea nitrogen, creatinine, aspartate aminotransferase, alanine aminotransferase, γ-glut-amyl transpeptidase, alkaline phosphatase, sodium, potassium, chlorine, calcium, and inorganic phosphorus). The levels of TNF-α was estimated in the serum using a Rat TNF-α Platinum enzyme linked immunosorbent assay (ELISA) kit (Bender MedSystems, Burlingame, CA, USA) while INF-γ was estimated using a Rat IFN-γ Platinum ELISA kit (Bender MedSystems). The 2-5-OAS1 was estimated using a 2’-5’-oligoadenylate synthetase 1 ELISA kit (Uscn Life Science, Houston, TX, USA) according to the manufacturers’ instructions.
Pre-porcine study in rats by CED infusions of fluorescently-labelled LipoTrust
Prior to commencement of the porcine study, we conducted a pilot study in rats to determine whether the liposomes were suitable for CED in grey and white matter. This experiment and the subsequent porcine experiment were performed at the School of Veterinary Sciences, University of Bristol, Bristol, UK.
Male Wistar rats (B & K, UK) weighing 225-275 g were anesthetized with ketamine and xylazine by intraperitoneal infusions. A stereotactic frame (Stoelting Co., Wood Dale, IL, USA) was fixed, and a skin incision was made between the glabella and the occiput. Burr holes (~2 mm) were made, and catheters (pre-primed with 1% bovine serum albumin [BSA]) were inserted into the grey (striatum) or white matter (corpus callosum). Then, CED infusions of the fluorescently-labelled cationic liposomes were performed at one of the following three different infusion rates for each rat: 0.5, 2.5, and 5 µL/min. After CED infusions, a thoracotomy was performed immediately, and animals were then perfusion-fixed with 100 mL of phosphate-buffered saline followed by 100 mL of 4% paraformaldeyhde (pH 7.4). The catheters were carefully withdrawn from the brain, and the brain was removed and fixed with 4% paraformaldeyhde (pH 7.4) for 48 h. The liposome distribution was analyzed under fluorescence microscopy. More detailed methods about CED in rats have been described previously [10-12].
Gadolinium with O6-methylguanine-DNA methyltransferase-siRNA/LipoTrust administration in a porcine brain
The protocol was reviewed and approved by the ethical committee at the University of Bristol.
An intramuscular ketamine dose (0.1 mg/kg body weight) was administered to each pig. General anesthesia was then induced and maintained with isoflurane (2-5%), and the animals were intubated with a cuffed endotracheal tube. Intravenous access was obtained using a catheter placed in the ear vein, and normal saline was infused at a rate of 250 mL/h. Pig head fixation was achieved using a custom-built fixation device designed to fit within an magnetic resonance imaging (MRI) foot/ankle coil (SENSE Coil; Philips, Amsterdam, The Netherlands). Following robust pig head fixation, a plate incorporating an N-shaped fiducial marker was attached to the head-fixation device, such that the fiducial plate lay flat over the top of the pig’s skull. The pig’s head and associated head fixation device were then placed inside the foot/ankle coil.
Animals were scanned in a 1.5 T MRI scanner (Intera, Phillips). Coronal T1-weighted MR images were obtained with a 2 mm slice thickness and 0.4 mm slice gap. Surgical planning to identify the stereotactic coordinates for catheter tip placement was performed using in-house software. The animal was transferred to the operating theater, and the MRI head-coil that was placed over the animal’s head was removed and positioned at a fixed location relative to the head fixation device. A midline incision was made, and the skull surface was exposed. Burr holes were drilled into the skull at the site of catheter entry, and guide tubes (Renishaw Plc., Wotton-Under-Edge, Gloucs., UK) push-fitted into the burr holes to facilitate catheter guidance into the target. A subacute catheter with 0.2 mm outer diameter fused silica tip (Renishaw) was inserted into the right frontal white matter. Following catheter implantation, the animal was removed from the stereotactic frame and repositioned in the foot/ankle coil and transferred to the MRI scanner. Infusions were then ramped from 0.5 µL/min to 1 µL/min and then to 2.5 µL/min and 5 µL/min after 10 min and 5 min intervals (total 140 µL) whilst serial T1-weighted MR images were obtained. After a 28-day recovery period, the pig was fixed transcardially with 10% formalin. The brain, kidney, liver, and spleen were harvested.
