Abstract
Tissue hypoxia is a common pathophysiological process. Since 1990s, numerous studies have focused on investigating cellular adaptation to experimental hypoxia. A modular incubator chamber made of solid materials has frequently been used in the experiments that require hypoxic conditions. Here, we introduce a novel and inflatable chamber for hypoxia experiments. In experiments detecting hypoxia-induced accumulation of hypoxia-inducible factor 1α (HIF-1α) and hypoxia-induced expression of HIF-1-regulated genes, the new chamber yielded reproducible and comparable results as the modular incubator chamber did. The new chamber did not create inner chamber pressure during its use. Other properties of the new chamber were low-cost, easy to use, and leakage-free. Moreover, the size of the new chamber was adjustable, and the smaller one could be placed onto an inverted microscope for real-time studies. The successful examples of real-time studies included the real-time recording of GFP-HIF-1α fusion nuclear translocation and endothelial cell tubular formation.
Keywords: Cell culture, hypoxia, hypoxia chamber, hypoxia-inducible factor 1
Introduction
Oxygen is essential for life, and an adequate oxygen tension in internal milieu is maintained by various cellular and molecular mechanisms. Both mammalian and non-mammalian cells can sense oxygen depletion and adapt to this new environment through a series of distinctive biological processes. Among these, the activation of hypoxia-inducible factor 1 (HIF-1) is considered to be the most critical event that triggers hypoxia adaptation [1]. HIF-1 is a heterodimeric transcription factor composed of α and β subunits [2]. The α subunit of HIF-1 (HIF-1α) is sensitive to oxygen tension and dominates HIF-1 transcriptional activity, whereas HIF-1β is a constitutively expressed subunit. Under normoxia, oxygen controls HIF-1α turnover by a post-translational modification process involving hydroxylation, ubiquitination and 26S proteasomal degradation [1,3,4]. Conversely under hypoxia, the rate of HIF-1α turnover is inhibited, resulting in an increased rate of the protein accumulation, nuclear translocation, and remarkably enhanced transcriptional activity [1].
Recent studies on cellular adaptation to hypoxia have been focusing more on regulating HIF-1α in cultured cells. A reliable experimental device that can create and maintain a hypoxic environment for cell culture is essential. There are several existing models for such a purpose. One is the modular incubator chamber that can be filled with low O2 gas containing 1% O2, 5% CO2 and 94% N2 [2]. The chamber is made of solid materials in a fixed shape and size, and is the most widely used hypoxia chamber in research laboratories in the past decades. Based on our experience, one of the common defects of this chamber is leakage. Although the leakage does not frequently occur, it does disrupt experimental processes and sometimes results an uncertainty about actual inner chamber air components. Besides, the chamber creates an inner pressure if the operation is inappropriate. Another hypoxia model is a cell culture incubator by displacing O2 with infusion of N2, which was supplied by an external high-pressure liquid nitrogen tank [5]. Third one is hypoxia workstation that can offer precise control of O2 and CO2 as well as temperature and relative humidity [6,7]. It is an idea workstation that provides a hypoxic environment for a long-term cell culture [6]. The second and third models are quite expensive, and they may be infeasible or inconvenient for small laboratories that do not perform hypoxia experiments on a daily basis. Hypoxia can be mimicked by treating cells or animals pharmacologically with cobalt chloride [8], a compound that may possess unknown effects in addition to induction of HIF-1α.
In the present study, we validated an alternative module for cell culture under hypoxia. It was an inflatable chamber made of transparent plastic materials. The new chamber yielded reproducible and comparable results in hypoxia-induced HIF-1α accumulation and HIF-1-regulated gene expression. Because it was inflatable, the new chamber was unlikely to create a pressurized environment during its use. It was cost-effective, easy to use, and leakage-free even during long-term experiments. More importantly, the new chamber was size-adjustable and could be used in real-time recording of the GFP-HIF-1α fusion undergoing nuclear translocation and dynamic endothelial cell tube formation under hypoxia.
