Abstract
Collapsin response mediator proteins (CRMPs) have been reported to control axonal guidance during neuronal development and degeneration. Among these proteins, CRMP-5 has been indicated to play an important role in growth cone development. However, the mechanisms underlying the linkage between growth cone development and the cytoskeleton remain to be elucidated. Here, we report that CRMP-5 interacts with tubulin to mediate growth cone development in cultured hippocampal neurons. We found that CRMP-5 physically interacted with tubulin in the growth cones of developing neurons. CRMP-5 colocalized with tubulin in lamellipodia in HEK293 cells and in the growth cones of cultured hippocampal neurons. Genetic silencing of CRMP-5 using RNA interference led to abnormal growth cone morphology in neurons. Overexpression of CRMP-5 led to significantly increased filopodial formation and enlarged growth cones. These results suggest that CRMP-5 interacts with tubulin to regulate growth cone dynamics, thus complying with the restrictive intracellular guidance cues.
Keywords: CRMP-5, tubulin, growth cone, hippocampal neuron
Introduction
During neural development, neurites undergo strictly defined routes and come into contact with precise target neurons, thus forming the functional neural circuit. Neuronal outgrowth and axonal guidance are highly regulated by extracellular and intracellular signaling mechanisms [1,2]. Neurites are tipped with motile growth cones that receive extracellular guidance cues to determine the extending direction [3]. However, how extracellular signals operate locally to control cytoskeletal elements within growth cones is not fully understood.
Collapsin response mediator proteins (CRMPs) are a family of five cytosolic proteins (CRMP 1-5) that are highly expressed in developing and adult nervous systems [4-6] and were originally identified as mediators of Semaphorin3A (Sema3A) signaling [7]. CRMP-5 was first identified as the CRMP-associated protein, designated CRAM, and it is the member of this protein family that has the lowest homology with other CRMP members [8]. CRMP-5 is highly expressed in post-mitotic neural precursors and the fasciculi of fibers in developing brains, but its expression decreases in adult brains [9]. Previous reports show that CRMP-5 can interact with tyrosine kinase Fes/Fps [10] and the mitochondrial protein septin [11], but the functional significance of these interactions remains unclear. The localization of CRMP-5 in the filopodia of growth cones suggests its role in mediating filopodial dynamics and growth cone development [12]. However, the detailed mechanisms of CRMP-5 regulation of growth cone development remain to be explored.
In this study, we found an interaction between tubulin and CRMP-5 using pull-down assays with the fusion protein GST-CRMP-5. When CRMP-5 and tubulin are coexpressed in HEK293 cells, these proteins will be immunoprecipitated together. Endogenous CRMP-5 and tubulin in neurons were also co-immunoprecipitated. CRMP-5 colocalized with tubulin in the lamellipodia of HEK293 cells and in the growth cones of cultured hippocampal neurons. Knockdown of CRMP-5 inhibited growth cone development, whereas overexpression of CRMP-5 enlarged growth cones. These findings suggest that CRMP-5 directly associated with tubulin, modulating the cytoskeleton to mediate growth cone development.
Materials and methods
Plasmids and constructs
Using PCR-based methods, the full-lengths of the cDNAs encoding rat CRMP-5 and tubulin were determined [13]. The full-length CRMP-5 cDNA was inserted into the pGEX-5x-3 (Amersham Pharmacia Biotech, Piscataway, NJ) and pCMV-Tag2 vectors (Stratagene, Santa Clara, CA). Tubulin cDNA was cloned into the pEGFP-C1 vector (Clontech, Mountain View, CA). All constructs were verified by sequencing. GST-CRMP-5 was used for GST fusion protein pull-down assays. GFP-tubulin and pCMV-Tag2-CRMP5 were used for co-expression experiments in HEK293 cells and neurons.
