Abstract
Primary mucinous lesions of the urinary system are extremely rare. We describe two cases of primary mucinous lesions of the urothelial tract. One case is of mucinous metaplasia in the bladder of a 40-year-old man presenting with frequent urination, urgency, and gross hematuria. The other case is of mucinous adenocarcinoma in the pelvis of an otherwise healthy 67-year-old man with left nephrolithiasis. The histological images of the two cases demonstrate a spectrum from benign mucinous metaplasia to malignant mucinous adenocarcinoma, and suggest that mucinous metaplasia in urothelial tract may be the precancerous lesion of mucinous adenocarcinoma.
Keywords: Mucinous metaplasia, urothelium, precancerous lesion
Introduction
Primary mucinous lesions of the urinary system are extremely rare and most of the previous case reports have been from Asian countries. The reported lesions include mucinous metaplasia, mucinous adenoma, and mucinous adenocarcinoma. Current hypothesis suggests that a mucinous adenoma-carcinoma sequence exist similar to colonic neoplasia; however, there are no studies exploring the evolution of these tumors. Here we report two cases of primary mucinous lesions of the urinary system and discuss previous findings in the literature.
Case report
The first case is of a 40-year-old man presenting with frequent micturition, urgency, and gross hematuria for 11 days. On physical examination, there was no tenderness or masses on palpation. Cystoscopy revealed rugged papillary lesions involving the roof, trigone and neck of the urinary bladder. Transurethral resection of bladder tumor (TURBT) was performed. The tumor was entirely resected and measured 4.5 × 3.0 × 1.5 cm in size. On microscopic examination, there were numerous glands lined by urothelium and intestinal type epithelium without atypia, consistent with cystitis cystica and cystitis glandularis of intestinal type (Figure 1). The “intestinal” appearance was striking. In some areas transition zones from urothelium to mucinous columnar epithelium were seen (Figure 2). The intestinal areas consist of glands lined by mucinous columnar epithelium (goblet cells) with basally located nuclei. There was no nuclear hyperchromasia, nuclear pleomorphism, or pseudostratification. Mitoses, necrosis, or signet ring cells were not seen. Prominent edema and mild inflammation were present and mucin accumulation was prominent. Focal mucin extravasation into the stroma was identified, with dissecting mucin pools (Figure 3). The epithelial cells showed no evidence of invasion. The diagnosis was cystitis cystica and cystitis glandularis of intestinal type.
Figure 1.
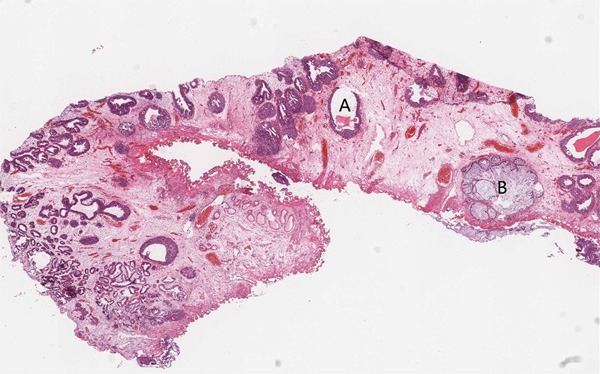
Histopathology of the bladder mucosa showing cystitis cystica (A), cystitis glandularis of intestinal type (B) in the bladder lamina propria (hematoxylin–eosin (HE), original magnification, ×10).
Figure 2.
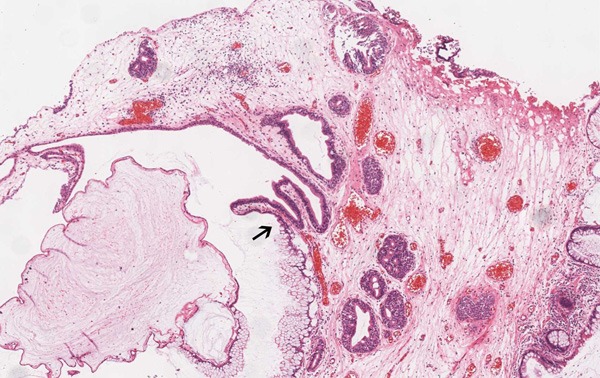
The transition zone (↑) from transitional epithelium to mucinous columnar epithelium (HE, ×40).
Figure 3.
