Abstract
The aim of this study was to measure changes in the cross-sectional area of the spinal canal and the area of the intervertebral foramen for each pedicle segment before and after the pedicle extension using computer-simulated transpedicular osteotomy to provide a theoretical basis for clinical decompression in the lumbar spinal canal. Using spiral CT scanning of the original lumbar spine, a finite element model was established. The pedicle was cut and extended by 2 mm, 4 mm, 6 mm, and 8 mm for respective modeling. The changes in the area of each plane of the vertebral canal and the area of the intervertebral foramen were measured. With the gradual extension of the pedicle, the areas of the spinal canal and intervertebral foramen also significantly increased compared with those of the original lumbar spine (P<0.05). The extension of the pedicle using transpedicular osteotomy can significantly increase the cross-sectional area of the lumbar canal and the area of the intervertebral foramen. This finding provides a new theoretically practicable method for the clinical decompression of the lumbar spinal canal.
Keywords: Spinal stenosis, pedicle length, finite element method, osteotomy
Introduction
In 1949, Verbiest first proposed the concept of stenosis in spinal canal, nerve root canal, and neural foramen; that is, lumbar spinal stenosis (LSS) [1]. Recent epidemiological studies have shown that the incidence of lumbar spinal stenosis is approximately 5.7% and increases with age, especially among 70 to 79-year-olds [2]. A narrow spinal canal or spinal lateral stenosis can compress the central canal spinal cord, cauda equina, or nerve root, causing axonal disruption as a result of the pressure, neurohormone function disorders, and expansion of nerve sheath, resulting in the obstruction of blood flow, venous restriction, tissue hypoxia, and localized stasis, which stimulate the nerve endings and generate the symptoms of low back pain.
The purpose of surgical treatment for lumbar spinal stenosis is to relieve nerve compression, ease back pain and increase spinal stability. The most common surgical method currently used is laminectomy, with complete decompression; this technique has a 5-year follow-up effective rate of 81.6% [3]. However, the technique is associated with considerable trauma, postoperative spinal instability, degeneration acceleration near the segment, and nerve adhesion [4]. In recent years, the newly developed surgical method of X-STOP interspinous dynamic stabilization system has been shown to significantly reduce the surgical trauma. Studies have shown that it can increase the anteroposterior diameter of the spinal canal by 18% and increase the intervertebral foramen area by 25% [5], but clinical efficacy studies with mid- and long-term follow-up are still lacking. We proposed a minimally invasive surgical technique to enlarge the spinal canal and intervertebral foramen area using percutaneous pedicle osteotomy laminoplasty to treat the spinal stenosis.
The volumes of lumbar spine vary greatly among individuals. For those with larger spinal canal volumes, degenerative changes may not cause spinal stenosis because of the larger space in the canal, whereas those with smaller spinal canal volumes may experience spinal stenosis after mild degenerative changes. Currently, there are few relevant studies of the relationship between the spinal pedicle length and the cross-sectional area or the intervertebral foramen area. In this study, CT scanning images and professional engineering software were used to establish a human lumbar spine model with a realistic appearance and accurate calculations, and the impact of the pedicle length on the spinal canal and intervertebral foramen area was analyzed and evaluated.
Materials and methods
Raw data collection
A 35-year-old healthy male volunteer was selected, and bone abnormalities were excluded using X-ray examination. Ll-L5 were continuously scanned along transects using a Philips 64-slice spiral CT. The scanning conditions were as follows: selected bone tissue window, voltage 120 kV, pixel 0.43 mm, layer distance 0.65 mm, and a total of 210 layers. The scanning data were directly saved on a disc using Dicom 3.0 Standard for later use.
Computer configuration of the modeling environment
The computer system included an Intel Xeon CPU with 2 G memory, a 19-inch LCD monitor, 64 M video memory, an ATI X550 video card, and Windows XP/Professional operating system. The software included Medical 3D image generation, Mimics 10.01 editing software, and reverse engineering software Geomagic Studio 10.