Statistical analyses
For comparison among groups, the mean and standard error were calculated in each group from the obtained numerical values. First, Bartlett test for equality of variance was performed. If the data showed homoscedasticity, one-way analysis of variance was performed. If the data showed unequal variance, a Kruskal-Wallis H test was performed. If they were significant, the mean value or average of the ranks was compared by Tukey’s test. The level of statistical significance was P<0.05 in the Bartlett test, one-way analysis of variance, and the Kruskal-Wallis H test. In the Tukey test, P<0.05 and P<0.01 were considered to be statistically significant.
Results
O6-methylguanine-DNA methyltransferase-siRNA/LipoTrust injection in normal rat brain with temozolomide administration
We evaluated the safety of the MGMT-siRNA/LipoTrust complex with or without TMZ by a single injection to normal rat brains.
In the 30-day follow-up period, all rats survived, and no apparent abnormalities in their general condition were observed.
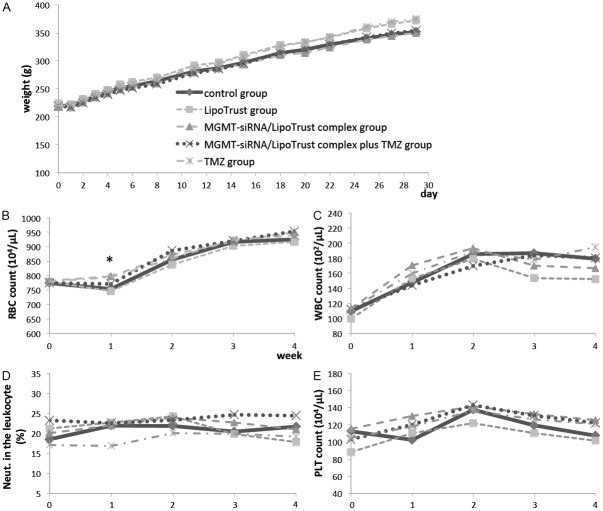
The MGMT-siRNA/LipoTrust complex group and MGMT-siRNA/LipoTrust complex plus TMZ group showed mild weight loss, but no significant difference was found between the groups (Figure 1A).
Figure 1.
A: Mean body weight of each group. B-E: Mean score of each hematological assessment of each group. B: Red blood cell count. C: White blood cell count. D: Neutrophil count. E: Blood platelet count. *a statistically significant difference (p<0.05).
In the hematological assessment, the red blood cell count at Day 7 was statistically higher in the MGMT-siRNA/LipoTrust complex group and TMZ group as compared to the control group (mean, 798±8, 794±10 vs. 753±14 (104/μL); P<0.05), but there were no significant differences in other cell counts between the control group and any other groups (Figure 1B-E). Serum chemical analysis showed no significant difference among all groups in all tests including TNF-α, IFN-γ, and 2-5-OAS1 (Table 1).
Table 1.