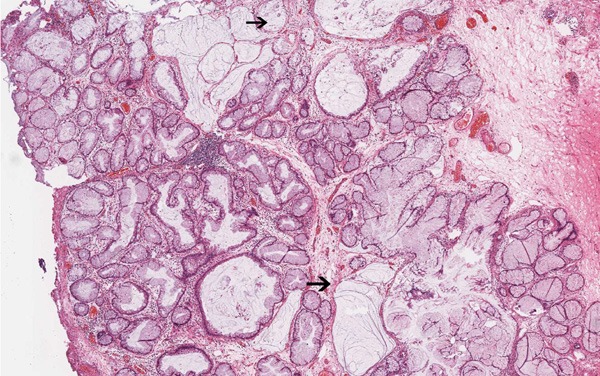
Remarkable “intestinal” appearance: focal mucin extravasated into the stroma with dissecting mucin pools (↑) (HE, ×40).
The second case was an otherwise healthy 67-year-old man with left nephrolithiasis found on routine physical examination for one month. There were no complaints of hematuria or pyuria. Computed tomography scan and ultrasonic study showed gross nephrolithiasis and hydronephrosis of the left kidney. Radionuclide renal dynamic imaging revealed a normal right kidney, and left kidney failure. Abdominal CT scan, pelvic ultrasound, and chest X-ray did not reveal any other abnormalities. Left nephrectomy was performed; the kidney was enlarged and showed hydronephrosis. The kidney also adhered to the surrounding tissue, and upon separation, large amounts of mucin was excreted. At the pathology laboratory we received an irregularly nodular kidney which measured 11.5 × 7.0 × 4.0 cm in size. Upon sectioning, there was significant hydronephrosis with dilatation of the pelvic-calyceal system, thinning of the cortex and loss of the corticomedullary demarcation. The normal structure was replaced by a multi-cystic mass filled with mucoid material and an irregular staghorn stone measuring 5.0 × 2.3 × 2.0 cm within the mucinous material. The inner walls were smooth with focally papillary excrescences. Microscopic examination revealed renal parenchyma atrophy, fibrous proliferation, and a cystic cavity. The cysts were dilated and lined by single-layer or pseudostratified columnar epithelium. In a focal area, the transition zone from urothelium to metaplastic columnar epithelium was seen (Figure 4). Parts of the columnar epithelium were single-layered without obvious atypia. The cells had vacuolated cytoplasm, and basally located small nuclei (Figure 5). Other parts of the columnar epithelium were arranged into pseudopapillary and glandular pattern with atypical cells containing round to ovoid nuclei that were enlarged and hyperchromatic (Figure 6). Nucleoli were not prominent, and mitotic figures were not easy identified. Necrosis was not observed. The mucin infiltrated into the fibrous stroma and focally formed mucin pools (Figure 5). The immunohistochemical stains showed strong expression of CK7, CK20, CDX-2 and P53 in the tumor cells, while CK34βE12 staining was negative. Ki-67 labeling index was very high. β-catenin was strongly positive with both cytoplasmic and cell membrane staining patterns (Figure 7). The histological features of cellular atypia and mucin infiltration are most consistent with the diagnosis of mucinous adenocarcinoma. No other tumors were found in the patient confirming a primary pelvis mucinous adenocarcinoma.
Figure 4.
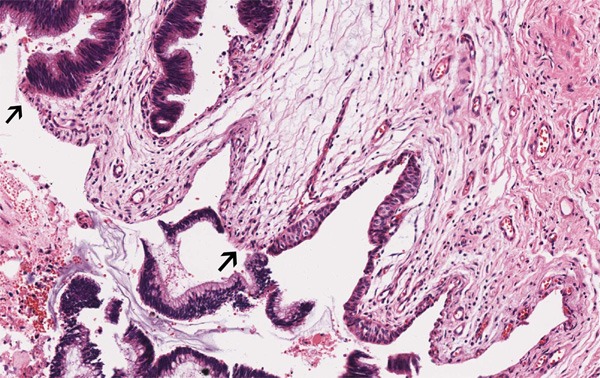
The transition zone (↑) from transitional epithelium to metaplastic columnar epithelium (HE, ×100).
Figure 5.
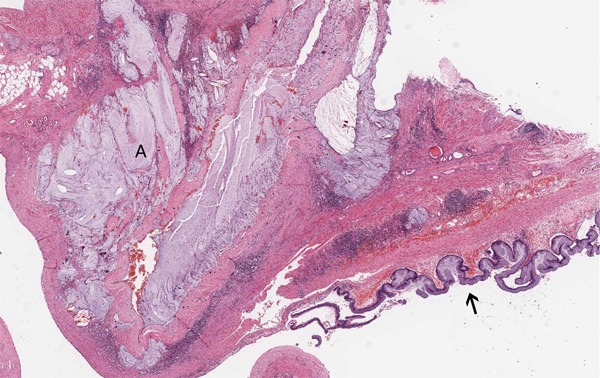
The mucin infiltrated into the fibro-stroma and abundant mucin gathered into pools (A), with low-grade intraepithelial neoplasia (↑) on the surface of the mucosa (HE, ×10).
