Abstract
Background: Cardiac syndrome X (CSX) is defined as normal coronary arteries with angina pectoris and a positive stress test. Epicardial adipose tissue (EAT) plays an important role in inflammatory process in cardiovascular system, therefore EAT may affect the pathogenesis of different cardiovascular disease. The aim of this study was to investigate the EAT thickness in patients with CSX and compare normal subjects. Methods: We prospectively enrolled 30 consecutive patients with CSX. The control group consisted of 30 age and sex-matched individuals with anginal chest pain and a negative treadmill or myocardial perfusion scan test. EAT thickness was measured by transthoracic echocardiography. Results: There were no differences in baseline clinical, biochemical and echocardiographic characteristics between CSX patients and the control group. Patients with CSX had significantly increased EAT thickness than those of the controls (3.43 ± 0.88 vs. 2.34 ± 0.89 mm, p=0.0001). Conclusion: We found that EAT thickness is increased in patients with CSX. This finding suggests that EAT may contribute to the etiopathogenesis of the CSX.
Keywords: Cardiac syndrome X, epicardial adipose tissue
Introduction
Cardiac syndrome X (CSX) is defined as angina-like chest pain, positive stres test and normal coronary arteriography [1,2]. These patients have objective signs of myocardial ischemia despite open epicardial coronary artery [2]. Endothelial dysfunction and coronary microvascular abnormalities have been proposed as potential mechanisms of the disease [3,4]. Recent studies suggested that inflamation may be responsible for the pathogenesis of CSX [5,6].
Epicardial adipose tissue (EAT) covers more than three quarters of the surface of the heart and is considered an cardiovascular risk predictor [7-9]. EAT is supplied by side-branches of the coronary arteries similar to the microcirculation of the myocardium [10]. Recent studies have identified EAT as an active organ, which secretes several mediators, such as adipokines [11]. Also EAT have high capacity of local proinflammatory activity [12]. Therefore, EAT can locally modulate both myocardium and coronary arteries. EAT can be measured by transthoracic echocardiography, magnetic resonance imaging (MRI) and multidetector computed tomography scanning [13]. Echocardio-graphic assessment of EAT is easily reproducible and showed an excellent reliability with the MRI measurements [14]. The association between CSX and inflammation is well known, however the potential pathophysiological role of EAT has not been well established in patients with CSX. Therefore, the aim of our study was to evaluate the EAT by transthoracic echocardiography in patients with CSX and to compare with normal control subjects.
Materials and methods
Study population
We prospectively studied 30 consecutive CSX patients who had diagnesed our clinic between June 2011 and June 2013. CSX is defined patients with typical chest pain, objective ischemia evidence, and normal coronary angiograms. The control group consisted of 30 age and sex-matched individuals with anginal chest pain and a negative treadmill or myocardial perfusion scan test in similar time period. Demographic, clinical and routine biochemical data were recorded. Echocardiographic examination was performed in CSX patients and control groups. The echocardiographic examinations were obtained by using GE VingMed System 7 (Norway). Left ventricular (LV) functions were measured by using the American Echocardiography Society guideline [15]. LV mass index was measured using the formula proposed by the Penn Convention [16]. Patients with hemolytic, hepatic, chronic renal diseases, collagenosis, thyroid dysfunction, moderate to severe valvular lesions, LV ejection fraction <45%, previous myocardial infarction, hypertrophic or dilated cardiomyopathy were excluded. Written informed consent was obtained from each subject, and the institutional ethics committee approved the study protocol.
Measurements of epicardial adipose tissue thickness
EAT was evaluated by transthoracic echocardiography. EAT thickness was measured by two cardiologists who were blinded the patient’s data according to the method previously described by Iacobellis et al. [14]. EAT was defined as echo-free space in front of the right ventricle free wall on transthoracic parasternal long-axis images. The measurement of EAT thickness was made to be perpendicular to the aortic anulus at the end-diastole. All measurements was performed for three consecutive cardiac cycles and an average value was obtained. Intra- and inter observer variability were calculated as 3.9% and 4.8%, respectively.
Statistical analyses
Continuous data were expressed as the mean ± SD; categorical variables were defined as percentages. The differences between normally distributed numeric variables were evaluated by Student’s t-test, while non-normally distributed variables were analyzed by Mann-Whitney U-test. The chi-square or Fischer’s exact test test were employed for the comparison of categorical variables. A p value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS software (Version 10.0, SPSS, Inc., Chicago, IL).