Serum chemical analysis including cytokine response
| GOT | GPT | BIL | GLU | Na | K | Cl | TP | GLB | UN | CRE | TNF-α | IFN-γ | 2-5-OAS1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| IU/L | IU/L | mg/dL | mg/dL | mEq/L | mEq/L | mEq/L | g/dL | g/dL | mg/dL | mg/dL | pg/mL | pg/mL | pg/mL | |
| control | 73 | 53 | 0.06 | 205 | 142.5 | 4.48 | 106.2 | 5.9 | 2.9 | 15.8 | 0.26 | 79.036 | 0 | 0 |
| ±6 | ±5 | ±0.01 | ±8 | ±0.3 | ±0.18 | ±0.4 | ±0.1 | ±0.1 | ±0.6 | ±0.01 | ±39.823 | ±0.000 | ±0.000 | |
| LipoTrust | 63 | 60 | 0.06 | 198 | 142.5 | 4.35 | 106.9 | 5.9 | 2.9 | 16.2 | 0.25 | 37.058 | 0 | 0 |
| ±3 | ±5 | ±0.01 | ±4 | ±0.2 | ±0.13 | ±0.6 | ±0.1 | ±0.1 | ±0.3 | ±0.01 | ±28.524 | ±0.000 | ±0.000 | |
| siRNA/LipoTrust | 66 | 64 | 0.06 | 212 | 142.6 | 4.29 | 105.8 | 5.9 | 2.9 | 16.3 | 0.26 | 3.425 | 0 | 0 |
| ±2 | ±3 | ±0.00 | ±13 | ±0.4 | ±0.13 | ±0.7 | ±0.1 | ±0.1 | ±0.6 | ±0.02 | ±3.425 | ±0.000 | ±0.000 | |
| siRNA/LipoTrust + TMZ | 89 | 78 | 0.07 | 206 | 142.2 | 4.18 | 105.2 | 6 | 2.9 | 15.5 | 0.27 | 0 | 0 | 0 |
| ±24 | ±15 | ±0.01 | ±5 | ±0.5 | ±0.10 | ±0.3 | ±0.1 | ±0.1 | ±0.7 | ±0.02 | ±0.000 | ±0.000 | ±0.000 | |
| TMZ | 70 | 67 | 0.06 | 194 | 142.3 | 4.54 | 105.3 | 6 | 2.9 | 16 | 0.26 | 0 | 0 | 0 |
| ±3 | ±6 | ±0.00 | ±5 | ±0.4 | ±0.12 | ±0.5 | ±0.1 | ±0.1 | ±0.6 | ±0.02 | ±0.000 | ±0.000 | ±0.000 | |
The number of each upper berth means the score of each test, and the number of the lower berth means standard deviation of each data.
The results of the histopathological examination after the observation period are shown in Figure 2 and Table 2. All abnormal cerebral findings were due to needle implant tracks (Figure 2); therefore, no local adverse events due to drugs were detected. Also, slight to moderate degree of abnormal findings were detected in other organs, and none of the rats showed any remarkable damage.
Figure 2.
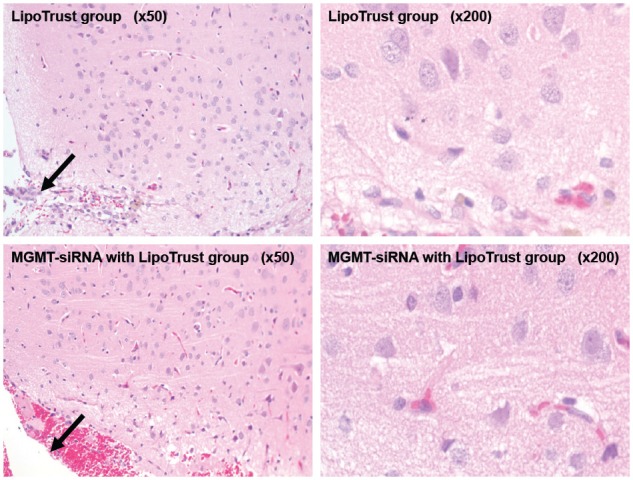
The histopathological examination in the rat brain after the observation period.
Table 2.