Materials and methods
Cell lines, culture conditions and reagents
All cell lines used in cell culture, except the C4-2 and ARCaP cell lines that were previously described [12], were purchased from the American Type Culture Collection (ATCC). Human prostate cancer cell lines LNCaP, C4-2, PC-3, PC3-M, DU145 and ARCaP were routinely maintained using the T-medium (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum and antibiotics (penicillin, 100 U/ml, and streptomycin, 100 μg/ml). HUV-EC-C cell line was maintained in Ham’s F12K medium with 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 0.1 mg/ml heparin, 0.03-0.05 mg/ml endothelial cell growth supplement, and 10% fetal bovine serum. HEK293 and COS-7 cell lines were maintained in Dulbecco’s modified Eagle’s medium with 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% fetal bovine serum. Cells were plated on cell culture dishes (100 mm or 60 mm) or 6-well plates at 50% confluence 24 hours before experiment. Cells were cultured either in normal conditions (37°C and 5% CO2 equilibrated with atmospheric O2 in a humidified incubator) that contains 21% O2 (hereafter referred as normoxia) or in the inflatable hypoxia chamber (1% O2, 5% CO2, balanced with N2 and humidified) that was placed in 37°C (hereafter referred as hypoxia).
Designing and assembling the inflatable chamber
Two pieces of transparent polyester barrier membranes of 4 Mil (Kapak, Minneapolis, MN) were cut into 16 × 16-inch dimensions and were used to construct the chamber that was basically formed as a pouch by using a heat impulse sealer (American International Electric, New York, NY) through sealing three sides and allowing one side free open that could be alternatively equipped with an airtight “zip-lock”. Two small holes (5-mm in diameter) were made at the two sealed corners. At one of the corners, the hole was connected with a plastic tube as an inlet port A, about 8-mm in outer diameter and 5-mm in inner diameter, by SuperGlue. The tube was used as a port for gas exchanges (Figure 1A). The other hole - Port B, was left open during gas filling. Each time before being used, the chamber was sterilized, which was accomplished by placing the chamber under germicidal UV irradiation for at least 2 hours. To precede a hypoxia experiment, a 16 cm × 16 cm (adjusted according to the chamber size) glass plate was placed inside the pouch as a supporting base. To maintain an environment with proper moisture similar to that of a common CO2 incubator, a clean sponge (4 cm × 6 cm × 2 cm) soaked with autoclaved water was put inside of the chamber before the chamber was sealed. Alternatively, a tissue culture plate containing autoclaved water was placed inside the chamber (Figure 1B). After the cell culture containers were placed, the free open side of the chamber was sealed with the heat impulse sealer or by closing the equipped airtight “zip-lock”. The chamber was filled with low O2 gas through the gas exchange Port A tube. The port B was controlled by a binder clip by periodically open and close. For hypoxia experiments, N2 equilibrated gas of 1% O2 and 5% CO2 (Specialty Gases Southeast, Union City, GA) was used. During gas filling, the gas flow was controlled by a single-stage regulator (Fisher) at 2 psi (pounds per square inch). After the chamber was filled to 80% of its capacity, the two Ports (A & B) were clamped or completely sealed by the heat impulse sealer. Finally, the chamber was placed into the 37°C incubator for a predetermined period of time.
Figure 1.
