Abstract
Numerous recent,epidemiological studies reveal that Western populations are growing more and more deficient in daily Mg intake which have been linked to etiology of cardiovascular (CV) diseases. A growing body of evidence suggests that a major missing link to this dilemma may reside within the sphingolipid-ceramide pathways. For the past 25 years , our labs have been focusing on these pathways in Mg-deficient mammals. The objective of this paper is two-fold: 1) to test various hypotheses and 2) to review the current status of the field and how protein kinase C isoforms may be pivotal to solving some of the CV attributes of Mg deficiency. Below, we test the hypotheses that: 1) short-term dietary deficiency of magnesium (MgD) would result in the upregulation of protein kinase C (PKC) isoforms in left ventricular (LV) and aortic smooth muscle (ASM) and serum; 2) MgD would result in a release of select cytokines and an upregulation of NF-kB in LV and ASM, and in primary cultured aortic smooth muscle cells (PCASMC); 3) MgD would result in an activation of the sphingolipid salvage pathway in LV and ASM, and in PCASMC; 4) MgD would result in a synthesis of sphingosine, but not sphinganine, in PCASMC which could be inhibited by fumonisin B1 (FB) an inhibitor of ceramide synthase (CS), but not scyphostatin an inhibitor of neutral sphingomyelinase (N-SMase); 5) incubation of PCASMC (in low Mg2+) with the PKC-mimic PMA would result in release and synthesis of NF-kB, cytokines, and ceramide but not sphingosine. The new data indicate that short-term MgD (10% normal dietary intake) result in an upregulation of all three classes of PKC isoforms in LV, aortic muscle and in serum coupled to the upregulation of ceramide, NF-kB activation, and cytokines. High degrees of linear correlation were found to exist between upregulation of PKC isoforms, p65 and cytokine release, suggesting cross-talk between these molecules and molecular pathways. Our experiments with PCASMCs demonstrated that MgD caused a pronounced synthesis of sphingosine (but not sphinganine), which could be inhibited with fumonisin B1, but not by scyphostatin; use of PMA stimulation released ceramide but not sphingosine suggesting a role for the “sphingolipid salvage pathway” in MgD vascular muscle. Use of different PKC pharmacological inhibitors suggested that although all three classes of PKC molecules, i.e., classical, novel, and atypical, play roles in MgD-induced synthesis/release of ceramide, sphingosine, and cytokines as well as activation of NF-kB, to varying degrees, PKC-zeta appears to play a greater role in these events than any of the other PKC isoforms; a specific PKC-zeta inhibitory peptide inhibited formation of sphingosine. Even low levels of water-borne Mg (e.g., 15 mg/l/day) either prevented or ameliorated the upregulation of all three classes of PKC isoforms. An attempt is made to integrate our new data with previous information in order to possibly explain many of the cardiovascular effects of MgD.
Keywords: Cardiac muscle, vascular muscle, p65, sphingosine, ceramide synthase, water-borne magnesium
Introduction
Animal and human studies indicate a statistical relationship between reduced dietary intake of magnesium (Mg) and cardiovascular disease [1-7]. Mg2+ has been demonstrated to modulate basal vascular tone, myogenic tone and contractile responsiveness of vascular smooth muscle (VSM) cells as well as cardiac contractility, coronary blood flow and excitability of cardiac muscle [8-17]. Low levels of extracellular Mg ([Mg2+]0) results in contraction , elevation of vascular tone and vasospasm in a variety of mammalian arteries and arterioles [8,9,11,13,15-19] as well as decreases in coronary blood flows, decreases in cardiac output, and disturbances in cardiac rhythm [1,10,15,18,20].
Numerous studies over the past five decades have demonstrated that a reduction in the dietary intake of Mg, as well as low Mg content of drinking water, is a risk factor for the development of hypertension, atherosclerosis, vasospasm, sudden cardiac death, stroke, and inflammatory conditions by ill-defined mechanisms [1-3,5,7-9,18,19,21-24]. Hypermagnesemic diets have been demonstrated to ameliorate hypertension, cardiac arrhythmias, and atherogenesis [1,7,9,12,13,18,19,23-26]. Moreover, the myocardial level of Mg has consistently been observed to be lower in subjects dying from ischemic heart disease in soft-water areas than in subjects living in hard-water areas [2,3,5,6,14,15,21,22]. At present, the average dietary intake of Mg has declined from 450-485 mg/day in 1900 to approximately 185-225 mg/day for large segments of the North American population [1,4,6]. A recent study of 10,000 people in the U.S.A. indicated that 79% of the individuals surveyed were not ingesting the RDA daily intake of Mg [27]. Studies of natives in Greenland, Bedouin tribes in the middle east, Aborigines in Australia, and the Bantu people of southern Africa demonstrate that the incidences of heart disease, hypertension, and stroke were low, being associated with high levels of Mg in their diets and drinking water [5,18]. However, when these peoples moved to urban and industrialized areas, and began eating modern Western diets (low in Mg), they developed incidences of high blood pressure, hypertension, and heart disease as often as peoples living in the industrialized Western countries.
With the advent of sensitive ion-specific Mg2+-selective electrodes, it has been found that patients with hypertension, ischemic heart disease, stroke, atherosclerosis, and certain inflammatory conditions exhibit a depletion of serum/plasma ionized, but not total, Mg [1,11,18,28,29]. Dietary deficiency of Mg in rats and rabbits has been demonstrated to cause vascular remodeling concomitant with hypertension and atherosclerosis (i.e., arteriolar wall hypertrophy and alterations in the matrices) of unknown origin [1,9,13,18,19,25,50]. It is now clear that multiple and complex signaling pathways participate in mechanisms controlling peripheral vasoconstriction (and vascular tone) as well as that of cardiac contractile force. Protein kinase C (PKC) and the thin filament - associated proteins have long-been considered to contribute in several ways to contractile regulation in all types of muscle cells [30]. Using various specific-pharmacological antagonists, our group has shown that low [Mg2+]0-induced arterial and arteriolar contractions (and coronary as well as cerebral vasospasms) can be attenuated, markedly, with a variety of PKC antagonists [16,31].
As early as 15 years ago, using several types of VSM cells in primary cultures, it was demonstrated that variation in free extracellular Mg2+ concentration causes sustained alterations in membrane phospholipids and second messengers as well as activation of several lipid signal transcription molecules (including PKC) concomitant with a significant activation of neutral sphingomyelinase (N-SMase) and release of ceramide [32,33]. In these studies, cellular levels of diacylglycerol (DAG), a major activator of protein kinase C isozymes, was also shown to be altered by low [Mg2+]0 levels [33]. Ceramide and other sphingolipids (e.g., sphingosine, sphingosine-1-phosphate) have been demonstrated to alter vascular tone and cardiac functions [1,34-40].
More recently, using a short-term model (21 days) of Mg deficiency in intact rats, we have found that low dietary Mg intake resulted in VSM and cardiac cell upregulation of N-SMase, ceramide synthase, sphingomyelin synthase, serine palmitoyl-transferases and nuclear-factor kB (NF-kB) [41-45] with concomitant apoptosis and synthesis/release of ceramide as well as release of mitochondrial cytochrome C and several cytokines and chemokines (e.g., interleukin-1, tumor necrosis factor-alpha, and interferon-gamma) [41-45] which are known to activate several PKC isoforms, NF-kB and N-SMase in several cell types [46-55]. Several lines of evidence have pointed to an important role for PKC isforms in regulation of activation of NF-kB in several tissue types [46,51-53,56,57]. Whether such a signaling pathway exists in cardiac and vascular tissues is not known , and what roles, if any, exist for ceramide and sphingosine in this proposed signaling pathway in the cardiovascular system has not been identified.
It has recently been suggested that another signaling pathway for recruitment of ceramide may play an important role in pathophysiological processes, termed the “salvage pathway” which is often referred to as the “sphingolipid recycling pathway” which involves a conversion of sphingosine to ceramide via ceramidase [58]. Interestingly, we very recently demonstrated that diets low in Mg result in an activation of ceramidase in rat ventricular, atrial and aortic smooth muscle [44].
Inflammatory conditions are now known to provide a milieu for generation of atherogenesis and hypertension [59]. Leukocytes and endothelial cells play a pivotal role in modulation of inflammatory conditions via the elaboration and release of cytokines and chemokines [59]. In this context, we have recently demonstrated that short-term dietary deficiency of Mg results in an upregulation of 12 different cytokines and chemokines, such as IL-1beta, IL-6, TNF-alpha, RANTES, and MCP-1 in cardiovascular tissues and VSM cells in MgD animals [44], key players in atherosclerosis and hypertensive vascular disease [59]. Interestingly, we have provided some evidence that there appears to be cross-talk between generation of these cytokines (and chemokines) and a key enzyme in the sphingolipid salvage pathway, viz., ceramide synthase [44].
Using a short-term model (21 days) of Mg deficiency in intact rats, and VSM cells in primary culture, we now present evidence that low levels of [Mg2+]0 result in increased cellular levels of VSM cell ceramide and sphingosine which are associated with activation and recruitment of different isoforms of PKC in cardiac and VSM cells concomitant with activation of NF-kB and elevated cardiac and aortic smooth muscle cytokine(and chemokine) levels; serum levels of the PKC isoforms showed similar changes. We also demonstrate that very low levels of water-borne Mg (i.e., 15 mg/l/day) either prevented or ameliorated the upregulation of all PKC isoforms (investigated in this study) in cardiac and aortic muscle tissues. In addition, we show evidence that 1) The PKC-zeta subspecies appears to play an important role in regulation of NF-kB activation in VSM cells, and 2) The PKC-zeta isoform appears to be involved in ceramide generation via the “salvage sphingolipid pathway” in Mg-deficient VSM cells.
Materials and methods
Animals, diets, sera, organ-tissue collections, treatment regimen, and measurement of serum ionized and total Mg
Mature male and female rats were used for all experiments. All experiments were approved by the Animal Use and Care Committee of the State University of New York Downstate Medical Center. Equal numbers of paired male and female animals were used for all nutrition experiments. Control (600 ppm Mg) and magnesium deficient (MgD; 60 ppm) pellet diets were obtained from DYETS (AIN-93 G diets, Bethlehem, PA). All animals were given their respective diets for 21 days as previously described [41-45]. MgD animals were allowed to drink triply distilled water (Mg2+<10-6 M) containing one of four different levels of Mg aspartate-HCl (0, 15, 40, or 100 mg/l Mg; Verla Pharm, Tutzing, Germany). All control animals received a normal Mg-containing diet (i.e., 600 ppm) as well as triply distilled water to drink. On the 22nd day, sera and tissues (the LVs and abdominal aorta between the superior mesenteric arteries, and renal arteries, cleaned of all connective tissues) were collected quickly after anesthesia (45 mg/kg im pentobarbital sodium). Tissues were stored rapidly under liquid nitrogen (-85°C) until use. Whole blood was collected under anaerobic conditions in red-stoppered (no anticoagulant present) tubes, allowed to clot under anaerobic conditions, and then centrifuged under anaerobic conditions in capped vacutainer tubes [41-45]. The sera were then collected into additional red-stoppered tubes under anaerobic conditions for processing shortly thereafter, similar to previously described methods [28,41-45]. Serum samples were then analyzed within 2 hr after collection, as previously described [41-45]. Total Mg levels were measured by standard techniques in our laboratory (Kodak DT-60 analyzer; Ektachem Colorimetric Instruments, Rochester, NY). The method compares favorably with atomic absorption techniques for total Mg [28]. A Mg2+-selective ion electrode with a novel neutral carrier-based membrane (NOVA 8 Analyzer; NOVA Biomedical Instruments, Waltham, MA) was utilized to measure the free divalent cation in the sera [28]. The ion-selective electrode was used in accordance with procedures developed in our laboratory having an accuracy and precision of 3% [28].