Cell culture and transfection
Hippocampi were dissected from postnatal rat pups (days 0 to 1, Sprague-Dawley), and dissociated hippocampal neurons were obtained using 0.125% trypsin and plated at a density of 1 × 104 cells/cm2 onto poly-D-lysine-coated glass coverslips. Cultures were maintained in Neurobasal-A medium containing 2% B27 and 0.5 mM glutamine supplement at 37°C in a 5% CO2 humidified incubator. One-half of the culture media was replaced every 3 days. Calcium phosphate transfections with different constructs were carried out on 9-10 days in vitro (DIV), and all experiments were performed on 11-12 DIV. Human embryonic kidney (HEK) 293 cells (a gift from Dr. Mingtao Li, Zhongshan School of Medicine, Sun Yat-Sen University, China) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and penicillin/streptomycin (Invitrogen, California, USA) in a 5% CO2 37°C incubator (Thermo, USA). Calcium phosphate was used to transfect the constructs into the HEK293 cells. Five micrograms of FLAG-CRMP-5 and GFP-tubulin (1:1) with the same ratio were used for immunoprecipitation assays. After transfection, cells were grown 36-48 h before harvesting.
Growth cone particle isolation
The methods were performed according to previous reports [14,15]. Briefly, brains were dissected from fetal rats at 18 days of gestation and homogenized by a Teflon-glass homogenizer in ~ 8 volumes (w/v) of 0.32 M sucrose containing 1 mM MgCl2, 1 mM Tes-NaOH, pH 7.3, and the following protease inhibitors: 3 I~M aprotinin (Calbiochem, San Diego, CA), 20 mM benzamidine, 1 mM leupeptin, 1 mM pepstatin A, 0.6 mM phenylmethylsulfonyl fluoride (all from Sigma). The homogenate was spun at 1300 r/min for 15 min. The low speed supernatant was loaded onto discontinuous sucrose density gradient consisting three layers: 0.75, 1.0 and 2.66 M; the gradients were spun to equilibrium at 35000 r/min for 200 min in a Beckman SW40Ti vertical rotor (Beckman Instruments, Palo Alto, CA). A-fraction was collected as growth cones for further analysis.
Recombinant protein expression and GST pull-down assay
GST fusion protein expression and pull-down assays were performed as previously described [16]. To purify GST-fused proteins, GST-CRMP-5 isoform constructs were transformed into the BL21 (DE3) strain of Escherichia coli (Invitrogen, Grand Island, NY). Production of fusion proteins was induced by incubation with 0.2 mmol/L isopropyl-1-thio-b-d-galac-topyranoside for 3 h at 30°C. Cells were spun down and resuspended in buffer containing (in mmol/L): 30 NaCl, 30 Tris, 0.2 EDTA, 1 DTT, pH 8.0, and a cocktail of protease inhibitors (Merck, Whitehouse Station, NJ). The cell suspension was treated with 0.1% lysozyme followed by 0.5% deoxycholic acid and placed on ice for 20 min. After sonication, the cell debris was removed by centrifugation (15,000 g for 30 min). Triton X-100 (1%) was added to the supernatant, and the GST fusion proteins were purified from this solution using glutathione-Sepharose beads.
Western blotting and antibodies
Western blot analysis was performed as previously described [17]. Briefly, lysates were separated using SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane. Membranes were blocked in Tris-buffered saline with 5% milk and 0.05% Tween and probed with primary antibodies at 4°C overnight. Antibodies against CRMP-5 and GFP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); FLAG and tubulin were purchased from Sigma (St. Louis, MO, USA). After washing, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit secondary antibodies (Jackson ImmunoResearch, West Grove, PA) and visualized using the ECL reagents.
Immunoprecipitation
Immunoprecipitation (IP) assays were performed as described previously [17,18]. For immunoprecipitation of hippocampal neurons, extracts were prepared by solubilization in 400 μl of cell lysis buffer (1% Triton X-100, 150 mM NaCl, 20 mM Tris-Cl (pH 7.4), 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 2.5 mM pyrophosphate, 1 mM glycerol phosphate, and protease inhibitor mixture) for 10 min at 4°C. After brief sonication, the lysates were cleared by centrifugation at 15,000 × g for 10 min at 4°C, the cell extract was immunoprecipitated with 4 μg of antibodies against CRMP-5 (Santa Cruz) or tubulin (Sigma), and then the samples were incubated with 60 μl of protein G plus protein A-agarose for 16 h at 4°C by continuous inversion. Immunocomplexes were pelleted and washed three times. The precipitated immunocomplexes were boiled in Laemmli buffer and assayed using Western blot analysis with anti-CRMP-5 or anti-tubulin antibodies. For HEK293 cell immunoprecipitation, 2.5 μg of GFP-tubulin and 2.5 μg of FLAG-CRMP-5 were co-transfected into HEK293 cells. Twenty-four hours after transfection, cells were lysed and immunoprecipitated with 2 μg of GFP (Santa Cruz) or FLAG (Sigma) antibodies. The precipitated immunocomplexes were assayed using Western blot analysis with anti-GFP or anti-FLAG antibodies.