Figure 6.
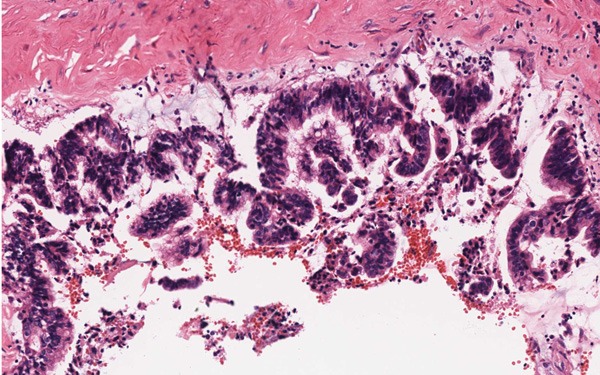
Some pseudostratified columnar epithelium in high-grade intraepithelial neoplasia (HE, ×200).
Figure 7.
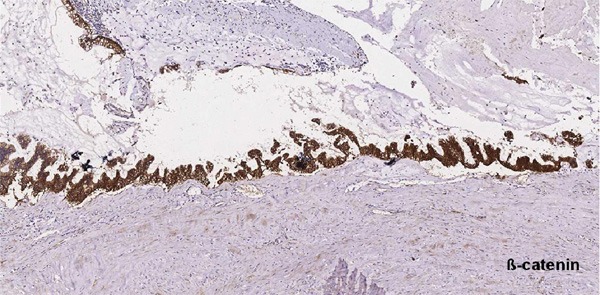
Immunohistochemistry showing ß-catenin was strongly positive in the cytoplasm and cell membrane (×100).
Discussion
The normal urological system is lined by urothelium. Chronic irritation often induces metaplasia of the urothelium but squamous metaplasia is much more common than mucinous metaplasia. It has been hypothesized that mucinous metaplasia of the urothelial tract is due to chronic stimuli such as nephrolithiasis, chronic inflammation, hydronephrosis [1]. In the first case, although mild inflammation was present, two types of epithelium were seen, urothelium and mucinous columnar epithelium, with the latter referred to as intestinal metaplasia. Extravasation of mucin into the stroma with dissecting mucin pools would generally create diagnostic difficulties. However, in the first case, an orderly arrangement of the glands, lack of more than mild atypia of the cells, and absence of invasion favored a diagnosis of cystis glandularis of intestinal type [2,3]. Similar to the first case, there was a number of mucinous epithelium present in the second case. The history of nephrolithiasis and presence of the transition zone between urothelium and mucinous type epithelium provide strong evidence for a close relationship between nephrolithiasis and mucinous metaplasia. Furthermore, frank atypia and invasive mucin pools were present and the diagnosis of mucinous adenocarcinoma was appropriate.
Mucin extravasation is a common finding in mucinous tumor of the urinary system. Shah VB et al. reported a mucinous adenocarcinoma of the renal pelvis leading to pseudomyxoma peritonei [4]. In the appendix, once the mucin infiltrates into the muscularis mucosa, it is necessary to diagnose the lesion as low-grade appendiceal mucinous neoplasm rather than mucinous adenoma. It is proposed that the same standards used in mucinous appendix tumors are more appropriate in the evaluation of mucinous tumors in the urinary system; thus, the first case may be diagnosed as a low-grade mucinous neoplasm.
When invasive mucinous carcinomas are identified in the urinary system, it is essential to rule out metastasis before labeling them as primary tumors. In the renal case, there are several lines of evidence that highly suggest a primary tumor. First, the patient had a history of nephrolithiasis. The nephrolithiasis acts as a chronic stimulator that triggers the metaplasia of the urothelium and eventually neoplasia. However, some authors regard the stone formation as the result of the neoplasm, and the glycoproteins secreted by the tumor combine with cations such as sodium, calcium, and magnesium, causing stone formation [5-7]. Secondly, in the transition zone from urothelium to metaplastic columnar epithelium, there is low-grade and high-grade intraepithelial neoplasia, suggesting a sequential evaluation process and supporting the diagnosis of a primary tumor. Thirdly, extensive post-operation work-up including GI endoscopy, chest and abdominal CT scan, chest X-ray, and pelvic ultrasound was negative, rule out the possibility of metastasis. Lastly, cytoplasmic-membranous staining of β-catenin was observed in 83.3% of primary bladder adenocarcinoma and only 25% of metastatic colonic adenocarcinoma [8]. Thus, the cytoplasmic-membranous staining of β-catenin in this case would be consistent with a primary tumor.