Biochemical measurements of PKC isoforms in LV, aortic smooth muscles and sera
PKC family members are a diverse group of proteins that phosphorylate a wide variety of protein targets known to be involved in multiple signaling pathways [50,53,60]. Most of these family members also serve as major receptors for phorbol esters [50,53,60]. Each PKC family member has a specific protein expression which is believed to play important role(s) in diverse cell types. Members of the PKC family can be subdivided into three categories: conventional (i.e., alpha, beta1, beta11, gamma), novel (delta, epsilon, eta, mu), and atypical (lambda, zeta). Using ELISA assays, we chose to measure PKC-alpha, PKC-beta1, PKC-gamma, PKC-delta, PKC-epsilon, PKC-mu, PKC-theta, and PKC-zeta, representative of all three categories. We chose to utilize recently developed ELISA assay kits for PKC subtypes (PRT KIN C [PKC] KT; EMD Millipore Chemicals, Billerica, MA) as our laboratory has a great deal of experience using ELISA assays [41-45]. The techniques employed for tissue preparation for the assays (using specific antibodies for each PKC subtype) were followed, similar to that described by EMD Millipore Corporation. The absorbance (optical density) of each well was read with a plate reader at 450 nm. Standard curves were used to measure the concentrations of the proteins.
Isolation of vascular muscles and primary culture of aortic VSM cells
Rat aortic VSM cells were isolated from anesthetized animals as we have described, previously, according to established methods in our laboratory (n=6-10 animals/group) and cultured in 1.2 mmol/l [Mg2+]0-normal Krebs-Ringer bicarbonate, FCS, and antibiotics at 37°C in a humidified atmosphere composed of 95% air-5% CO2 [17,40,61-63]. After confluence had been reached, VSM cells were placed in normal Krebs-Ringer bicarbonate media containing 0.3 or 1.2 mmol/l [Mg2+]0 for varying periods of time (i.e., 2 or 24 hr). It should be stressed here that the experiments using cell cultures and those below on primary VSM cells in culture were never part of the whole animal nutritional experiments (described above); these experiments and others were separate from the nutritional experiments.
Influence of 4-beta-phorbol 12-myristate 13-acetate (PMA) on ceramide, sphingosine and NF-kB levels in aortic VSM cells in primary culture
Inasmuch as that the classical group of PKC isozymes are activated by 1,2-diacylglycerol (DAG) and calcium, and that PMA is a mimic, we hypothesized that: 1) Prolonged stimulation of VSM cells in culture with PMA (200 nM; CalBiochem, CA) in normal [Mg2+]0 would result in some hydrolysis of SM and release of ceramide concomitant with activation of NF-kB as determined from activation of the p65 subunit of the DNA-binding proteins; 2) Doing the latter experiment in low [Mg2+]0 would result in increased levels of ceramide and greater activation of NF-kB; 3) Exposure of the VSM cells (in normal Mg2+) to both PMA (200 nM) and scyphostatin (75 uM; Sigma Aldrich, St. Louis, MO) (an inhibitor of N-SMase) would attenuate the PMA-induced rise in total cellular ceramide and NF-kB activation either not at all or only slightly, but repetition of the latter protocol in low Mg2+ would result in considerable inhibition of rises in VSM cell ceramide and pulsed-labeled ceramide as well as NF-kB activation; 4) doing the latter experiments in the presence of fumonisin B1 (75 uM, Sigma Aldrich, St Louis, MO), an inhibitor of ceramide synthase (via action on the precursor of ceramide, viz., sphingosine, in the “salvage pathway”) [58] would result in considerable inhibition of pulsed-labeled ceramide, sphingosine levels and NF-kB activation.
Measurement of cellular levels of ceramide, sphingosine and [3H]-labeled ceramide
Cellular levels of pulse-labeled ceramide were measured utilizing methods we have published previously [43-45]. Briefly, the aortic cells, in primary cultures, were exposed to [3H] palmitic acid (4-20 uCi/ml) at 37°C, rinsed with fresh normal Krebs-Ringer bicarbonate (NKRB) solution and transferred to NKRB (under 95%-5% CO2) for 3 hr. The labeled cells were washed for 5-10 min with unlabeled medium, transferred to culture medium containing either 0.3 or 1.2 mmol/l Mg2+ and either 200 nM PMA, 75 uM scyphostatin, both PMA and scyphostatin, or no additions (controls) for 18 hr. Lipid extractions were performed for phospholipid analysis using techniques similar to those we have reported previously. Total ceramide cellular levels were determined similar to methods we have used recently employing conversion of ceramide into ceramide-1-[32P] phosphate by Escherichica coli DAG kinase followed by lipid separation on high-performance TLC plates [43-45]. After autoradiography, spots corresponding to ceramide-1-phosphate were carefully scraped into vials, and the radioactivity counted in a scintillation counter (LS-6500; Beckman). Quantitation of ceramide levels and results as picomoles per 106 cells were determined. For determination of sphingosine levels, we employed the methodology of Merrill et al [64], modified by Riley et al [65] which utilizes an HPLC procedure for micro-quantities of tissue/cell samples as modified by Yoo et al [66]. Free sphingosine was extracted from the VSM cells following the methods of Yoo et al [66]. Sphingosine was quantitated by using recovery of a C20 sphinganine internal standard [65]. The results were expressed as pmol/ug protein using a Bradford reagent for total protein (Bio-Rad Laboratories, Hercules, CA) following the manufacturer’s protocol using a 96-well plate.
Differentiation of origin of free sphingosine in VSM cells: de novo biosynthesis vs.complex sphingolipids (i.e., salvage pathway)
In order to determine whether the free sphingosine base generated in cultured VSM cells is derived from de novo biosynthesis or from complex sphingolipids, we employed several techniques: 1) We labeled cells with either [3H] serine (10-50 uCi) or [3H] palmitate (10-20 uCi) and measured the amount of free sphingosine base formed over 6h after the VSM cells were first exposed to low [Mg2+]0 (0.3 mM) for 2 h; 2) These experiments were repeated in the presence of either scyphostatin (75 uM) or fumonisin B1 (75 uM). We rationalized that no or little [3H] serine labeling would appear in the sphingosine whereas [3H] palmitate labeling would appear in the sphingosine. Moreover, our hypothesis, if proven, would demonstrate that scyphostatin incubation would not affect the levels of sphingosine generated by the low [Mg2+]0 but incubation with fumonisinB1 would either greatly reduce the amount of sphingosine formed or inhibit its formation.
Analysis of NF-kB expression in cardiovascular tissues and primary cultured VSMCs exposed to low [Mg2+]0 with and without inhibitors
For analysis of NF-kB expression, in the primary cultured VSMCs, we employed EMSA techniques similar to those we have reported previously [61,62]. Briefly, for the aortic VSM cells, in culture, nuclear protein extracts were separated by SDS/PAGE on 7.5% (wt/vol) polyacrylamide gels made in a buffer containing a Tris/boric acid/EDTA mixture followed by electrophoresis at a constant voltage of 100 V for 90 min [61,62]. For the supershift assays, nuclear proteins were incubated with antibodies specific for p65 (Santa Cruz Biotechnology, Santa Cruz, CA) before gamma-32P labeled. NF-kB oligonucleotides were added as described previously [61,62]. These experiments employed an identical regimen we used for evaluation of the effects of low [Mg2+]0 in absence or presence of PMA (with and without scyphostatin) or in the presence of fumonisinB1, as in the above studies for SM, sphingosine and ceramide.
For measurement of NF-kB expression in ventricular and aortic tissue obtained from MgD animals, we utilized a highly sensitive ELISA kit (TransAM NF-kB family transcription factor assay kit; Active Motif North America, Carlsbad, CA) to measure the expression of p65 which our group recently reported on [44].
Influence of pharmacological inhibitors of PKC activation on ceramide and sphingosine levels and expression of p65 in VSM cells exposed to low [Mg2+]0
The classical and novel PKCs when activated by phorbol esters signal primarily through proteins with C1 domains. In order to investigate the possible involvement of one or more PKCs in hydrolysis of SM (activated by low [Mg2+]0), thus releasing ceramide, we used a panel of inhibitors that block different classes of PKCs, i.e., bisindolemaleimide I (2 uM, Sigma, St Louis, MO) an inhibitor of classical and novel PKCs; rottlerin (10 uM, Sigma St, MO) an inhibitor of PKC-delta; GO 6976 (5 uM, Sigma, St Louis, MO) an inhibitor of classical PKCs (e.g., alpha and beta1), and an isoform-specific inhibitory myristoylated peptide derived from the pseudosubstrate (PS) region of PKC-zeta (10 uM; Biomol) to inhibit PKC-zeta. All of the VSMs, in culture, were first exposed to NKRB (with normal Mg2+) for 2 hr as done previously containing either one of the four PKC antagonists or a comparable volume of saline (controls). We then exposed the cells to either 0.3 or 1.2 mmol/l Mg2+, containing one of the four PKC antagonists, for 18 hr.
In one set of experiments, we extracted the lipids in the cells as we have detailed elsewhere [43,44]. The ceramide was next converted into ceramide-1-[32P] phosphate by Escherichia coli DAG kinase, and the lipids separated on high-performance TLC plates as described elsewhere [43,44]. After autoradiography and counting of the radioactivity (in a scintillation counter, LS-6500; Beckman), the ceramide levels were quantified and results reported as picomoles per 108 cells.
In the second set of experiments, VSM cells, in culture, were exposed to NKRB for 2 hr, and we then determined the expression of p65 in cells exposed to either 0.3 or 1.2 mmol/l Mg2+ containing one of the four PKC inhibitors (above) for 18 hr, as above. The NF-kB p65 DNA-binding protein was then measured in the cells with and without the PKC inhibitors.
In the third set of experiments, VSM cells, in culture, were exposed to NKRB for 2 hr, and we then determined the sphingosine level in cells exposed to either 0.3 or 1.2 mmol/l Mg2+ containing one of the four PKC inhibitors (above) for 2 hr, as above.
Assay of cytokines and chemokines in cardiovascular tissues, sera and VSM cells in culture exposed to low Mg2+ with and without inhibitors
For the assays in sera and cardiovascular tissues, we utilized methods recently employed in our laboratory [44]. Sera and tissues were harvested from the control and MgD animals, as described above, and kept frozen (-85°C) until biochemical analysis [44]. Multi-Analyte ELISArray kits were used for the quantitative measurement of different cytokines (i.e., IL-1beta, IL-2, IL-6, IL-10, IL-12, IFN-gamma and TNF-alpha) [44]. We read the absorbances at 450 nm within 30 min of stopping the reaction. Standards were used, standard curves were plotted, and the experimental cytokine values were calculated.
For analysis of select cytokine (IL-1beta, IL-6, IL-8, and TNF-alpha) and chemokine [monocyte chemoattractant protein-1 (MCP-1)] concentrations in aortic VSM cells in culture, we used select antibodies similar to those reported recently [44]. Briefly, the VSM cell experiments employed an identical regimen we used for evaluation of the effects of low [Mg2+]0 in the presence or absence of PMA (with and without scyphostatin) or in the presence of fumonisin B1, as in the above studies for SM, sphingosine and ceramide.
Statistical analyses
Where appropriate, means and means ± S.E.M.s were calculated. Differences between means were assessed for statistical significance by Student’s t-test and ANOVA followed by a Newman-Keuls test. In some cases, correlation coefficients were calculated by the method of least squares. P values of<0.05 were considered significant.
Results
Serum total and ionized Mg levels
Feeding the animals the synthetic pellet diet (n=18-20 animals/group) resulted in a total serum Mg level of 1.05 ± 0.03 mM/l, whereas the animals that received the MgD diet demonstrated a serum total Mg level of 0.40 ± 0.004 mM/l (P<0.01). The serum level of ionized Mg in the normal, control group was 0.58 ± 0.002 mM/l, whereas in the MgD group the mean serum ionized Mg level was lowered to 0.28 ± 0.004 mM/l (P<0.01). Feeding the animals various levels of Mg in their drinking water (as shown previously, [42-45]) resulted in concentration-dependent rises in both the total and ionized levels of serum Mg. Use of 15 and 40 mg/l/day of Mg2+ in their drinking water elevated the total Mg to 68 (0.69 ± 0.005 mM/l) and 85% (0.84 ± 0.007 mM/l), respectively, of normal whereas feeding 100 mg/l/day raised the total serum Mg level to 0.98 ± 0.004 mM/l (n=18-20/group). With respect to the serum ionized levels , feeding animals 100 mg/l/day of Mg2+ restored the level of ionized Mg to normal while feeding 15 and 40 mg/l/day of Mg2+ in the drinking water raised the serum ionized Mg levels to 68% and 72%, respectively , of normal (n=16-18; P<0.05).
