The authors examined the protective effects of adipose-derived stem cells and adipose-derived stem cell-conditioned medium (ASC-CM) against retinal damage and identified the neuroprotective factors in ASC-CM. The findings suggest that ASC-CM and progranulin have neuroprotective effects in the light-induced retinal-damage model. Progranulin may be a potential target for the treatment of the degenerative diseases of the retina.
Keywords: Progranulin, Adipose-derived stem cell, Photoreceptor, Retina, Light-induced photoreceptor cell death
Abstract
Adipose tissue stromal vascular fraction contains mesenchymal stem cells, which show protective effects when administered to damaged tissues, mainly through secreted trophic factors. We examined the protective effects of adipose-derived stem cells (ASCs) and ASC-conditioned medium (ASC-CM) against retinal damage and identified the neuroprotective factors in ASC-CM. ASCs and mature adipocytes were isolated from mouse subcutaneous tissue. ASCs were injected intravitreally in a mouse model of light-induced retinal damage, and ASC injection recovered retinal function as measured by electroretinogram and inhibited outer nuclear layer, thinning, without engraftment of ASCs. ASC-CM and mature adipocyte-conditioned medium were collected after 72 hours of culture. In vitro, H2O2- and light-induced cell death was reduced in a photoreceptor cell line with ASC-CM but not with mature adipocyte-conditioned medium. In vivo, light-induced photoreceptor damage was evaluated by measurement of outer nuclear layer thickness at 5 days after light exposure and by electroretinogram recording. ASC-CM significantly inhibited photoreceptor degeneration and retinal dysfunction after light exposure. Progranulin was identified as a major secreted protein of ASCs that showed protective effects against retinal damage in vitro and in vivo. Furthermore, progranulin phosphorylated extracellular signal-regulated kinase, cAMP response element binding protein, and hepatocyte growth factor receptor, and protein kinase C signaling pathways were involved in the protective effects of progranulin. These findings suggest that ASC-CM and progranulin have neuroprotective effects in the light-induced retinal-damage model. Progranulin may be a potential target for the treatment of the degenerative diseases of the retina.
Introduction
Excessive light exposure leads to photoreceptor degeneration [1], and several epidemiological studies have suggested that long-term history of exposure to light may have some impact on the incidence of age-related macular degeneration [2]. Photoreceptor loss is the primary cause of blindness in degenerative diseases such as age-related macular degeneration and retinitis pigmentosa. However, there are few effective therapeutic strategies for these diseases. Therefore, meaningful therapeutic methods such as transplantation, regenerative therapy, and photoreceptor-protective agents are required.
Recently, transplanted bone marrow-derived stem cells (BMSCs) have been shown to exert significant neuroprotection in several central nervous system degenerative models [3–6]. In the retina, intravitreal BMSC transplantation resulted in a significant decrease in the rate of retinal ganglion cell axon loss normalized to cumulative intraocular pressure exposure [7]. BMSC transplantation could inhibit photoreceptor apoptosis and slow down retinal damage in light-damaged rat eyes [8]. Despite the improvements observed, only a few integrated into the neural retina, and the majority of the transplanted cells survived in the vitreous cavity because of glial reactivity. Moreover, no engrafted cells differentiated into neural or retinal cells [7–9]. It is possible that these improvements are the result of trophic support provided to host cells from factors released by mesenchymal stem cells (MSCs).
Adipose-derived stem cells (ASCs) are MSCs within the subcutaneous adipose tissue that self-renew and display multilineage developmental plasticity [10, 11]. ASCs can be obtained repeatedly in large quantities under local anesthesia. A comparative analysis of MSCs obtained from bone marrow, adipose tissue, and umbilical cord clearly showed no differences between MSCs and ASCs in terms of morphology, immune phenotype, colony frequency, and differentiation capacity [12, 13]. ASCs secrete several potentially beneficial growth factors such as vascular endothelial growth factor, hepatocyte growth factor (HGF), basic fibroblast growth factor, and insulin-like growth factor 1 [14, 15], which may protect retinal neurons from injury as well as promote endogenous repair. Some evidence supports the protective effect of cytokines during oxidative stress; for example, basic fibroblast growth factor has been shown to protect photoreceptor cells from light-induced retinal damage in mice [16], and HGF has been shown to have protective effects against retinal ischemia-reperfusion [17]. These reports indicate the retinal protective effects of stem cells and cytokines. Recently, we have reported that ASC-conditioned medium (ASC-CM) has a neuronal protective effect [18]. Therefore, we evaluated the protective effects of ASCs and ASC-CM against retinal degeneration in vitro and in vivo and the various factors included in ASC-CM.
Progranulin is a 593-amino-acid, cysteine-rich protein that was originally identified as the precursor of smaller related peptides, referred to as “granulin.” Progranulin is expressed in a variety of peripheral tissues as well as in the adult central nervous system, including cortical and hippocampal pyramidal cells [19]. It is particularly prominent in epithelial and hematopoietic cells and tends to be more highly expressed in tissues with high turnover rates [20, 21]. Moreover, progranulin also promotes tumor cell invasiveness [22–24], and it is upregulated during wound healing and stimulates neutrophil, macrophage infiltration, and neovascularization of wound tissue [25, 26]. In the central nervous system, mutations in progranulin have been reported to cause tau-negative frontotemporal dementia, showing the important function of progranulin in neuronal survival [27]. Neurons from progranulin-deficient mice were more vulnerable to damage by activated microglia and by depletion of oxygen and glucose [28]. Recently, tumor necrosis factor (TNF) receptors have been revealed to be receptors for progranulin [29]. In the retina, “progranulin-a” was expressed in microglia at the site of light-induced photoreceptor injury in zebrafish [30]. Consequently, progranulin may play an important role in retinal neuroprotection.
In the present study, we identified progranulin as a major secreted factor from ASC-CM and examined the protective effects of ASC-CM and progranulin against retinal damage in vitro and in vivo.
Materials and Methods
Animals
Male adult ddY mice and C57BL/6-Tg (CAG-EGFP) mice were purchased from Japan SLC, Inc. (Hamamatsu, Japan, http://www.jslc.co.jp/). They were kept under controlled lighting conditions (12-hour light/12-hour dark). Nine- or 10-week-old ddY mice were used in the experiments. All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved and monitored by the institutional animal care and use committee of Gifu Pharmaceutical University.
Isolation and Culture of ASCs and Mature Adipocytes
Murine ASCs were obtained from a C57BL/6-Tg (CAG-EGFP) mouse that ubiquitously expresses enhanced green fluorescent protein (EGFP). Cells were obtained from a 16-week-old female mouse.
Adipose tissue was dissected from a subcutaneous site. For ASC harvest, the inguinal fat pads were removed, and a cell pellet containing ASCs was obtained, as described previously [31]. Briefly, the fat tissue was minced, digested with 0.15% collagenase (Wako Pure Chemical Industries, Ltd., Osaka, Japan, http://www.wako-chem.co.jp/egaiyo/), and centrifuged. After this procedure, most of the mature adipocytes remained in the supernatant. The floating adipocytes were collected and washed twice in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). The cell pellet was resuspended in 10% fetal bovine serum (FBS)/DMEM and plated in a 100-mm culture dish. ASCs were maintained in 10% FBS/DMEM, 100 U/ml penicillin (Meiji Seika Pharma Co., Ltd., Tokyo, Japan, http://www.meiji-seika-pharma.co.jp/english/index.html), and 100 μg/ml streptomycin (Meiji Seika) under a humidified atmosphere of 95% air and 5% carbon dioxide (CO2) at 37°C. The cells were passaged by trypsinization every 3–4 days and were used in the experiments from passages 4–8. ASCs were characterized by MSC markers and a leukocyte marker (supplemental online Fig. 1). The multipotency of ASCs was confirmed, as described in our previous report [31].
Collection of ASC-CM and Mature Adipocyte-Conditioned Medium
ASCs and mature adipocytes (4 × 105 cells) were cultured in FBS-free DMEM. ASC-CM and mature adipocyte-conditioned medium were collected after 72 hours of culture, centrifuged at 300g for 5 minutes, and filtered using a 0.22-μm syringe filter. The media were concentrated by centrifugation at 2,600g using the Amicon Ultra-15 (Millipore, Billerica, MA, http://www.millipore.com; molecular weight cutoff: 3,000).
