Abstract
Objectives
To describe diet, alcohol, physical activity and tobacco use prospectively, that is, before and 10 months after colonoscopy for patients and their partners.
Design
Prospective cohort study of health behaviour change in patients and partners. Comparison groups are patients receiving a normal result notification (NRN) versus patients receiving an abnormal result notification (ARN). Patients and partners (controls) are also compared.
Setting
5 Scottish hospitals.
Participants
Of 5798 colonoscopy registrations, 2577 (44%) patients met the eligibility criteria of whom 565 (22%) were recruited; 460 partners were also recruited.
Measures
International Physical Activity Questionnaire, Scottish Collaborative Group Food Frequency Questionnaire (includes alcohol), smoking status, sociodemographic characteristics, body mass index, medical conditions, colonoscopy result, Multidimensional Health Locus of Control Scale, behaviour-specific self-efficacy scales.
Results
57% of patients were men, with a mean age of 60.8 years (SE 0.5) and 43% were from more affluent areas. 72% (n=387) of patients received an ARN and 28% (n=149) received an NRN. Response rate of the second questionnaire was 68.9%. Overall, 27% of patients consumed <5 measures of fruit and vegetables/day, 20% exceeded alcohol limits, 50% had low levels of physical activity and 21% were obese. At 10-month follow-up, a 5% reduction in excessive alcohol consumption and an 8% increase in low levels of physical activity were observed among patients; no significant changes occurred in partners. Baseline high alcohol consumption and low physical activity were the strongest predictors of these behaviours at follow-up. Low alcohol self-efficacy and increasing age were associated with poorer health-related behaviours at follow-up for alcohol consumption and physical activity, respectively.
Conclusions
Colonoscopy is associated with marginal beneficial changes in some behaviours but not others. Further work is needed to explore how services can optimise increases in beneficial behaviours and mitigate increases in harmful ones.
Registrations
REC REF 10/S0709/24, UKCRN 9911.
Keywords: Epidemiology, Public Health
Strengths and limitations of this study.
This is the first prospective cohort study on health behaviours in colonoscopy patients in the UK.
We obtained a large sample size and high follow-up rate and were able to use patients’ partners as controls.
Selection biases may have led to our sample comprising healthier patients than in the general colonoscopy population.
Introduction
Illnesses and interactions with health services may motivate patients to change their health behaviours for the better. Such ‘teachable moments’ have been described for changes in smoking behaviour following life transitions, such as pregnancy, and health events, such as screening for lung cancer.1–10 A recent review of the literature concludes that while the term ‘teachable moment’ is used imprecisely—to describe either an opportunity for behavioural change or an event associated with actual change—there is evidence that beneficial behavioural changes can be actively created through appropriate clinician–patient interactions.11 To our knowledge, evidence from the UK that patients who undergo screening for cancer are likely to make improvements in health behaviour is limited to a single pilot study of a health promotion intervention delivered during colorectal cancer screening.12 The evidence from UK observational research that there are spontaneous health behaviour changes after a cancer diagnosis is limited to analyses of population surveys that depend on self-reported cancer diagnosis and found little evidence that a cancer diagnosis motivates health-protective changes.13 Relatives of patients with cancer may also change their behaviour in response to a familial diagnosis, but the evidence is limited to one study on breast cancer.14
Colonoscopy is mainly performed for the diagnosis of colon and rectal cancer and the current UK rates of 80 procedures/10 000 population are likely to increase in the future as screening by flexible sigmoidoscopy is introduced.15 The number of colonoscopies performed through the Scottish bowel screening programme almost doubled from 5358 in 2009 to 9296 in 201116; in England, the number of colonoscopies performed increased by 38% between 2006–2007 and 2009–2010.17 We hypothesised that colonoscopy represents both concepts of a teachable moment for beneficial health behaviour change—it may be a time of concern about a cancer diagnosis and thus be associated with spontaneous change1 18 and it may also present an opportunity for clinicians to promote improvements in behaviours. An abnormal colonoscopy result may be more likely to trigger beneficial changes in behaviour, so we compared behaviours between participants with normal and abnormal test results. Our aim was to describe health-related behaviours before and after a colonoscopy among patients and their partners. Our objective was to carry out a prospective observational study on patients with colonoscopy and their partners using self-reported questionnaire survey data immediately before and 10 months after colonoscopy. As self-efficacy and locus of control are central to several health behaviour theories, including the Health Belief Model,19 Social Cognitive Theory20 and Protection Motivation Theory,21 we assessed their roles using validated questionnaires and also recorded physical activity (PA), tobacco smoking, diet and alcohol consumption. We hypothesised that study participants with high scores of self-efficacy and with ‘internal’ locus of control would change health behaviours for the better following colonoscopy. Although there is no cut-off score to define persons as being high or low self-efficacious Ralph Schwarzer,22 an expert in self-efficacy measurement, recommends conducting a median split, which is to dichotomise the study sample. ‘Health-internals’ believe that the locus of control for health is internal and that one stays or becomes healthy or sick as a result of his or her behaviour, whereas ‘health-externals’ believe that factors which determine their health are such things as ‘powerful others’ and ‘chance’ over which they have a little control.
Methods
Study design
We conducted a prospective cohort study of health behaviour change in patients and their partners before and 10 months after colonoscopy. Comparison groups are patients receiving a normal result notification (NRN) versus patients receiving an abnormal result notification (ARN), such as colorectal cancer, polyps or diverticulitis. Health behaviour change of patients and partners (controls) are also compared.
Setting
Colonoscopy clinics in five hospitals within three Health Board areas in Scotland, UK. Recruitment occurred between September 2010 and August 2011.