Influence of dietary Mg intake on PKC isozyme levels in cardiac and vascular smooth muscles and in sera: relationship to serum ionized Mg
Figure 1 shows that feeding rats a MgD diet for 21 days resulted in 300-400% rises in all of the PKC isoforms in LV muscle, except for PKC-epsilon, while aortic smooth muscle demonstrated 300-500% elevations in the levels of all isoforms, except for PKC-epsilon (Figure 2). Interestingly, feeding the MgD animals as little as 15 mg/l/day of Mg in the drinking water in LV and aortic smooth muscles reduced the rises in some of the PKC isoforms, but not all , whereas feeding 100 mg/l/day of Mg2+ restored all of the PKC isoform levels to normal (P<0.01, ANOVA; see Figures 1 and 2). From linear regression analysis of the data (Figures 3 and 4), it is clear that the lower the serum level of ionized Mg, the greater the rises in the levels of all PKC isoforms, except for PKC-epsilon (r=0.73-0.92, P<0.01). There were either no or weak correlations (e.g., 0.3-0.4) between total serum Mg and the PKC isoform data (data not shown). Figure 5 indicates that the sera levels of the eight PKC isoforms paralleled the relative levels in the LV. From linear regression analysis of these data (Figure 6), it is clear that the lower the serum ionized Mg, the higher the levels of PKC isoforms (r=0.70-0.96, P<0.01) with PKC zeta showing the highest inverse correlation to the ionized Mg level.
Figure 1.
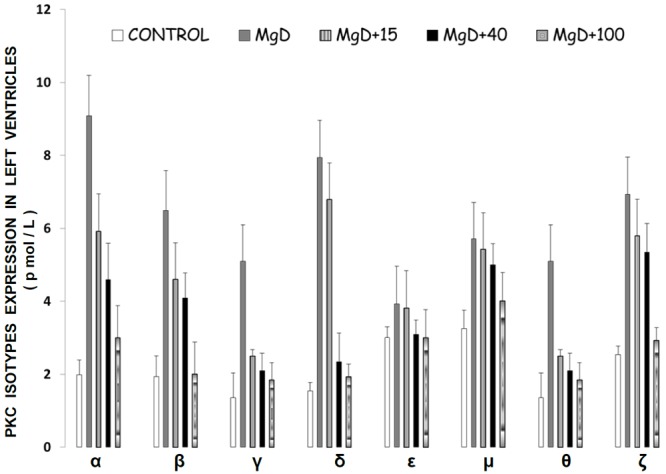
PKC isoform levels in left ventricular (LV) muscle in normal and Mg-deficient (MgD) rats with and without Mg added to the drinking water. N=10-14 animals per group. Mean values (± S.E.) for MgD animals are significantly different from all other groups for all PKC isoforms, except PKC-epsilon (ANOVA, P<0.01).
Figure 2.
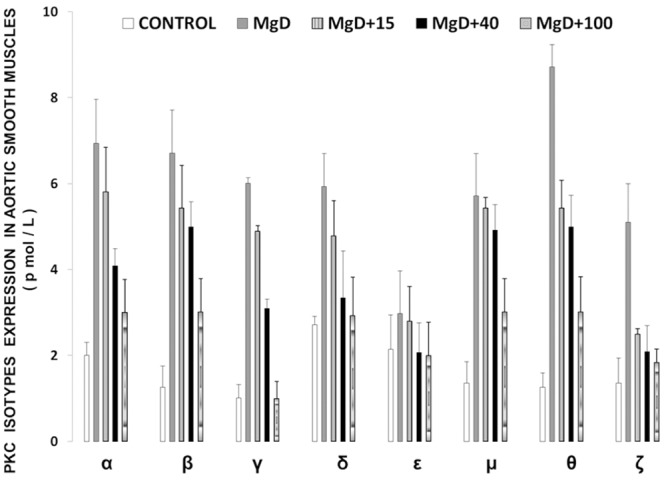
PKC isoform levels in abdominal aortic smooth muscle in normal and MgD rats with and without Mg added to the drinking water. Mean values (± SE) for MgD animals are significantly different from all other groups for all isoforms except for PKC-epsilon (ANOVA, P<0.01).
Figure 3.
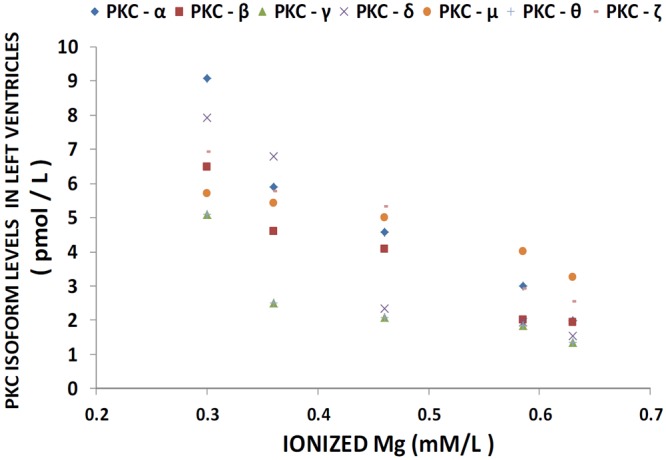
Linear correlation between LV PKC isoform levels and ionized Mg in normal and MgD rats with and without Mg added to the drinking water; n=10-12 animals per group. PKC-alpha is diamond (linear regression equation: y=-0.367x + 0.5121; r=0.9237); PKC-beta is square (linear regression equation: y=-0.241x + 0.9349; r=0.8326); PKC-gamma is triangle (linear regression equation: y=-0.1868x + 0.3371; r=0.8513); PKC-delta is x (linear regression equation: y=-0.3555x + 0.1568; r=0.7329); PKC-mu is circle(linear regression equation: y=-0.1292x + 3.1296; r=0.8365); and PKC-zeta is minus (linear regression equation: y=-0.2339x + 1.0013; r=0.8005). PKC-epsilon is not shown as there were no significant differences in levels of this isoform in animals fed MgD diets (P>0.05).
Figure 4.
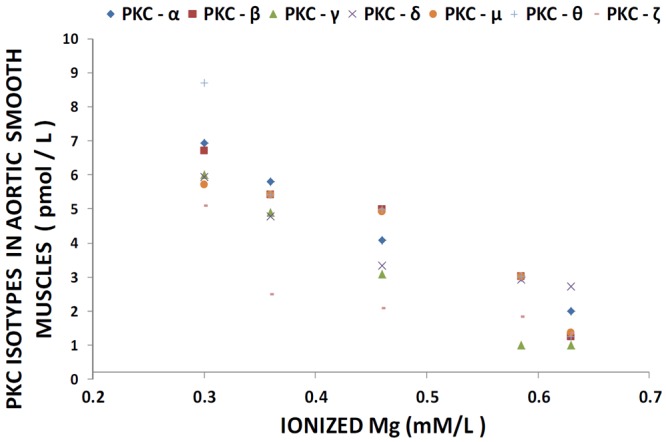
Linear correlation between aortic smooth muscle PKC isoform levels and ionized Mg in normal and MgD rats with and without Mg added to the drinking water; n=10-12. PKC alpha is diamond (linear regression equation: y=-0.262x +1.2252; r=0.892); PKC-beta is square (linear regression equation: y=-0.2805x + 0.9159; r=0.8946); PKC-gamma is triangle (near regression equation: y=-0.2747x + 0.0943; r=0.7784); PKC-delta is x (linear regression equation: y=-0.1731 + 1.8653; r=0.8303); PKC-mu is circle (linear regression equation : y=- 0.2273x + 1.3598; r=0.7901); and PKC-zeta is minus (linear regression equation: y=- 0.1868 + 0.3371; r=0.8513). PKC-epsilon is not shown as there were no significant differences in levels of this isoform in animals fed MgD diets (P>0.05).
Figure 5.
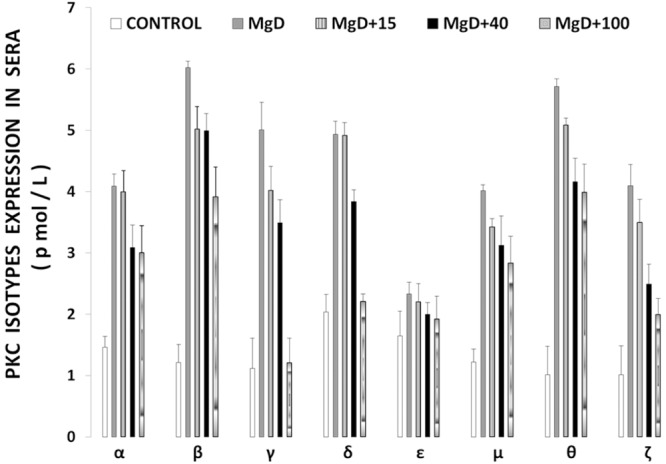
PKC isoform levels in sera of control and MgD rats with and without Mg added to the drinking water. Mean values (± SE) for MgD animals are significantly different from control groups except for PKC epsilon.
Figure 6.
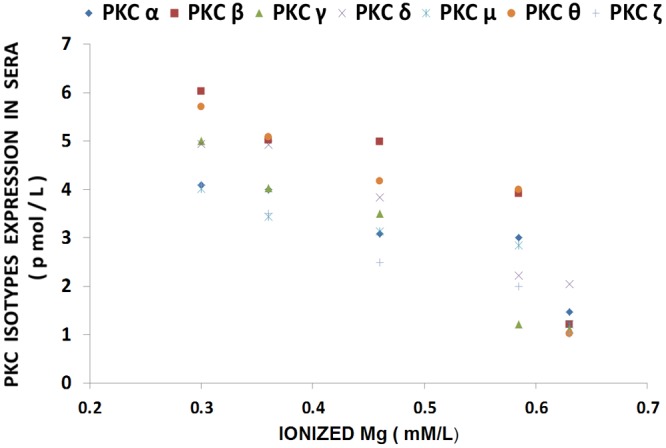
Linear correlation between serum PKC isoform levels and serum ionized Mg in normal MgD rats with and without Mg added to the drinking water. n=10-12. PKC-alpha is diamond (linear regression equation: y=-18.816x + 13.701; r=0.9214); PKC-beta is square (linear regression equation: y= -13.183x + 9.9808; r=0.9461); PKC-gamma is triangle (linear regression equation: y=-8.6959 + 6.6374; r=0.7008); PKC-delta is x (linear regression equation: y=-19.891 + 13.395; r=-0.871); PKC-mu is star (linear regression equation: y=-7.0848x + 7.9879; r=0.9544); PKC=theta is circle (linear regression equation: y=- 8.6959x + 6.6374; r=0.7008); and PKC-zeta is plus (linear regression equation: y=13.206x + 10.873; r=0.9685). PKC-epsilon is not shown as there were no significant differences in levels of this isoform in animals fed MgD diets (P>0.05).
Influence of PMA on ceramide, [3H] ceramide, sphingosine and expression of NF-kB levels in primary cultured aortic cells: effects of scyphostatin and fumonisin B1
The data presented in Table 1 indicate that prolonged incubation of single aortic VSM cells with PMA (in NKRB solution, containing normal Mg2+) caused significant rises in the content of ceramide and expression of p65; levels of sphingosine were not altered significantly. However, the addition of the N-SMase inhibitor (i.e., scyphostatin) to the cell cultures only slightly attenuated the PMA-induced rises in total ceramide, [3H]-labeled ceramide, and p65. A repeat of the same experimental protocol (with PMA) using the CS inhibitor fumonisin B1, in place of scyphostatin, resulted in considerable inhibition of the rises in total cellular ceramide, and [3H]-labeled ceramide as well as NF-kB activation (Table 1); sphingosine levels were not altered significantly. Incubation of the single cells in low Mg (in absence of PMA) resulted in rises in total ceramide, [3H]-ceramide, sphingosine, and p65 (Table 2) compared to results in normal Mg-containing-NKRB (see Table 1). Using low [Mg2+]0-KRB (containing PMA) in place of NKRB resulted in greater rises in cellular levels of total ceramide and [3H]-labeled ceramide as well as steep rises in NF-kB activation (Table 2); sphingosine levels were, however, not elevated over and above that seen in absence of PMA.
Table 1.