Results
The baseline demographic and biochemical parameters of the patient and the control groups are demonstrated in Table 1. There were no significant differences in age, gender, body mass index, hemoglobin, thrombosit count, serum creatinine, glucose and LDL cholesterol levels between the groups. The medications were statistically similar between the groups (Table 1). EAT thickness and baseline echocardiographic parameters of both groups were given in Table 2. There were no significant differences in LV diameters, LV volumes, ejection fraction, LV wall thickness and LV mass index between the groups (Table 2). EAT thickness was significantly higher (3.43 ± 0.88 vs 2.34 ± 0.89 mm, p=0.0001) in patients with CSX than controls (Table 2, Figure 1).
Table 1.
Baseline characteristics of study populations
| Control group (n=30) | CSX group (n=30) | p | |
|---|---|---|---|
| Age (years) | 53.6 ± 8 | 52.4 ± 8 | 0.56 |
| Male (%) | 53 | 46 | 0.4 |
| Smoking (%) | 38 | 78 | 0.2 |
| Diabetes Mellitus (%) | 4 | 16 | 0.1 |
| Hypertension (%) | 30 | 40 | 0.3 |
| Body mass index (kg/m2) | 29 ± 7 | 29 ± 4.5 | 0.8 |
| Glucose (mg/dL) | 91.5 ± 8 | 99.4 ± 20 | 0.1 |
| LDL cholesterol (mg/dL) | 123 ± 36 | 126 ± 36 | 0.6 |
| P count (x 103/uL) | 236 ± 37 | 239 ± 61 | 0.85 |
| Hemoglobin (g/dL) | 14.5 ± 1.3 | 14.2 ± 1.4 | 0.21 |
| Creatinine (mg/dL) | 0.77 ± 0.16 | 0.75 ± 0.14 | 0.64 |
| Pharmacological Therapy | |||
| ACE-I or ARB (%) | 20 | 23.3 | 0.5 |
| B Blocker (%) | 6.7 | 23.3 | 0.08 |
| CCBs (%) | 10 | 3.3 | 0.3 |
| OAD (%) | 4 | 16 | 0.1 |
| Statin (%) | 6.7 | 6.7 | 1 |
LDL: Low-density lipoprotein, P: Platelet, ACE-I: Angiotensin-converting enzyme inhibitors, ARB: Angiotensin receptor blockers, CCBs: Calcium channel blockers, OAD: Oral Anti-diabetic.
Table 2.
Echocardiographic measurement of study populations
| Control group (n=30) | CSX group (n=30) | p | |
|---|---|---|---|
| LVEDD (mm) | 46.3 ± 3.2 | 47.6 ± 4.2 | 0.16 |
| LVESD (mm) | 28 ± 3.6 | 29.4 ± 3.9 | 0.12 |
| LVEDV (ml) | 86.4 ± 23.2 | 84.7 ± 23.8 | 0.77 |
| LVESV (ml) | 30.2 ± 10 | 29.7 ± 9.3 | 0.83 |
| EF (%) | 65.1 ± 4.1 | 65 ± 4.3 | 0.9 |
| LVMI (g/m2) | 91 ± 17.5 | 96 ± 17 | 0.23 |
| IVS (mm) | 11.2 ± 1.8 | 10.7 ± 1.2 | 0.16 |
| PW (mm) | 10.1 ± 1.2 | 10.5 ± 1 | 0.21 |
| LA (mm) | 34.7 ± 2.9 | 35.1 ± 3.4 | 0.65 |
| EAT Thickness (mm) | 2.34 ± 0.89 | 3.43 ± 0.88 | 0.0001 |
LVEDD: Left ventricular end-diastolic diameter, LVESD: Left ventricular end-systolic diameter, LVEDV: Left ventricular end-diastolic volume, LVESV: Left ventricular end-systolic volume, EF: Ejection fraction, LVMI: Left ventricular mass index, IVS: Interventricular septum, PW: Posterior wall, LA: Left atrium, EAT: Epicardial adipose tissue.
Figure 1.