The types of abnormal findings and the numbers of rats which showed each abnormity in histopathological examination
| control | LipoTrust | siRNA/LipoTrust | siRNA/LipoTrust + TMZ | TMZ | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||||||||
| - | ± | + | ++ | - | ± | + | ++ | - | ± | + | ++ | - | ± | + | ++ | - | ± | + | ++ | ||
| Brain | Needle tract | 5 | 3 | 1 | 1 | 4 | 1 | 4 | 5 | ||||||||||||
| Liver | Focal cell infiltration of Glisson’s capsule | 4 | 1 | 4 | 1 | 4 | 5 | 5 | |||||||||||||
| Bile duct proliferation | 5 | 4 | 1 | 5 | 5 | 4 | 1 | ||||||||||||||
| Pancreas | Microvacuolation in secretion | 4 | 1 | 5 | 5 | 5 | 5 | ||||||||||||||
| Kidneys | Eosinophilic body in renal proximal tubular epithelial cells | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 3 | 1 | 2 | 2 | 1 | 2 | 3 | ||||||
| Focal basophilic renal tubule | 4 | 1 | 4 | 1 | 3 | 2 | 3 | 2 | 3 | 2 | |||||||||||
| Vacuolar degeneration of renal proximal tubular cells | 4 | 1 | 5 | 5 | 5 | 5 | |||||||||||||||
| Focal interstitial cell infiltration | 5 | 4 | 1 | 5 | 5 | 5 | |||||||||||||||
| Heart | Focal myocardial degeneration or focal cell accumulation | 5 | 4 | 1 | 5 | 5 | 5 | ||||||||||||||
| Lungs and Bronchi | Focal mineral deposition of artery wall | 4 | 1 | 5 | 4 | 1 | 4 | 1 | 5 | ||||||||||||
| Focal pneumonia | 4 | 1 | 5 | 5 | 5 | 5 | |||||||||||||||
- (normal), ± (minor change), + (mild change), ++ (moderate change). All other tested organs showed only normal findings.
Fluorescently-labelled LipoTrust administration by convection-enhanced delivery into the white and grey matter of rats
Microscopic analysis of the rat brain tissue sections demonstrated that CED of these liposomes into grey matter did not lead to sufficient distribution beyond the needle track and the needle tip. However, in the white matter, there was some evidence of distribution and less tissue damage at the rates of 0.5 µL/min and 2.5 µL/min (Figure 3). At high flow rates (5 µL/min), there was evidence of significant reflux of infusate in the white matter.
Figure 3.
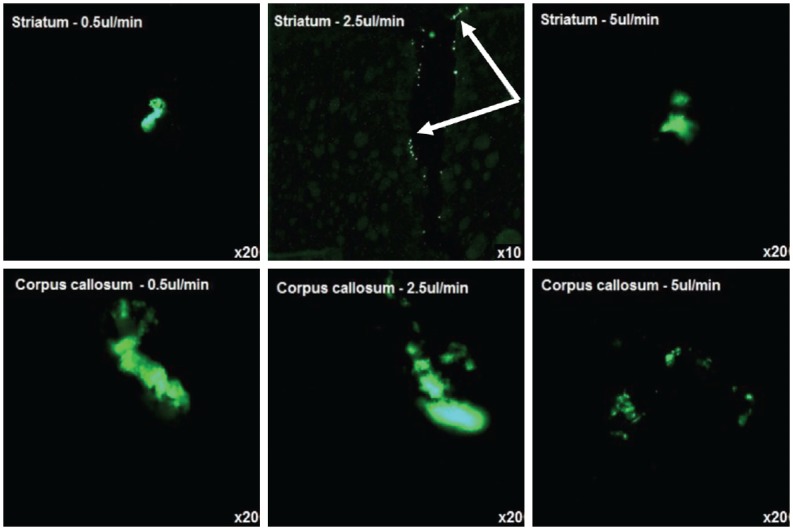
Fluorescently-labelled liposome administration by CED into the white matter (corpus callosum) and grey matter (striatum) of rats. CED of liposomes in to the striatum showed no distribution beyond the needle tip. CED into the corpus callosum showed some distribution, but at high flow rates it showed reflux of infusate.