Influence of PMA on total ceramide (pmol/108 cells), [3H]-labeled ceramide (cpm x 103/mg wet wt), sphingosine (pmol/ug protein), and p65 DNA-binding protein of NF-kB (% control) in single aortic smooth muscle cells in the presence and absence of either scyphostatin or fumonisin B1 in Mg2+-NKRB
| Total ceramide | [3H]-ceramide | sphingosine | p65 | |
|---|---|---|---|---|
| Controls | 33 ± 3.3 | 0.52 ± 0.05 | 0.0015 ± 0.0003 | 100 |
| PMA | 48 ± 3.6* | 0.70 ± 0.08* | 0.0018 ± 0.0005 | 147 ± 12* |
| PMA + Scyph | 42 ± 3.1* | 0.66 ± 0.04* | 0.0020 ± 0.0006 | 140 ± 14* |
| PMA + Fum | 37 ± 3.3 | 0.58 ± 0.04 | 0.0022 ± 0.0006 | 124 ± 8* |
Values are means ± SE; n=6-8 animals per group.
P<0.01 compared to controls.
Table 2.
Influence of PMA on total ceramide (pmol/108 cells), [3H]-labeled ceramide (cpm x 103/mg wet wt.), sphingosine (pmol/ug protein), and p65 DNA-binding protein of NF-kB (% control) in single aortic smooth muscle cells in the presence and absence of either scyphostatin or fumonisin B1 in low [Mg2+]0-NKRB
| Total ceramide | [3H]-ceramide | sphingosine | p65 | |
|---|---|---|---|---|
| Controls (low Mg) | 62 ± 5.8* | 1.04 ± 0.08* | 0.0038 ± 0.0008* | 192 ± 16* |
| PMA (+ low Mg) | 82 ± 5.2** | 1.32 ± 0.12** | 0.0042 ± 0.0010* | 198 ± 18* |
| PMA (+ low Mg) w/scy | 76 ± 5.2** | 1.28 ± 0.10** | 0.0036 ± 0.0008* | 188 ± 18* |
| PMA (+ low Mg) w/fum | 74 ± 5.2** | 1.08 ± 0.06** | 0.0044 ± 0.0010* | 146 ± 12* |
Values are means ± SE; n=6-8.
Significantly different from mean values in normal-NKRB (P<0.01; Table 1).
Significantly different from mean values in low [Mg2+]0 (P<0.01).
Influence of pharmacological inhibitors of PKC activation on total cellular ceramide levels, sphingosine, [3H]-labeled ceramide and expression of p65 in VSM cells exposed to low [Mg2+]0
Exposure of aortic VSM cells, in culture (with 0.3 mM/l Mg2+), for 18 hr in the presence of a variety of both classical and novel PKC inhibitors, resulted in varying falls in the elevated levels of total cellular ceramide and expression of the p65 DNA-binding protein (subunit of NF-kB) which has been demonstrated recently to be expressed in cultured VSM cells incubated in low [Mg2+]0 [44,61,62] (Table 3); the [3H]-labeled fraction of ceramide was only moderately attenuated in the presence of the classical and novel PKC antagonists. However, exposure of the VSM cells, in low [Mg2+]0-KRB, to the PS-PKC-zeta inhibitor resulted in the greatest attenuation in the [3H]-labeled rises in ceramide and p65; sphingosine levels also appeared to be lowered (Table 4).
Table 3.
Influence of pharmacological inhibitors of PKC isozyme activation on total ceramide (pmol/108 cells), [3H]-labeled ceramide (cpm x 103/mg wet wt.), and sphingosine (pmol/ug protein) in single aortic VSM cells exposed to low [Mg2+]0
| Total ceramide | [3H]-ceramide | sphingosine | |
|---|---|---|---|
| Controls-low Mg2 | 65 ± 5.3* | 0.98 ± 0.06* | 0.0036 ± 0.0008* |
| Bisindolem I | 42 ± 3.8** | 0.88 ± 0.04** | 0.0032 ± 0.0008* |
| Rottlerin | 58 ± 5.6* | 1.02 ± 0.08* | 0.0040 ± 0.0010* |
| GO 6976 | 52 ± 5.0** | 0.90 ± 0.06** | 0.0038 ± 0.0006* |
| PKCzeta pept | 60 ± 5.8* | 0.74 ± 0.04** | 0.0026 ± 0.0004** |
Values are means ± SE; n=6-8 per group.
Significantly different from mean values in normal Mg2+-containing NKRB (compared to values in Table 1; P<0.01).
Significantly different from mean control values in low Mg2+.
Table 4.
Influence of pharmacological inhibitors of PKC isozymes on expression of p65 DNA-binding protein of NF-kB in single aortic VSM cells exposed to low [Mg2+]0
In contrast to the effects of the PKC inhibitors on the pulsed-labeled ceramide levels, the four different PKC antagonists exerted varying effects on the cellular levels of expression of p65 in VSMCs exposed to low [Mg2+]0. For example, bisindolemaleimide I reduced the level of the stimulated (in low Mg)-active form of p65 approximately 40%, whereas exposure of the MgD cells to rottlerin reduced p65 levels by approximately 12-18% (Table 4). Incubation of the MgD VSMCs with Go6976 resulted in a 15-20% reduction in active p65, whereas exposure of the MgD cells to the isoform-specific inhibitory peptide against PKC zeta resulted in a 30-35% reduction in the activation of p65.
Correlation of PKC isoform levels in LV and aortic muscle tissues with levels of activated p65
Inasmuch as the above suggested that that there may be cross-talk between activated levels of PKC isoforms and NF-kB activation, we hypothesized that several of the PKC isoforms (e.g., alpha, beta, delta, gamma, mu, and zeta) would be correlated to different degrees to activated levels of p65 in LV and aortic muscle obtained from the MgD rats. As indicated in Figure 7, the LV and aortic smooth muscles, obtained from the MgD animals, demonstrate marked elevations in p65 levels, similar to that reported previously, while feeding the animals as little as 15 mg/l Mg2+ in the drinking water reduced the expression of p65. The data shown in Figures 8 and 9 seem to support our hypothesis that correlations between the PKC isoforms and p65 levels in MgD animals are quite high (i.e., r=0.81-0.94, P<0.01).
Figure 7.
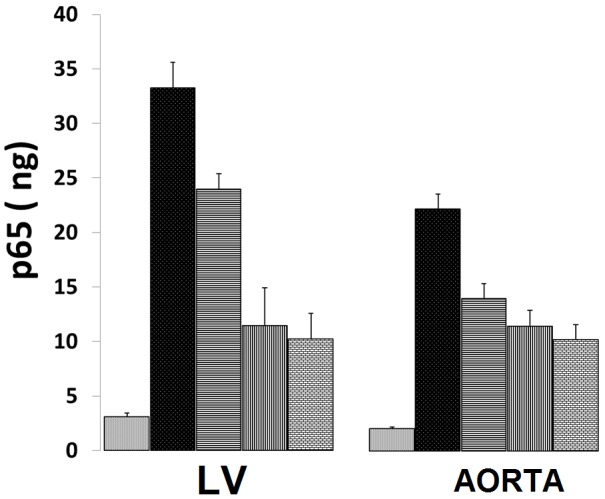
p65 levels in LV and aortic vascular muscle in normal and MgD rats with and without Mg2+ added to the drinking water; n=8-12 animals per group. Bars are SEs. Open bar ( ) = control; dark filled-in bar = MgD; horizontal lines = MgD + 15 mg/l/day of Mg2+; vertical lines = MgD + 40 mg/l/day Mg2+; and cross-hatched = MgD + 100 mg/l/day.
Figure 8.
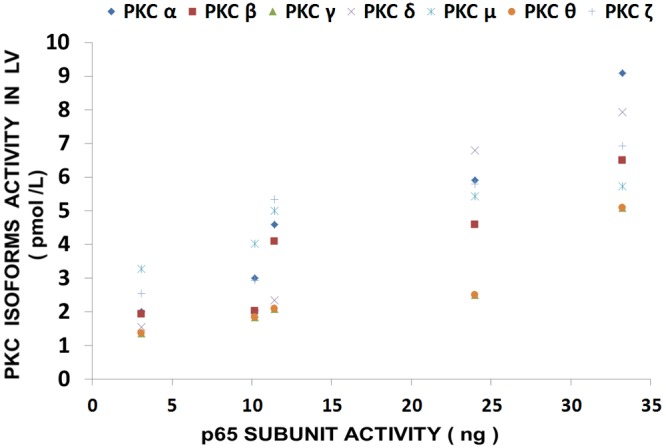
Linear correlation between PKC isoform levels in LV and p65 in normal and MgD rats with and without Mg2+ added to the drinking water; n=8-12 animals per group. PKC-alpha is diamond (linear regression equation: y=0.224x + 1.249; r=0.9405); PKC-beta is square (linear regression equation: y=0.1478x + 1.4016; r=0.8651); PKC-gamma is triangle (linear regression equation: y=0.1173x + 0.7038; r=0.837); PKC-delta is x (linear regression equation: y=0.243x + 0.1241; r=0.9452); PKC-mu is star (linear regression equation: y= 0.0768x + 0.8153; r=0.8153); PKC-theta is (linear regression equation: y=0.1119x + 0.7424; r=0.844); circle and PKC-zeta is plus (linear regression equation: y=0.1418x +2.3825; r=0.812). PKC-epsilon is not shown as there were no significant differences in levels of this isoform in animals fed MgD diets (P>0.05).
Figure 9.
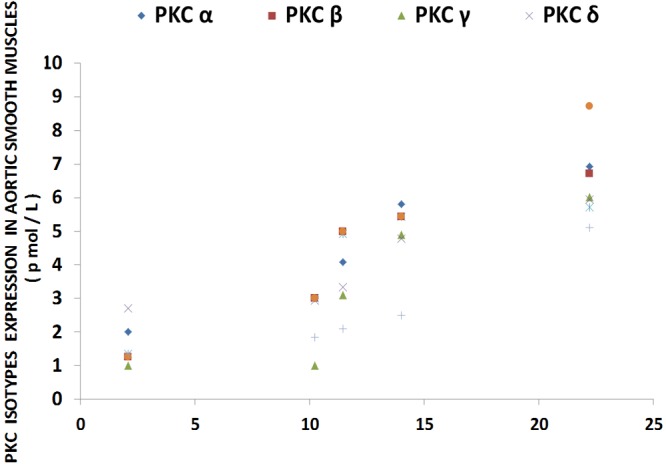
Linear correlation between PKC isoform levels in aortic vascular muscle in normal and MgD rats with and without Mg added to the drinking water; n=8-12 animals per group. PKC-alpha is diamond (linear regression equation: y=0.262x + 1.2252; r=0.8924); PKC-beta is square (linear regression equation: y=0.2805x + 0.9159; r=0.8946); PKC-gamma is triangle (linear regression equation: y=0.2747x + 0.0943; r=0.7784); PKC-delta is x (linear regression equation: y=0.1731x + 1.8653; r=0.8303); PKC-mu is star (linear regression equation: y=0.2273x + 1.3598; r=0.7901); PKC-theta is circle (linear regression equation: y= 0.3779x + 0.1487; r=0.9554); and PKC-zeta is plus (linear regression equation: y= 0.1868x + 0.3371; r= 0.8513).
Determination of origin of free sphingosine in VSM cells: de novo biosynthesis vs. complex sphingolipids
The results shown in Figures 10 and 11 indicate that when aortic VSM cells in culture are exposed to low [Mg2+]0, there is a gradual rise in [3H] palmitate-labeled sphingosine, but not [3H] serine-labeled sphingosine, over 6 hr, suggesting that the sphingosine arises from complex sphingolpids. Repeating these experiments in the presence of fumonisin B1 demonstrates inhibition of the [3H] palmitate-labeled sphingosine, seeming to confirm this hypothesis. Use of scyphostatin under the same protocol, in low [Mg2+]0, failed to inhibit the formation of [3H] palmitate-labeled sphingosine.
Figure 10.
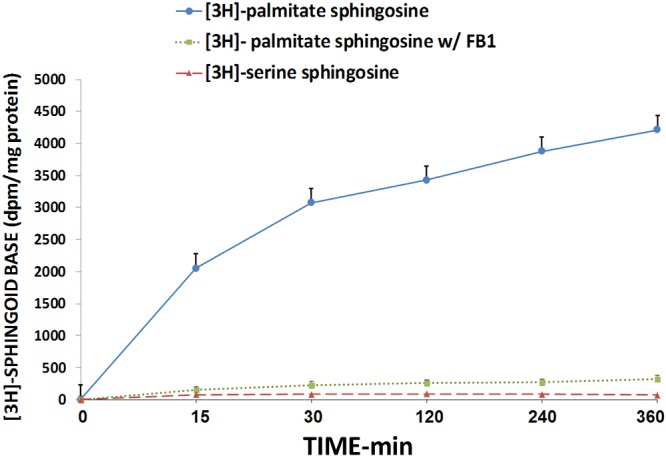
[3H]-palmitate and [3H]-serine incorporation into free sphingosine free base in primary cultured single aortic VSM cells exposed to 0.3 mM [Mg] (with and without fumonisin B1 (FB1) - see Methods) over time; n=6-8.