Participants
Patients were invited by an advertisement for the study enclosed along with their colonoscopy appointment letter from the hospital. On attendance for colonoscopy, clinic staff obtained verbal consent from patients (and, if present, their partners) to allow the research assistant (RA) to approach them. If a patient did not wish to be approached by the RA, consent was sought to retain non-identifiable data (age, sex and sector level of the postcode) to assess selection bias. The RA asked the consenting participants to complete the baseline (T1) questionnaire and written consent form at home and return both in a prepaid addressed envelope. Patients whose partners were not present were asked to provide the name and contact details of their partner who was subsequently contacted by the RA by telephone about the study.
Eligibility criteria
Patient inclusion criteria were: (1) referred for colonoscopy, (2) no past history of cancer, (3) ≥18 years old and (4) has a partner. Partner inclusion criteria: (1) ≥18 years old and (2) no past history of cancer.
Primary outcome
The primary outcome measure was the extent of change in individual health behaviours before colonoscopy and 10 months later. Consequently, we selected measures that ranked individuals along a distribution of amount of PA, dietary and alcohol intake and tobacco use.
Variables, measures and data sources
Information was self-reported by participants or obtained from medical records.
Heath-related behaviour variables
The International Physical Activity Questionnaire23 24 was used to categorise participants into low, moderate or high levels of PA using metabolic equivalent of task (MET) scores. The Scottish Collaborative Group Food Frequency Questionnaire (SCG FFQ)25 26 was used to measure intakes of foods and alcohol. Current smokers were defined as those who had smoked at least 100 cigarettes in their entire life and currently smoke.
An aggregate risk behaviour score was calculated based on generally accepted requirements for risk factor reduction for most of the main chronic diseases and specifically for prevention of colorectal cancer because colonoscopy is a main investigation for colorectal cancer symptoms.27 28 Specifically, we defined high-risk behaviour using the Scottish government recommendations29 30 as follows: <5 measures/day of fruit and vegetables; <3 portions/day of bread; >4 (men) and >3 (women) units/day of alcohol; current smoker and low PA (equivalent to less than 30 min of moderate intensity activity on at least 5 days of the week). For the purpose of this study, a ‘measure’ as defined in the SCG FFQ was assumed to be equal to a ‘portion’ of fruit or vegetables.
Demographic variables
Participants self-reported their age, sex, level of education, household income, employment status and postcode (to calculate Scottish Index Multiple Deprivation, SIMD31).
Clinical variables
Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2) using self-reported values. Participants were categorised as obese if BMI ≥30 kg/m2. Participants self-reported their medical conditions currently being treated by a doctor. Results of the colonoscopy were obtained from patients’ medical records. Patients were categorised as receiving an NRN or ARN such as colorectal cancer, polyps or diverticulitis.
Psychological variables
Participants’ perceived control over their health-related behaviours was measured using the Multidimensional Health Locus of Control (MHLC) Scale.32 33 The instrument measures three dimensions of control: internal, powerful others and chance. Participants’ self-efficacy was measured using four separate self-efficacy scales (smoking, diet, alcohol and PA) recommended by Schwarzer and colleagues.22 There is no cut-off score to define persons as being high or low self-efficacious, so we split the sample at the median, as recommended by Schwarzer.22
Data collection
The baseline (T1) questionnaire was administered to patients who attended a colonoscopy clinic between September 2010 and August 2011. A prepaid envelope was provided for participants to return the questionnaire after completing it at home. Participants were requested to report health behaviours before colonoscopy. There was no cut-off date for returning the baseline questionnaire. The follow-up questionnaire (T2) was posted 10 months after the clinic date for colonoscopy, between July 2011 and June 2012.
Bias
We attempted to minimise information biases by using, where possible, validated questionnaires and to minimise selection biases by inviting all patients with colonoscopy to participate.
Statistical analysis
Statistical analysis was carried out using Stata/SE V.11.2 software. Means±SEs or medians (IQR) were presented as appropriate following visual assessment of histograms of continuous variables. Differences in characteristics and behaviours between groups were assessed using Pearson's χ2 test for categorical measures and either Student t tests or Mann-Whitney tests for continuous variables as appropriate. Differences in continuous variables between T1 and T2 were assessed using a paired t test or the Wilcoxon signed-rank test. Differences in categorical variables between T1 and T2 were assessed in SPSS V.20 using the McNemar-Bowker test for variables with three or more categories, and the McNemar test for binary variables. To predict health-related behaviour at T2, logistic regression models were undertaken.
Statistical power
Our sample size was based on answering the research question “Do health behaviours change after a major health threat?” We used the variable PA to calculate the statistical power because it has an association with colorectal cancer survival, and detecting colorectal cancer is one of the main reasons for colonoscopy referral. This paper reports a comparison of changes in health behaviours of patients receiving NRN and ARN following colonoscopy. However, the study had initially aimed to detect an increase in PA of 25 or greater MET hours in patients diagnosed with cancer at colonoscopy compared with patients with no cancer, and samples of 46 patients with cancer and 46 patients with no cancer would have been required. The effect size was derived from Satia et al,34 and sample size calculations assumed conventional values of α=0.05 and β=0.20 (giving a power, or 1−β, of 80%). Thus, our study numbers exceeded those required by the initial sample size calculation.