Gadolinium with O6-methylguanine-DNA methyltransferase-siRNA/LipoTrust administration by convection-enhanced delivery into porcine brain
Real-time MR images demonstrated that gadolinium with MGMT-siRNA/LipoTrust (180 µL) infused into the right frontal white matter showed two different intensity areas: two aggregated areas with relatively high intensity and a widely-distributed relatively low intensity area. The former coincided with the positions of the catheter tip and guide tube tip. The latter was seen to distribute widely but did not correspond to the white matter anatomy. From our past experience, they seemed to represent the refluxed gadolinium encapsulated by LipoTrust. [12] (Figure 4A). Histological investigation of the brain showed no observable tissue damage including the right frontal white matter (Figure 4B). The liver, kidneys, and spleen also showed no histological abnormality.
Figure 4.
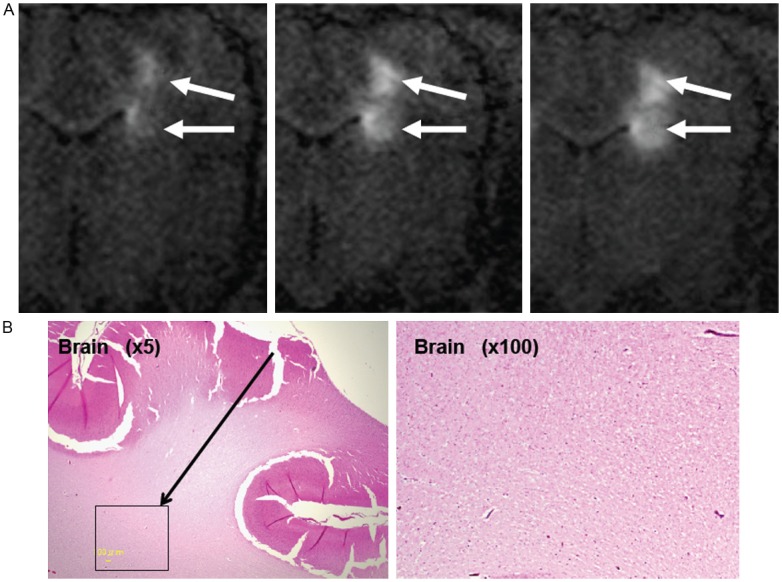
A: An intraoperative MR image of porcine brain infused with MGMTsiRNA/LipoTrust complex conjugated with gadolinium. B: Histopathological examination of the pig brain after gadolinium with MGMT-siRNA/LipoTrust administration.
Discussion
In this study, no significant adverse events were observed in rats receiving infusions of MGMT-siRNA/LipoTrust complex directly into the brain with or without TMZ administration. The pig that was administered with MGMT-siRNA/LipoTrust complex by CED also showed no evidence of neurological dysfunction or histological abnormalities. However, the MGMT-siRNA/LipoTrust complex did not appear to achieve effective distribution by CED in either the rat or the porcine brain tissue.
Intracranial glioma possesses unique characteristics when compared to other cancers. The brain is separated from the other organs by the blood-brain barrier (BBB), whose neighboring normal neural cells do not usually divide, and intracranial gliomas rarely metastasize to extra-cranial organs. These characteristics seem to be advantageous for gene therapy, because local intra-cranial administrations are likely to have an effect on confined tumors, and the induced genes or injected delivery systems such as siRNA or liposomes are less likely to influence other organs. For these reasons, gene therapy for malignant glioma has been studied from an early stage [13], but there is no established gene therapy clinical treatment available at present.