Immunofluorescence
Hippocampal neurons or HEK293 cells were grown on coverslips (Fisher) and processed for immunofluorescence according to the standard protocol described previously [19]. Cells were fixed with 4% (w/v) paraformaldehyde (Sigma, St. Louis, MO) for 5 min at room temperature and permeabilized with 0.1% Triton X-100 in PBS for 20 min. The cells were blocked in 3% normal donkey serum in TBS + 0.1% Triton X-100 for 1 h at room temperature and incubated with rabbit anti-CRMP-5 antibody (Santa Cruz) and mouse anti-tubulin (Sigma) or anti-FLAG tag antibody (Sigma) at 4°C overnight. The cells were washed 3 times for 10 min with PBS + 0.1% Tween20, and incubated with monoclonal donkey anti-rabbit IgG Dylight 549 (Jackson ImmunoResearch) or monoclonal donkey anti-mouse IgG Dylight 488 (Jackson ImmunoResearch) for 2 h at room temperature. After three washes, cells were mounted on glass slides with Fluoro Gel II containing DAPI (EMS, Hatfield, PA). Microscopy and image analysis were carried out using the same optical slice thickness for every channel (488-nm laser 1 AU=0.7 μm, 543-nm laser 0.71 AU=0.7 μm) using a confocal microscope (LSM 710; Carl Zeiss, Germany).
RNA interference
A validated CRMP-5 siRNA (siCRMP-5) fragment and NC (scrambled sequence, negative control) were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China) [12,13]. To determine the efficacy and specificity of the siRNA, co-transfection was performed using NC or siCRMP-5 together with rat FLAG-CRMP-5 plasmids into HEK293 cells. Expression of FLAG-CRMP-5 protein was examined by Western blot analysis using a FLAG antibody. Hippocampal neurons were transfected with siRNA using a calcium phosphate protocol [19]. To transfect neurons in 24-well tissue culture plates, 100 pmol of siRNA was combined with 37 μl of 2 M CaCl2 solution in sterile, deionized water to a final volume of 300 μl and then mixed well with 300 μl of 2 × HEPES-buffered saline. The mixtures were vortexed and incubated at 25°C for approximately 4 min. In each well, 30 μl mixture was added drop-wise to the cells and allowed to incubate for another 25 min. The GFP expression plasmid was co-transfected with the siRNAs to mark the transfected cells. Transfection efficiency in the neurons was determined by calculating the percentages of GFP-positive cells out of the total cell numbers. The total number of neurons counted for each treatment group was more than 100.
Statistical analysis
Data are presented as the mean ± SEM. Significant differences were assessed with one-way ANOVA followed by Bonferroni or Tamhane post hoc tests. P<0.05 was considered to be statistically significant.
Results
CRMP-5 interacts with tubulin in growth cones
CRMP-5 has been reported to be distributed in the filopodia of growth cones in a manner that is independent of the filamentous actin [12]; thus, functional roles for CRMP-5 binding proteins within growth cones remain to be explored. In mouse brain extracts, MAP2 and tubulin associate with CRMP-5 [13]. Thus, we asked whether CRMP-5 would interact with tubulin in the filopodia and promote growth cone development. To test this idea, reciprocal co-expression/immunoprecipitation experiments were conducted using lysates of HEK293 cells co-transfected with FLAG-CRMP-5 and GFP-Tubulin. We detected FLAG-CRMP-5 signals in the complexes associated with GFP antibody but not with the rabbit IgG (Figure 1A). Similarly GFP-Tubulin was also detected in the complex associated with FLAG antibody, but not the mouse IgG (Figure 1B). These results are consistent with a previous report [13]. We next applied a GST pull-down assay. Growth cones from rat brain extracts were collected and enriched as previously described [14,15]. As shown in Figure 1C, recombinant GST-CRMP-5 was expressed and purified. Tubulin was detected in the lysates of enriched rat brain growth cones using GST-CRMP-5 immobilized on glutathione-Sepharose beads (Figure 1D), suggesting their co-assembly in vivo. To obtain additional biochemical evidence for an in vivo interaction between CRMP-5 and tubulin in growth cones, a co-immunoprecipitation assay was carried out. Growth cone lysates were incubated with the CRMP-5 antibody. The immune complex was purified and immunoblotted with the tubulin antibody. As shown in Figure 1E, tubulin was detected in complexes immunoprecipitated by the CRMP-5 antibody. Moreover, CRMP-5 was also present in the complexes immunoprecipitated by the tubulin antibody (Figure 1F). These data indicated that CRMP-5 physically interacts with tubulin in growth cones.