Establishment and measurement of the model
The CT images in Dicom format were directly read using Mimics software (Figure 1A). After positioning and organizing the images and processing the interpolation, the lumbar spine bone tissues were segmented for regional growth. A three-dimensional geometric surface mesh model for the thoracolumbar spine was established using 3D calculation; the generated surface mesh model was imported into Geomagic Studio 10 to establish the preliminary three-dimensional digital model and meshed after the appropriate unit type was selected and the appropriate constants were defined (Figure 1B). After the model was meshed with grid, it was broken into points to generate the cloud map (Figure 1C). After readjustment, a polygonal three-dimensional map was obtained. After local modification, a complete three-dimensional polygon model of the lumbar spine was obtained (Figure 1D). The model position was adjusted (Figure 2A, 2E) to locate each lumbar pedicle (Figure 2B, 2F), which was truncated to extend rearward by 2 mm, 4 mm, 6 mm, and 8 mm with successive modeling to form four lumbar spine models with successive increments of pedicle lengths (Figure 2C, 2G). The area of the intervertebral foramen was measured for each model (Figure 2G). At the same time, the L1-L5 vertebrae were segmented along the cross-section of the lumbar vertebrae to expose the canal plane of each vertebra (Figure 2D), and the areas were successively measured.
Figure 1.
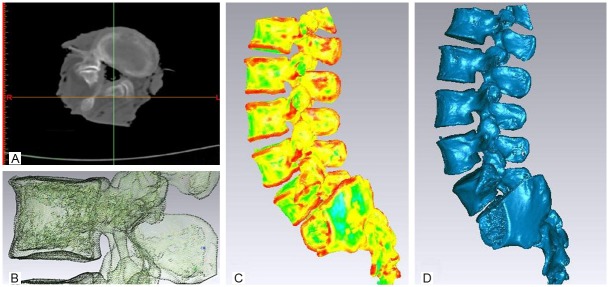
Model Construction. A: The CT images in Dicom format. B: A three-dimensional geometric surface mesh model. C: The model was broken into points to generate the cloud map. D: A complete three-dimensional polygon lumbar model.
Figure 2.
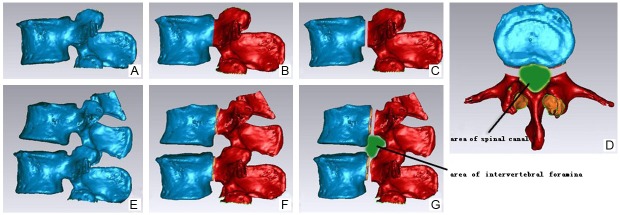
Pedicle Lengthening and Area Measurement. A and E: The model position was adjusted in standard sagittal position. B and F: The root of each lumbar pedicle was located. C and G: The lumbar pedicle was truncated to extend rearward. And the area of the intervertebral foramen was measured. D: The cross-sectional areas of lumbar canal were measured after pedicle lengthening.
Statistical analysis
All data were analyzed by the first author using the SPSS 19.0 software package (SPSS, Inc., Chicago, IL, USA) using a t-test for paired samples for the statistical analysis. P<0.05 was considered statistically significant.
Results
This study is based on CT images in Dicom format. Using medical image processing and software for reverse analysis, L1-S1 models with successive increment of pedicle length were established. The cross-sectional canal area of each vertebra in L1-L5 (Table 1) and the area of the intervertebral foramen in L1-S1 (Table 2) were measured.
Table 1.
The Change on Canal Area of Each Vertebra in L1-L5
| Pedicle Lengthening (mm) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Control | 2 mm | 4 mm | 6 mm | 8 mm | |
| L1 Canal Area (mm2) | 289.5718 | 314.1795 | 339.0393 | 356.6109 | 441.4728 |
| Percent of Increase | - | 108.50% | 117.08% | 123.15% | 152.46% |
| L2 Canal Area (mm2) | 305.4862 | 358.5207 | 408.223 | 457.4879 | 507.2519 |
| Percent of Increase | - | 117.36% | 133.63% | 149.76% | 166.05% |
| L3 Canal Area (mm2) | 318.6712 | 363.5621 | 414.7315 | 465.7756 | 516.9144 |
| Percent of Increase | - | 114.09% | 130.14% | 146.16% | 162.21% |
| L4 Canal Area (mm2) | 331.5416 | 372.5684 | 425.7357 | 479.0196 | 532.2568 |
| Percent of Increase | - | 112.37% | 128.41% | 144.48% | 160.54% |
| L5 Canal Area (mm2) | 438.2487 | 513.3594 | 580.7175 | 648.3467 | 714.8475 |
| Percent of Increase | - | 117.14% | 132.51% | 147.94% | 163.11% |
Table 2.