Figure 11.
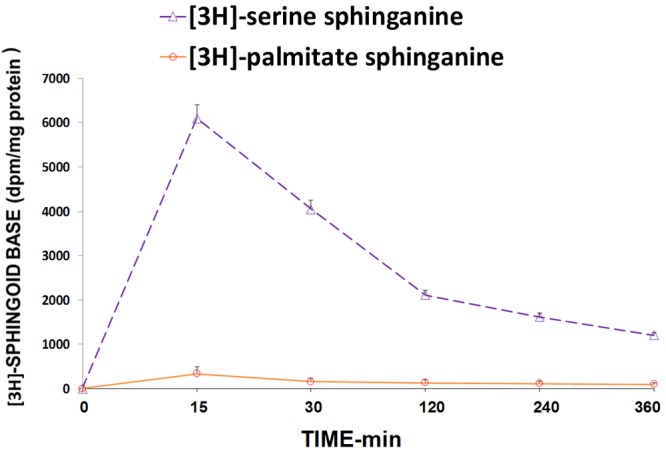
[3H]-serine and [3H]-palmitate incorporation into free sphinganine base in primary cultured single aortic VSM cells exposed to 0.3 mM [Mg] - see Methods over time. N=6-8.
Assay of cytokines and chemokines in cardiovascular tissues, sera and primary cultured VSM cells with and without inhibitors: correlation of PKC isoform levels in LV and aortic muscle tissues with levels of cytokines
The results shown in Table 5 demonstrate that pre-incubation of the primary cells (exposed to low Mg2+) with bisindolemaleimide I resulted in significant reductions in the cellular levels of the cytokines IL-1beta, IL-6, IL-8, and TNF-alpha as well as the chemokine MCP-1. However, pre-incubation of the cultured cells with either rottlerin or GO 6976 also resulted in some reductions in all of the cytokines as well as MCP-1, although at somewhat less inhibitory levels (Table 5). Interestingly, pre-incubation of the VSM cells (exposed to low Mg2+) with the PKC zeta inhibitor, i.e., isoform-specific-myristoylated peptide (see Methods), also resulted in significant reductions in the cellular levels of the cytokines and chemokine (Table 5).
Table 5.
Influence of PKC isozyme enzyme inhibitors on cytokine/chemokine levels released from aortic VSM cells (in culture) exposed to low [Mg2+]0
| [Mg], mM | IL-1beta, pg/ml | IL-6, pg/ml | IL-8, pg/ml | TNF, pg/ml | MCP-1, ng/ml |
|---|---|---|---|---|---|
| Control 1.2 mM | 4.8 ± 0.4 | 224 ± 28 | 0.22 ± 0.06 | 6.2 ± 1.0 | 0.32 ± 0.06 |
| 0.3 | 38.4 ± 3.2* | 622 ± 44* | 2.28 ± 0.24* | 26.4 ± 4.2* | 2.88 ± 0.4* |
| w/Bisindole | 24.2 ± 2.6** | 428 ± 36** | 1.56 ± 0.14** | 16.8 ± 2.4** | 1.98 ± 0.2** |
| w/Rottlerin | 32.2 ± 2.8* | 518 ± 38* | 1.98 ± 0.18* | 22.4 ± 1.8* | 2.42 ± 0.4* |
| w/GO 6976 | 30.4 ± 2.2** | 484 ± 32** | 1.76 ± 0.14** | 20.6 ± 1.6* | 2.22 ± 0.2** |
| w/PKCzetpep | 26.8 ± 2.2** | 460 ± 34** | 1.64 ± 0.12** | 18.6 ± 2.2** | 2.08 ± 0.2** |
Values are means ± SE; n=6-10 per group.
Significantly different from mean values in 1.2 mM Mg2+ (P<0.01).
Significantly different from mean values in 1.2 and 0.3 mM Mg2+ (P<0.01; ANOVA).
The results shown in Figure 12 confirm recently reported data [44] which indicate that cardiovascular tissues harvested from MgD animals show 3-10 fold rises in cytokine and chemokine levels whereas MgD animals fed as little as 15-40 mg/l Mg in their drinking water largely prevented the rises in levels of cytokines and chemokines. The data summarized in Figures 13, 14 and 15, using multiple regression analysis, indicates high degrees of correlation (0.69-0.99, P<0.001) of LV and aortic muscle PKC isoform levels to cytokine levels in tissues obtained from the MgD animals. Although not shown, all of the cytokines assayed in this study demonstrated a high degree of linear correlation (0.72-0.99, P<0.01) to all the PKC isoforms, except for PKC-epsilon.
Figure 12.
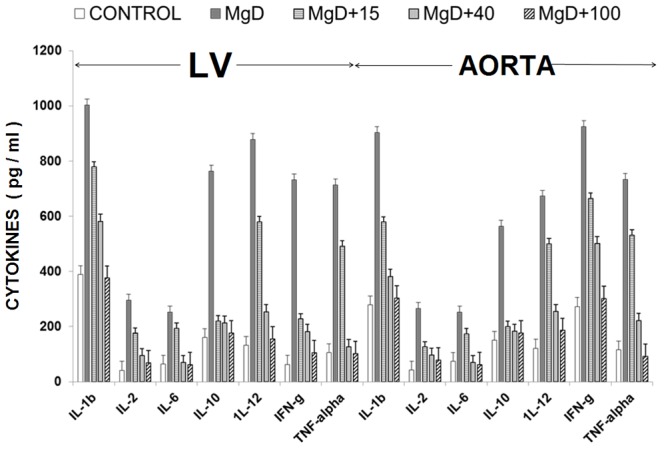
Cytokine levels in LV and aortic vascular muscle in normal and MgD rats with and without Mg2+ added to the drinking water; n=8-12 animals per group.
Figure 13.
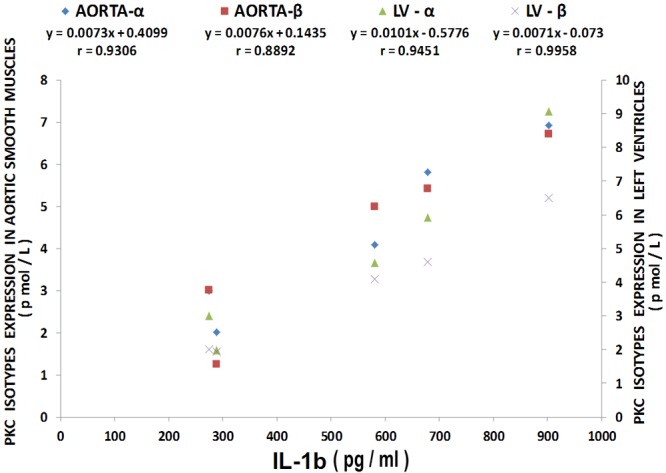
Multiple regression analysis of left ventricular (LV) and aortic vascular muscle PKC-alpha and beta isoform levels with IL-1beta levels. Regression equations with r-values are shown in the figure. n=10-12.
Figure 14.
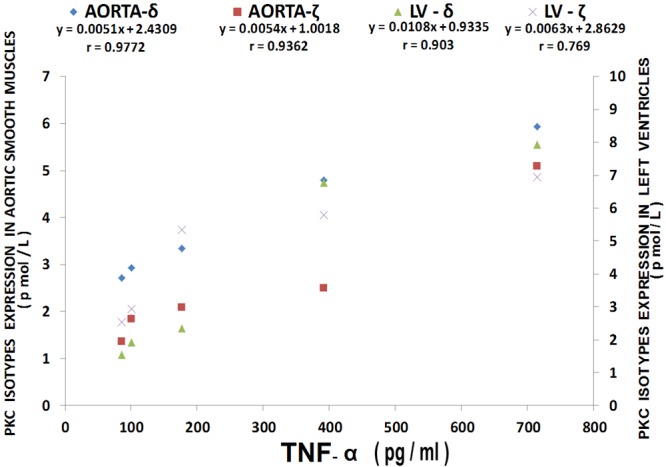
Multiple regression analysis of LV and aortic vascular muscle PKC-delta and zet isoform levels with TNF-alpha levels . Regression equations with r-values are shown in the figure. n=10-12.
Figure 15.
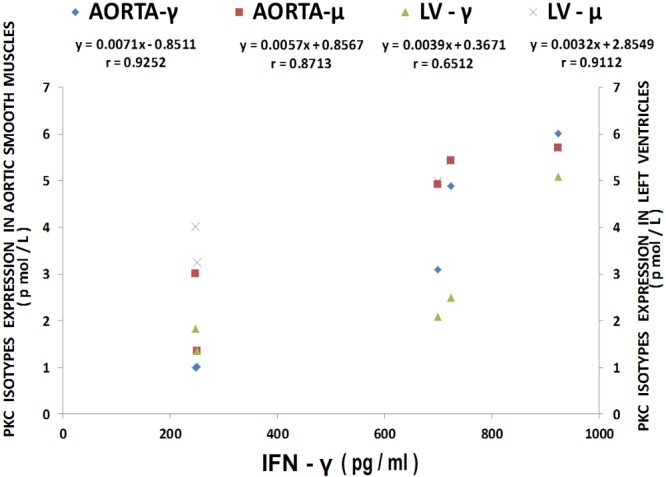
Multiple regression analysis of LV and aortic vascular muscle PKC-gamma and mu isoform levels with IFN-gamma levels. Regression equations with r-values are shown in the figure. n=10-12.
Discussion
The results reported, herein, are the first demonstration that short-term deficiency of Mg in an intact mammal results in activation of all classes of PKC isoforms (i.e., classical, novel, and atypical PKCs) in cardiac and VSM tissues and cells. To our knowledge, this the first time anyone has shown an upregulation of all classes of PKC isoforms by MgD in any cell type in any species. We also demonstrate for the first time that there is cross-talk between the ceramide, sphingosine, NF-kB, cytokine and PKC pathways in cardiovascular cells in MgD animals. In addition, we demonstrate that as little as 15 mg/l of Mg2+ added to drinking water prevents the upregulation of the PKC isoforms in both intact cardiac and VSM produced by low [Mg2+]0. We also demonstrate that blood levels, in the MgD animals, of the PKC isoforms parallel those found in the LV (the significance of this is discussed below). Lastly, we provide evidence for a role for the “sphingolipid salvage pathway” in VSM cells.
Molecular pathways for synthesis of ceramides and low Mg
The molecular pathways via which ceramides are synthesized are known to be highly conserved between yeast and mammals [67-69]. The de novo synthesis of SM is brought about via the action of serine palmitoyl-CoA transferase (SPT), 3-ketosphinganine reductase, CS, dihydroceramide desaturase and SM synthase (SMS) [35,64,68-74]. This reaction directly affects SM, phosphatidylcholine (PC), and ceramide as well as DAG levels. Three of us have previously noted, using primary cerebral and peripheral VSM cells in culture, that a variation in [Mg2+]0 influences the cellular levels of SM, PC, DAG and ceramide [32,33]. Ceramide, either released as a consequence of SMase acting on SM and/or activation of either SPT or SMS or activation of CS is now thought to play important roles in fundamental pathological processes such as cell proliferation, angiogenesis, immune inflammatory responses, cell adhesion, atherogenesis, microcirculatory functions, membrane-receptor functions, senescence, and programmed cell death as well as transport and distribution of Mg2+ in VSM cells [32,33,35,38,42-45,66-68,71,72,74-81].