Figure 1.
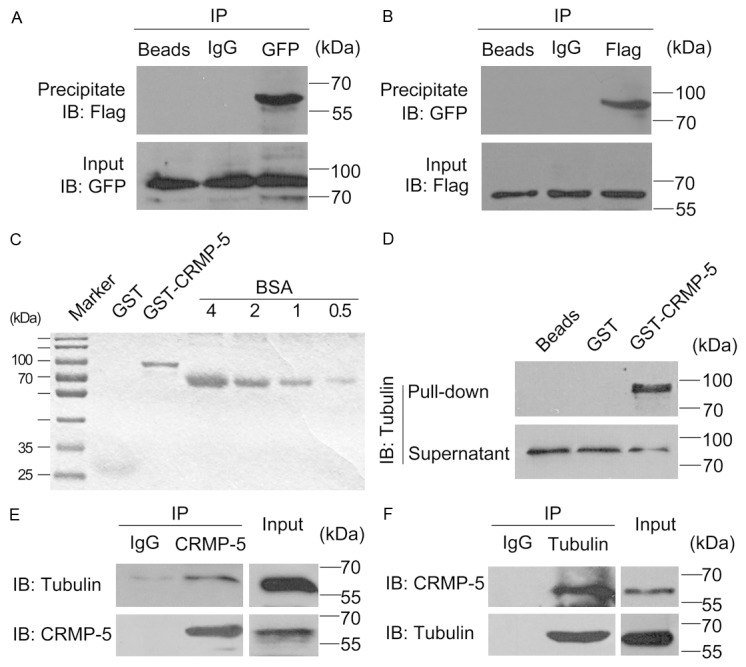
CRMP-5 physically interacts with tubulin. A, B: FLAG-CRMP-5 and GFP-Tubulin were transiently transfected into HEK293 cells. FLAG-CRMP-5 or GFP-Tubulin was immunoprecipitated with FLAG or GFP antibodies tagged with, respectively, in samples containing equal amounts of protein. Immunoprecipitates were separated by SDS-PAGE and detected by Western blot. A: Blot results were obtained using an anti-GFP antibody for the IP assay, and the blots were detected with anti-Flag or anti-GFP antibodies. B: Blot results obtained using and an anti-Flag antibody for the IP assay and detected with an anti-Flag or anti-GFP antibody. Similar results were obtained in three independent experiments. C: GST-CRMP-5 expression and purification. D: Pull-down of tubulin from enriched brain growth cone lysates by GST or GST-CRMP-5 conjugated to glutathione-Sepharose beads. Western blots of the pellet were probed for tubulin immunoreactivity. Each result is representative of three to five separate experiments with similar results. E: Tubulin was detected in complexes immunoprecipitated by the CRMP-5 antibody. F: CRMP-5 was also present in the complexes immunoprecipitated by the tubulin antibody.