The Change on Area of Intervertebral Foramen in L1-S1
| Pedicle Lengthening (mm) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Control | 2 mm | 4 mm | 6 mm | 8 mm | |
| L1-2 Left Area (mm2) | 53.83247 | 68.84867 | 83.86486 | 98.88106 | 113.8973 |
| Percent of Increase | - | 127.89% | 155.79% | 183.68% | 211.58% |
| L1-2 Right Area (mm2) | 77.66435 | 101.2815 | 124.8987 | 148.5159 | 172.1331 |
| Percent of Increase | - | 130.41% | 160.82% | 191.23% | 221.64% |
| L2-3 Left Area (mm2) | 51.00065 | 70.98665 | 90.9726 | 110.9587 | 130.9447 |
| Percent of Increase | - | 139.19% | 178.38% | 217.56% | 256.75% |
| L2-3 Right Area (mm2) | 66.40518 | 89.41898 | 112.4328 | 135.4466 | 158.4604 |
| Percent of Increase | - | 134.66% | 169.31% | 203.97% | 238.63% |
| L3-4 Left Area (mm2) | 83.37431 | 108.0263 | 132.6783 | 157.3303 | 181.9823 |
| Percent of Increase | - | 129.57% | 159.14% | 188.70% | 218.27% |
| L3-4 Right Area (mm2) | 72.20558 | 96.59358 | 120.9816 | 145.3696 | 169.7576 |
| Percent of Increase | - | 133.78% | 167.55% | 201.33% | 235.10% |
| L4-5 Left Area (mm2) | 105.2215 | 133.7822 | 162.3429 | 190.9037 | 219.4644 |
| Percent of Increase | - | 127.14% | 154.29% | 181.43% | 208.57% |
| L4-5 Right Area (mm2) | 88.44273 | 115.5217 | 142.6006 | 169.6795 | 196.7584 |
| Percent of Increase | - | 130.62% | 161.23% | 191.85% | 222.47% |
| L5-S1 Left Area (mm2) | 64.88391 | 86.40991 | 107.9359 | 129.4619 | 150.9879 |
| Percent of Increase | - | 133.18% | 166.35% | 199.53% | 232.70% |
| L5-S1 Right Area (mm2) | 42.05878 | 62.26079 | 82.46279 | 102.6648 | 122.8668 |
| Percent of Increase | - | 148.03% | 196.07% | 244.10% | 292.13% |
When the pedicle length of each vertebrae increased by 2 mm, the area of spinal canal increased by 47.73 mm2 on average (24.61 mm2-75.11 mm2), with t=-5.777 and P<0.01 compared with the areas before the extension, and the spinal canal increased by 13.89% on average (8.50%-17.36%). When the pedicle length of each vertebrae increased by 4 mm, the spinal canal area increased by 96.99 mm2 on average (49.47 mm2-142.47 mm2), with t=-6.562 and P<0.01 compared with the areas before the extension, and the spinal canal increased by 28.35% on average (17.08%-33.63%). When the pedicle length of each vertebrae increased by 6 mm, the spinal canal area increased by 144.74 mm2 on average (67.04 mm2-210.10 mm2), with t=-6.355 and P<0.01 compared with the area before the extension, and the spinal canal increased by 42.30% on average (23.15%-49.76%); When the pedicle length of each vertebrae increased by 8 mm, the spinal canal area increased by 205.84 mm2 on average (151.90 mm2-276.60 mm2), with t=-10.281 and P<0.01 compared with the area before the extension, and the spinal canal increased by 60.87% on average (52.46%-66.05%).