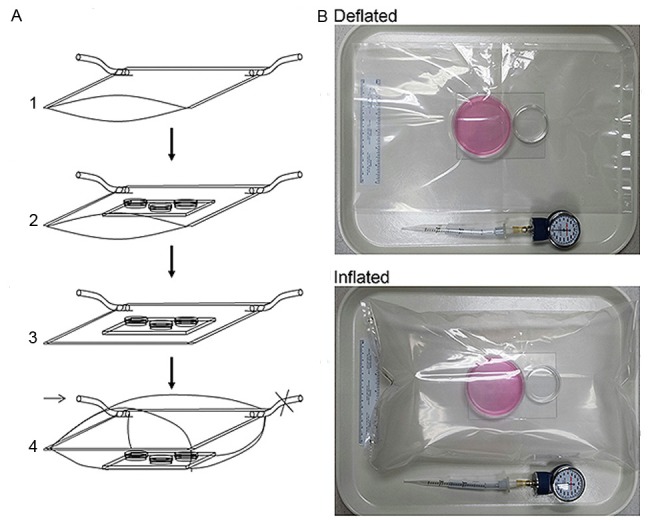
Assembly of the inflatable chamber. A. Showing an inflatable chamber in size of 16 × 16 inches. A1. An air-tight plastic pouch is prepared with two gas ports attached to the bottom corners. A2. A glass plate is placed inside the pouch as a support for culture containers. A sponge soaked with autoclaved water was placed inside of the chamber. A3. The pouch was sealed to form an insulated chamber and gently vacuumed via the gas port. A4. The chamber is filled with specified gas through the Port A (arrow) to about 80% of its maximal capacity. The gas Port B is controlled by a clamp (denoted as ×). The chamber is ready for incubation at 37°C after both ports are either closed by clamps or directly sealed at chamber corners by heat impulse sealer. B. Showing an inflatable chamber in adjusted small size that is in sealed and deflated or inflated status. Two cell culture dishes are placed on a glass plate. The large dish (60 mm) is for cell culture, and the small one (3.5 cm) contains autoclaved water. A manometer used for monitoring inner chamber atmospheric pressure after each experiment is shown.
Protein extraction and immunoblotting
The inflatable chamber was opened by incision along one side. Cells were immediately washed with ice-cold PBS and treated with the triple detergent lysis buffer freshly supplemented with protease inhibitors cocktail (Roche, Indianapolis, IN). Aliquots of 30-μg protein from whole cell lysates were fractionated onto 7.5% SDS-PAGE, and blotted onto nitrocellulose membrane (BioRad Laboratories, Hercules, CA). Monoclonal antibody to human HIF-1α (BD Transduction Laboratories, Lexington, KY) was used in 1:250 dilution. After incubation with the secondary antibody, an HRP-conjugated sheep anti-mouse IgG at 1:5000 dilution (Amersham Biosciences, Piscataway, NJ), HIF-1α was detected with an enhanced chemiluminescence kit (Amersham Biosciences). The nitrocellulose membrane was re-blotted with the antibody against β-actin.
Transient transfection and reporter gene assays
Monolayer 75% confluent cells were grown in 6-well plates and transfected in triplicate with reporter plasmids pBI-GLV6L or p2.1 (0.1 μg/well for HEK293 cells and 1 μg/well for PC-3 cells) plus control reporter pTK-RL (0.05 μg/well for HEK293 cells and 0.5 μg/well for PC-3 cells), using the Gene-Porter transfection reagent (Gene Therapy Systems, San Diego, CA). After 5 hours post transfection, the cells were allowed to recover overnight in medium containing 10% FBS. The cells were subjected to normoxia or hypoxia or for 16 hours. Plates with cells treated in hypoxia were placed inside the inflatable chamber. Luciferase activity was determined using a Dual-Luciferase Reporter System (Promega, Madison, WI) in a LUMIstar Galaxy luminometer (BMG Labtechnologies, Offenburg, Germany). Relative luciferase activity was documented by normalizing the activity of the experimental reporter (Firefly) to the activity of the control reporter (Renilla) per μg protein. Data points were statistically expressed as mean ± standard deviation.
Vascular endothelial growth factor (VEGF) ELISA
Cell culture media were used for determination of VEGF production. The DuoSet ELISA Development System for human VEGF (R & D Systems, Minneapolis, MN) was used by following with the manufacturer’s recommended protocol. The VEGF was quantified with a colorimetric assay using the substrate reagent pack (R & D Systems). Average VEGF concentration in each sample was determined with six readings. The amount of VEGF in the culture media was normalized by the amount of cellular protein, which was determined from the whole cell lysates with the BCA Protein Assay Kit (PIERCE, Rockford, IL). Data points were statistically expressed as mean ± standard deviation of two triplicate assays.