CRMP-5 colocalizes with tubulin at the lamellipodia of HEK293 cells and at growth cones in hippocampal neurons
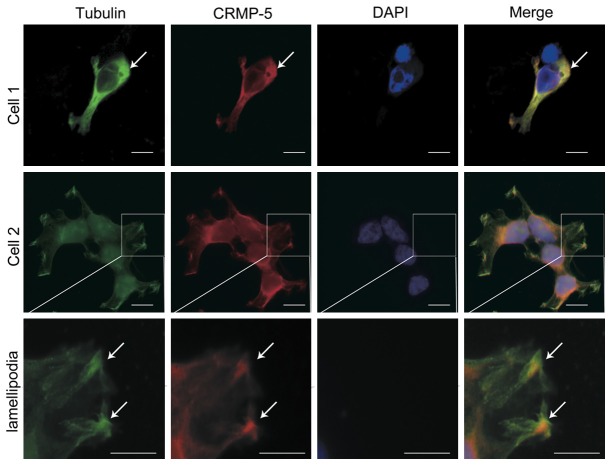
To visualize the distribution of CRMP-5 and tubulin, immunofluorescence was used. In HEK293 cells immuno-labeled anti-CRMP-5 and anti-tubulin antibodies, the immunofluorescence showed that both CRMP-5 and tubulin were distributed in the cytoplasm but not in the nucleus. Moreover, CRMP-5 and tubulin immunofluorescence signals were intense at the tips of the lamellipodia. CRMP-5 was clearly colocalized with tubulin in the cytoplasm and tips of the lamellipodia (Figure 2). In hippocampal neurons, most of the CRMP-5 and tubulin immunofluorescence was distributed throughout the cells (Figure 3A). Detailed confocal microscopic analysis of the growth cones showed that CRMP-5 immunofluorescence was distributed mostly in the C-domain, revealing that CRMP-5 signaling colocalized with tubulin signaling (Figure 3B). These data indicated that CRMP-5 colocalized with tubulin in lamellipodia in HEK293 cells and in growth cones in cultured hippocampal neurons.
Figure 2.
Colocalization of CRMP-5 and tubulin in HEK293 cells. Anti-tubulin and anti-CRMP-5 were used to detect endogenous tubulin and CRMP-5 proteins in HEK293 cells; DAPI was used to stain the nuclei. The merged images show the colocalization (yellow spots) of tubulin (green) with CRMP-5 (red). Enlarged images of cells show details of the lamellipodia. Scale bar, 20 μm.
Figure 3.
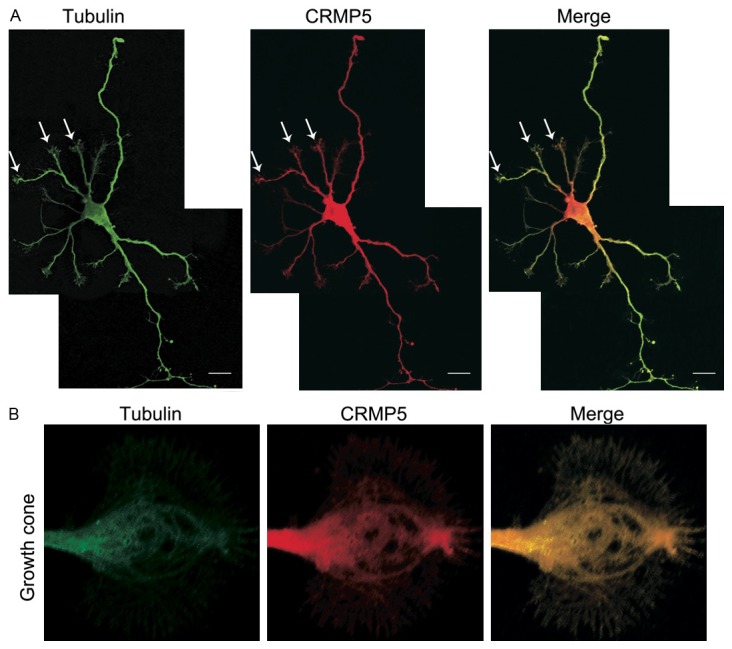
Colocalization of CRMP-5 and tubulin in hippocampal neurons. A: Anti-tubulin and anti-CRMP-5 antibodies were used to detect endogenous tubulin and CRMP-5 proteins, respectively, in hippocampal neurons cultured for 72 h. The merged images show the colocalization (yellow) of tubulin (green) and CRMP-5 (red). B: Confocal microscope scans of growth cones in cultured hippocampal neurons. The merged images show the colocalization (yellow) of tubulin (green) and CRMP-5 (red). Scale bar, 10 μm.