When the pedicle length of each vertebrae increased by 2 mm, the area of the intervertebral foramen increased by 22.80 mm2 on average (15.02 mm2-28.56 mm2), with t=-18.617 and P<0.01 compared with the area before the extension, and the intervertebral foramen increased by 33.46% on average (27.14%-48.03%). When the pedicle length of each vertebrae increased by 4 mm, the area of the intervertebral foramen increased by 45.61 mm2 on average (30.03 mm2-57.12 mm2), with t=-18.617 and P<0.01 compared with the area before the extension, and the intervertebral foramen increased by 66.89% on average (54.29%-96.07%). When the pedicle length of each vertebrae increased by 6 mm, the area of the intervertebral foramen increased by 68.41 mm2 on average (45.05 mm2-85.68 mm2), with t=-18.617 and P<0.01 compared with the area before the extension, and the intervertebral foramen increased by 100.34% on average (81.43%-144.10%). When the pedicle length of each vertebrae increased by 8 mm, the area of the intervertebral foramen increased by 91.22 mm2 on average (60.06 mm2-114.24 mm2), with t=-18.617 and P<0.01 compared with the area before the extension, and the intervertebral foramen increased by 133.79% on average (108.57%-192.13%).
Discussion
Currently, the most common clinical surgical treatments for symptomatic lumbar spinal stenosis are laminectomy and decompression. The traditional decompression surgery removes part of the lamina, the yellow ligaments, and facet joints. When spondylolisthesis or scoliosis occurs, interbody fusion is needed. Traditional decompressive laminectomy can thoroughly decompress and is still the most widely used standard procedure. However, it may cause a series of intraoperative and postoperative complications, including large surgical trauma, blood loss, slow recovery, postoperative spinal instability, residual back pain, accelerated degeneration in adjacent segments, and scar adhesions with recurring neurological symptoms that require further surgery. Turner et al. [6] showed an excellent success rate of 64% after decompression based on a meta-analysis, and Jansson et al. [7] indicated that the reoperation rate within 10 years after spinal decompression is 11%, according to a statistical study with a large number of samples. A less-invasive surgical approach is clinically needed. The commercially available X-Stop interspinous dynamic stabilization system was approved by the FDA in 2005 and is currently widely used. It can maintain the integrity of the spinal canal and the stability of the spine and distributes pressure among the vertebrae. It allows a slight flexion in the lumbar spine with restricting straightness. Patients who undergo this treatment are able to maintain a relatively normal position rather than excessive flexion. The symptoms of lumbar spinal stenosis can be reduced after the implantation. However, the X-Stop system is still under debate because observations of its mid-term and long-term efficacy are lacking. The results of a recent study [8] showed that, for the patients with degenerative lumbar spinal stenosis associated with intermittent neurogenic claudication, the surgical efficacies of the X-Stop system and decompressive laminectomy were comparable, but patients who undergo treatment with the X-Stop system may have a greater risk of reoperation. Therefore, the X-Stop system is not the preferred method of treatment for patients with degenerative lumbar spinal stenosis associated with neurogenic intermittent claudication.
The purpose of surgery for the patients with lumbar spinal stenosis is to decompress on the nerve tissue and blood vessels in the spinal canal, the nerve root canal or the intervertebral foramen; restore the nerve function; relieve the symptoms; and increase the stability of the spine. The study by Yamazaki et al. showed that the postoperative clinical outcomes were correlated with the expansion of the cross-sectional area of the dural tube [9]. Hermansen et al. found that the cross-sectional area of the dural sac after decompression increased by 101% compared with the preoperative measurement [10]. Decompressive laminectomy can significantly increase the spinal area, but not the area of the intervertebral foramen; in addition, decompressive laminectomy is associated with large traumas and many postoperative complications. In comparison, the interspinous fusion method can improve the area of both the intervertebral foramen and the spine. Wan et al. found that after fixation with the X-Stop system, the width of the intervertebral foramen increased by 24.4%, and the area of the intervertebral foramen increased by 32.9% compared with the preoperative measurement [11]. The literature indicates that the expansion of the spinal canal has a very positive impact on improving the symptoms of patients with neurological symptoms. Meves et al. [12] reported a significant correlation between nerve injury and spinal stenosis in patients with thoracolumbar burst fracture; Vaccaro et al. [13] also believed that the spinal morphology, and not just the sagittal diameter of the spinal canal, after thoracolumbar burst fracture injury is related to the nerve injury and prognosis. Hamanishi et al. reported that the cross-sectional area of the dural sac of patients with spinal stenosis and intermittent claudication symptoms is less than the key value of 100 mm2 [14]. Danielson et al. reported that the spinal area of the patients with lumbar canal stenosis is significantly small, and the cross-sectional area of the spinal canal in some patients is even less than 100 mm2 [15].