Monitoring GFP-HIF-1α translocation
Full coding sequence of the human HIF1α was cloned by RT-PCR and confirmed by DNA sequencing analysis. It was cloned in frame to the pEGFPc1 vector (Clontech), and transfected to COS-7 cells in 6-well plates using the above mentioned method. Expression of the fusion protein was confirmed by western blotting with the anti-HIF1α antibody subsequent to transfection. To study the translocation of the GFP-HIF1α fusion, the transfected cells were treated with hypoxia in the inflatable chamber which was modified in smaller size capable of 3 liters of low O2 gas; the smaller chamber was fitted on the platform of an inverted fluorescence microscope. The hypoxia treatments started at 24 hours after transfection. At different time points after hypoxic treatment, cells inside hypoxia chamber were subjected to microscopic studies without discontinuing the hypoxic environment, followed by fluorescence imaging through the plastic chamber.
HUV-EC-C tubular formation assay
HUV-EC-C cells were seeded onto 24-well plate pre-coated with Matrigel (1:1 diluted) in 3 × 105 cells/well for 2 hours, followed by normoxia or hypoxia treatment. Cells subjected to hypoxia were placed into the inflatable chamber which was modified in smaller one capable of 3 liters of low O2 gas to fit on the platform of an inverted microscope. Cell morphology was observed, and the pictures were taken at different time points between 20 minutes to 6 hours without disrupting the hypoxic environment.
Results
Description of the inflatable hypoxia chamber
The major components of the new chamber were a transparent plastic bag and soft tubes as gas exchange ports (Figure 1A & 1B). The maximal volume capacity of a 16 × 16 inches size chamber was 8 liters, and the chamber contained about 6.5 liters of gas when it was filled to about 80% of its maximal volume capacity. The chamber was able to sustain the ratio of gas components for long-term cell culture experiments, such as 10 days of continuous hypoxia. After the chamber was inflated to 80% of its maximal capacity with the low O2 gas and was incubated at 37°C, O2 and CO2 contents inside the chamber did not change (1% and 5%, respectively) during and after a 10-day incubation. This new hypoxia chamber was airtight, inflatable, easy to use, low-cost, and highly compliant. In addition, its size was adjustable for different purposes though it was about in 16 × 16 inches in dimension at complete deflation for most experiments. As measured by a manometer, the atmospheric pressure inside the new chamber remained at 0 mmHg by multiple tests throughout the 24 hours of incubation at 37°C.
The inflatable chamber effectively created a hypoxia environment
We next assessed the application of the inflatable chamber in hypoxia experiments. It is well known that hypoxia induces a rapid accumulation of HIF-1α, resulting the transactivation of HIF-1 target genes including the one encoding VEGF. The hypoxia-induced accumulation of HIF-1α, HIF-1-dependent transcriptional activity, and HIF-1-regulated VEGF expression were thus severed as markers to determine the effectiveness of the chamber. In a panel of human prostate cancer cell lines, HIF-1α was markedly induced after 24-h hypoxia by using the inflatable chamber (Figure 2A) although the basal HIF-1α levels were different among these cell lines. Similarly, significant increase in HIF-1-dependent transcriptional activity was demonstrated in PC-3 and HEK293 cells in hypoxia compared to normoxia as determined by a HIF-1/luciferase reporter that contains HRE repeats as a promoter upstream of the luciferase gene (Figure 2B). Additionally, secretion of VEGF in culture media was significantly increased by hypoxia in the majority of prostate cancer cell lines tested (Figure 2C).
Figure 2.