In a previous study, our MGMT-siRNA/LipoTrust complex used in combination with TMZ showed desirable synergistic effects on glioma cells in vivo, and no significant adverse events were observed on the mouse xenograft models. [9] As for siRNA therapy, off-target effects [14] and immune responses are two important issues preventing the widespread adoption of this therapy. To avoid off-target effects, we used three types of MGMT-siRNA in the previous study [9], which showed the same effect based on MGMT depletion. However, it is difficult to strictly prove the non-existence of an off-target effect, at least in the rats and pig infused with MGMT-siRNA/LipoTrust complex in this study. No serious adverse events occurred in general observations, blood examinations, or histological examinations in these animal models. Moreover, even if off-target effects occur in our therapy, side effects may be more limited than in systemic administrations because of local brain infusions.
Occasionally, immune responses occur against siRNA or vectors in gene therapy. We used the liposome, LipoTrust EX Oligo, as a vector to form siRNA-lipoplex nanoparticles, which have shown significant potential in other clinical applications [15-17]. Synthetic non-viral vectors represent potentially safer delivery systems than viral delivery systems because of their tendency to elicit lower innate immunity and toxicity responses. Sato et al. used vitamin-A coupled LipoTrust Oligo to achieve functional delivery of anti-gp46 siRNA for the treatment of liver cirrhosis in a mice model and reported on its safety [18]. Rai et al. used LipoTrust Oligo to deliver miR-7 expressing plasmid for mouse xenograft models of EGFR-TKI-resistant lung cancer and reported no serious complications [19]. In our previous study, though LipoTrust Oligo itself showed mild cytotoxicity in vitro, LipoTrust or MGMT-siRNA/LipoTrust complex in combination with TMZ showed no remarkable toxicity in vivo [9]. In the current study, TNF-α, IFN-γ, 2-5-OAS1, or other indicators of immune responses did not differ significantly in MGMT-siRNA/LipoTrust complex-administered rat groups as compared to the control group. In addition, histological examination did not reveal serious abnormalities such as tissue degeneration (suggestive of inflammation or intense infiltration of inflammatory cells) in all tested organs of the rats and pig, including their brains. We conclude from this that MGMT-siRNA/LipoTrust complex alone and MGMT-siRNA/LipoTrust complex in combination with TMZ did not trigger a cytokine response.
We adopted CED as the drug delivery system of choice for our current study. One of the limiting factors in the use of advanced therapeutics for brain tumors is the presence of the BBB that consists of a layer of tight conjunct endothelial cells and has strong efflux pumps, which actively remove toxic substances from the brain and reduce effective drug penetration [17]. Most drugs are inhibited transport to the brain, and even if drug transmission through the BBB is achieved, systemic complications may occur before the required drug concentration within the target brain tumor is obtained [18]. Therefore, direct intra-cranial administration has been studied for the treatment of glioma in the field of gene therapy. For example, direct intraoperative injection or multiple infusions via an indwelling catheter have been used for gene therapy. However, these methods were not satisfactory due to the short duration of gene expression, inefficient spreading of the vectors, and movement of the vectors into off-target sites. In the CED method, drugs or vectors can be administrated directly into the brain interstitium by using a fine catheter and controlled infusion rates. This administration process is performed very slowly and continuously over several hours or several days in order to achieve wider drug distribution. CED relies on a pressure gradient to establish bulk flow over time, which leads to widespreadand uniform distribution throughout the targeted brain tissue without forming an injectate pool or causing mechanical injury. In this study, the intraoperative MR images of the pig revealed that the gadolinium encapsulated by LipoTrust aggregated in two discrete areas, the catheter tip and catheter step, resulting in limited radial distribution from the site of infusion. The pre-porcine rat study also showed very limited distribution in grey matter and minimal distribution in the white matter. Considering these results together, we concluded that insufficient distribution of LipoTrust liposomes was achieved for tumor treatment by CED.