Low Mg, PKC isoforms and ceramide via sphingolipid salvage pathway
Although the activation of N-SMase, SPT-1, SPT-2 as well as SMS by low [Mg2+]0 results in (and ensures) ceramide production in cardiovascular tissues [42-45], the activation of CS (by low Mg2+) via the “salvage pathway” could result in additional levels of de novo ceramide along with elevated levels of sphingosine [58]. The present studies, we believe, provide new evidence for the existence of the latter (“salvage”) pathway in cardiovascular tissues via activation of several isoforms of PKC. Our present experiments employing 4-beta-phorbol 12-myristate 13-acetate to stimulate both the novel and classical PKC pathways in vascular tissue, with and without specific antagonists of all three classes of PKC isoforms, suggest that activation of PKC-zeta appears to play a dominant role in de novo formation of ceramide (along with sphingosine) via the “salvage pathway”. The failure of PMA stimulation on palmitate-labeling of ceramide seems to demonstrate that PMA, in our experiments, must perforce result in the accumulation of ceramide from pre-labeled precursors other than sphingosine, particularly as PMA does not stimulate PKC-zeta. We hypothesize such a scenario implicates the salvage pathway. We believe the present work is the first to implicate the salvage pathway in cardiovascular tissues via cross-talk with the PKC isoform-PKC-zeta. If confirmed, our results could be suggestive of new therapeutic and prophylactic approaches in the management of some cardiovascular diseases, e.g., hypertension, coronary ischemic syndromes and atherosclerosis.
Since we have reported, previously, that low [Mg2+]0 environments can result in activation of N-SMases, SPT-1, and SPT-2 which result in production of elevated ceramide levels in cardiac and VSM cells [42-45], the results in this study, when taken together with our previous data suggest that the resultant production of ceramide is, most likely, recruiting all three classes of PKC molecules, with a great effect on the recruitment of PKC zeta. A number of previously published studies, using a variety of cell types (including VSM), have found that the atypical isoforms of the PKC superfamily, e.g., PKC zeta, is directly or indirectly regulated by several lipids including ceramides [25,27,32,40,48,53,54,67,73]. Over the past decade a number of studies have accumulated to suggest that PKC zeta plays fundamental roles in cardiac ischemic-reperfusion events, the cardiac endothelial synthesis of nitric oxide (NO), release of reactive oxygen species, proatherosclerotic events, mitogenesis and proliferation in VSM cells, diabetic-vascular events, and apoptosis [48,49,54-57,82-85]. It is important to note, here, that we have shown that MgD environments result in synthesis of NO in cardiac, vascular smooth muscle as well as microvessels [86] concomitant with release of reactive oxygen species, proatherosclerotic events, and apoptosis [42-45,86]. In view of the present findings, it is of interest to point out, here, that In vitro studies on working and perfused rat hearts obtained from MgD animals clearly demonstrate that even short-term MgD results in reduction in a variety of hemodynamic functions, e.g., falls in cardiac output, coronary flow, stroke volume, developed pressures and ischemia concomitant with a lowering of high-energy phosphates [10,20,24]. Obviously, such findings would be compatible with a release/synthesis of ceramides and activation of PKC isoforms (e.g., zeta) along with several other PKC isoforms suggested by the present studies. In this context, it has been demonstrated, recently, that specific inhibition of PKC zeta exerts protective effects on ischemia-reperfusion injury events in rat heart preparations [84]. The fact that our new results demonstrate a parallel rise of PKC isoforms in the sera of the MgD animals, similar to those in the highly-muscularized LV, indicates that the dietary deficiency of Mg used herein (and previously) must perforce damage the cardiac cells to allow PKC isozymes (MW=70-100 kD) to leak out of the cells into the blood stream. Previously, using the same model of MgD, we reported leakage of lactic acid dehydrogenase (MW=140-230 kD), creatine phosphokinase (80-85 kD), and SGOT (MW=90-95 kD) [45].
Potential role of PKC isoforms as biomarkers in Mg deficiency
At present, there is no way to positively identify the presence of a MgD in patients or animals other than subjecting the hosts to a Mg-load test or a serum ionized Mg test. Since neither of these is in clinical use, as yet, we believe our new findings of high levels of several PKC isoforms in sera of MgD animals may point to a new method for determining the presence of a MgD, clinically. This hypothesis should be put to rigorous testing in the future for its potential implications.
Potential role of PKC zeta activation linked to NF-kB in MgD-, programmed cell death, cell transformation and differentiation
Recently, it was shown that MgD results in apoptotic events in both cardiac and VSM cells [41,87,88]. Since it has been shown that the ceramide-mediated PKC zeta activation can lead to inhibition of cell growth in some cells [50,54,55,57], it is tempting to speculate that our findings of MgD-induced programmed cell death in both cardiac and vascular smooth muscles may be, at least, in part, a result of ceramide-mediated PKC zeta activation.
A prime function of ceramide’s role in pathophysiological events is its ability to induce cell transformation and differentiation [35,67,71,75,79]. Abnormal cell differentiation, transformation, and growth are major factors in the development of both atherogenesis and hypertension [59,89-92]. Hyperplasia and cardiovascular hypertrophy are common events in hypertension and key elements in target organ damage [59,90,91,93]. But, the precise mechanisms regulating alterations in tissue mass, transformation of VSM cell phenotypes, plaque formation in vascular walls, and lipid deposition are not completely understood [59,89,90,92]. Activation of NF-kB DNA-binding proteins is known to play important roles in atherogenesis and hypertension [59,93]. NF-kB is a transcription factor and a pleiotrophic regulator of numer ous genes important in inflammatory processes involved in atherogenesis and hypertension. It is not, as yet, clear as to what factor(s) initiate these molecular and cellular events. Recently, we demonstrated in the same model of MgD used, herein, that the key NF-kB DNA-binding proteins, p65 and cRel, are activated along with ceramide [44], a number of cytokines/chemokines [44], and p53 [43]; the latter tumor suppressor protein is known to play key roles in regulation of cell growth and apoptosis [92,93], important events in generation of atherogenesis and hypertension. The present studies confirm the association of NF-kB activation with simultaneous elevation in ceramide levels. The present work shows that the former (i.e., NF-kB activation) is associated with an activation of several PKC isoforms, with PKC zeta having a large role in these events. Moreover, our findings indicate that this activation of PKC zeta is, in large measure, dependent upon a rise in pulsed-labeled ceramide, which, to us, suggests that the activation of PKC zeta, at least in primary cultured rat aortic cells, is most likely dependent on generation of ceramide (and probably sphingosine via “the salvage pathway”). It is important to point out, here, that ceramide and activation of NF-kB (and p53; see [43]) are known to induce cell cycle arrest (and senescence), induce programmed cell death, and are associated with DNA damage (genotoxic events) [59,67,75-77,93]. MgD can induce all of these pathophysiological phenomena in multiple cell types [1,18,42-45,87,88,90]. Our present findings could be used to suggest that MgD environments drive ceramide synthesis, at least, in part, in VSM cells via the CS and “salvage pathways”.
MgD results in activation of sphingolipids, vasospasm and inflammation: relation to cytokine and chemokine release
It should be recalled here that Mg2+-deficient environments, both in vivo and in vitro, have been repeatedly shown to produce arterial and arteriolar contraction and vasospasm (see [1-5,8,16,17,24]). Up to the present studies, it has generally been thought that these contractile response of blood vessels are dependent, primarily, on both an influx of [Ca2+ 0 and an intracellular release of [Ca2+]i [1,8,11,16-18,63]. Since ceramides, sphingosine, and sphingosine-1-phosphate have been demonstrated to induce contraction of diverse VSM as well as vasospasm of blood vessels [1,34,36,37,39,40], we hypothesize, based on the studies presented herein, that activation of several PKC isoforms, including PKC-zeta probably play major roles in coronary and cerebral vasospasm seen under clinical conditions as well as in hypertensive and stroke events. Several recent studies have implicated PKC zeta in atherosclerotic plaque formation (and rupture), monocyte- and endothelial-oxidation of LDL, and fibroblast proliferation (see [57] for review). All of these events are inflammatory in nature and involve synthesis and release of different cytokines and chemokines. In this context, our present data, when viewed in light of other recent studies (see [44]), show clear relationships to generation of these cytokines (and chemokines) and activation of PKC isoforms (particularly PKC zeta) coupled to release and generation of ceramide and sphingosine in MgD animals. We, thus, believe prolonged MgD states result in inflammatory conditions which can give rise to hyperlipidemic states [1,14,18,23,25,32,33,45], atherogenesis [25], hypertension [9,13,19,26,50], and cardiac damage, possibly in large measure due to cross-talk between PKC isoforms, activation of de novo biosynthetic pathways for ceramide release, generation of ceramide and sphingosine via the salvage pathway coupled to release of cytokines and chemokines and activation of NF-kB.
MgD states result in upregulation of NF-kB linked to PKC activation
NF-kB is kept in the cytosol of quiescent cells via the inhibitor proteins IkB; the latter being degraded with cell activation via a diverse number of stimuli [51,52,57], of which MgD states appears to be one [1,11,44,61,62]. These stimuli are known to include cytokines and chemokines, including IL-2, IL4, TNF-alpha, and MCP-1 [51,52], which we have shown herein and elsewhere to be upregulated by MgD states [44]. Degradation of IkB occurs when it undergoes ubiquination via a proteasome system [57]. Atypical PKCs, particularly PKC zeta, have been suggested to be important mediators in control of cell survival via activation of NF-kB [51,57]. We, herein, clearly demonstrate that upregulation of PKC zeta by MgD in cardiovascular tissues and cells is linked to activation of NFkB. Recently, we reported that MgD in cardiovascular tissues and cells leads to phosphorylation of IkB by the IKK complex [44,61,62], a pivotal step in the release and translocation of NF-kB to the nucleus [57]. PKC-zeta has been demonstrated recently in other types of tissues and cells to control phosphorylation of the RelA subunit (e.g., p65) of the NF-kB complex, thus enabling transcription and subsequent gene expression [51,52,57]. Our experiments reveal that MgD upregulates p65. We, thus, believe our present findings, together with those published by our group recently [44,61,62], are compatible with ,and support this hypothesis. Since MgD appears to be prevalent in the North American population, adequate dietary intake of Mg would seem to us to be pivotal in preventing and alleviation of the potential activation (trigerring?) of these diverse interacting pathways which cross-talk to one another.
Is Mg vasodilator action linked to PKC zeta activation?
Although it has time and again been demonstrated that prolonged administration of Mg2+ (oral and intravenous) can lower arterial blood pressure in both experimental and clinical forms of hypertension, as well as stabilize and prevent cardiac arrhythmias [1,12,13,18,24,94], the precise mechanisms are not known. It has, often, been suggested that Mg2+ lowers blood pressure by promoting vasodilation and decreasing work load on the heart via direct actions on Ca2+ channels (and cellular redistribution) in vascular and cardiac muscle cells. In view of the present work, and other studies recently published [1,8,40,42-45], we believe that ceramide (and sphingolipid metabolism) and activation of PKC-zeta must now be taken into consideration in helping to explain the blood pressure-lowering actions of this divalent cation.
Cardiovascular death rates linked to water hardness and Mg levels
Approximately 55 years ago, Kobayashi demonstrated in an epidemiological study that where the hardness of drinking water was elevated, the rate of death from cardiovascular diseases decreased [95]. This notion has gained considerable credibility over the past five decades from a large number of epidemiological studies from different parts of the globe; the death rates from sudden cardiac death are lower in hard-water areas than in soft-water areas [1-6,15,18,21,22]. Even though the hardness of water is due to the concentration of Ca2+ and Mg2+, the overwhelming evidence, to date, supports the idea that it is the Mg content that is responsible for the protective effects of hard-water. Approximately 30 years ago, it was suggested (on the basis of epidemiological data) that as little as 15-30 mg/l/day of Mg2+ in drinking water should be cardioprotective [5,22]. Recently, using the same model of dietary deficiency of Mg as in the present study (21 days of MgD), we demonstrated, for the first time, in well-controlled experiments that as little as 15 mg Mg2+/l/day in drinking water, either prevented or ameliorated the formation of reactive oxygen species, DNA fragmentation, caspase-3 activation, activation of p53, mitochondrial release of cytochrome c, lipid peroxidation, activation of apoptosis, hydrolysis of membrane sphingomyelin, upregulation of SPT-1 and SPT-2, activation of SMS, as well as activation of CS [42-45]. Although the present work indicates that as little as 15 mg/l/day of Mg2+ in water can prevent/ameliorate the upregulation/synthesis of a variety of numerous PKC isoforms, including PKC-zeta, in both cardiac and VSM, in some cases something between 15 and 40 mg/l/day of Mg2+ in water must be imbibed to prevent the upregulation of certain PKC isoforms. From the present findings, and previously published studies, we hypothesize that between 15 and 40 mg/l/day of Mg2+ of water-borne Mg2+ should be both cardioprotective and vascular protective.