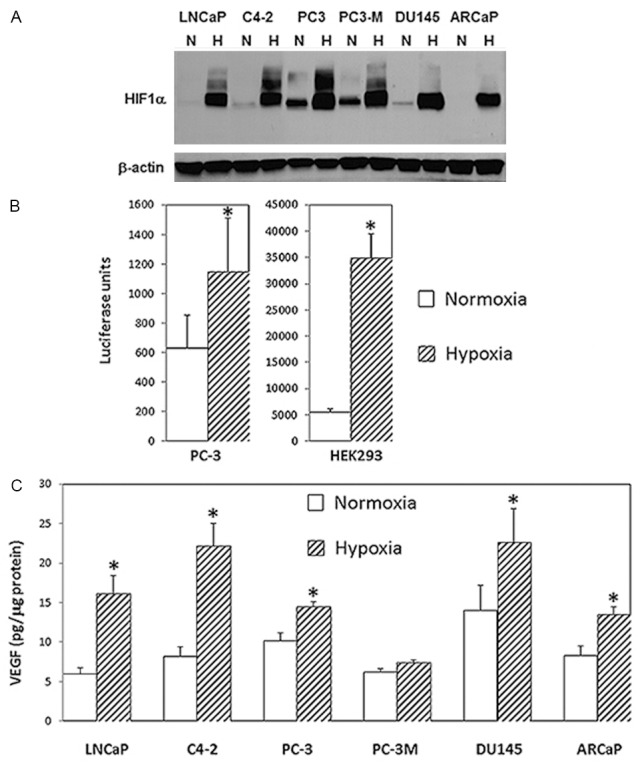
HIF-1-associated biological changes in in cells under hypoxia. A. HIF-1α as determined by western blotting in several prostate cancer cell lines in response to hypoxic treatment with the new inflatable chamber. Whole cell lysates from cells under normoxic (N) or hypoxic (H) culture conditions for 24 hours were prepared and fractionated on 7.5% SDS-PAGE before proceeding to western blotting for HIF-1α (upper panel). Western blotting for β-actin was for loading control (lower panel). B. Relative report gene activity showing in luciferase units from PC-3 and HEK293 cells in normoxia and hypoxia (new inflatable chamber). Data points were statistically expressed as mean ± standard deviation from triplicate. The (*) denotes that the luciferase units were significantly different in statistical analysis (two sample t test; A p<0.05 was considered statistically significant) between normoxia and hypoxia. C. VEGF production in culture media from prostate cancer cells in 24 hours normoxia or hypoxia (new inflatable chamber). Data in the histogram were the mean ± standard deviation from two triplicate assays (6 data points). The results generated by ELISA were normalized by the amount of cellular protein from cultured cells. The (*) denotes significantly different VEGF levels between normoxia and hypoxia in statistical analysis (two sample t test; A p<0.05 was considered statistically significant).
The inflatable chamber was applicable in hypoxia experiments for real-time studies
It is always desirable to study the cellular response to hypoxia or other specific environments on living cells at real time. Since the size of the inflatable chamber is adjustable, a smaller-sized chamber provides a good platform for such a purpose (Figure 1B). In a series of transient transfection assays by using a GFP-HIF1α expression plasmid, enhanced nuclear translocation of GFP-HIF-1α fusion was repeatedly demonstrated in COS7 cells treated within the inflatable hypoxia chamber in a time-dependent fashion. The enhancing signals could be observed at as early as 30 minute after placing the transfected cells under hypoxia, and the obvious enhancement in GFP-HIF-1α nuclear translocation was demonstrated at 1 hour post hypoxia treatment (Figure 3). Accordingly, in cells under normoxia and at 24 hours post transfection, GFP-HIF-1α signals vaguely appeared to be more centrally located (Figure 3A), whereas in cells at 30 minutes under hypoxia, GFP-HIF-1α signals started to shift into nuclei (Figure 3B). The signals became coarse granular and were condensed within nuclei after 1 hour under hypoxia (Figure 3C & 3D). In contract, cells transfected control vector showed uniformed green fluorescence signals throughout cytoplasm and nuclei, showing similar pattern in cells under normoxia and under hypoxia (Data not shown).
Figure 3.
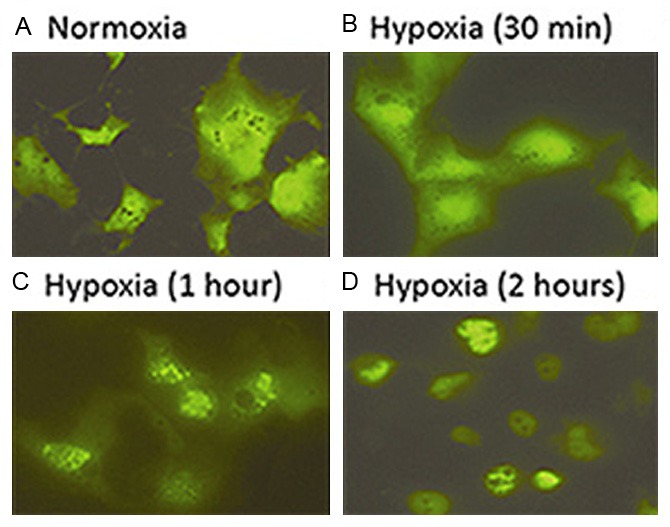
Real-time recording hypoxia-enhanced GFP-HIF-1α nuclear translocation using the small sized new chamber. Dynamic differences in cellular distribution of GFP-HIF-1α fusion were shown in normoxia (A) and different time points of hypoxia (B to D).