Cell Culture
Mouse photoreceptor-derived 661W cells were a kind gift from Dr. Muayyad R. Al-Ubaidi (Department of Cell Biology, University of Oklahoma Health Sciences Center, Oklahoma City, OK). The cells were maintained in 10% FBS/DMEM, 100 U/ml penicillin, and 100 μg/ml streptomycin under a humidified atmosphere of 95% air and 5% CO2 at 37°C. The cells were passaged by trypsinization every 3–4 days and were used in the experiments from passage 5 to passage 15.
H2O2-Induced and Light-Induced Cell Death in 661W Cell Cultures
The 661W cells were seeded at 2 × 103 (H2O2 study) or 3 × 103 (light irradiation study) cells per well in 96-well plates and then incubated for 24 hours. The medium of the experimental groups was then replaced with 1% FBS ASC-CM, and the cells were incubated for 12 hours. Recombinant mouse progranulin (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com) was dissolved in phosphate buffered saline (PBS) and added to the medium. Pretreatment with 100 μM Trolox (Sigma-Aldrich), a vitamin E analog and an antioxidant regent, was done as a positive control. Then, H2O2 (Wako) was added at a final concentration of 0.3 mM. Nuclear staining assays were carried out after 27 hours. In the light-induced cell death assay, at 1 hour before progranulin treatment, the cells were treated with U0126 (Promega, Madison, WI, http://www.promega.com), a mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor, H-89 (Merck & Co., Whitehouse Station, NY, http://www.merck.com), a protein kinase A inhibitor, and Gö 6976 (Merck), a protein kinase C (PKC) inhibitor. Thereafter, the cells, in the absence or presence of ASC-CM or recombinant mouse progranulin, were exposed to 2,500 lux (lx) of light using a white fluorescent lamp (Nikon, Tokyo, Japan, http://www.nikon.com) for 24 hours under a humidified atmosphere of 95% air and 5% CO2 at 37¡C. The luminance was measured using a light meter LM-332 (As One Corporation, Osaka, Japan, http://www.as-1.co.jp/), and the temperature of the cell surface was measured using a noncontact thermometer MT-7 (As One). Dark control cells and light-stressed 661W cells were all from the same stock, eliminating any preexisting bias (e.g., light, temperature). as previously described by Kanan et al. [32]. The experiments were always started at around 9 a.m. Nuclear staining assays were carried out after light exposure.
Hoechst 33342 and Propidium Iodide Staining
Cell death was observed by using combination staining with two fluorescent dyes, Hoechst 33342 and propidium iodide (PI; both from Invitrogen, Carlsbad, CA, http://www.invitrogen.com). At the end of the culture period, Hoechst 33342 and PI were added to the culture medium for 15 minutes at final concentrations of 8.1 μM and 1.5 μM, respectively. Images were collected using an Olympus IX70 inverted epifluorescence microscope (Olympus, Tokyo, Japan, http://www.olympus-global.com). The total number of cells (not fewer than 500 cells in each group) was counted in a blind manner (M.Y.), and the percentage of PI-positive cells was calculated.
Cytokine Array
RayBio Biotin Label-based Mouse Antibody Array I (RayBiotech, Inc., Norcross, GA, http://www.raybiotech.com) was used to investigate cytokines secreted by ASCs. Briefly, the membranes were blocked with blocking buffer and then incubated with biotin-labeled medium from ASCs or mature adipocytes cultures at room temperature for 2 hours. The membranes were washed, horseradish peroxidase-conjugated streptavidin was added, and the membranes were incubated at room temperature for 2 hours. The membranes were developed by using enhanced chemiluminescence-type solution and visualized using a LAS-4000 luminescent image analyzer (Fuji Film, Tokyo, Japan, http://www.fujifilm.com/).
Western Blot Analysis
ASC-CM was supplemented with protease inhibitor cocktail (Sigma-Aldrich), phosphate inhibitor cocktails 2 and 3 (Sigma-Aldrich), and sample buffer (Wako). In vitro, 661W cells were washed with PBS, harvested, and lysed in RIPA buffer (Sigma-Aldrich) supplemented with protease inhibitor cocktail and phosphate inhibitor cocktails 2 and 3. Lysates were centrifuged at 12,000g for 15 minutes at 4°C. Protein concentrations were measured by comparison with a known concentration of bovine serum albumin, using a bicinchoninic acid protein assay kit. Thereafter, sample buffer was added and boiled for 5 minutes. The samples were subjected to 5%–20% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes (Immobilon-P; Millipore). For immunoblotting, the following primary antibodies were used: monoclonal anti-mouse progranulin antibody (R&D Systems), anti-β-actin, (Sigma-Aldrich), anti-ERK1/2, anti-phosphorylated ERK1/2, anti-HGF receptor (MET), anti-phosphorylated MET (Cell Signaling Technology, Danvers, MA, http://www.cellsignal.com), anti-cAMP response element binding protein (Cell Signaling Technology), and anti-phosphorylated CREB (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, http://www.scbt.com). The primary antibodies were diluted 1:1,000 using Can Get Signal Solution 1 (Toyobo, Osaka, Japan, http://www.toyobo.co.jp/e), and incubated for overnight at 4°C. Anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibody was used. The secondary antibodies were diluted 1:2,000 using Can Get Signal Solution 2 (Toyobo) and incubated for 45 minutes at room temperature. The immunoreactive bands were visualized using Immunostar-LD (Wako) and an LAS-4000 luminescent image analyzer.
Confirmation of ASC Intravitreous Injection
Mice were anesthetized with 3.0% isoflurane (Merck Animal Health, Boxmeer, The Netherlands, http://www.merck-animal-health.com/) and maintained with 1.5% isoflurane in 70% nitrous oxide and 30% oxygen by an animal general anesthesia apparatus (Soft Lander; Sin-ei Industry Co., Ltd., Saitama, Japan, http://www.shinei.me). ASCs (1 × 103 cells per eye; total injected volume was 2 μl) contained in DMEM were then intravitreously injected in the left eyes. After 2 days of ASCs injection, ocular fundus images were obtained using a retinal imaging microscope (Micron III; Phoenix Research Laboratories Inc., Pleasanton, CA, http://www.phoenixreslabs.com/). Five microliters of ophthalmic solution containing 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Mydrin-P; Santen, Osaka, Japan, http://www.santen.com/en/) was applied topically 10 minutes before anesthesia to dilate the pupil, and then mice were anesthetized intraperitoneally with a mixture of ketamine (120 mg/kg; Daiichi-Sankyo, Tokyo, Japan, http://www.daiichisankyo.com/) and xylazine (6 mg/kg; Bayer Health Care, Tokyo, Japan, http://www.bayer.com/en/Homepage.aspx). A few minutes later, 0.1% purified sodium hyaluronate (Hyalein; Santen) was applied topically to prevent desiccation and to keep the surface smooth, and fundus images were captured.
Exposure to Light
After dark adaptation for 24 hours, the pupils of the mice were dilated with 1% cyclopentolate hydrochloride eye drops (Santen) at 30 minutes before exposure to light. Nonanesthetized mice were exposed to 8,000 lx of white fluorescent light (Toshiba, Tokyo, Japan, https://www.toshiba.co.jp/worldwide/index.html) for 3 hours in cages with a reflective interior. The temperature during exposure to light was maintained at 25°C ± 1.5°C. After exposure to light, all mice were returned to darkness for 24 hours and then placed in a normal light/dark cycle.
ASCs (103 cells per eye) or DMEM (as a vehicle) was injected into the vitreous chamber of ddY mice at 24 hours after light exposure in order to avoid damage to ASCs by light. In addition, we prepared dead ASCs as a control for electroretinogram (ERG). ASCs were killed by heating at 80°C for 30 minutes and injected into the vitreous chamber. ASC-CM concentrated 150-fold or recombinant mouse progranulin (100 μg/ml) was intravitreally injected (1 μl per eye) at 6 hours before exposure to light.