Results
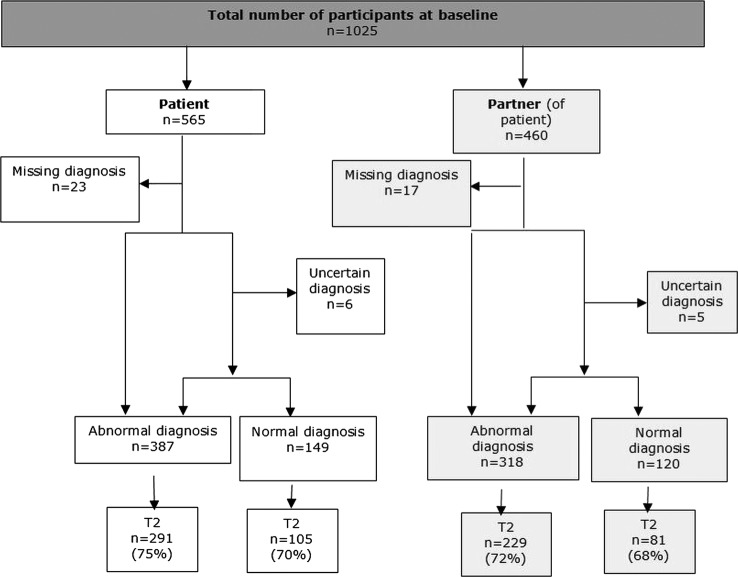
Of 5798 colonoscopy registrations, 2577 patients (44%) met the eligibility criteria for the study of whom 565 (22%) eligible patients and 460 partners were recruited (figure 1). Recruited patients compared with non-recruited patients were significantly older (60.3±0.5 vs 57.2±0.4), more affluent (Carstairs decile 1 and 2, 43% vs 29%) and had a higher proportion of men (57% men vs 50% men). Seventy-two per cent (n=387) of patients received an ARN and 28% (n=149) received an NRN. Overall, 68.9% (n=706) of participants returned a second follow-up questionnaire at T2. The median times for patients and partners to return the baseline questionnaire was 12 days.
Figure 1.
Recruitment of participants.
At baseline (T1), a higher proportion of patients with ARN were men, older, retired and with a household income under £20 000 compared with patients with NRN (table 1). Overall, 27% consumed less than five measures a day of fruit/vegetable, 20% exceeded the recommended alcohol limits, 50% had low levels of PA and 21% were obese. A higher proportion of patients with ARN were not meeting these recommendations but the difference was only significant for PA. Only 11% of patients reported current smoking. When combined, overall 52% of participants had two or more risk behaviours, which comprised 55% of ARN and 42% of NRN patients.
Table 1.
Patient and partner baseline characteristics
| Patients (n=536) |
Partners of patients (n=438) |
||||||
|---|---|---|---|---|---|---|---|
| All (n=536) | ARN (n=387) | NRN (n=149) | p Value* | ARN (n=318) | NRN (n=120) | p Value† | |
| Sex | |||||||
| Male | 308 (57) | 236 (61) | 72 (48) | 0.008 | 121 (38) | 56 (47) | 0.101 |
| Female | 228 (43) | 151 (39) | 77 (52) | 197 (62) | 64 (53) | ||
| Age (years): mean±SE | 60.8±0.5 | 62.1±0.6 | 57.6±1.1 | <0.001 | 62.0±0.6 | 56.8±1.2 | <0.001 |
| SIMD quintile | |||||||
| 1 (most deprived) | 41 (8) | 31 (8) | 10 (7) | 0.979 | 26 (9) | 9 (8) | 0.425 |
| 2 | 76 (14) | 56 (15) | 20 (14) | 40 (13) | 9 (8) | ||
| 3 | 91 (17) | 65 (17) | 26 (18) | 48 (16) | 25 (22) | ||
| 4 | 131 (25) | 93 (25) | 38 (26) | 78 (26) | 28 (24) | ||
| 5 (least deprived) | 186 (35) | 134 (35) | 52 (36) | 113 (37) | 44 (38) | ||
| Highest level of education | |||||||
| School completed | 282 (53) | 208 (54) | 74 (50) | 0.578 | 171 (54) | 51 (43) | 0.092 |
| College/university | 197 (37) | 137 (35) | 60 (40) | 120 (38) | 55 (46) | ||
| Postgraduation degree completed | 57 (11) | 42 (11) | 15 (10) | 26 (8) | 14 (12) | ||
| Employment status | |||||||
| Self-employed/paid employment | 231 (43) | 153 (40) | 78 (52) | 0.010 | 128 (41) | 71 (59) | 0.001 |
| Not employed | 14 (3) | 11 (3) | 3 (2) | 3 (1) | 5 (4) | ||
| Retired from paid work | 252 (47) | 196 (51) | 56 (38) | 154 (49) | 38 (32) | ||
| Looking after family-home | 22 (4) | 12 (3) | 10 (7) | 24 (8) | 4 (3) | ||
| Long-term sick or disabled | 15 (3) | 13 (3) | 2 (1) | 7 (2) | 2 (2) | ||
| Household income | |||||||
| Under £20 000 | 138 (30) | 108 (32) | 30 (25) | 0.040 | 85 (31) | 29 (28) | 0.002 |
| £20 000–29 999 | 99 (21) | 79 (23) | 20 (17) | 71 (26) | 12 (12) | ||
| £30 000–39 000 | 60 (13) | 46 (13) | 14 (12) | 43 (16) | 13 (13) | ||
| £40 000–49 000 | 61 (13) | 42 (12) | 19 (16) | 25 (9) | 13 (13) | ||
| £50 000 and above | 104 (23) | 66 (19) | 38 (31) | 53 (19) | 37 (36) | ||
| Physical activity (MET-min/week) | 4152 (1997, 7668) | 4381 (2157, 7338) | 0.801 | 4250 (1980, 8406) | 4134 (1964, 8178) | 0.917 | |
| Risk behaviour | |||||||
| <5 measures/day of fruit and vegetables | 93 (27) | 70 (28) | 23 (25) | 0.493 | 62 (29) | 20 (27) | 0.764 |
| <3 measures/day of bread and consume white bread only | 75 (15) | 54 (15) | 21 (15) | 0.989 | 49 (16) | 12 (10) | 0.126 |
| >4 (men) and >3 (women) units/day of alcohol | 88 (20) | 67 (22) | 21 (17) | 0.267 | 36 (14) | 26 (28) | 0.002 |
| Current smoker | 54 (11) | 36 (10) | 18 (13) | 0.320 | 36 (12) | 19 (17) | 0.169 |
| Low physical activity level | 203 (50) | 159 (54) | 44 (40) | 0.009 | 135 (53) | 40 (41) | 0.050 |
| Obese (BMI ≥30 kg/m2) | 92 (21) | 70 (23) | 22 (18) | 0.355 | 44 (19) | 25 (26) | 0.205 |
| Risk behaviour score group | |||||||
| 1 or less | 154 (48) | 102 (45) | 52 (58) | 0.026 | 88 (45) | 31 (44) | 0.930 |
| 2 or more | 164 (52) | 127 (55) | 37 (42) | 108 (55) | 39 (56) | ||
Bold font represents p values of ≤0.05. Italic font are p>0.05.