CRMP-5 is critical for growth cone development in cultured hippocampal neurons
The localization of CRMP-5 in growth cones encouraged us to investigate the role of CRMP-5 in growth cone development. First, we studied growth cone development after CRMP-5 knockdown using siRNA. The target sequence used against CRMP-5 for these experiments was selected from previous reports [12,13]. FLAG-CRMP-5 together with siRNA fragments or NC was co-transfected into HEK293 cells to confirm the siRNA efficacy. As shown in Figure 4C, the FLAG antibody revealed the FLAG-CRMP-5 protein, demonstrating that transfection with CRMP-5-targeted siRNAs significantly suppressed the expression of FLAG-CRMP-5, whereas the level of FLAG-CRMP-5 in the NC group or the level of an unrelated gene (GAPDH) was unaffected. In hippocampal neurons, the use of this siRNA fragment also resulted in a 60-80% knockdown of endogenous CRMP-5 (Figure 4A). After transfection with CRMP-5 siRNAs, we observed that silencing CRMP-5 impaired growth cone development in neurons. The average area of the growth cone in the CRMP-5 siRNA group was reduced by approximately 50%. To further understand the effect of CRMP-5 expression on growth cone morphology, FLAG-CRMP-5 was overexpressed in hippocampal neurons. As shown in Figure 5A, anti-FLAG immunoblotting signals showed clear expression of CRMP-5 after transfection of the FLAG-CRMP-5 plasmid but not with the control vector. When FLAG-CRMP-5 was co-transfected, neurons exhibited enlarged growth cones compared to the control. The average area of the growth cone was markedly increased (Figure 5B, 5C). Taken together, these results suggest that CRMP-5 is necessary and sufficient for neuronal growth cone development, which is consistent with a previous report [12].
Figure 4.
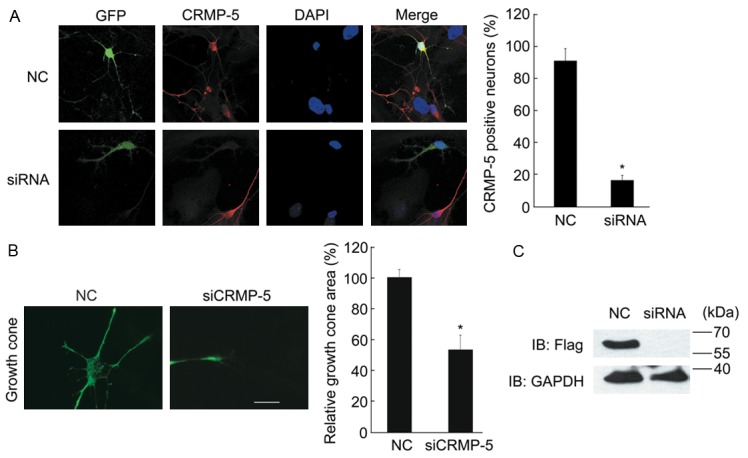
CRMP-5 is necessary for growth cone development in cultured hippocampal neurons. A: Hippocampal neurons cultured 48 h were co-transfected with scrambled siRNA (NC) or CRMP-5-siRNA together with a GFP-encoding plasmid; after 24 h, the neurons were fixed and subjected to the immunocytochemistry protocol. Endogenous CRMP-5 was detected by an anti-CRMP-5 antibody. Typical images of the CRMP-5 immunostaining are shown (left panel). A non-targeting siRNA was used as the negative control (NC). The percentages of CRMP-5-positive neurons with each treatment were quantified (right panel) as the mean ± SEM for three independent experiments. *Denotes P<0.05; Scale bar, 10 μm. B: Typical growth cone morphology in neurons with each treatment (left panel). The growth cone area of transfected cells was determined and plotted. Comparisons were made using one-way ANOVA. *Denotes P<0.05 (n=30-40 cells from 3 independent experiments). Error bars indicate SEM. Scale bar, 10 μm. C: HEK293 cells were co-transfected with scrambled siRNA (NC) or siCRMP5 together with the FLAG-CRMP-5 expression plasmid. Lysates were probed with anti-CRMP5 antibody. The GAPDH antibody was used as a loading control.
Figure 5.

CRMP-5 is sufficient for growth cone development in cultured hippocampal neurons. A: HEK293 cells were transfected with the FLAG-CRMP-5 expression plasmid or the control vector. CRMP-5 expression was detected by immunoblot using an anti-FLAG antibody. The GAPDH antibody was used as a loading control. B: Representative images of growth cones from neurons transfected with the FLAG-CRMP-5 expression plasmid or the control vector are shown. The growth cone area of transfected cells was measured and plotted. C: Comparisons were made using one-way ANOVA. Data are shown as the mean ± SEM from three independent experiments. *Denotes P<0.05; Scale bar, 10 μm.