ERG
ERGs were recorded at 5 days (ASC-CM injected group) or 27 days (ASC- or dead-ASC-injected group) after light exposure (Mayo, Aichi, Japan, http://homepage3.nifty.com/mayo) as described previously [33]. Briefly, mice were anesthetized and their pupils were dilated. Flash ERG was recorded in the left eyes of dark-adapted mice. The amplitude of the a-wave was measured from the baseline to the maximum a-wave peak, and the b-wave was measured from the maximum a-wave peak to the maximum b-wave peak.
Histological Analysis
Both eyes of each mouse were enucleated under sodium pentobarbital anesthesia (80 mg/kg i.p.; Nakalai Tesque, Kyoto, Japan, http://www.nacalai.co.jp/global/) and kept immersed in a fixative solution containing 4% paraformaldehyde for at least 24 hours at 4°C. Six paraffin-embedded sections (5 μm thick) cut through the optic disc of each eye were prepared in a standard manner and stained with hematoxylin and eosin. The damage induced by light exposure was then evaluated, with six sections from each eye used for the morphometric analysis, as described below. Light microscopy images were acquired, and the thickness of the outer nuclear layer (ONL) from the optic disc was measured at 240-μm intervals by photographic imaging in a masked fashion by two observers (S.S. and Y.O.). Data were averaged for each eye.
Immunostaining
EGFP transgenic mouse-derived ASCs (103 cells per eye or dead ASCs) were injected into the vitreous chamber. At 28 days after injection, mice were euthanized, and the eyeballs were quickly extracted. The extracted eyes were fixed overnight in 4% paraformaldehyde and immersed for 2 days in 25% sucrose with PBS. The eyes were then embedded in a supporting medium for frozen-tissue specimens (Tissue-Tek O.C.T. compound; Sakura Finetek Japan, Tokyo, Japan, http://www.sakura-finetek.com/top_e.html). Retinal sections (10 μm) were cut on a cryostat at −25°C. Then, the sections were preincubated with 10% normal goat serum for 1 hour. For immunofluorescence double staining, the sections were incubated overnight at 4°C with the primary antibody rabbit anti-green fluorescent protein polyclonal antibody (1:1,000; Medical & Biological Laboratories Co. Ltd., Nagoya, Japan, http://www.mbl.co.jp/e/index.html). Next, they were incubated for 1 hour with Alexa Fluor 488 F(ab′)2 fragment of goat anti-rabbit IgG (H+L) antibody and were further incubated for 30 minutes with Hoechst 33342 for nuclear staining.
Statistical Analysis
Data are presented as means ± SEM. Statistical comparisons were made using Student’s t test and one-way ANOVA followed by Dunnett’s test (using STAT VIEW version 5.0; SAS Institute, Cary, NC, http://www.sas.com). A p value <.05 was considered to indicate statistical significance.
Further information is available in the supplemental online data.
Results
Photoreceptor Protection by ASCs Without Engraftment
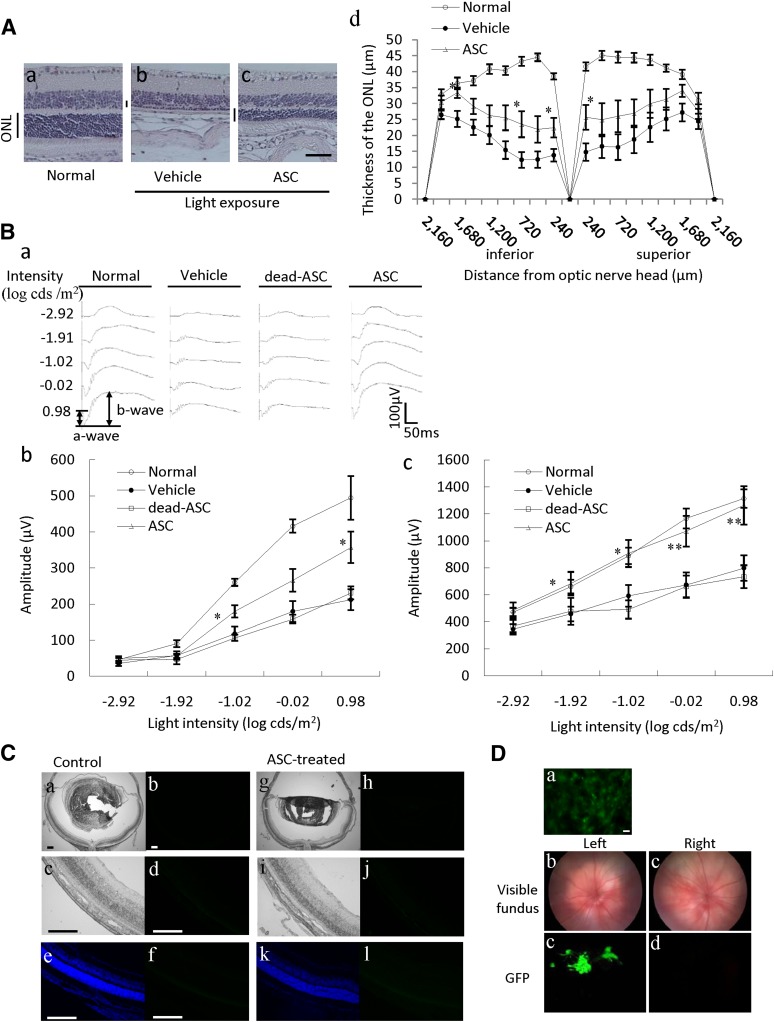
The effects of ASCs on light-induced retinal damage were investigated by histological and functional analyses. Representative retinal images from the optic nerve were taken at 28 days after light exposure (Fig. 1A). The ONL was markedly thinned in the vehicle group (Fig. 1Ab) versus the normal group (Fig. 1Aa). The ASC-injected group showed suppression of the damage (Fig. 1Ac) without changing rhodopsin localization (supplemental online Fig. 2). The thickness of the outer nuclear layer was measured in 240-μm steps in Figure 1Ad. ASCs significantly protected the retinal superior area and the inferior area. The functional consequences of ASCs were evaluated by recording the ERG response. The a-wave shows the function of the photoreceptors, and the b-wave reflects bipolar cells and Müller cell function (Fig. 1B). Consequently, decreases in a- and b-wave amplitudes indicate retinal dysfunction. Both a- and b-wave amplitudes were significantly reduced at 28 days after 8,000 lx of light exposure for 3 hours, and in the ASC-injected group, a decrease in the a- and b-wave amplitudes was significantly prevented compared with the vehicle group (Fig. 1Bb, 1Bc). In the group treated with dead ASCs (killed by heating for 30 minutes at 80°C), no effect was observed on the decrease in the a- and b-waves by light exposure. To evaluate whether injected ASCs are integrated into the host’s retina, we prepared retinal cross-sections at 28 days after ASC injection. Representative images from the nontreated group and the ASC-treated (103 cells per eye) group are given in Figure 1C, respectively, Figures 1Ca–1Cd and 1Cg–1Cj. EGFP fluorescence was not observed in either the EGFP transgenic mouse-derived ASCs injected or in the control groups, although EGFP fluorescence was detected in the cultured ASCs and in the vitreous body at 2 days after injection (Fig. 1D). In immunostaining with anti-green fluorescent protein antibody, green fluorescent protein-positive cells were not observed in both groups (Fig. 1C, 1Cf, 1Cl). In the retina, immune response by ASCs was not observed 2–10 days after injection (supplemental online Fig. 3).
Figure 1.