In an earlier study, it was found that changes in the intervertebral foramen area caused by activity or degeneration can affect the nerve root and cause pain [16]. Based on CT measurements, Infusa et al. indicated that the cross-sectional area of the spinal canal and the intervertebral foramen area in an anteflexion position were higher than those in a neutral position by 10.9% and 11.8%, respectively, while the cross-sectional area of the spinal canal and the intervertebral foramen area in a posterior extension position were lower than those in a neutral position by 11.2% and 15.3%, respectively [17]. Fujiwara et al. studied the changes in the intervertebral foramen area in different states using in vitro biomechanical experiments and indicated an 11.3% increase in the flexion position, a 12.0% decrease in the extension position; an 8.0% increase in concavity and an 8.4% decrease in convexity in the lateral flexion position; and a 5.7% decrease in area in the axial rotation position and an 6.5% increase in the contralateral position [18].
The results of this study showed that the longer the lumbar pedicle is, the larger the areas of the spinal canal and the intervertebral foramen are. According to this principle, we can design a novel surgical approach: percutaneous pedicle osteotomy laminoplasty, which truncates the bilateral pedicle at the junction of the posterior vertebral wall through a minimally invasive approach and moves the rear structures, such as the pedicle and lamina, rearward to enlarge the spinal canal and release the spinal nerve compression. Currently, only few relevant studies have been reported. Based on in vitro experiments on cadavers, Ali et al. proved that the surgical method for pedicle extension and fixation with pedicle osteotomy can expand the spinal canal and intervertebral foramen areas for the long term and maintain spinal stability without altering the biomechanical properties of the spine [19]. Serger et al. analyzed the 12-month follow-up results for 19 patients who underwent pedicle extending surgery and found that this surgery is safe and effective and offers satisfactory clinical efficacy [20]. As a clinical innovative surgical approach, however, it presents many problems that remain to be solved. Further studies of its safety, suitability, and effectiveness are still needed. The results of this study provided the preliminary theoretical basis for implementing this innovative surgery.
References
- 1.Verbiest H. Primary stenosis of the lumbar spinal canal in adults, a new syndrome. Ned Tijdschr Geneeskd. 1950;94:2415–2433. [PubMed] [Google Scholar]
- 2.Yabuki S, Fukumori N, Takegami M, Onishi Y, Otani K, Sekiguchi M, Wakita T, Kikuchi SI, Fukuhara S, Konno SI. Prevalence of lumbar spinal stenosis, using the diagnostic support tool, and correlated factors in Japan: a population-based study. J Orthop Sci. 2013;18:893–900. doi: 10.1007/s00776-013-0455-5. doi: 10.1007/s00776-013-0455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouras T, Stranjalis G, Loufardaki M, Sourtzis I, Stavrinou LC, Sakas DE. Predictors of long-term outcome in an elderly group after laminectomy for lumbar stenosis. J Neurosurg Spine. 2010;59:329–34. doi: 10.3171/2010.3.SPINE09487. [DOI] [PubMed] [Google Scholar]
- 4.Gelalis ID, Stafilas KS, Korompilias AV, Zacharis KC, Beris AE, Xenakis TA. Decompressive surgery for degenerative lumbar spinal stenosis: long term results. Int Orthop. 2006;30:59–63. doi: 10.1007/s00264-005-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen M. X-STOP surgical implant for the treatment of lumbar spinal stenosis: clinical practice recommendations for neurosurgical nurse practitioners. J Neurosci Nurs. 2013;45:44–51. doi: 10.1097/JNN.0b013e318275b1e4. [DOI] [PubMed] [Google Scholar]