Discussion
CRMPs are reported to function in axonal guidance, neuronal polarity and dendritic growth. A previous report shows that CRMP-5, an isoform distinct from the other four CRMPs, localizes in the filopodia of growth cones, independently from actin assembly [12], leaving the role of CRMP-5-associated proteins in regulating growth cones to be further elucidated. All of the CRMP proteins (CRMP 1-5) have been implicated to associate with tubulin [13,20]. Thus, in this study, we provided evidence for a CRMP-5 interaction with tubulin to mediate growth cone development. We enriched growth cones from hippocampal extracts, and using GST pull-down and co-immunoprecipitation; we found that CRMP-5 directly interacts with tubulin. Furthermore, in HEK293 cells, CRMP-5 colocalized with tubulin in the growing lamellipodia cells and also in the developing growth cones in hippocampal neurons. Genetic knockdown of endogenous CRMP-5 disrupted growth cone formation, whereas the overexpression of CRMP-5 enlarged growth cones, indicating a critical role for CRMP-5 in regulating growth cone development.
In the CRMP family of proteins, the role of CRMP-2 in regulating axonal elongation and neuronal polarity has been studied extensively. CRMP-2 localizes in the growth cones of hippocampal neurons at stage 3, and the overexpression of CRMP-2 promotes axonal growth [21,22]. The expression of CRMP-5 is high in fetal and neonatal rat brains, and its expression decreases to very low levels in adult brains [8]. The spatiotemporal distribution of CRMP-5 in cultured hippocampal neurons varies at different developmental stages in the dendrites and axons. At stage 3 after neuronal polarity occurs, CRMP-5 is present in growth cones. After stage 5, neurons display a polarized morphology and CRMP-5 signaling decreases to low levels. The distribution of CRMP-5 contributes to the dynamic regulation of the development of dendrites, axons and neuronal polarity [13]. Our results are consistent with a previous report [12] showing that CRMP-5 distributed in the growth cone is necessary and sufficient for growth cone development. Our results suggest that CRMP-5 functions through its interaction with tubulin. In hippocampal neurons, CRMP-5 has been reported to inhibit dendritic growth [13]. However, in cerebellar Purkinje cells, CRMP-5 promotes dendritic development and synaptic plasticity, as shown in studies using crmp5-/- mice, where aberrant dendrite morphology is revealed [23]. The differences in the results from these previous studies suggest that CRMP-5 actions may be cell-type specific or may be due to differences in its efferent distribution. These suggestions are worthy of further investigation. Taken together, we have provided new evidence for CRMP-5 regulation of growth cone development.
Acknowledgements
The authors declare no conflict of interest. This work was supported by the National Natural Science Foundation of China (No. 31170941), the Fundamental Research Funds for the Central Universities (No. 21612424) and the Scientific Research and Training Foundation of the First Affiliated Hospital of Jinan University (No. 2013101).
Disclosure of conflict of interest
None.
References
- 1.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 2.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 3.Nieto MA. Molecular biology of axon guidance. Neuron. 1996;17:1039–1048. doi: 10.1016/s0896-6273(00)80237-8. [DOI] [PubMed] [Google Scholar]
- 4.Minturn JE, Fryer HJ, Geschwind DH, Hockfield S. TOAD-64, a gene expressed early in neuronal differentiation in the rat, is related to unc-33, a C. elegans gene involved in axon outgrowth. J Neurosci. 1995;15:6757–6766. doi: 10.1523/JNEUROSCI.15-10-06757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukada M, Watakabe I, Yuasa-Kawada J, Kawachi H, Kuroiwa A, Matsuda Y, Noda M. Molecular characterization of CRMP5, a novel member of the collapsin response mediator protein family. J Biol Chem. 2000;275:37957–37965. doi: 10.1074/jbc.M003277200. [DOI] [PubMed] [Google Scholar]
- 6.Yuasa-Kawada J, Suzuki R, Kano F, Ohkawara T, Murata M, Noda M. Axonal morphogenesis controlled by antagonistic roles of two CRMP subtypes in microtubule organization. Eur J Neurosci. 2003;17:2329–2343. doi: 10.1046/j.1460-9568.2003.02664.x. [DOI] [PubMed] [Google Scholar]