ASCs reduced retinal damage induced by exposure to light in mice without engraftment. (A): Light-induced retinal degeneration was reduced by ASCs. Representative photographs of hematoxylin and eosin staining are as follows: nontreated group (Aa), light exposure (8,000 lx) plus vehicle-treated group (Ab), and light exposure plus ASC-treated (103 cells per eye, intravitreal administration) group (Ac) at 28 days after light exposure in mice. (Ad): Thickness of the ONL was measured at 28 days after light exposure. The ONL was measured at 240-μm intervals from the optic disc. Scale bar = 50 µm. (B): Light-induced retinal dysfunction was reduced by ASCs. (Ba): Typical traces of dark-adapted electroretinogram responses measured at 28 days after exposure to light. Stimulus flashes were used from −2.92 to 0.98 log-candela seconds/m2. Amplitudes of a- and b-waves of the light exposure (8,000 lx) plus vehicle-treated group (Bb) versus the light exposure plus ASC-treated group (103 cells per eye) group (Bc) are shown. (C): ASCs were not engrafted into the retina. (Ca–Cf): Nontreated. (Cg–Cl): ASCs were injected into the vitreous chamber. (Ca, Cc, Cg, Ci): Images of retinal cross-section. (Cb, Cd, Ch, Cj): Bright-field images. (Ce, Cf, Ck, Cl): Fluorescence images to detect GFP transgenic mouse-derived ASCs. Images of retinal cross-sections showing Hoechst staining (Ce, Ck) and immunostaining with GFP antibody (Cf, Cl). Scale bars = 200 µm. (D): Fluorescence of CAG-EGFP mouse-derived ASCs. (Da): The cultured ASCs were investigated using a fluorescence microscope. Scale bars = 50 µm. The ASCs were injected into the left vitreous, and the ocular fundus (Db, Dc) and the fluorescence of ASCs in the vitreous body (Dd, De) were investigated using a fluorescence retinal microscope. Data are shown as mean ± SEM, n = 7 or n = 8. *, p < .05 versus the light exposure plus vehicle-treated group (Student’s t test). Abbreviations: ASC, adipose-derived stem cell; GFP, green fluorescent protein; ONL, outer nuclear layer.
Protective Effects of ASC-CM Against Cell Damage Induced by H2O2 and Visible Light in 661W Cells
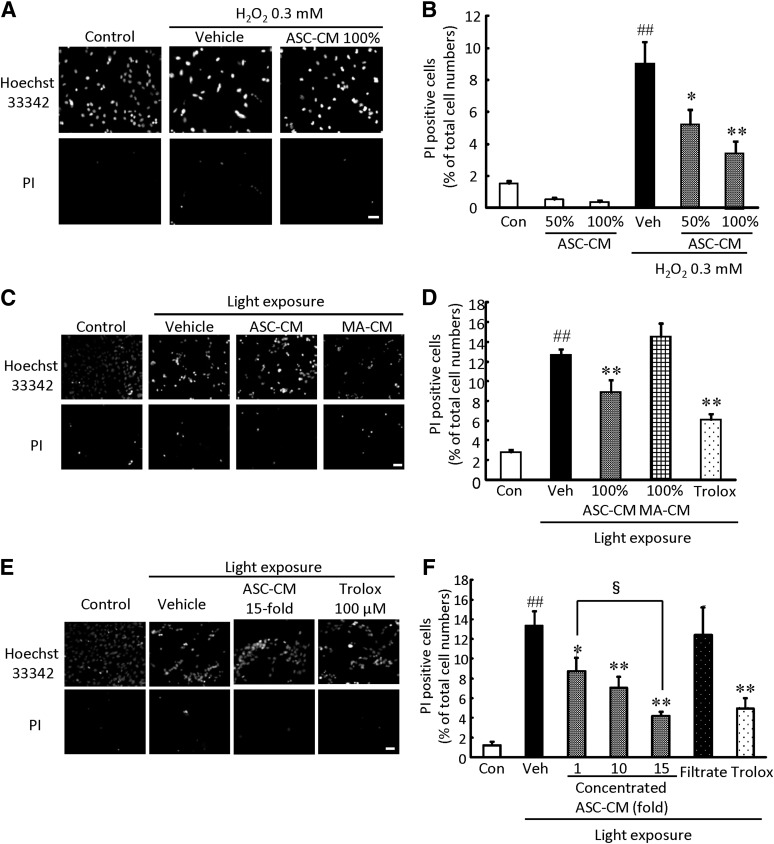
To investigate whether factors secreted from ASCs are associated with retinal protection, we used ASC-CM in vitro. Representative photographs of Hoechst 33342 and PI staining are shown in Figures 2A, 2C, and 2E and in supplemental online Figure 4. Hoechst 33342 stains both live and dead cells, whereas PI stains only dead cells. Pretreatment with ASC-CM at 50% and 100% protected against H2O2-induced cell death in a concentration-dependent manner (Fig. 2B). Pretreatment with ASC-CM at 100% also protected against visible light-induced cell death, whereas mature adipocyte-conditioned medium at 100% did not inhibit cell death (Fig. 2D).
Figure 2.
ASC-CM suppressed H2O2- and light-induced cultured photoreceptor cell death. Representative fluorescence microscopy images showing nuclear staining for Hoechst 33342 and PI after 27 hours of H2O2 (0.3 mM) treatment (A) or 24 hours of light irradiation (2,500 lx) (C, E). (B, D, F): The number of cells exhibiting PI fluorescence was counted, and positive cells were expressed as the percentage of PI- to Hoechst 33342-positive cells. (B): ASC-CM exerted a protective effect against H2O2-induced cell death in a concentration-dependent manner. (D): ASC-CM, but not MA-CM, reduced visible light-induced cell death. (F): Concentrated ASC-CM reduced light-induced cell death in a concentration-dependent manner. Scale bars = 50 µm. Data are shown as mean ± SEM (n = 6). *, p < .05 versus vehicle; **, p < .01; ##, p, < .01 versus control; §, p, < .05. Abbreviations: ASC-CM, adipose-derived stem cell-conditioned medium; Con, control; H2O2, hydrogen peroxide; MA-CM, mature adipocyte-conditioned medium; PI, propidium iodide; Veh, vehicle.
Moreover, we examined whether the neuroprotective effect might be potentiated by concentrated ASC-CM. Pretreatment with ASC-CM protected against visible light-induced cell death in a concentration-dependent manner. ASC-CM concentrated 15-fold significantly decreased the rate of cell death compared with ASC-CM concentrated 1-fold. However, the filtrate (molecular weight cutoff, 3,000) did not affect cell death (Fig. 2F). Cell viability was reduced by H2O2 treatment or light irradiation, and treatment with 10-fold ASC-CM attenuated these reductions (supplemental online Fig. 5). Trolox at 100 μM inhibited cell death and decrease of cell viability.
Protective Effects of ASC-CM Against Light-Induced Retinal Histological Change and Functional Damage in Mice
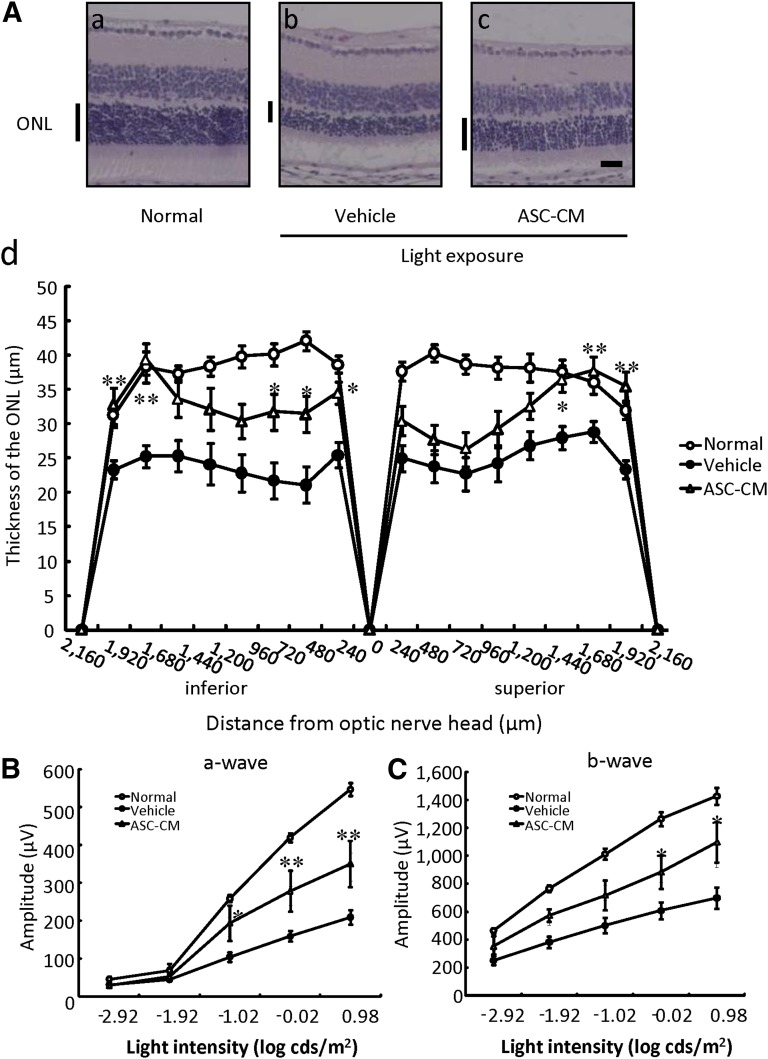
Because ASC-CM showed protective effects in vitro, we investigated its effects in an in vivo model. A typical vitreous volume for mice is considered to be approximately 10 μl; therefore, 1 μl of 150-fold concentrated ASC-CM was injected in vitreous to be equal to in vitro experimental concentration. In the histological evaluation, representative retinal images from the optic nerve were taken at 5 days after light exposure (Fig. 3A). The ONL was markedly thinned in the vehicle group (Fig. 3Ab) versus the normal group (Fig. 3Aa). The ASC-CM-treated group showed suppression of the damage (Fig. 3Ac). The thickness of the outer nuclear layer was measured in 240-μm steps in Figure 3Ad. ASC-CM significantly protected the retinal superior area from 1,680 μm to 2,160 μm and the inferior area from 240 to 720 μm and from 1,920 to 2,160 μm.