- 6.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Jansson KA, Nemeth G, Granath F, Blomqvist P. Spinal stenosis re-operation rate in Sweden is 11% at 10 years - a national analysis of 9,664 operations. Eur Spine J. 2005;14:659–663. doi: 10.1007/s00586-004-0851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strömqvist BH, Berg S, Gerdhem P, Johnsson R. X-Stop Versus Decompressive Surgery for Lumbar Neurogenic Intermittent Claudication: Randomized Controlled Trial With 2-Year Follow-up. Spine. 2013;38:1436–42. doi: 10.1097/BRS.0b013e31828ba413. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki K, Yoshida S, Ito T, Toba T, Kato S, Shimamura T. Postoperative outcome of lumbar spinal canal stenosis after fenestration: correlation with changes in intradural and extradural tube on magnetic resonance imaging. J Orthop Surg (Hong Kong) 2002;10:136–143. doi: 10.1177/230949900201000206. [DOI] [PubMed] [Google Scholar]
- 10.Hermansen E, Moen G, Barstad J, Birketvedt R, Indrekvam K. Laminarthrectomy as a surgical approach for decompressing the spinal canal: assessment of preoperative versus postoperative dural sac cross-sectional areal. Eur Spine J. 2013;22:1913–9. doi: 10.1007/s00586-013-2737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan Z, Wang S, Kozanek M, Xia Q, Mansfield FL, Lü G, Wood KB, Li G. The effect of the X-Stop implantation on intervertebral foramen, segmental spinal canal length and disc space in elderly patients with lumbar spinal stenosis. Eur Spine J. 2012;21:400–10. doi: 10.1007/s00586-011-2021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meves R, Avanzi O. Correlation among canal compromise, neurologic deficit, and injury severity in thoracolumbar burst fractures. Spine. 2006;31:2137–41. doi: 10.1097/01.brs.0000231730.34754.9e. [DOI] [PubMed] [Google Scholar]
- 13.Vaccaro AR, Nachwalter RS, Klein GR, Sewards JM, Albert TJ, Garfin SR. The significance of thoracolumbar spinal canal size in spinal cord injury patients. Spine. 2001;26:371–6. doi: 10.1097/00007632-200102150-00013. [DOI] [PubMed] [Google Scholar]
- 14.Hamanishi C, Matukura N, Fujita M, Tomihara M, Tanaka S. Cross-sectional area of the stenotic lumbar dural tube measured from the transverse views of magnetic resonance imaging. J Spinal Disord. 1994;7:388–93. [PubMed] [Google Scholar]
- 15.Danielson BI, Willén J, Gaulitz A, Niklason T, Hansson TH. Axial loading of the spine during CT and MR in patients with suspected lumbar spinal stenosis. Acta Radiol. 1998;39:604–11. doi: 10.3109/02841859809175484. [DOI] [PubMed] [Google Scholar]
- 16.Panjabi MM, Takata K, Goel VK. Kinematics of the lumbar intervertebral foramen. Spine. 1983;8:348–57. doi: 10.1097/00007632-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Inufusa A, An HS, Lim TH, Hasegawa T, Haughton VM, Nowicki BH. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine. 1996;21:2412–20. doi: 10.1097/00007632-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara A, An HS, Lim TH, Haughton VM. Morphologic changes in the lumbar intervertebral foramina due to flexion-extension lateral bending, and axial rotation: an in-vitro anatomic and biomechanical study. Spine. 2001;26:876–82. doi: 10.1097/00007632-200104150-00010. [DOI] [PubMed] [Google Scholar]
- 19.Kiapour A, Anderson DG, Spenciner DB, Ferrara L, Goel VK. Kinematic effects of a pedicle-lengthening osteotomy for the treatment of lumbar spinal stenosis. J Neurosurg Spine. 2012;17:314–320. doi: 10.3171/2012.6.SPINE11518. [DOI] [PubMed] [Google Scholar]
- 20.Mlyavykh S, Ludwig SC, Mobasser JP, Kepler CK, Anderson DG. Twelve-month results of a clinical pilot study utilizing pedicle-lengthening osteotomy for the treatment of lumbar spinal stenosis. J Neurosurg Spine. 2013;18:347–355. doi: 10.3171/2012.11.SPINE12402. [DOI] [PubMed] [Google Scholar]