In another real-time study, the endothelial cell tube formation assay was performed using the inflatable chamber to create a hypoxic environment (Figure 4). An inverted microscope through the small sized and transparent inflatable chamber (Figure 1B) was used to observe HUV-EC-C cell tubular formation that was gradually enhanced in hypoxia, and the different degree in tubular formation between normoxic and hypoxic HUV-EC-C cells became obvious at 40 minutes after different treatments (Figure 4).
Figure 4.
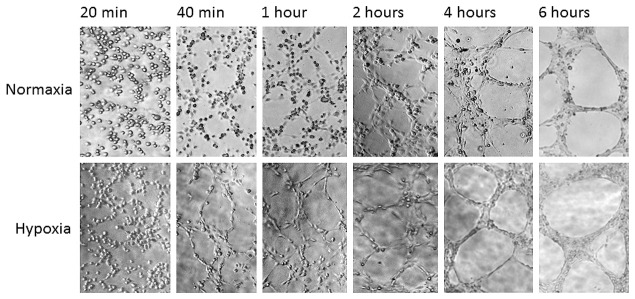
Real-time recording hypoxia-enhanced tubular formation of HUV-EC-C cells using the small sized new chamber. Cells were treated in normoxia or hypoxia (new inflatable chamber) and microscopically observed at different time points between 20 minutes to 6 hours. Photos showing dynamic cytomorphology were taken at the same locations and under the same magnification.
These results indicate that the newly designed chamber does create and maintain a hypoxic environment for cell culture, and it is an alternative tool for real-time studies of cellular response to hypoxia at both cytomorphological and molecular levels.
Discussion
An ideal hypoxia chamber should be easy to use, reliable and leakage-free. An effective but low-cost chamber should be attractive to all laboratories, especially those that are new to the research field or that do not perform hypoxia experiments on a routine basis. The chamber is expected to only create an accurate low O2 experimental setting but not to cause other environmental changes, such as an increased inner chamber pressure. According to the literature, most laboratories have utilized the modular incubator chamber in hypoxia cell culture while few laboratories have used a tissue culture incubator constantly infused with specified gas [5] or a hypoxia workstation to create a hypoxic environment [6,7]. Compared to the tissue culture incubator or the hypoxia workstation, the modular incubator chambers are less expensive for daily use, but it could produce inner chamber pressure or sometimes air leakage. The additional unwanted effect or air leakage is not easy to recognize and could result in unreliable data. In addition, none of the existing modules can be used for real-time study or continuous observation of cytomorphological or molecular dynamics in live cells under hypoxia. In the present study, we validated a novel and inflatable chamber for hypoxia experiments. The new chamber yields comparable results as the existing chambers usually do, and it shows additional properties that the existing chambers do not have.
The data obtained by using the new chamber were basically in agreement with those previously reported by using different hypoxia cell culture systems. For example, many previous studies have shown that HIF-1α, HIF-1 transcriptional activity and HIF-1 regulated gene expression in cultured cells under hypoxia are remarkably induced in a variety of cancer cell lines in comparison with cells under normoxia [1]. In the present study, similar expressing patterns between normoxic and hypoxic cells were reproduced by using the inflatable novel chamber (Figure 2).