Figure 3.
ASC-CM suppressed light-induced retinal damage after light exposure. (A): Light-induced retinal degeneration was reduced by ASC-CM. Representative photographs of hematoxylin and eosin staining are as follows: nontreated group (Aa), light exposure (8,000 lx) plus vehicle-treated group (Ab), and light exposure plus ASC-CM-treated (150-fold concentrated, intravitreal administration) group (Ac) at 5 days after light exposure in mice. (Ad): ONL thickness was measured at 5 days after light exposure. The ONL was measured at 240-μm intervals from the optic disc. (B, C): Representative electroretinogram recording in the nontreated group, the light exposure (8,000 lx) plus vehicle-treated group, and the light exposure plus ASC-CM-treated (150-fold concentrated, intravitreal administration) group. Intensity response functions for dark-adapted a-wave (B) and b-wave (C) amplitudes. The ASC-CM-treated group shows significantly preserved a- and b-wave amplitudes compared with the vehicle group. Scale bar = 25 µm. Data are shown as mean ± SEM (normal [nontreated]: n = 13; vehicle: n = 17; ASC-CM: n = 8). *, p < .05 versus the light exposure plus vehicle-treated group (vehicle); **, p < .01. Abbreviations: ASC-CM, adipose-derived stem cell-conditioned medium; ONL, outer nuclear layer.
In the functional analysis using ERG, both a- and b-wave amplitudes were significantly reduced at 5 days after 8,000 lx of light exposure for 3 hours, and in the ASC-CM-treated group, a decrease in the a- and b-wave amplitudes was significantly prevented compared with the vehicle group (Fig. 3B, 3C).
Cytokine Protein Array From ASC-CM and Mature Adipocyte-Conditioned Medium
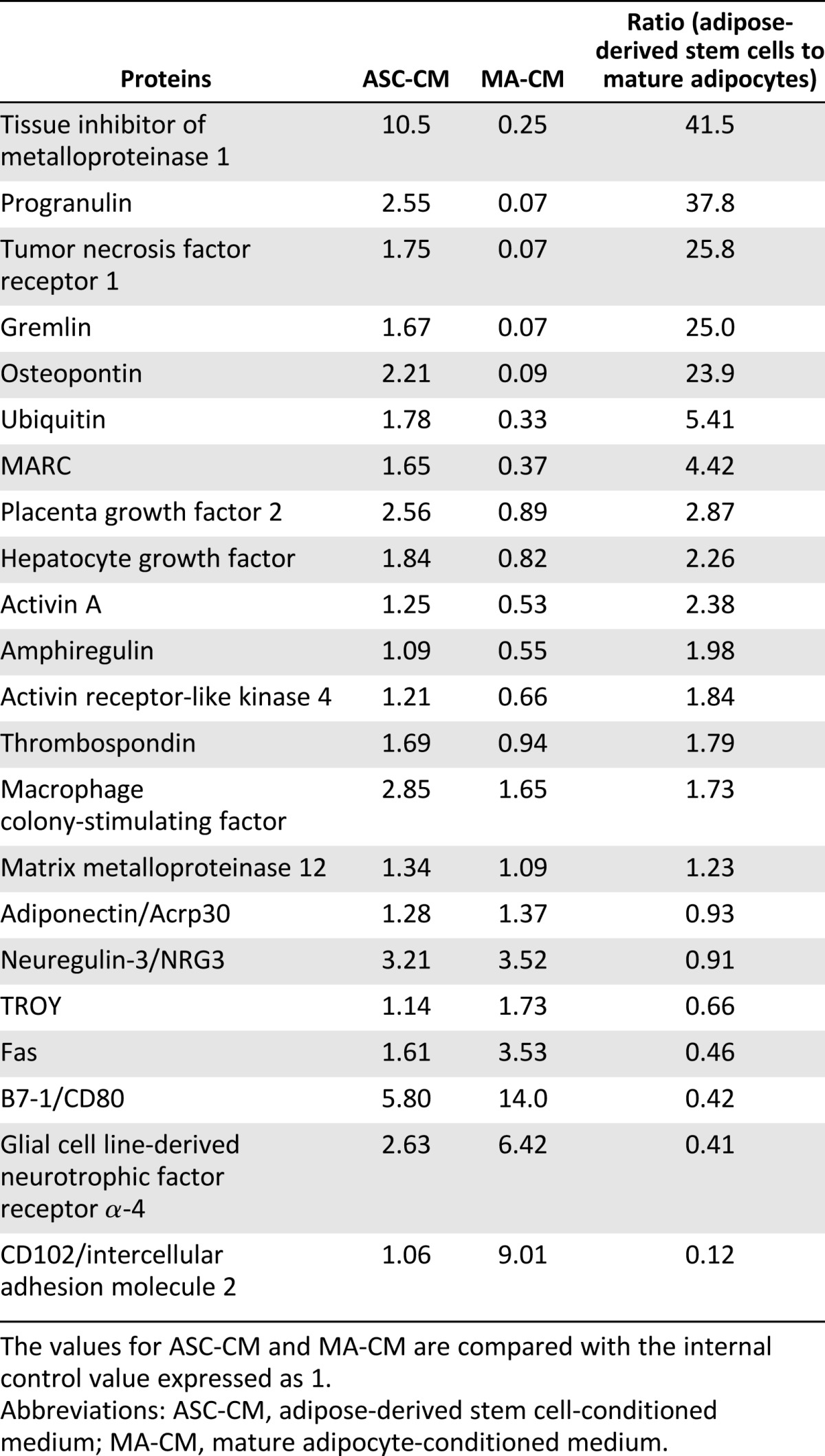
To identify the active factors in ASC-CM and mature adipocyte-conditioned medium, we used the RayBio biotin label-based cytokine antibody array (RayBiotech). First, 22 factors in ASC-CM were selected and showed higher intensity than negative control. As shown in Figures 2 and 3, ASC-CM, but not mature adipocyte-conditioned medium, inhibited retinal cell death in vitro and in vivo. Consequently, we focused on ASC-CM-specific factors (Table 1). Tissue inhibitor of metalloproteinase 1 (TIMP-1) and progranulin were secreted 30-fold in ASCs compared with mature adipocytes.
Table 1.