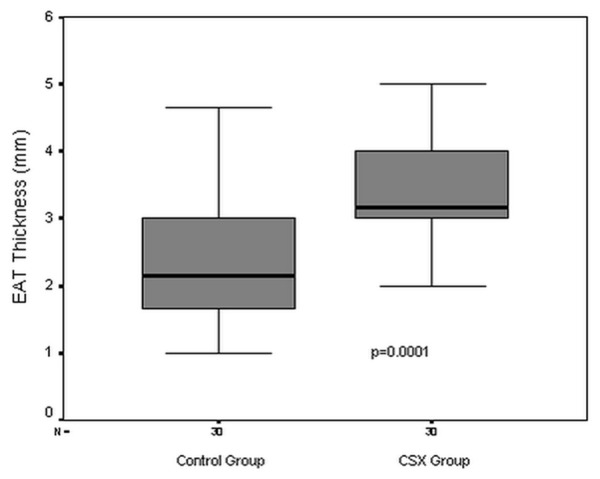
Epicardial adipose tissue thickness in the study groups. EAT: Epicardial adipose tissue, CSX: Cardiac syndrome X.
Discussion
In the present study we have found that EAT thickness was significantly higher in patients with CSX than control subjects.
Although the pathophysiology of CSX has not been well established yet, some mechanisms have been proposed to be responsible for CSX. Quyyumi et al suggested an endothelial dysfunction of the coronary microvasculature in patients with chest pain and angiographically normal epicardial coronary arteries [4]. Egashira et al. reported that endothelium-dependent dilatation of the resistance coronary arteries is impaired in patients with CSX [3]. In addition, other some abnormalities including insulin resistance, abnormal autonomic control, enhanced sodium hydrogen exchange activity, abnormal cardiac sensitivity have been reported [17]. Numerous studies indicated that inflamation may be responsible for the pathogenesis of CSX [5,6,18,19]. It has been shown that markers of inflammation such as C-reactive protein (CRP), pentraxin-3, vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 are increased in these patients [6,18,19]. In a recent study, Recio et al demonstrated that CSX patients with C-reactive protein (CRP) levels >3 mg/l had more ischemic events during adenosine stress and a more severe reduction in corrected coronary flow reserve compared with patients with CRP ≤3 mg/l [5]. Whereas coronary flow reserve was similar between CSX patients with CRP ≤3 mg/l and control. They suggested that inflammation is most important modulator of microvascular function in patients with CSX.
EAT is a true visceral adipose tissue. It is also closely associated with myocardium and coronary arteries [7]. EAT and myocardium share the same microcirculation. EAT plays an important role in inflammatory process in cardiovascular system because of it relases several bioactive molecules including adiponectin and proinflammatory cytokines such as interleukin-1, interleukin-6 and tumor necrosis factor [8-11]. Increased EAT promote inflammatory markers that impair coronary microvascular fuction, and may thus contribute to pathogenesis of CSX. On the other hand EAT may affect the pathogenesis of several cardiovascular disease. In the last decade, many studies suggested that EAT was associated with several cardiovascular advers effects such as atherosclerosis [20], arterial stiffness [21], impaired coronary flow reserve [22], enlarged atrial and ventricular dimension [23,24], increased LV mass index [25], diastolic dysfunction [23] and atrial fibrillation [26]. In addition to these clinical situation, increased EAT may be responsible for the development of CSX.
Previous study has reported the relation between EAT and CSX [22]. Sade et al studied the relation of EAT and coronary microvascular function in patients with women with chest pain and angiographically normal coronary arteries [22]. They found that EAT is independent predictor of reduced coronary flow reserve. However their study did not include control group and male patients with CSX. Also their study groups include patients with normal and indetermined stress test.
Limitations of study
Firstly, modest number of patients is a potential limitation of this study which warrants the necessity of large prospective studies to establish the relationship between EAT and CSX. In this study we measured EAT thickness by transthoracic echocardiography. Iacobellis et al showed that echocardiographic measurement of EAT was strongly correlated with MRI measurements of EAT [14]. However, echocardiographic EAT measurement may not reflect the total epicardial fat volume. But, echocardiography is accurate, easier, and less expensive than MR and computed tomography imaging. We did not analyse markers of inflammation such as CRP in the current study, although the role of inlammation has been previously reported in patients CSX [5,6,18,19].
In conclusion, EAT thickness is increased in patients with CSX. Increased EAT may have impact on the pathogenesis of CSX.
Disclosure of conflict of interest
None.