*p Values for difference between patients.
†p Values for difference between partners.
ARN, abnormal result notification; BMI, body mass index; MET, metabolic equivalent of task; NRN, normal result notification and SIMD, Scottish Index Multiple Deprivation.
There were no significant differences between patients with abnormal and normal results with respect to self-efficacy for smoking cessation, PA, diet and alcohol (table 2). Partners of patients with normal results were more likely to have low self-efficacy for smoking cessation.
Table 2.
Self-efficacy and scores at baseline (patients on ARN and NRN and their partners): median (IQR)
| Patients |
Partners of patients |
|||||
|---|---|---|---|---|---|---|
| ARN (n=387) | NRN (n=149) | p Value* | ARN (n=318) | NRN (n=120) | p Value† | |
| Smoking cessation self-efficacy‡ | ||||||
| Score (range 5–20) | 12 (9, 16) | 13 (9, 18) | 0.355 | 11 (9, 14) | 10 (7, 12) | 0.055 |
| Low (5–12): n (%) | 20 (51) | 8 (42) | 0.512 | 20 (54) | 14 (82) | 0.045 |
| High (13–20): n (%) | 19 (49) | 11 (58) | 17 (46) | 3 (18) | ||
| Physical activity self-efficacy | ||||||
| Score (range 5–20) | 14 (11, 15) | 14 (12, 18) | 0.073 | 14 (11, 17) | 14 (10, 16) | 0.948 |
| Low (5–14): n (%) | 205 (55) | 76 (52) | 0.530 | 163 (53) | 65 (56) | 0.588 |
| High (15–20): n (%) | 167 (45) | 70 (48) | 144 (47) | 51 (44) | ||
| Diet self-efficacy | ||||||
| Score (range 5–20) | 15 (14, 18) | 15 (14, 20) | 0.121 | 15 (14, 19) | 15 (12, 20) | 0.875 |
| Low (5–15): n (%) | 230 (62) | 84 (59) | 0.566 | 191 (62) | 73 (64) | 0.731 |
| High (16–20): n (%) | 144 (39) | 59 (41) | 116 (38) | 41 (36) | ||
| Alcohol resistance self-efficacy | ||||||
| Score (range 3–12) | 9 (7, 12) | 10 (7, 12) | 0.657 | 11 (8, 12) | 10 (7, 12) | 0.530 |
| Low (3–10): n (%) | 187 (55) | 71 (55) | 0.956 | 133 (48) | 55 (51) | 0.511 |
| High (11–12): n (%) | 151 (45) | 58 (45) | 146 (52) | 52 (49) | ||
| MHLC Internal | ||||||
| Score (range 6–36) | 25 (22, 28) | 26 (21, 29) | 0.381 | 26 (22, 28) | 26 (22, 29) | 0.696 |
| Low (6–25): n (%) | 203 (53) | 72 (49) | 0.415 | 152 (48) | 59 (50) | 0.783 |
| High (26–36): n (%) | 183 (47) | 76 (51) | 164 (52) | 60 (50) | ||
| MHLC Chance | ||||||
| Score (range 6–36) | 17 (13, 21) | 17 (13, 21) | 0.335 | 18 (14, 22) | 17 (13, 22) | 0.788 |
| Low (6–17): n (%) | 197 (51) | 83 (56) | 0.332 | 154 (49) | 61 (51) | 0.638 |
| High (18–36): n (%) | 189 (49) | 66 (44) | 162 (51) | 58 (49) | ||
| MHLC Powerful Others | ||||||
| Score (range 6–36) | 18 (14, 23) | 17 (12, 21) | 0.032 | 18 (13, 22) | 15 (11, 20) | 0.018 |
| Low (6–17): n (%) | 186 (48) | 77 (52) | 0.442 | 154 (49) | 75 (63) | 0.008 |
| High (18–36): n (%) | 199 (52) | 71 (48) | 162 (51) | 44 (37) | ||
Bold font represents p values of ≤0.05. Italic font are p≥0.05.
*p values for difference between patients.
†p values for difference between partners.
‡Current smokers only.
ARN, abnormal result notification; MHLC, Multidimensional Health Locus of Control; NRN, normal result notification.