As suggested recently [42-45], we believe that water intake (e.g., from tap waters, well waters, bottled waters, and beverages using tap/well/spring waters) in humans varying between 1 and 2 l/day, with Mg2+ intakes varying from <5 to >100 mg/l, may represent an excellent way to overcome and control marginal intakes of Mg obtained with most Western diets. In view of the present results, and other data shown elsewhere, it is probably propitious to suggest that all desalinated-purified recovered/recycled waters, harvested rain waters, as well as well-waters, tap waters, and all bottled waters given to humans should be supplemented with bioavailable Mg2+ to ameliorate/prevent the induction of cardiovascular risk factors and disease processes worldwide. In the past few months, based on our studies and others, a pilot study has been initiated, in a large population, in Southern Israel ,to determine if Mg2+ added to the area’s desalinated drinking water will be cardioprotective (e.g., see March 2012 issue of The Jerusalem Post).
Acknowledgements
This work was supported, in part, by a research grant from Regalware Worldwide to BMA and NIH grants to B. M. Altura. We appreciate the gratis supply of magnesium aspartate-HCl that was provided to us by Dr. Angela Weigert of Verla Pharm (Tutzing, Germany).
References
- 1.Altura BM, Altura BT. Magnesium: forgotten mineral and cardiovascular biology and atherogenesis. In: Nishizawa N, Morii H, Durlach J, editors. New Perspectives in Magnesium Research. New York: Springer; 2007. pp. 239–260. [Google Scholar]
- 2.Durlach J, Bara M, Guilet-Bara A. Magnesium level in drinking water and cardiovascular risk factor: a hypothesis. Magnesium. 1985;4:5–15. [PubMed] [Google Scholar]
- 3.Eisenberg MJ. Magnesium deficiency and sudden death. Am Heart J. 1992;124:544–549. doi: 10.1016/0002-8703(92)90633-7. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;121:2879–2882. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 5.Marier JR, Neri LC. Quantifying the role of magnesium in the interrelationship between human mortality/morbidity and water hardness. Magnesium. 1985;4:53–59. [PubMed] [Google Scholar]
- 6.Marx A, Neutra RR. Magnesium in drinking water and deaths from ischemic heart disease. Epidemiol Rev. 1997;19:258–272. doi: 10.1093/oxfordjournals.epirev.a017957. [DOI] [PubMed] [Google Scholar]
- 7.Rubenowitz E, Molin L, Axellson G, Rylander R. Magnesium in drinking water in relation to morbidity and mortality from acute myocardial infarction. Epidemiology. 2000;11:416–421. doi: 10.1097/00001648-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Altura BM, Altura BT. Magnesium and contraction of arterial muscle. Microvasc Res. 1974;7:145–155. doi: 10.1016/0026-2862(74)90001-6. [DOI] [PubMed] [Google Scholar]
- 9.Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. Magnesium deficiency and hypertension: correlation between magnesium deficient diets and microcirculatory changes in situ. Science. 1984;223:1315–1317. doi: 10.1126/science.6701524. [DOI] [PubMed] [Google Scholar]
- 10.Altura BM, Barbour RL, Dowd TL, Wu F, Altura BT, Gupta RK. Low extracellular magnesium induces intracellular free Mg deficits, depletion of high-energy phosphates and cardiac failure in intact working hearts; A 31P-NMR study. Biochim Biophys Acta. 1993;1182:329–332. doi: 10.1016/0925-4439(93)90077-e. [DOI] [PubMed] [Google Scholar]
- 11.Altura BT, Memon ZI, Zhang A, Cracco RQ, Altura BM. Low levels of ionized magnesium are found in patients early after stoke which result in rapid elevation in cytosolic free calcium and spasm in cerebral vascular smooth muscle cells. Neurosci Lett. 1997;230:37–40. doi: 10.1016/s0304-3940(97)00471-0. [DOI] [PubMed] [Google Scholar]
- 12.Berthelot A, Luthringer C, Myers E, Exinger A. Disturbances of magnesium metabolism in the spontaneously hypertensive rat. J Am Coll Nutr. 1997;6:329–332. doi: 10.1080/07315724.1987.10720195. [DOI] [PubMed] [Google Scholar]
- 13.Laurant P, Dalle M, Berthelot A, Rayssiguier Y. Time course of the change in blood pressure level in magnesium-deficient Wistar rats. Br J Nutr. 1999;82:243–251. [PubMed] [Google Scholar]
- 14.Luthringer C, Rayssiguier Y, Gueux E, Berthelot A. Effect of moderate magnesium deficiency on serum lipids, blood pressure and cardiovascular reactivity in normotensive rats. Br J Nutr. 1988;59:243–250. doi: 10.1079/bjn19880031. [DOI] [PubMed] [Google Scholar]
- 15.Turlapaty PDMV, Altura BM. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science. 1980;208:198–200. doi: 10.1126/science.7361117. [DOI] [PubMed] [Google Scholar]
- 16.Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Low extracellular magnesium contraction of arterial muscle: role of protein kinase C and protein tyrosine phosphorylation. Eur J Pharmacol. 1999;378:273–281. doi: 10.1016/s0014-2999(99)00474-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhang A, Cheng TPO, Altura BM. Magnesium regulates intracellular free ionized calcium concentration and cell geometry in vascular smooth muscle cells. Biochim Biophys Acta. 1992;1134:25–29. doi: 10.1016/0167-4889(92)90024-6. [DOI] [PubMed] [Google Scholar]
- 18.Altura BM, Altura BT. Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res. 1995;41:347–359. [PubMed] [Google Scholar]
- 19.Altura BM, Altura BT, Gebrewold A, Gunther T, Ising H. Noise-induced hypertension and magnesium: Relationship to microcirculation and magnesium. J Appl Physiol. 1992;72:194–202. doi: 10.1152/jappl.1992.72.1.194. [DOI] [PubMed] [Google Scholar]
- 20.Wu F, Altura BT, Gao J, Barbour RL, Altura BM. Ferrylmyoglobin formation induced by acute magnesium deficiency in perfused rat heart causes cardiac failure. Biochim Biophys Acta. 1994;1225:158–164. doi: 10.1016/0925-4439(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 21.Chipperfield B, Chipperfield JR. Relation of myocardial metal concentrations to water hardness and death from ischemic heart disease. Lancet. 1979;2:709–712. doi: 10.1016/s0140-6736(79)90641-x. [DOI] [PubMed] [Google Scholar]
- 22.Leary WP. Content of magnesium in drinking water and deaths from ischemic heart disease in White South Africans. Magnesium. 1986;5:150–153. [PubMed] [Google Scholar]
- 23.Rayssiguier Y, Gueux E, Bussiere I, Durlach J, Mazur A. Dietary Magnesium affects susceptibility of lipoproteins and tissues to peroxidation in rats. J Am Coll Nutr. 1993;12:133–137. doi: 10.1080/07315724.1993.10718293. [DOI] [PubMed] [Google Scholar]
- 24.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 25.Altura BT, Brust M, Barbour RL, Stempak J, Bloom S, Altura BM. Magnesium dietary intake modulates blood lipid levels and atherogenesis. Proc Natl Acad Sci U S A. 1990;87:1840–1844. doi: 10.1073/pnas.87.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joffres MR, Reed DM, Yano K. Relationship of magnesium intake and other dietary factors to blood pressure: the Honolulu heart study. Am J Clin Nutr. 1987;45:469–475. doi: 10.1093/ajcn/45.2.469. [DOI] [PubMed] [Google Scholar]
- 27.Seelig MS, Rosanoff A. The Magnesium Factor. New York: Penguin Group; 2003. [Google Scholar]
- 28.Altura BT, Altura BM. Measurement of ionized magnesium in whole blood, plasma and serum with a new ion-selective electrode in healthy and diseased human subjects. Magnes Trace Elem. 1991;10:90–98. [PubMed] [Google Scholar]
- 29.Handwerker S, Altura BT, Royo B, Altura BM. Ionized magnesium and calcium levels in umbilical cord serum of pregnant women with transient hypertension during labor. Am J Hypertens. 1993;6:542–545. doi: 10.1093/ajh/6.6.542. [DOI] [PubMed] [Google Scholar]
- 30.Christova T, Duridanova D, Setchenska M. Protein kinase C and smooth muscle contraction. Biomed Rev. 1997;8:87–100. [Google Scholar]
- 31.Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Low extracellular Mg induces contraction and Ca2+ rises in cerebral arteries: roles of Ca2+, PKC and P-I-3 kinases. Am J Physiol Heart Circ Physiol. 2000;272:H2898–H2907. doi: 10.1152/ajpheart.2000.279.6.H2898. [DOI] [PubMed] [Google Scholar]
- 32.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Lett. 1997;408:191–197. doi: 10.1016/s0014-5793(97)00420-1. [DOI] [PubMed] [Google Scholar]
- 33.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane sphingolipids and lipid second messengers in vascular smooth muscle cells. FEBS Lett. 1998;440:167–171. doi: 10.1016/s0014-5793(98)01446-x. [DOI] [PubMed] [Google Scholar]
- 34.Altura BM, Gebrewold A, Zheng T, Altura BT. Sphingomyelinase and ceramide analogs induce vasoconstriction and leukocyte-endothelial interactions in cerebral venules in the intact brain: insight into mechanisms and possible relation to brain injury and stroke. Brain Res Bull. 2002;58:271–278. doi: 10.1016/s0361-9230(02)00772-4. [DOI] [PubMed] [Google Scholar]
- 35.Auge N, Andrieu N, Negre-Salvayre R, Thiers JC, Levade T, Salvayre R. Sphingomyelin metabolites in vascular signaling and atherogenesis. Prog Lipid Res. 2002;39:207–229. doi: 10.1016/s0163-7827(00)00007-2. [DOI] [PubMed] [Google Scholar]
- 36.Bischoff A, Czyborra P, Fetscher C, Meyer Zu Heringdorf D, Jacobs KH, Michel MC. Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br J Pharmacol. 2000;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi SK, Ahn DS, Lee YH. Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery. Cardiovasc Res. 2002;82:324–332. doi: 10.1093/cvr/cvp054. [DOI] [PubMed] [Google Scholar]
- 38.Houck KL. Sphingolipid and phospholipid metabolites regulate the phenotype of vascular smooth muscle cells. PhD Thesis, Penn State Univ; 2008. [Google Scholar]
- 39.Michel MC, Mulders ACM, Jonsma M, Alewijinse AE, Peters SLM. Vascular effects of sphingolipids. Acta Paediatr. 2007;96:44–48. doi: 10.1111/j.1651-2227.2007.00207.x. [DOI] [PubMed] [Google Scholar]
- 40.Zheng T, Li W, Wang J, Altura BT, Altura BM. Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca2+] in canine cerebral vascular muscle. Am J Physiol Heart Circ Physiol. 2000;278:H1421–H1428. doi: 10.1152/ajpheart.2000.278.5.H1421. [DOI] [PubMed] [Google Scholar]
- 41.Altura BM, Shah NC, Jiang XC, Li Z, Perez-Albela JL, Altura BT. Short-term magnesium deficiency results in decreased levels of serum sphingomyelin, lipid peroxidation, and apoptosis in cardiovascular tissues. Am J Physiol Heart Circ Physiol. 2009;297:H86–H92. doi: 10.1152/ajpheart.01154.2008. [DOI] [PubMed] [Google Scholar]
- 42.Altura BM, Shah NC, Li Z, Jiang XC, Perez-Albela JL, Altura BT. Magnesium deficiency upregulates serine palmitoyltransferase (SPT 1 and SPT 2) in cardiovascular tissues: relationship to serum ionized Mg and cytochrome C. Am J Physiol Heart Circ Physiol. 2010;299:H932–H938. doi: 10.1152/ajpheart.01076.2009. [DOI] [PubMed] [Google Scholar]
- 43.Altura BM, Shah NC, Li Z, Jiang XC, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term magnesium deficiency upregulates sphingomyelin synthase and p53 in cardiovascular tissues and cells: relevance to the de novo synthesis of ceramide. Am J Physiol Heart Circ Physiol. 2010;299:H2046–2055. doi: 10.1152/ajpheart.00671.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altura BM, Shah NC, Shah G, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term magnesium deficiency upregulates ceramide synthase in cardiovascular tissues and cells: cross-talk among cytokines, Mg2+, NFkB and de novo ceramide. Am J Physiol Heart Circ Physiol. 2012;302:H319–332. doi: 10.1152/ajpheart.00453.2011. [DOI] [PubMed] [Google Scholar]