Although intestinal metaplasia often coexists with adenocarcinoma of the urinary bladder, it is unclear whether intestinal metaplasia of the bladder is a premalignant lesion. Morton MJ et al [9] used quantitative fluorescent in situ hybridization (FISH) to measure telomere length and UroVysion FISH to detect cytogenetic abnormalities in urinary bladder specimens with intestinal metaplasia. The results showed significant telomere shortening in intestinal metaplasia of the urinary bladder and cytogenetic abnormalities associated with urothelial carcinoma, suggesting that intestinal metaplasia is a precursor to adenocarcinoma of the bladder. However, many others retrospectively evaluated the association among Intestinal metaplasia and bladder carcinoma, and found mucinous metaplasia is not a risk factor for bladder adenocarcinoma [10]. From the morphology, our cases revealed the spectrum from normal mucinous epithelium, low-grade and high-grade dysplasia, which may suggesting a sequential evaluation process of mucinous epithelium from benign to malignant. At the latest follow-up (9 and 10 months, respectively), no signs of recurrence or metastasis were seen in either patient from the two cases reported here. However, it is also proposed that the persistence of mucinous metaplasia, which was seen in the first case, has the potential to progress to mucinous adenocarcinoma after a long duration. However, the genetics mechanism of the evolution remains to be further research.
Acknowledgements
We thank Prof. Jiaoti Huang in department of pathology, UCLA, for help with consultation of the two cases. This research is funded by Maixin pathological research fund (m1008).
Disclosure of conflict of interest
None.
References
- 1.Spires SE, Banks ER, Cibull ML, Munch L, Delworth M, Alexander NJ. Adenocarcinoma of renal pelvis. Arch Pathol Lab Med. 1993 Nov;117:1156–60. [PubMed] [Google Scholar]
- 2.Young RH. Pseudoneoplastic lesions of the urinary bladder and urethra: a selective review with emphasis on recent information. Semin Diagn Pathol. 1997;14:133–146. [PubMed] [Google Scholar]
- 3.Jacobs LB, Brooks JD, Epstein JI. Differentiation of colonic metaplasia from adenocarcinoma of urinary bladder. Hum Pathol. 1997;28:1152–1157. doi: 10.1016/s0046-8177(97)90253-7. [DOI] [PubMed] [Google Scholar]
- 4.Shah VB, Amonkar GP, Deshpande JR, Bhalekar H. Mucinous adenocarcinoma of the renal pelvis with pseudomyxoma peritonei. Indian J Pathol Microbiol. 2008;51:536–7. doi: 10.4103/0377-4929.43753. [DOI] [PubMed] [Google Scholar]
- 5.Torres Gómez FJ, Torres Olivera FJ. Renal pelvis mucinous carcinoma. Case report. Arch Esp Urol. 2006;59:300–2. doi: 10.4321/s0004-06142006000300015. [DOI] [PubMed] [Google Scholar]
- 6.Fareghi M, Mohammadi A, Madaen K. Primary mucinous cystadenocarcinoma of the renal pelvis: a case report. Cases J. 2009;2:9395. doi: 10.1186/1757-1626-2-9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xambre L, Cerqueira M, Cardoso A, Correia T, Macedo Dias A, Carreira F, Galán T. Primary mucinous adenocarcinoma of the renal pelvis--additional case report. Act Urol Esp. 2009;33:200–204. doi: 10.1016/s0210-4806(09)74124-5. [DOI] [PubMed] [Google Scholar]
- 8.Roy S, Smith MA, Cieply KM, Acquafondata MB, Parwani AV. Primary bladder adenocarcinoma versus metastatic colorectal adenocarcinoma: a persisting diagnostic challenge. Diagn Pathol. 2012;7:151. doi: 10.1186/1746-1596-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morton MJ, Zhang S, Lopez-Beltran A, Maclennan GT, Eble JN, Montironi R, Sung MT, Tan PH, Zheng S, Zhou H, Cheng L. Telomere shortening and chromosomal abnormalities in intestinal metaplasia of the urinary bladder. Clin Cancer Res. 2007 Oct 15;13:6232–6236. doi: 10.1158/1078-0432.CCR-07-0121. [DOI] [PubMed] [Google Scholar]
- 10.Smith AK, Hansel DE, Jones JS. Role of cystitis cystica et glandularis and intestinal metaplasia in development of bladder carcinoma. Urology. 2008 May;71:915–918. doi: 10.1016/j.urology.2007.11.079. [DOI] [PubMed] [Google Scholar]