The most critical determinant for unsatisfactory distribution by CED may be the positive charge of the LipoTrust. In general, cell membranes are negatively charged; therefore, cationic liposomes are adsorbed by cell membranes at an early stage after infusion. This may be advantageous for gene transduction against single cells in vitro, but this poses a disadvantage for large tissues in vivo, because widespread distribution by CED was not achieved. To avoid early adsorption, we had to adopt a relatively high flow rate, which may have resulted in injectant reflux. There is some published data that demonstrate that cationic liposomes are not suitable for delivery to the brain by CED. Mackay et al. investigated the relationship between liposome size or charge and the extent of distribution in the brain delivered by CED and reported that cationic lipoplexes are inadequate to allow large volumes of distribution by CED [20]. Saito et al. investigated the effect of tissue affinity on the distribution of infusates delivered by CED and concluded that cationic liposomes result in poorer brain distribution [21]. In our study, we could also see surrounding faintly-high signals that did not follow normal white matter anatomy; this was presumed to be free gadolinium infused with the gadolinium encapsulated by LipoTrust. This kind of non-anatomic distribution is sometimes seen with tissue disruption caused by the liposomes [12], but histological examination of the pig brain showed no noticeable evidence of tissue damage in this study, nor did the brains of rats directly injected with LipoTrust.
We chose LipoTrust EX Oligo as a vector for our recent studies, because it showed good cell transduction efficiency, was relatively safe in past studies, and was commercially available under adaptive quality for clinical use. However, we conclude it is not a suitable liposome for CED. Despite these findings, MGMT-siRNA combined with TMZ therapy has demonstrated significant potential for the treatment of malignant glioma, as no serious adverse events were detected in this study and desirable effects were observed in our previous study. Currently, there are many studies focused on vectors including viruses, neutral or anionic liposomes, and polymer micelles. Miller et al. put forward an idea called ABCD nanoparticles, in which specific functions could be imparted to lipid-based nanoparticles [22]. These techniques may achieve a good balance between widespread distribution by CED, effective gene introduction, and drug safety for clinical use.
We believe our MGMT-siRNA combined therapy has immense potential for the treatment of malignant glioma. However, further studies are warranted to investigate the application of MGMT-siRNA for human clinical studies.
Acknowledgements
This work was supported by Japan Ministry of Education, Culture, Sports, Science and Technology’s Grant-in-Aid for Scientific Research on Innovative Areas (AN). We have no conflict of interest to declare in this study.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Day RS 3rd, Ziolkowski CH, Scudiero DA, Meyer SA, Lubiniecki AS, Girardi AJ, Galloway SM, Bynum GD. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980;288:724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- 3.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 4.Fruehauf JP, Brem H, Brem S, Sloan A, Barger G, Huang W, Parker R. In vitro drug response and molecular markers associated with drug resistance in malignant gliomas. Clin Cancer Res. 2006;12:4523–4532. doi: 10.1158/1078-0432.CCR-05-1830. [DOI] [PubMed] [Google Scholar]
- 5.Ma J, Murphy M, O’Dwyer PJ, Berman E, Reed K, Gallo JM. Biochemical changes associated with a multidrug-resistant phenotype of a human glioma cell line with temozolomide-acquired resistance. Biochem Pharmacol. 2002;63:1219–1228. doi: 10.1016/s0006-2952(02)00876-6. [DOI] [PubMed] [Google Scholar]