Overall, there was a 5% reduction in respondents who exceeded alcohol consumption guidance and an 8% increase in the proportion with low PA (table 3). The proportionate increase in low PA was similar in patients with abnormal and normal colonoscopy results but statistically significant only among those with abnormal findings (which might be explained by the larger sample size in the abnormal category). When risk behaviours were aggregated, there was no overall change in behaviours in any patient group. We found no significant change in health behaviours of partners, irrespective of the colonoscopy result of the patient (data not shown).
Table 3.
Change in patient risk behaviours between T1 and T2: n (%)
| All patients (n=418)* |
ARN (n=387) |
NRN (n=149) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | p Value† | T1 | T2 | p Value† | T1 | T2 | p Value‡ | |
| Risk behaviour | |||||||||
| <5 measures/day of fruit and vegetables | 38 (23) | 40 (24) | 0.845 | 28 (24) | 30 (26) | 0.815 | 9 (20) | 7 (15) | 0.687 |
| <3 measures/day of bread and consume white bread only | 46 (12) | 43 (11) | 0.766 | 34 (13) | 30 (11) | 0.585 | 11 (11) | 10 (10) | 1.000 |
| >4 (men) and >3 (women) units/day of alcohol | 58 (21) | 45 (16) | 0.047 | 41 (22) | 31 (17) | 0.064 | 14 (17) | 13 (16) | 1.000 |
| Current smoker | 27 (8) | 24 (7) | 0.250 | 15 (6) | 13 (5) | 0.500 | 10 (10) | 9 (9) | 1.000 |
| Low physical activity level | 131 (50) | 150 (58) | 0.003 | 98 (54) | 112 (61) | 0.019 | 25 (40) | 29 (46) | 0.055 |
| Moderate physical activity | 37 (14) | 41 (16) | 24 (13) | 28 (15) | 9 (14) | 11 (17) | |||
| High physical activity | 92 (35) | 69 (27) | 61 (33) | 43 (24) | 29 (46) | 23 (37) | |||
| Obese (BMI≥30 kg/m2) | 56 (19) | 64 (22) | 0.152 | 43 (21) | 49 (24) | 0.238 | 10 (14) | 11 (15) | 1.000 |
| Risk behaviour score group (number of risk behaviours) | |||||||||
| 1 or less | 84 (54) | 80 (51) | 0.572 | 52 (49) | 47 (44) | 0.359 | 29 (67) | 30 (70) | 1.000 |
| 2 or more | 73 (47) | 77 (49) | 55 (51) | 60 (56) | 14 (33) | 13 (30) | |||
Bold font represents p values of ≤0.05. Italic font are p>0.05.
*Includes patients with missing diagnosis.
†p values for difference between T1 and T2 in patients with an abnormal diagnosis
‡p values for difference between T1 and T2 in patients with a normal diagnosis
ARN, abnormal result notification; BMI, body mass index; NRN, normal result notification.
We used multiple logistic regression analyses to explore the predictors of the two behaviours that changed significantly over time: low PA and high alcohol intake (table 4). Low PA at baseline was the strongest predictor of low PA at T2 among patients, and increasing age was also associated with lower PA. Sex, socioeconomic circumstances, comorbidities, colonoscopy findings and self-efficacy did not predict changes in PA over time.
Table 4.
Predictors of low PA at T2 in patients (n=268)
| OR | 95% CI | p Value | |
|---|---|---|---|
| Low PA at T1 (vs high) | 26.2 | (9.57 to 71.7) | <0.001 |
| Age (years) | 1.07 | (1.02 to 1.12) | 0.005 |
| Female sex (vs male) | 0.73 | (0.29 to 1.81) | 0.495 |
| SIMD quintile | |||
| 1 | 1.00 | ||
| 2 | 0.98 | (0.14 to 6.67) | 0.981 |
| 3 | 1.65 | (0.23 to 11.8) | 0.618 |
| 4 | 4.93 | (0.77 to 31.6) | 0.093 |
| 5 | 1.70 | (0.29 to 10.1) | 0.558 |
| Any medical conditions (vs none) | 1.13 | (0.44 to 2.93) | 0.801 |
| ARN (vs NRN) | 1.17 | (0.44 to 3.13) | 0.749 |
| Raw MHLC Internal score | 0.96 | (0.88 to 1.05) | 0.421 |
| Raw MHLC Chance score | 0.99 | (0.91 to 1.07) | 0.775 |
| Raw MHLC Powerful Others score | 1.06 | (0.98 to 1.15) | 0.126 |
| Raw PA self-efficacy score | 0.99 | (0.96 to 1.02) | 0.517 |
| Raw time spent sitting per day (including motor vehicle) | 1.00 | (0.99 to 1.00) | 0.922 |
| Constant | 0.004 | (0.00 to 0.26) | 0.009 |
Bold font represents p values of ≤0.05. Italic font are p>0.05.
ARN, abnormal result notification; MHLC, Multidimensional Health Locus of Control; NRN, normal result notification; PA, physical activity; SIMD, Scottish Index Multiple Deprivation.
Excessive alcohol consumption at T2 was most strongly determined by excessive consumption at baseline (table 5). Lower self-efficacy at T1 was associated with higher alcohol consumption at T2. Age, sex, socioeconomic circumstances, comorbidities and colonoscopy findings were not associated with higher alcohol intake at follow-up.
Table 5.