The newly designed hypoxia chamber possesses several important features that are relatively unique. First, the new chamber is inflatable and deflatable since it is made of soft plastic materials, so that the air in the chamber can be completely removed before the chamber is inflated with the low O2 gas. As a result, the chamber with a precise O2 concentration is guaranteed. Second, the chamber would never reach an abnormal pressure if the input gas volume was well-controlled during inflating. When the rigid wall based chamber is being used, a normal chamber pressure is not ensured but is often dependent on individual experience. The inner chamber pressure is in fact increased at 37°C in the rigid wall based chamber, and the inner pressure can differ among separate experiments (data not shown). An elevated chamber pressure could be a factor, directly or indirectly, altering HIF-1α and HIF-1 transcriptional activity. As demonstrated in prior studies, environmental air or hydrostatic pressure does alter gene expression profile or transcriptional activity [9-11]. Another important feature of the newly designed inflatable chamber is that it can be used for real-time studies of hypoxic cells under microscopy. On the contrary, other hypoxia chambers are impossible to adapt for such a purpose. In addition, the new chamber is easy to use, and even easier if it is manufactured to equip with an air-tight “zip-lock”. The new chamber is leakage-free even for a long-term experiment, which has been evidenced in several prolonged hypoxia experiments up to 10 days long (data not shown). Alternatively, the inflatable chamber could be used in studying culture cells that require a specific environmental setting other than hypoxia. The new chamber is lab-made and low-cost, and it is disposable whenever harmful contamination becomes a concern. Therefore, the inflatable chamber not only provides an accurate and worry-free experimental hypoxic setting, but also has wider applications in contrast to other hypoxia designs.
In brief summary, a new and inflatable chamber was invented for cell culture in hypoxia. It shows various advantages in comparison to those preexisting ones.
Acknowledgements
The authors thank Dr. Lorna Rodriguez (Rutgers Cancer Institute of New Jersey) for critical reading of the manuscript.
Disclosure of conflict of interest
The authors declare there is no conflict of interests regarding the publication of this article.
References
- 1.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 4.Safran M, Kaelin WG Jr. HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Post DE, Van Meir EG. A novel hypoxia-inducible factor (HIF) activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–2072. doi: 10.1038/sj.onc.1206464. [DOI] [PubMed] [Google Scholar]
- 6.Edin NJ, Olsen DR, Sandvik JA, Malinen E, Pettersen EO. Low dose hyper-radiosensitivity is eliminated during exposure to cycling hypoxia but returns after reoxygenation. Int J Radiat Biol. 2012;88:311–319. doi: 10.3109/09553002.2012.646046. [DOI] [PubMed] [Google Scholar]
- 7.Esteban MA, Maxwell PH. Manipulation of oxygen tensions for in vitro cell culture using a hypoxic workstation. Expert Rev Proteomics. 2005;2:307–314. doi: 10.1586/14789450.2.3.307. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers GM, Fisher JW, George WJ. The role of renal adenosine 3’,5’-monophosphate in the control of erythropoietin production. Am J Med. 1975;58:31–38. doi: 10.1016/0002-9343(75)90530-6. [DOI] [PubMed] [Google Scholar]
- 9.Morin SM, Stotz-Potter EH, DiMicco JA. Injection of muscimol in dorsomedial hypothalamus and stress-induced Fos expression in paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1276–1284. doi: 10.1152/ajpregu.2001.280.5.R1276. [DOI] [PubMed] [Google Scholar]
- 10.Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- 11.Yang P, Agapova O, Parker A, Shannon W, Pecen P, Duncan J, Salvador-Silva M, Hernandez MR. DNA microarray analysis of gene expression in human optic nerve head astrocytes in response to hydrostatic pressure. Physiol Genomics. 2004;17:157–169. doi: 10.1152/physiolgenomics.00182.2003. [DOI] [PubMed] [Google Scholar]
- 12.Wang R, Xu J, Saramäki O, Visakorpi T, Sutherland WM, Zhou J, Sen B, Lim SD, Mabjeesh N, Amin M, Dong JT, Petros JA, Nelson PS, Marshall FF, Zhau HE, Chung LWK. PrLZ, a novel prostate-specific and androgen-responsive gene of the TPD52 Family, amplified in chromosome 8q21.1 and overexpressed in human prostate cancer. Cancer Res. 2004;64:1589–1594. doi: 10.1158/0008-5472.can-03-3331. [DOI] [PubMed] [Google Scholar]