Protein expression levels in ASC-CM and MA-CM
Protective Effects of Progranulin Against Cell Damage Induced by H2O2 and Visible Light Exposure in 661W Cells
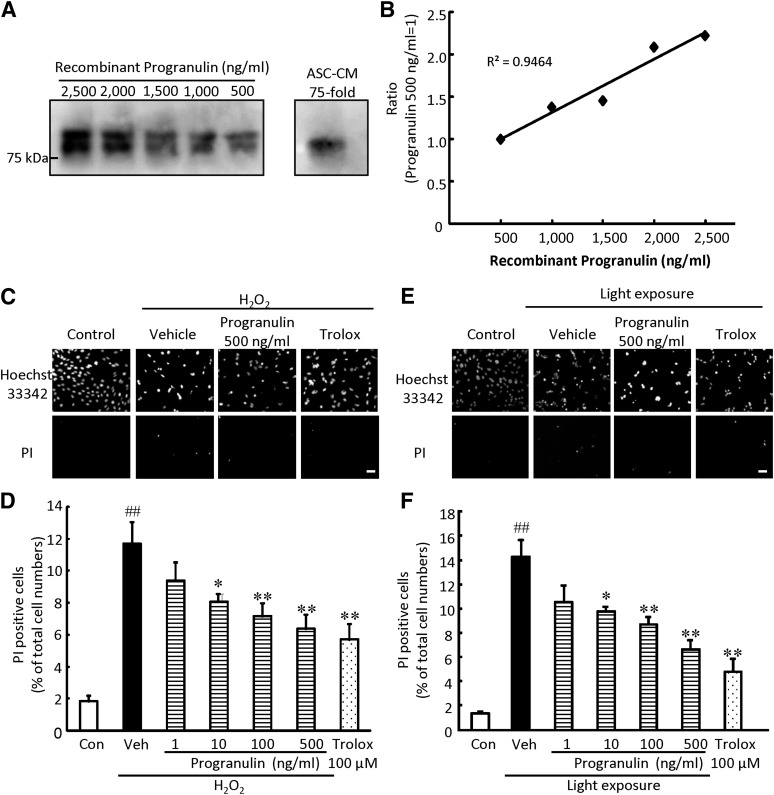
We investigated the protective effect of progranulin against retinal damage. To estimate the quantity of progranulin in ASC-CM, we performed Western blot analysis (Fig. 4A). We electrophoresed the recombinant mouse progranulin at 500 to 2,500 ng/ml and constructed a standard curve by luminescence intensity and confirmed linearity (Fig. 4B). The intensity of 75-fold concentrated ASC-CM was 1.05; therefore, 75-fold concentrated ASC-CM was estimated to contain 574.15 ± 290.07 ng/ml progranulin (n = 5). This result indicates that the ASC-CM contains 7.66 ng/ml of progranulin.
Figure 4.
Progranulin suppressed H2O2- or light-induced cultured photoreceptor cell death. (A, B): Quantitative determination of progranulin in ASC-CM by Western blot. (A): Representative immunoblots showing progranulin protein levels in ASC-CM and recombinant mouse progranulin. The recombinant progranulin was expressed in a mouse myeloma cell line, and post-translational modifications such as glycosylation may contribute to various molecular weights. (B): Standard curve was generated from the density of mouse recombinant progranulin. (C, E): Representative fluorescence microscopy images showing nuclear staining for Hoechst 33342 and PI after 27 hours of H2O2 (0.3 mM) treatment (C) or 24 hours of light exposure (E). (D, F): The number of cells exhibiting PI fluorescence was counted, and positive cells were expressed as the percentage of PI- to Hoechst 33342-positive cells. The number of PI-positive cells increased after H2O2 treatment or light exposure. Progranulin significantly reduced cell death in a concentration-dependent manner. Scale bars = 50 µm. Data are shown as mean ± SEM (n = 6 or n = 9). *, p < .05 versus vehicle; **, p < .01; ##, p < .01 versus control. Abbreviations: ASC-CM, adipose-derived stem cell-conditioned medium; Con, control; H2O2, hydrogen peroxide; PI, propidium iodide; Veh, vehicle.
Representative photographs of Hoechst 33342 and PI staining are shown in Figures 4C and 4E and in supplemental online Figure 4. Pretreatment with progranulin at 1 to 500 ng/ml protected against H2O2- and light-induced cell death in a concentration-dependent manner; the effect was significant for the 10-to-500 ng/ml concentrations (Fig. 4D, 4F). The reduction of cell viability by H2O2 or light irradiation was attenuated by progranulin (supplemental online Fig. 5). Trolox at 100 μM inhibited cell death.
Protective Effect of Progranulin Against Light-Induced Retinal Histological Change
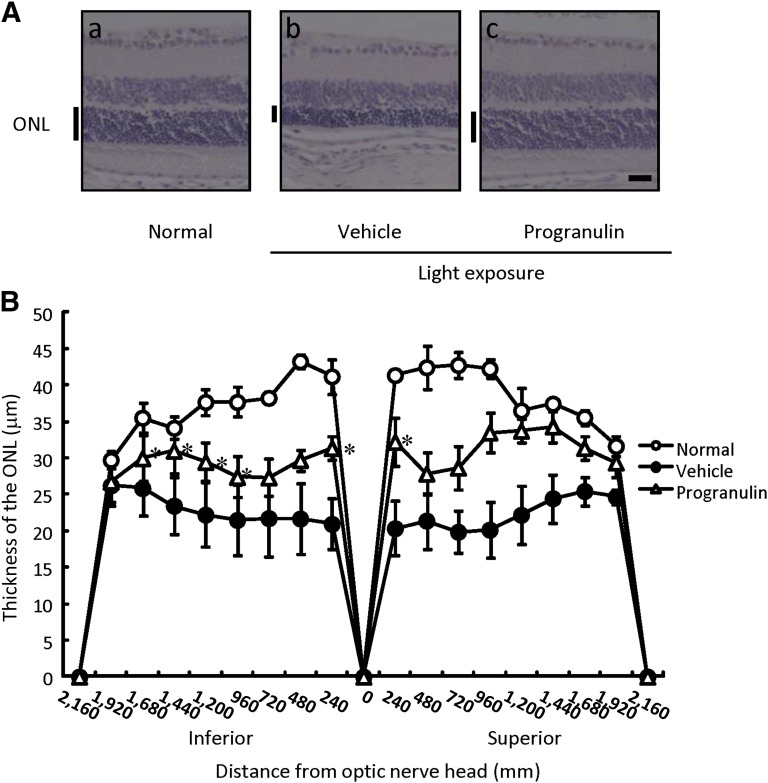
Progranulin has been shown to have a protective effect against light-induced photoreceptor cell death in vitro. We also investigated the protective effect of progranulin in vivo. In the histological evaluation, representative retinal images from the optic nerve were taken at 5 days after light exposure (Fig. 5A). The thickness of the outer nuclear layer was reduced by light exposure, and pretreatment with progranulin significantly suppressed this thinning (Fig. 5B).
Figure 5.
Progranulin reduced retinal damage induced by exposure to light in mice. (A): Representative photographs of hematoxylin and eosin staining are as follows: nontreated group (Aa), light exposure (8,000 lx) plus vehicle-treated group (Ab), and light exposure plus progranulin-treated (200 ng per eye) group (Ac) at 5 days after light exposure. (B): ONL thickness was measured at 5 days after light exposure. The ONL was measured at 240-μm intervals from the optic disc. Scale bar = 25 µm. Data are shown as mean ± SEM (n = 7 or n = 8). *, p < .05 versus the light exposure plus vehicle-treated group (vehicle). Abbreviation: ONL, outer nuclear layer.