- 7.Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 8.Inatome R, Tsujimura T, Hitomi T, Mitsui N, Hermann P, Kuroda S, Yamamura H, Yanagi S. Identification of CRAM, a novel unc-33 gene family protein that associates with CRMP3 and protein-tyrosine kinase(s) in the developing rat brain. J Biol Chem. 2000;275:27291–27302. doi: 10.1074/jbc.M910126199. [DOI] [PubMed] [Google Scholar]
- 9.Ricard D, Rogemond V, Charrier E, Aguera M, Bagnard D, Belin MF, Thomasset N, Honnorat J. Isolation and expression pattern of human Unc-33-like phosphoprotein 6/collapsin response mediator protein 5 (Ulip6/CRMP5): coexistence with Ulip2/CRMP2 in Sema3a- sensitive oligodendrocytes. J Neurosci. 2001;21:7203–7214. doi: 10.1523/JNEUROSCI.21-18-07203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsui N, Inatome R, Takahashi S, Goshima Y, Yamamura H, Yanagi S. Involvement of Fes/Fps tyrosine kinase in semaphorin3A signaling. EMBO J. 2002;21:3274–3285. doi: 10.1093/emboj/cdf328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi S, Inatome R, Yamamura H, Yanagi S. Isolation and expression of a novel mitochondrial septin that interacts with CRMP/CRAM in the developing neurones. Genes Cells. 2003;8:81–93. doi: 10.1046/j.1365-2443.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- 12.Hotta A, Inatome R, Yuasa-Kawada J, Qin Q, Yamamura H, Yanagi S. Critical role of collapsin response mediator protein-associated molecule CRAM for filopodia and growth cone development in neurons. Mol Biol Cell. 2005;16:32–39. doi: 10.1091/mbc.E04-08-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brot S, Rogemond V, Perrot V, Chounlamountri N, Auger C, Honnorat J, Moradi-Ameli M. CRMP5 interacts with tubulin to inhibit neurite outgrowth, thereby modulating the function of CRMP2. J Neurosci. 2010;30:10639–10654. doi: 10.1523/JNEUROSCI.0059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfenninger KH, Ellis L, Johnson MP, Friedman LB, Somlo S. Nerve growth cones isolated from fetal rat brain: subcellular fractionation and characterization. Cell. 1983;35:573–584. doi: 10.1016/0092-8674(83)90191-5. [DOI] [PubMed] [Google Scholar]
- 15.Lohse K, Helmke SM, Wood MR, Quiroga S, de la Houssaye BA, Miller VE, Negre-Aminou P, Pfenninger KH. Axonal origin and purity of growth cones isolated from fetal rat brain. Brain Res Dev Brain Res. 1996;96:83–96. doi: 10.1016/0165-3806(96)00076-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Deng L, Maeno-Hikichi Y, Lai M, Chang S, Chen G, Zhang JF. Formation of an endophilin-Ca2+ channel complex is critical for clathrin-mediated synaptic vesicle endocytosis. Cell. 2003;115:37–48. doi: 10.1016/s0092-8674(03)00726-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Fan J, Tian Q, Song Z, Zhang JF, Chen Y. Characterization of two distinct modes of endophilin in clathrin-mediated endocytosis. Cell Signal. 2012;24:2043–2050. doi: 10.1016/j.cellsig.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Tian Q, Zhang JF, Fan J, Song Z, Chen Y. Endophilin isoforms have distinct characteristics in interactions with N-type Ca2+ channels and dynamin I. Neurosci Bull. 2012;28:483–492. doi: 10.1007/s12264-012-1257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan M, Ma S, Huang Q, Hu K, Song B, Li M. GSK-3alpha/beta-mediated phosphorylation of CRMP-2 regulates activity-dependent dendritic growth. J Neurochem. 2013;125:685–697. doi: 10.1111/jnc.12230. [DOI] [PubMed] [Google Scholar]
- 20.Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4:583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Arimura N, Menager C, Fukata Y, Kaibuchi K. Role of CRMP-2 in neuronal polarity. J Neurobiol. 2004;58:34–47. doi: 10.1002/neu.10269. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita N, Mosinger B, Roy A, Miyazaki M, Ugajin K, Nakamura F, Sasaki Y, Yamaguchi K, Kolattukudy P, Goshima Y. CRMP5 (collapsin response mediator protein 5) regulates dendritic development and synaptic plasticity in the cerebellar Purkinje cells. J Neurosci. 2011;31:1773–1779. doi: 10.1523/JNEUROSCI.5337-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]