Predictors of high alcohol intake at T2 in patients (n=232)
| OR | 95% CI | p Value | |
|---|---|---|---|
| >4 (men) and >3 (women) units/day alcohol at T1 | 12.4 | (4.83 to 31.5) | <0.001 |
| Age (years) | 0.99 | (0.95 to 1.03) | 0.601 |
| Female sex (vs male) | 1.02 | (0.39 to 2.63) | 0.975 |
| SIMD quintile | |||
| 1 | 1.00 | ||
| 2 | 4.87 | (0.48 to 49.5) | 0.181 |
| 3 | 3.23 | (0.33 to 31.5) | 0.313 |
| 4 | 1.23 | (0.15 to 10.1) | 0.847 |
| 5 | 1.96 | (0.24 to 15.8) | 0.525 |
| Any medical conditions (vs none) | 0.66 | (0.23 to 1.89) | 0.441 |
| Abnormal diagnosis (vs normal) | 0.83 | (0.29 to 2.34) | 0.725 |
| Raw MHLC Internal score | 1.00 | (0.92 to 1.08) | 0.988 |
| Raw MHLC Chance score | 0.97 | (0.88 to 1.07) | 0.556 |
| Raw MHLC Powerful Others score | 1.00 | (0.93 to 1.08) | 0.905 |
| Raw alcohol self-efficacy score | 0.70 | (0.58 to 0.84) | <0.001 |
| Constant | 3.74 | (0.04 to 360.3) | 0.572 |
Bold font represents p values of ≤0.05. Italic font are p>0.05.
MHLC, Multidimensional Health Locus of Control; SIMD, Scottish Index Multiple Deprivation.
Discussion
Colonoscopy was associated with marginal spontaneous changes (ie, without a behavioural intervention) in some health-related behaviours. We found that there was a 5% reduction in excessive alcohol consumption (a beneficial change) but an 8% increase in low levels of PA (a change for the worse) 10 months after colonoscopy. There were no significant changes in health behaviours among patients’ partners, suggesting that behavioural changes in patients with colonoscopy were not necessarily part of wider trends that might influence the health behaviours. No behavioural advice was given to patients as part of their investigations, and thus colonoscopy might be regarded as a teachable moment in which spontaneous changes are triggered. However, colonoscopy also represents an interaction with health services that might be optimised to encourage improvements in health-related behaviours. At baseline, we found that patients receiving ARN and their partners scored significantly higher than patients receiving NRN and their partners on the MHLC Powerful Others scale, indicating that patients with ARN and their partners believed more strongly that health professionals were responsible for their health and health outcomes. Thus, patients receiving ARN, in particular, may be receptive to health promotion advice from health professionals.
Our conclusion that colonoscopy may be regarded as a teachable moment is similar to a recent systematic review of 11 articles about the impact of cancer screening (9 of which investigated the impact of lung screening on smoking cessation) which concluded that cancer screening ‘might’ be a teachable moment for health behaviour change.35 Research about change in health behaviours after diagnosis of chronic health conditions indicates, at best, only modest changes.36 Thus, based on the current evidence, it remains uncertain whether and the extent to which major health events represent teachable moments. Health behaviours are likely to be influenced by a complex mix of factors, so that in addition to any beneficial teachable moment effects, ongoing symptoms that prompted colonoscopy may also affect behaviour. These may explain reductions in high levels of PA and concomitant increases in low levels.
We found that baseline health behaviours for low PA and excessive alcohol consumption were the strongest predictors of the same behaviours 10 months after colonoscopy but, additionally, increasing age predicted lower PA and lower self-efficacy around alcohol-predicted excessive alcohol consumption at follow-up. Among our patient sample, 11% smoked, which is much lower than the Scottish general adult population prevalence of 23%.37
This study found that a low level of PA at the time of colonoscopy and increasing age were predictive of a low level of PA 10 months postcolonoscopy. Furthermore, high alcohol intake at the time of colonoscopy and low alcohol self-efficacy were predictive of high alcohol intake postcolonoscopy. Of note, fatalism (MHLC Chance score) was not predictive of any differential health behaviour change. ARN was not predictive of health behaviour change following this health event, which is in contrast to studies of smokers undergoing screening for lung disease.38 39 A cross-sectional study of over 10 000 smokers at 2–3 years postscreening for chronic obstructive pulmonary disease found that those with a first-time positive result were significantly more likely to stop smoking than those with a negative result.38 A cross-sectional study of 134 active smokers who underwent spiral CT screening found that 62% of those with a positive result either stopped or decreased smoking, whereas only 46% with a negative result did so.39 Thus, the effect of test results following screening for disease on health-related behaviour appears to vary according to type of screen (eg, colonoscopy vs spiral CT), reason for screening (eg, colon disease vs lung disease) and health-related behaviour (eg, alcohol vs smoking).
Our study has strengths and limitations. This is the first prospective observational study to report health-related behaviours of patients and their partners before and 10 months after colonoscopy. We used validated questionnaire survey tools, obtained a relatively large sample for a prospective cohort study and achieved high follow-up rates of 69%. There are, however, several study limitations. First, the sample may not be representative of all patients undergoing colonoscopy. The response rate of eligible patients was 22%, and there was some selection bias towards a more affluent, older and male population. Socioeconomic affluence may explain why our sample had a lower smoking prevalence than the general population. Patients who participate in the newly introduced national colorectal cancer screening programme also may be more likely to be motivated to change their behaviour. Second, while participants were requested to self-report health behaviours before the colonoscopy and as soon as possible thereafter, the baseline questionnaire was completed after the colonoscopy when participants may already have been influenced and starting to make some changes and there was no cut-off date for returning the baseline questionnaire. Thus, the observed changes for the better or worse in health behaviours may be an underestimation of the extent of change. Third, although valid and reliable survey instruments were used, self-reported data are susceptible to expectation biases and other misclassification effects.40 However, our principal interest was in behavioural change rather than absolute prevalences of behaviours, and thus over-reporting or under-reporting of certain behaviours would not necessarily invalidate our findings on whether they changed after colonoscopy. The observed decreases in excessive alcohol consumption and in PA following colonoscopy may not be caused by the procedure but by other confounding factors that we have not identified. We are not aware of any health promotion activity associated with colonoscopy as it was delivered within the participating hospitals and our questionnaires were designed not to imply any favourable behaviours. The fact that there were no observed changes in partners and no differences between patients receiving ARN and NRN suggests that the observed behavioural changes in patients were related to the colonoscopy.