Protective Effect of Progranulin by PKC Pathways
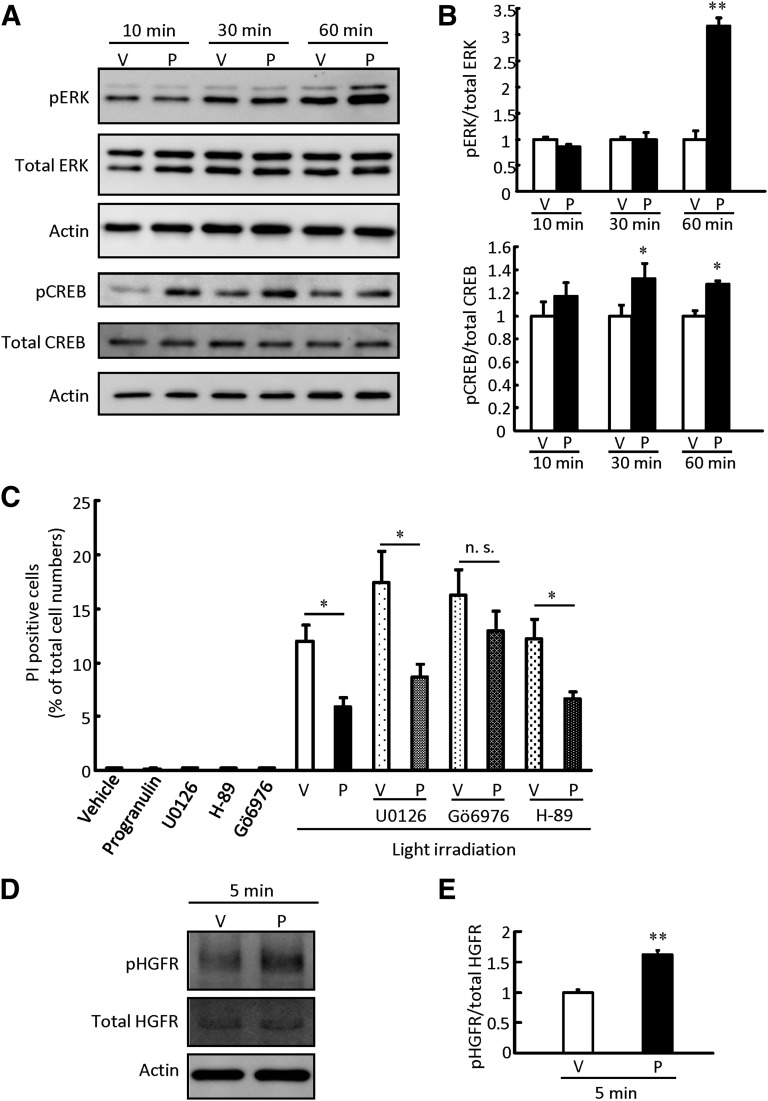
Progranulin showed photoreceptor protective effects in vitro and in vivo, thus we investigated the signal pathway and receptor of progranulin. Progranulin has been reported to activate ERK [34]. In the present study, ERK phosphorylation was enhanced at 60 minutes after progranulin treatment (Fig. 6A, 6B). In contrast, cAMP response element binding protein (CREB), a downstream target of ERK, was phosphorylated faster than ERK (Fig. 6A, 6B). ERK, protein kinase A, and PKC lie upstream of CREB; therefore, we treated 661W cells with each specific inhibitor (U0126, H-89, and Gö 6976, respectively) to identify the progranulin signaling pathway. Gö 6976 attenuated the protective effect of progranulin against light-induced 661W cell death, whereas U0126 and H-89 had no effect (Fig. 6C). Moreover, we investigated the receptor for progranulin using a Mouse Phospho-RTK Array Kit (R&D Systems) and Western blotting. HGF receptor was phosphorylated by progranulin in 5 minutes (Fig. 6D, 6E; supplemental online Table 1), and neurotrophic tyrosine kinase receptor type 3 and macrophage stimulating protein (MSP) receptor were slightly phosphorylated by progranulin (supplemental online Table 1).
Figure 6.
Progranulin exerted a photoreceptor protective effect through the protein kinase C pathway. Representative band images show immunoreactivity against pERK, total ERK, pCREB, and total CREB (A) and phosphorylated HGFR and total HGFR (D) after progranulin treatment (500 ng/ml) at 5, 10, 30, and 60 minutes. (B, E): Quantitative analysis of band densities. Data are shown as mean ± SEM (n = 3). (C): Progranulin (500 ng/ml), U0126 (1,000 nM), H-87 (500 nM), and Gö 6976 (500 nM) were added to 661W cells, and the cells were irradiated with white light (2,500 lx) for 24 hours. Cell death rate was calculated as described in Materials and Methods. Data are shown as mean ± SEM (n = 6). *, p < .05 versus vehicle; **, p < .01. Abbreviations: CREB, cAMP response element binding protein; HGFR, hepatocyte growth factor receptor; n.s., not significant; P, progranulin; pCREB, phosphorylated CREB; pERK, phosphorylated ERK; V, vehicle.
Discussion
In the present study, we demonstrated that ASC-CM, but not mature adipocyte-conditioned medium, suppressed both H2O2- and visible light-induced cell death in 661W cells and that 150-fold concentrated ASC-CM significantly protected against light-induced retinal damage in mice. This study is the first to report that ASC-CM effectively and potently blocks neuronal damage, tissue loss, and functional impairment in a model of retinal degeneration in vitro and in vivo.
Excessive light exposure leads to photoreceptor degeneration in many animals [1, 35]. In the present study, ASCs reduced photoreceptor degeneration without engraftment, concordant with the results of previous studies using BMSCs [7–9]. ASCs can be obtained repeatedly in large quantities in a less invasive manner compared with BMSCs. Moreover, a single injection of ASCs after light exposure reduced light-induced retinal degeneration. Hence, ASCs may be a therapeutic tool for retinal degeneration. In the present study, ASCs were injected into the vitreous and were evaluated at 28 days after light irradiation with expectation of engraftment and long-term effects. In contrast, retinal damage is mostly caused within 5 days after light irradiation, and there are few histological and functional differences compared with 28 days after light irradiation in this experimental model [36]. Consequently, the effects of ASC-CM and progranulin in vivo were investigated at 5 days after light irradiation. An injection of ASCs into the subretinal space may be more effective to engraft and/or to protect by their secretions. Some different effects, especially retinal function, between ASCs and ASC-CM on light-irradiation damage may be attributed to direct cellular interaction.
Light-induced photoreceptor cell death is reportedly induced by various factors [37], such as calcium levels, nitric oxide, reactive oxygen species, mitochondria, and rhodopsin mutation. In vitro, light stress-activated caspases, calpain 2, cathepsin D, and mitochondria-dependent apoptotic pathways, leading to cell death [32]. ASC-CM may inhibit these pathways through factors secreted by ASCs and exert neuroprotective effects.
We investigated the factors secreted by ASCs using a biotin label-based cytokine mouse antibody array kit. The expression levels of 308 mouse target proteins can be detected simultaneously, including cytokines, chemokines, adipokines, growth factors, proteases, soluble receptors, and other proteins. In this study, we collected ASC-CM and mature adipocyte-conditioned medium derived from the same mouse and analyzed the secreted molecules. The obtained results are concordant with previous reports showing that ASCs secreted HGF [14, 15] and macrophage colony-stimulating factor [38]. ASC-CM, but not mature adipocyte-conditioned medium, suppressed retinal cell death; therefore, we selected 22 factors that were often found in ASC-CM to identify the active factors in ASC-CM. The result showed 5 factors (TIMP-1, progranulin, TNF receptor 1, gremlin, and osteopontin; Table 1) to be ASC-specific.
Progranulin is expressed in a variety of peripheral tissues as well as in the adult central nervous system, including cortical and hippocampal pyramidal cells [19]. In the retina, progranulin-a was closely associated with photoreceptors that were lesioned by light exposure and expressed exclusively by microglia in zebrafish [30]. Therefore, progranulin may contribute to the retinal neuroprotective effect of ASC-CM. In the present study, progranulin at 10 ng/ml inhibited both H2O2- and light-induced cell death. It has been reported that pretreatment with HGF at 15 ng/ml increased the cell viability of primary cortical neurons damaged by H2O2. [39] Ciliary neurotrophic factor at 50 ng/ml inhibited serum-deprived retinal ganglion cell death [40]. Taken together, these findings indicate that the cell protective effect of progranulin may be comparable to that of other growth factors in vitro. In the present study, ASC-CM contained 7.66 ng/ml progranulin, a value that is concordant with the observed effective concentrations of progranulin (10–500 ng/ml, Figure 4). TIMP-1, which is the most secreted protease inhibitor of ASCs, showed significant neuroprotective effects at 500 nM (14.3 μg/ml) against in vitro ischemia-reperfusion injury [41]. Hence, progranulin may be one of the key factors in the retinal protective effect of ASC-CM, although other factors may additively contribute to its protective effect. Moreover, progranulin enhanced phosphorylation of HGF receptor, and may activate CREB via the PKC pathways (Fig. 6). Progranulin has been reported to be associated with HGF signaling [42 In contrast, tyrosine kinase receptor type 3 and receptor for macrophage stimulating protein, which has considerable homology with HGF [43], were also phosphorylated by progranulin treatment (supplemental online Table 1). Progranulin may have multiple effects through various receptors, including sortilin [44], and intracellular signaling. Recently, progranulin was reported to be a negative regulator of TNF signaling [29]. Light irradiation induced expression of TNFα [45] in the retina is associated with microglial activation and inflammation, leading to photoreceptor degeneration. We also investigated the association between TNFα and light-induced cell death using a neutralizing antibody for TNF receptor; however, the neutralizing TNF receptor had no effect on light-induced cell death (data not shown). Although TNF signaling was not directly associated with light-induced photoreceptor cell death, ASC-CM and progranulin may contribute to the reduction of harmful inflammatory response in vivo through TNF signaling modulation.