- 6.Hermisson M, Klumpp A, Wick W, Wischhusen J, Nagel G, Roos W, Kaina B, Weller M. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem. 2006;96:766–776. doi: 10.1111/j.1471-4159.2005.03583.x. [DOI] [PubMed] [Google Scholar]
- 7.Kokkinakis DM, Bocangel DB, Schold SC, Moschel RC, Pegg AE. Thresholds of O6-alkylguanine-DNA alkyltransferase which confer significant resistance of human glial tumor xenografts to treatment with 1,3-bis(2-chloroe- thyl)-1-nitrosourea or temozolomide. Clin Cancer Res. 2001;7:421–428. [PubMed] [Google Scholar]
- 8.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 9.Kato T, Natsume A, Toda H, Iwamizu H, Sugita T, Hachisu R, Watanabe R, Yuki K, Motomura K, Bankiewicz K, Wakabayashi T. Efficient delivery of liposome-mediated MGMT-siRNA reinforces the cytotoxity of temozolomide in GBM-initiating cells. Gene Ther. 2010;17:1363–1371. doi: 10.1038/gt.2010.88. [DOI] [PubMed] [Google Scholar]
- 10.White E, Bienemann A, Megraw L, Bunnun C, Gill S. Evaluation and optimization of the administration of a selectively replicating herpes simplex viral vector to the brain by convection-enhanced delivery. Cancer Gene Ther. 2011;18:358–369. doi: 10.1038/cgt.2011.2. [DOI] [PubMed] [Google Scholar]
- 11.White E, Bienemann A, Sena-Esteves M, Taylor H, Bunnun C, Castrique E, Gill S. Evaluation and optimization of the administration of recombinant adeno-associated viral vectors (serotypes 2/1, 2/2, 2/rh8, 2/9, and 2/rh10) by convection-enhanced delivery to the striatum. Hum Gene Ther. 2011;22:237–251. doi: 10.1089/hum.2010.129. [DOI] [PubMed] [Google Scholar]
- 12.White E, Bienemann A, Malone J, Megraw L, Bunnun C, Wyatt M, Gill S. An evaluation of the relationships between catheter design and tissue mechanics in achieving high-flow convection-enhanced delivery. J Neurosci Methods. 2011;199:87–97. doi: 10.1016/j.jneumeth.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Iwami K, Natsume A, Wakabayashi T. Gene therapy for high-grade glioma. Neurol Med Chir (Tokyo) 2010;50:727–736. doi: 10.2176/nmc.50.727. [DOI] [PubMed] [Google Scholar]
- 14.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi A, Aoki Y, Sugaya S, Serikawa T, Takakuwa K, Tanaka K, Suzuki N, Kikuchi H. Development of novel cationic liposomes for efficient gene transfer into peritoneal disseminated tumor. Hum Gene Ther. 1999;10:947–955. doi: 10.1089/10430349950018346. [DOI] [PubMed] [Google Scholar]
- 17.Tachibana R, Harashima H, Ide N, Ukitsu S, Ohta Y, Suzuki N, Kikuchi H, Shinohara Y, Kiwada H. Quantitative analysis of correlation between number of nuclear plasmids and gene expression activity after transfection with cationic liposomes. Pharm Res. 2002;19:377–381. doi: 10.1023/a:1015162722295. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, Takayama T, Niitsu Y. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- 19.Rai K, Takigawa N, Ito S, Kashihara H, Ichihara E, Yasuda T, Shimizu K, Tanimoto M, Kiura K. Liposomal delivery of MicroRNA-7-expressing plasmid overcomes epidermal growth factor receptor tyrosine kinase inhibitor-resistance in lung cancer cells. Mol Cancer Ther. 2011;10:1720–1727. doi: 10.1158/1535-7163.MCT-11-0220. [DOI] [PubMed] [Google Scholar]
- 20.MacKay JA, Deen DF, Szoka FC Jr. Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005;1035:139–153. doi: 10.1016/j.brainres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Saito R, Krauze MT, Noble CO, Tamas M, Drummond DC, Kirpotin DB, Berger MS, Park JW, Bankiewicz KS. Tissue affinity of the infusate affects the distribution volume during convection-enhanced delivery into rodent brains: implications for local drug delivery. J Neurosci Methods. 2006;154:225–232. doi: 10.1016/j.jneumeth.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Miller AD. Lipid-based nanoparticles in cancer diagnosis and therapy. J Drug Deliv. 2013;2013:165981. doi: 10.1155/2013/165981. [DOI] [PMC free article] [PubMed] [Google Scholar]