Healthcare settings are recognised as important loci for promoting health behaviour change41–43 and health events have been conceptualised as a ‘teachable moment’, particularly in relation to smoking cessation.1–10 Conceptualising health events as teachable moments may be appealing to policymakers and clinicians because they represent an opportunity to introduce low-intensity interventions to change modifiable risk of health-related behaviours to prevent disease, and a recent review has identified nine lifestyle interventions at the point of cancer screening to take advantage of this health event as a ‘teachable moment’, including two studies of a multiple lifestyle intervention offered to people who had undergone colonoscopy and had adenomas removed.44 45
For future research, developing and testing the effect of low-intensity interventions (eg, self-efficacy enhancement) to further reduce alcohol consumption may be appropriate because patients appear to spontaneously reduce alcohol consumption following colonoscopy, whereas more intense interventions may be required for health-related behaviours that do not change (eg, diet) or change for the worse (eg, PA). A qualitative study to understand why patients spontaneously change some health behaviours but not others following a major health event will add to understanding about the utility of teachable moments for public health.
Supplementary Material
Acknowledgments
The authors take this opportunity to thank hospital clinical staff for assisting in recruitment and patients and partners who participated in the study.
Footnotes
Contributors: GH designed and managed the study, obtained ethical approval and drafted the paper. DM designed and managed the study, obtained ethical approval and revised the paper. ROC, NC, AC, KS, BD and AB designed the study and revised the paper. LFM designed the study, wrote the statistical analysis plan, cleaned and analysed the data and revised the paper. SF advised on the statistical analysis plan and analysed the data.
Funding: This work was funded by Chief Scientist Office, Scotland (grant number: CZH/4/567).
Competing interests: None.
Ethics approval: The study received West of Scotland Medical Research Ethics Committee approval (REC REF 10/S0709/24).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The relevant anonymised patient-level data are available on reasonable request from the corresponding author.
References
- 1.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res 2003;18:156–70 [DOI] [PubMed] [Google Scholar]
- 2.Gritz ER, Fingeret MC, Vidrine DJ, et al. Successes and failures of the teachable moment: smoking cessation in cancer patients. Cancer 2006;106:17–27 [DOI] [PubMed] [Google Scholar]
- 3.Sharp L, Johansson H, Fagerström K, et al. Smoking cessation among patients with head and neck cancer: cancer as a ‘teachable moment. Eur J of Cancer Care (Engl) 2008;17:114–19 [DOI] [PubMed] [Google Scholar]
- 4.McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Control 2003;10:325–33 [DOI] [PubMed] [Google Scholar]
- 5.Simmons VN, Vidrine JI, Brandon TH. Smoking cessation counseling as a teachable moment for skin cancer prevention: pilot studies. Am J Health Behav 2008;32:137–45 [DOI] [PubMed] [Google Scholar]
- 6.Pollak KI, Denman S, Gordon KC, et al. Is pregnancy a teachable moment for smoking cessation among US Latino expectant fathers? A pilot study. Ethn Health 2010;15:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Warner DO. Surgery as a teachable moment for smoking cessation. Anesthesiology 2010;112:102–7 [DOI] [PubMed] [Google Scholar]
- 8.Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol 2010;202:135.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi L, Iguchi MY. ‘Risk homeostasis’ or ‘teachable moment’? The interaction between smoking behavior and lung cancer screening in the Mayo Lung Project. Tob Induc Dis 2011;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dohnke B, Ziemann C, Will KE, et al. Do hospital treatments represent a ‘teachable moment’ for quitting smoking? A study from a stage-theoretical perspective. Psychol Health 2012;27:1291–307 [DOI] [PubMed] [Google Scholar]
- 11.Lawson PJ, Flocke SA. Teachable moments for health behavior change: a concept analysis. Patient Educ Couns 2009;76:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robb KA, Power E, Kralj-Hans I, et al. The impact of individually-tailored lifestyle advice in the colorectal cancer screening context: a randomised pilot study in North-West London. Prev Med 2010;51:505–8 [DOI] [PubMed] [Google Scholar]
- 13.Williams K, Steptoe A, Wardle J. Is a cancer diagnosis a trigger for health behaviour change? Findings from a prospective, population-based study. Br J Cancer 2013;108:2407–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemon SC, Zapka JG, Clemow L. Health behavior change among women with recent familial diagnosis of breast cancer. Prev Med 2004;39:253–62 [DOI] [PubMed] [Google Scholar]
- 15.NHS Atlas of Variation in Healthcare 2011 Cancer and Tumours. Map 1: Rate of colonoscopy procedures and flexisigmoidoscopy procedures per population by PCT. http://www.rightcare.nhs.uk/atlas/downloads/CancerMaps_AoV_2011.pdf (accessed 4 Jun 2013).
- 16.http://www.isdscotland.org/Health-Topics/Hospital-Care/Publications/2012-09-25/IP_DC_OP_Procedures_Sep12.xls (accessed 18 Jan 2013).