Conclusion
A single injection of ASCs reduced retinal damage induced by light exposure in vivo, and ASC-CM inhibited the retinal damage induced by H2O2 and visible light in vitro and in vivo. Moreover, progranulin, found in ASC-CM, may play a pivotal role in the retinal protective effect against light-induced damage. Taken together, these results suggest that ASCs, ASC-CM, and progranulin may offer more options for different therapeutic approaches, and progranulin may be a potential target for the treatment of degenerative diseases of the retina such as age-related macular degeneration and retinitis pigmentosa.
Supplementary Material
Footnotes
Contributed equally as first authors.
Author Contributions
K.T. and M.Y.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.S., T.O., Y.O., and Y.N.: collection and/or assembly of data, data analysis and interpretation; Y.I., M.S., S.Y., and T.I.: conception and design, provision of study material or patients; H.H.: conception and design, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Noell WK, Walker VS, Kang BS, et al. Retinal damage by light in rats. Invest Ophthalmol. 1966;5:450–473. [PubMed] [Google Scholar]
- 2.Taylor HR, West S, Muñoz B, et al. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110:99–104. doi: 10.1001/archopht.1992.01080130101035. [DOI] [PubMed] [Google Scholar]
- 3.Kang SK, Lee DH, Bae YC, et al. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003;183:355–366. doi: 10.1016/s0014-4886(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 4.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 5.Karussis D, Kassis I, Kurkalli BG, et al. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): A proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008;265:131–135. doi: 10.1016/j.jns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Torrente Y, Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant. 2008;17:1103–1113. doi: 10.3727/096368908787236576. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TV, Bull ND, Hunt DP, et al. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Wang W. Effects of bone marrow mesenchymal stem cell transplantation on light-damaged retina. Invest Ophthalmol Vis Sci. 2010;51:3742–3748. doi: 10.1167/iovs.08-3314. [DOI] [PubMed] [Google Scholar]
- 9.Johnson TV, Bull ND, Martin KR. Identification of barriers to retinal engraftment of transplanted stem cells. Invest Ophthalmol Vis Sci. 2010;51:960–970. doi: 10.1167/iovs.09-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 12.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 13.Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 15.Nakagami H, Maeda K, Morishita R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 16.O’Driscoll C, O’Connor J, O’Brien CJ, et al. Basic fibroblast growth factor-induced protection from light damage in the mouse retina in vivo. J Neurochem. 2008;105:524–536. doi: 10.1111/j.1471-4159.2007.05189.x. [DOI] [PubMed] [Google Scholar]
- 17.Shibuki H, Katai N, Kuroiwa S, et al. Expression and neuroprotective effect of hepatocyte growth factor in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2002;43:528–536. [PubMed] [Google Scholar]
- 18.Egashira Y, Sugitani S, Suzuki Y, et al. The conditioned medium of murine and human adipose-derived stem cells exerts neuroprotective effects against experimental stroke model. Brain Res. 2012;1461:87–95. doi: 10.1016/j.brainres.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed Z, Mackenzie IR, Hutton ML, et al. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation. 2007;4:7. doi: 10.1186/1742-2094-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel R, Daniels E, He Z, et al. Progranulin (acrogranin/PC cell-derived growth factor/granulin-epithelin precursor) is expressed in the placenta, epidermis, microvasculature, and brain during murine development. Dev Dyn. 2003;227:593–599. doi: 10.1002/dvdy.10341. [DOI] [PubMed] [Google Scholar]
- 21.Serrero G, Ioffe OB. Expression of PC-cell-derived growth factor in benign and malignant human breast epithelium. Hum Pathol. 2003;34:1148–1154. doi: 10.1016/s0046-8177(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 22.He Z, Ismail A, Kriazhev L, et al. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res. 2002;62:5590–5596. [PubMed] [Google Scholar]
- 23.Tangkeangsirisin W, Serrero G. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis. 2004;25:1587–1592. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Gao G, Crabb JW, et al. Purification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell line. J Biol Chem. 1993;268:10863–10869. [PubMed] [Google Scholar]
- 25.Zanocco-Marani T, Bateman A, Romano G, et al. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59:5331–5340. [PubMed] [Google Scholar]
- 26.Zhu J, Nathan C, Jin W, et al. Conversion of proepithelin to epithelins: Roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 27.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 28.Yin F, Banerjee R, Thomas B, et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010;207:117–128. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craig SE, Calinescu AA, Hitchcock PF. Identification of the molecular signatures integral to regenerating photoreceptors in the retina of the zebra fish. J Ocul Biol Dis Infor. 2008;1:73–84. doi: 10.1007/s12177-008-9011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikegame Y, Yamashita K, Hayashi S, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13:675–685. doi: 10.3109/14653249.2010.549122. [DOI] [PubMed] [Google Scholar]
- 32.Kanan Y, Moiseyev G, Agarwal N, et al. Light induces programmed cell death by activating multiple independent proteases in a cone photoreceptor cell line. Invest Ophthalmol Vis Sci. 2007;48:40–51. doi: 10.1167/iovs.06-0592. [DOI] [PubMed] [Google Scholar]
- 33.Imai S, Inokuchi Y, Nakamura S, et al. Systemic administration of a free radical scavenger, edaravone, protects against light-induced photoreceptor degeneration in the mouse retina. Eur J Pharmacol. 2010;642:77–85. doi: 10.1016/j.ejphar.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 34.Monami G, Gonzalez EM, Hellman M, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–7110. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- 35.Nickells RW, Zack DJ. Apoptosis in ocular disease: A molecular overview. Ophthalmic Genet. 1996;17:145–165. doi: 10.3109/13816819609057889. [DOI] [PubMed] [Google Scholar]
- 36.Inoue Y, Tsuruma K, Nakanishi T, et al. Role of heparin-binding epidermal growth factor-like growth factor in light-induced photoreceptor degeneration in mouse retina. Invest Ophthalmol Vis Sci. 2013;54:3815–3829. doi: 10.1167/iovs.12-11236. [DOI] [PubMed] [Google Scholar]
- 37.Wenzel A, Grimm C, Samardzija M, et al. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Park BS, Kim WS, Choi JS, et al. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: Evidence of increased growth factor secretion. Biomed Res. 2010;31:27–34. doi: 10.2220/biomedres.31.27. [DOI] [PubMed] [Google Scholar]
- 39.Hu ZX, Geng JM, Liang DM, et al. Protection of hepatocyte growth factor against hydrogen peroxide-induced mitochondria-mediated apoptosis in rat cortical neurons [in Chinese] Sheng Li Xue Bao. 2009;61:247–254. [PubMed] [Google Scholar]
- 40.Lingor P, Tönges L, Pieper N, et al. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- 41.Tejima E, Guo S, Murata Y, et al. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma. 2009;26:1935–1941. doi: 10.1089/neu.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li YH, Chen MH, Gong HY, et al. Progranulin A-mediated MET signaling is essential for liver morphogenesis in zebrafish. J Biol Chem. 2010;285:41001–41009. doi: 10.1074/jbc.M110.138743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshimura T, Yuhki N, Wang MH, et al. Cloning, sequencing, and expression of human macrophage stimulating protein (MSP, MST1) confirms MSP as a member of the family of kringle proteins and locates the MSP gene on chromosome 3. J Biol Chem. 1993;268:15461–15468. [PubMed] [Google Scholar]
- 44.Zheng Y, Brady OA, Meng PS, et al. C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking. PLoS One. 2011;6:e21023. doi: 10.1371/journal.pone.0021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng TF, Turpie B, Masli S. Thrombospondin-1-mediated regulation of microglia activation after retinal injury. Invest Ophthalmol Vis Sci. 2009;50:5472–5478. doi: 10.1167/iovs.08-2877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.