References
- 1.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 2.Kemp HG Jr. Syndrome X revisited. J Am Coll Cardiol. 1991;17:507–8. doi: 10.1016/s0735-1097(10)80123-8. [DOI] [PubMed] [Google Scholar]
- 3.Egashira K, Inou T, Hirooka Y, Yamada A, Urabe Y, Takeshita A. Evidence of impaired endothelium-dependent coronary va-sodilatation in patients with angina pectoris and normal coro-nary angiograms. N Engl J Med. 1993;328:1659–64. doi: 10.1056/NEJM199306103282302. [DOI] [PubMed] [Google Scholar]
- 4.Quyyumi AA, Cannon RO 3rd, Panza JA, Diodati JG, Epstein SE. Endothelial dysfunction in patients with chest pain and normal coronary arteries. Circulation. 1992;86:1864–71. doi: 10.1161/01.cir.86.6.1864. [DOI] [PubMed] [Google Scholar]
- 5.Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome x patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging. 2013;6:660–7. doi: 10.1016/j.jcmg.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Buyukkaya E, Karakau MF, Kurt M, Motor S, Akcay AB, Buyukkaya S, Karakas E, Sen N. The serum pentraxin-3 is elevated in patients with cardiac syndrome X. Turk Kardiyol Dern Ars. 2013;41:290–5. doi: 10.5543/tkda.2013.20025. [DOI] [PubMed] [Google Scholar]
- 7.Roy PE. Lipid droplets in the heart interstitium: concentration and distribution. Recent Adv Stud Cardiac Struct Metab. 1975;10:17–27. [PubMed] [Google Scholar]
- 8.Company JM, Booth FW, Laughlin MH, Arce-Esquivel AA, Sacks HS, Bahouth SW, Fain JN. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: relationship to visceral and subcutaneous fat. J Appl Physiol. 2010;109:1904–12. doi: 10.1152/japplphysiol.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Curr Pharm Des. 2007;13:2180–4. doi: 10.2174/138161207781039670. [DOI] [PubMed] [Google Scholar]
- 10.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–7. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacobellis G, di Gioia CR, Cotesta D, Petramala L, Travaglini C, De Santis V, Vitale D, Tritapepe L, Letizia C. Epicardial adipose tissue adiponectin expression is related to intracoronary adiponectin levels. Horm Metab Res. 2009;41:227–31. doi: 10.1055/s-0028-1100412. [DOI] [PubMed] [Google Scholar]
- 12.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 13.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–8. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J of Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Shub C, Klein AL, Zachariah PK, Bailey KR, Tajik AJ. Determination of left ventricular mass by echocardiography in a normal population: effect of age and sex in addition to body size. Mayo Clin Proc. 1994;69:205–211. doi: 10.1016/s0025-6196(12)61058-1. [DOI] [PubMed] [Google Scholar]
- 17.Al Suwaidi J, Higano ST, Holmes DR Jr, Lerman A. Pathophysiology, diagnosis, and current management strategies for chest pain in patients with normal findings on angiography. Mayo Clin Proc. 2001;76:22. [PubMed] [Google Scholar]
- 18.Arroyo-Espliguero R. Chronic inflammation and increased arterial stiffness in patients with cardiac syndrome X. Eur Heart J. 2003;24:2006–2011. doi: 10.1016/j.ehj.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Tousoulis D, Davies G, Asimakopoulos G, Homaei H, Zouridakis E, Ahmed N, Kaski JC. Vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 serum level in patients with chest pain and normal coronary arteries (syndrome X) Clin Cardiol. 2001;24:301–304. doi: 10.1002/clc.4960240409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eroglu S, Sade LE, Yildirir A, Bal U, Ozbicer S, Ozgul AS, Bozbas H, Aydinalp A, Muderrisoglu H. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis. 2009;19:211–7. doi: 10.1016/j.numecd.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Natale F, Tedesco MA, Mocerino R, de Simone V, Di Marco GM, Aronne L, Credendino M, Siniscalchi C, Calabrò P, Cotrufo M, Calabrò R. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr. 2009;10:549–55. doi: 10.1093/ejechocard/jep002. [DOI] [PubMed] [Google Scholar]
- 22.Sade LE, Eroglu S, Bozbaş H, Ozbiçer S, Hayran M, Haberal A, Müderrisoğlu H. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204:580–5. doi: 10.1016/j.atherosclerosis.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis G, Leonetti F, Singh N, M Sharma A. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007;115:272–3. doi: 10.1016/j.ijcard.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Iacobellis G. Relation of epicardial fat thickness to right ventricular cavity size in obese subjects. Am J Cardiol. 2009;104:1601–2. doi: 10.1016/j.amjcard.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94:1084–7. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 26.Batal O, Schoenhagen P, Shao M, Ayyad AE, Van Wagoner DR, Halliburton SS, Tchou PJ, Chung MK. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:230–6. doi: 10.1161/CIRCEP.110.957241. [DOI] [PMC free article] [PubMed] [Google Scholar]