- 17.Department of Health. 2010. Further Analysis of HES Endoscopy Data, DH Knowledge & Intelligence Team, November.
- 18.McBride CM, Puleo E, Pollak KI, et al. Understanding the role of cancer worry in creating a ‘teachable moment’ for multiple risk factor reduction. Soc Sci Med 2008;66:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glanz K, Rimer BK, Lewis FM (eds). Health Behavior and Health Education: Theory, Research and Practice. 3rd edition. San Francisco: Jossey Bass, 2002.
- 20.Bandura A. Self-efficacy: the exercise of control. New York: WH Freeman and Company, 1997 [Google Scholar]
- 21.Rogers RW. A protection motivation theory of fear appeals and attitude change. J Psychol 1975;91:93–114 [DOI] [PubMed] [Google Scholar]
- 22.Schwarzer R. Everything you wanted to know about the General Self-Efficacy Scale but were afraid to ask. 2011. http://userpage.fu-berlin.de/%7Ehealth/faq_gse.pdf (accessed 27 Jan 2013).
- 23.http://www.ipaq.ki.se/downloads.htm (accessed 11 Jan 2013).
- 24.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95 [DOI] [PubMed] [Google Scholar]
- 25.http://www.foodfrequency.org.uk (accessed 11 Jan 2013).
- 26.Masson LF, McNeill G, Tomany JO, et al. Statistical approaches for assessing the relative validity of a food frequency questionnaire: use of correlation coefficients and the kappa statistic. Public Health Nutr 2003;6:313–21 [DOI] [PubMed] [Google Scholar]
- 27.http://www.who.int/dietphysicalactivity/en/ (accessed 11 Jan 2013).
- 28.World Cancer Research Fund/American Institute for Cancer Research Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: AICR, 2007 [Google Scholar]
- 29.Scottish Government Healthy eating, active living: an action plan to improve diet, increase physical activity and tackle obesity (2008–2011). Edinburgh: Scottish Government, 2008. http://www.scotland.gov.uk/Resource/Doc/228860/0061963.pdf (accessed 27 Sep 2013). [Google Scholar]
- 30.Scottish Government Changing Scotland's relationship with alcohol: a framework for action. Edinburgh: Scottish Government, 2009. http://www.scotland.gov.uk/Resource/Doc/262905/0078610.pdf (accessed 27 Sep 2013). [Google Scholar]
- 31.http://simd.scotland.gov.uk/publication-2012/ (accessed 11 Jan 2013).
- 32.Wallston KA. The validity of the multidimensional health locus of control scales. J Health Psychol 2005;10:623–31 [DOI] [PubMed] [Google Scholar]
- 33.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) scales. Health Educ Monogr 1978;6:160–70 [DOI] [PubMed] [Google Scholar]
- 34.Satia JA, Campbell MK, Galanko JA, et al. Longitudinal changes in lifestyle behaviours and health status in colon cancer survivors. Cancer Epidemiol Biomarker Prev 2004;13:1022–31 [PubMed] [Google Scholar]
- 35.van der Aalst CM, van Klaveren RJ, de Koning HJ. Does participation to screening unintentionally influence lifestyle behavior and thus lifestyle-related morbidity? Best Pract Res Clin Gastroenterol 2010;24:465–78 [DOI] [PubMed] [Google Scholar]
- 36.Newson JT, Huguet N, Ramage-Morin PL, et al. Health behaviour changes after diagnosis of chronic illness among Canadians aged 50 or older. Statistics Canada, Catalogue no. 82–003-XPE • Health Reports, Vol. 23, no. 4, December 2012 [PMC free article] [PubMed]
- 37.Scottish Government The Scottish Health Survey: summary of key findings. Edinburgh: Scottish Government, 2012 [Google Scholar]
- 38.Hepper NG, Drage CW, Davies SF, et al. Chronic obstructive pulmonary disease: a community-oriented program including professional education and screening by a voluntary health agency. Am Rev Respir Dis 1980;121:97–104 [DOI] [PubMed] [Google Scholar]
- 39.Ostroff JS, Buckshee N, Mancuso CA, et al. Smoking cessation following CT screening for early detection of lung cancer. Prev Med 2001;33:613–21 [DOI] [PubMed] [Google Scholar]
- 40.Brener ND, Billy JO, Grady WR. Assessment of factors affecting the validity of self-reported health-risk behavior among adolescents: evidence from the scientific literature. J Adolesc Health 2003;33:436–57 [DOI] [PubMed] [Google Scholar]
- 41.http://www.healthscotland.com/topics/settings/health/index.aspx#hphs (accessed 19 Jan 2013).
- 42.Backer V, Nelbom BM, Duus BR, et al. Introduction of new guidelines for emergency patients: motivational counseling among smokers . Clin Respir J 2007;1:37–41 [DOI] [PubMed] [Google Scholar]
- 43.Bernstein SL, Boudreaux ED, Cydulka RK, et al. Tobacco control interventions in the emergency department: a joint statement of emergency medicine organizations. Ann Emerg Med 2006;48:e417–26 [DOI] [PubMed] [Google Scholar]
- 44.Anderson A, Mackison D, Boath C, et al. Promoting changes in diet and physical activity in breast and colorectal cancer screening settings: an unexplored opportunity for endorsing healthy behaviors. Cancer Prev Res 2013;6:165–72 [DOI] [PubMed] [Google Scholar]
- 45.Stead M, Eadie D, Caswell S, et al. The BeWEL Team Understanding the potential and challenges of adenoma treatment as a prevention opportunity: insights from the BeWEL formative study. Prev Med 2012;54:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.