- 45.Shah NC, Liu JP, Iqbal J, Hussain M, Jiang XC, Li Z, Li Y, Zheng T, Li W, Sica AC, Perez-Albela JL, Altura BT, Altura BM. Mg deficiency results in modulation of serum lipids, glutathione, and NO synthase isozyme activation in cardiovascular tissues: relevance to de novo synthesis of ceramide, serum Mg and atherogenesis. Int J Clin Exp Med. 2011;4:103–118. [PMC free article] [PubMed] [Google Scholar]
- 46.Andrather J, Csizmadia V, Soares MP, Winkler H. Regulation of NF-kB RelA phosphorylation and transcription activity by p21 and protein kinase C zeta in primary endothelial cells. J Biol Chem. 1999;274:13594–13603. doi: 10.1074/jbc.274.19.13594. [DOI] [PubMed] [Google Scholar]
- 47.Becker KP, Kitakani K, Idkowiak-Baldys J, Bielawaski J, Hannun YA. Selective inhibition of juxtanuclear translocation of protein kinase C BIII by a negative feedback mechanism involving ceramide formed from the salvage pathway. J Biol Chem. 2005;280:2606–2612. doi: 10.1074/jbc.M409066200. [DOI] [PubMed] [Google Scholar]
- 48.Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling pathway. J Biol Chem. 2000;275:35617–35623. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- 49.Carlin S, Yang KXF, Donnelly R, Black JL. Protein kinase C isoforms in human airway smooth muscle cells: activation of PKC-zeta during proliferation. Am J Physiol. 1999;276:L506–L512. doi: 10.1152/ajplung.1999.276.3.L506. [DOI] [PubMed] [Google Scholar]
- 50.Hirai T, Chida K. Protein kinase C zeta (PKCzeta): activation mechanisms and cellular functions. J Biochem. 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- 51.Lallena MJ, Diaz-Meco MT, Bren G, Paya CV, Moscat J. Activation of IkB kinase beta by protein kinase C isoforms. Mol Cell Biol. 1999;19:2180–2188. doi: 10.1128/mcb.19.3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lozano J, Berra E, Munico MM, Diaz-Meco MT, Dominguez I, Sanz L, Moscat J. Protein kinase C zeta isoform is critical for kB-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 53.Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012;11:937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muller G, Ayoouh M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K. PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protin kinase C zeta before the formation of a pro-apoptotic complex with PAR-4 differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 56.Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. PKC isozymes in chronic disease: possible therapeutic targets. Annu Rev Pharmacol Toxicol. 2008;48:569–599. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 57.Diaz-Meco MT, Moscat J. The atypical PKCs in inflammation: NF-kB and beyond. Immunol Rev. 2012;246:154–167. doi: 10.1111/j.1600-065X.2012.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. Eighth Ed. Philadelphia: Saunders; 2010. pp. 487–506. [Google Scholar]
- 60.Steinberg SF. Cardiac actions of protein kinase C isoforms. Physiology (Bethesda) 2012;27:130–139. doi: 10.1152/physiol.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Altura BM, Gebrewold A, Zhang A, Altura BT. Low extracellular magnesium ions induces lipid peroxidation and activation of nuclear factor kappa B in canine cerebral vascular smooth muscle: possible relation to traumatic brain injury and strokes. Neurosci Lett. 2003;341:189–192. doi: 10.1016/s0304-3940(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 62.Altura BM, Kostellow AB, Zhang A, Li W, Morrill GA, Gupta RK, Altura BT. Expression of the nuclear factor-kappa B and proto-oncogenes c-fos and c-jun are induced by low extracellular Mg2+ in aortic and cerebral vascular smooth muscle cells: possible links to hypertension, atherogenesis and stroke. Am J Hypertens. 2003;16:701–707. doi: 10.1016/s0895-7061(03)00987-7. [DOI] [PubMed] [Google Scholar]
- 63.Zheng T, Li W, Wang J, Altura BT, Altura BM. Effects of neutral sphingomyelinase on phenylephrine - induced vasoconstriction and Ca2+ mobilization in rat aortic smooth muscle. Eur J Pharmacol. 2000;391:127–135. doi: 10.1016/s0014-2999(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 64.Merrill AH Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrophotometry. Methods. 2005;36:207–224. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 65.He Q, Riley RT, Sharma RP. Fumonisin-induced tumor necrosis factor-alpha expression in a porcine kidney cell line is independent of sphingold base accumulation induced by ceramide synthase inhibition. Toxicol Appl Pharmacol. 2001;174:69–77. doi: 10.1006/taap.2001.9189. [DOI] [PubMed] [Google Scholar]
- 66.Yoo HS, Norred WP, Showker J, Riley RT. Elevated sphingoid bases and complex sphingolipid depletion as contributing factors in fumonisin-induced cytotoxicity. Toxicol Appl Pharmacol. 2001;138:211–218. doi: 10.1006/taap.1996.0119. [DOI] [PubMed] [Google Scholar]
- 67.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 68.Kolesnick R. Signal transduction through the sphingomyelin pathway. Mol Chem Neuropathol. 1994;21:287–297. doi: 10.1007/BF02815356. [DOI] [PubMed] [Google Scholar]
- 69.Villani M, Subathra M, Im YB, Choi Y, Signorelli P, Del Poeta M, Luberto C. Sphingomyelin synthases regulate production of diacylglycerol at the Golgi. Biochem J. 2008;414:31–41. doi: 10.1042/BJ20071240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jaffrezou JP, Maestre N, de Mas-Mansat V, Bezombes C, Levade T, Laurent G. Positive feedback control of sphingomyelinase activity by ceramide. FASEB J. 1998;12:999–1006. doi: 10.1096/fasebj.12.11.999. [DOI] [PubMed] [Google Scholar]
- 71.Luberto C, Hannun YA. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. J Biol Chem. 1998;273:14550–9. doi: 10.1074/jbc.273.23.14550. [DOI] [PubMed] [Google Scholar]
- 72.Pandey S, Murphy RF, Agarawal DK. Recent advances in the immunobiology of ceramide. Exp Mol Pathol. 2007;82:298–309. doi: 10.1016/j.yexmp.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perry DK, Carton J, Shah AK, Meredith F, Uhlingeri DJ, Hannun YA. Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J Biol Chem. 2000;275:9078–9084. doi: 10.1074/jbc.275.12.9078. [DOI] [PubMed] [Google Scholar]
- 74.Vandrager AB, Houweling M. Effect of ceramides on phospholipid biosynthesis and its implications for apoptosis. In: Quinn PJ, Kagan PJ, editors. Phospholipid Metabolism in Apoptosis. New York: Kluwer Academic; 2002. pp. 207–227. [DOI] [PubMed] [Google Scholar]
- 75.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Rad Biol Med. 2001;31:717–728. doi: 10.1016/s0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 76.Birbes H, Bawab SE, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 2001;14:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 77.Haimovitz-Friedman A, Kolesnick RN, Fuks Z. Ceramide signaling in apoptosis. Br Med Bull. 1997;53:539–553. doi: 10.1093/oxfordjournals.bmb.a011629. [DOI] [PubMed] [Google Scholar]
- 78.Meng A, Luberto C, Meier P, Bai A, Yang X, Hannun YA, Zhou D. Sphingomyelin synthase as a potential target for D609-induced apoptosis in U937 humanmonocytic leukemia cells. Exp Cell Res. 2004;292:385–392. doi: 10.1016/j.yexcr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Ohta H, Sweeney EA, Masamune A, Yatomi Y, Hakomori SI, Igarashi Y. Induction of apoptosis by sphingosine in human leukemic HL-60 cells: A possible endogenous modulator of apoptotic DNA fragmentation occurring during phorbol ester-induced differentiation. Anal Biochem. 1994;222:489–494. [PubMed] [Google Scholar]
- 80.Panjarian S, Kozhaya L, Arayssi S, Bielawski YM, Usta J, Hannun YA, Obeid LM, Dbalbo GS. De novo N-palmitoylsphingosine synthesis is the major biochemical mechanism of ceramide accumulation following p53 up-regulation. Prostaglandins Other Lipid Mediat. 2008;86:41–48. doi: 10.1016/j.prostaglandins.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Voelker DR, Kennedy EP. Cellular and enzymatic synthesis of sphingomyelin. Biochem. 1992;21:2753–2759. doi: 10.1021/bi00540a027. [DOI] [PubMed] [Google Scholar]
- 82.Krishnamurthy K, Wang G, Siva J, Condie G, Bieberich E. Ceramide regulates atypical PKCzeta-mediated cell polarity in primitive ectoderm cells. J Biol Chem. 2007;282:3379–3390. doi: 10.1074/jbc.M607779200. [DOI] [PubMed] [Google Scholar]
- 83.Parmentier JH, Smelcer P, Pavicevic Z, Basic E, Idrizvic A, Estes A. PKC-zeta mediates norepinephrine-induced phospholipase D activation and cell proliferation in VSMC. Hypertension. 2003;41:794–800. doi: 10.1161/01.HYP.0000047873.76255.0B. [DOI] [PubMed] [Google Scholar]
- 84.Phillipson A, Petermann EE, Taormina P, Harvey M, Brue RJ, Atkinson N, Omiyi D, Chukwu C, Young LH. Protein kinase C-zeta inhibition exerts cardioprotective effects in ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;289:H898–H907. doi: 10.1152/ajpheart.00883.2003. [DOI] [PubMed] [Google Scholar]
- 85.Shin HG, Barnett JV, Chang P, Reddy S, Drinkwater DC, Pierson RN, Wiley RG, Murray KT. Molecular heterogeneity of protein kinase C expression in human ventricle. Cardiovasc Res. 2000;48:285–299. doi: 10.1016/s0008-6363(00)00185-1. [DOI] [PubMed] [Google Scholar]
- 86.Yang ZW, Gebrewold A, Nowakowski M, Altura BT, Altura BM. Mg2+ induced endothelial-dependent relaxation of blood vessels and blood pressure lowering: role of NO. Am J Physiol Regul Integr Comp Physiol. 2000;278:R628–R639. doi: 10.1152/ajpregu.2000.278.3.R628. [DOI] [PubMed] [Google Scholar]
- 87.Li JF, Li W, Altura BT, Altura BM. Peroxynitrite induces apoptosis and decline of intracellular free Mg2+ with concomitant elevation in [Ca2+] in rat aortic smooth muscle cells: possible roles of extracellular and intracellular magnesium ions in peroxynitrite-induced cell death. Drug Metab Lett. 2007;1:85–89. doi: 10.2174/187231207780363651. [DOI] [PubMed] [Google Scholar]
- 88.Tejero-taldo ML, Chmelinska JJ, Weglicki WB. Chronic dietary Mg2+ deficiency induces cardiac apoptosis in the rat heart. Magnes Res. 2007;20:208–212. [PubMed] [Google Scholar]
- 89.Campbell JH, Campbell GR. Smooth muscle phenotype modulation-A personal experience. Arterioscl Thromb Vasc Biol. 2012;32:1784–1789. doi: 10.1161/ATVBAHA.111.243212. [DOI] [PubMed] [Google Scholar]
- 90.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 91.Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci. 2002;17:105–109. doi: 10.1152/nips.01366.2001. [DOI] [PubMed] [Google Scholar]
- 92.Andreassi M. Coronary atherosclerosis and somatic mutations: an overview of the contributive factors for oxidative DNA damage. Mutat Res. 2003;543:67–86. doi: 10.1016/s1383-5742(02)00089-3. [DOI] [PubMed] [Google Scholar]
- 93.Mercer J, Mahmoudi M, Bennett M. DNA damage, p53, apoptosis and vascular disease. Mutat Res. 2007;621:75–86. doi: 10.1016/j.mrfmmm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 94.Rosannoff A. Magnesium supplements may enhance the effect of antihypertensive medications in stage 1 hypertensive subjects. Magnes Res. 2010;23:27–40. doi: 10.1684/mrh.2010.0198. [DOI] [PubMed] [Google Scholar]
- 95.Kobayashi J. On geographical relationship between the chemical nature of river water and death from apoplexy. Berichte des Ohara Inst fur Landwirtsch Biol. 1957;11:12–21. [Google Scholar]


